Published online Nov 15, 2017. doi: 10.4291/wjgp.v8.i4.161
Peer-review started: February 13, 2017
First decision: July 10, 2017
Revised: July 25, 2017
Accepted: September 4, 2017
Article in press: September 5, 2017
Published online: November 15, 2017
Processing time: 287 Days and 13.4 Hours
To examine the role that enzyme Acyl-CoA:diacylglycerol acyltransferase-1 (DGAT1) plays in postprandial gut peptide secretion and signaling.
The standard experimental paradigm utilized to evaluate the incretin response was a lipid challenge. Following a lipid challenge, plasma was collected via cardiac puncture at each time point from a cohort of 5-8 mice per group from baseline at time zero to 10 h. Incretin hormones [glucagon like peptide-1 (GLP-1), peptide tyrosine-tyrosine (PYY) and glucose dependent insulinotropic polypeptide (GIP)] were then quantitated. The impact of pharmacological inhibition of DGAT1 on the incretin effect was evaluated in WT mice. Additionally, a comparison of loss of DGAT1 function either by genetic ablation or pharmacological inhibition. To further elucidate the pathways and mechanisms involved in the incretin response to DGAT1 inhibition, other interventions [inhibitors of dipeptidyl peptidase-IV (sitagliptin), pancreatic lipase (Orlistat), GPR119 knockout mice] were evaluated.
DGAT1 deficient mice and wildtype C57/BL6J mice were lipid challenged and levels of both active and total GLP-1 in the plasma were increased. This response was further augmented with DGAT1 inhibitor PF-04620110 treated wildtype mice. Furthermore, PF-04620110 was able to dose responsively increase GLP-1 and PYY, but blunt GIP at all doses of PF-04620110 during lipid challenge. Combination treatment of PF-04620110 and Sitagliptin in wildtype mice during a lipid challenge synergistically enhanced postprandial levels of active GLP-1. In contrast, in a combination study with Orlistat, the ability of PF-04620110 to elicit an enhanced incretin response was abrogated. To further explore this observation, GPR119 knockout mice were evaluated. In response to a lipid challenge, GPR119 knockout mice exhibited no increase in active or total GLP-1 and PYY. However, PF-04620110 was able to increase total GLP-1 and PYY in GPR119 knockout mice as compared to vehicle treated wildtype mice.
Collectively, these data provide some insight into the mechanism by which inhibition of DGAT1 enhances intestinal hormone release.
Core tip: Pharmacological Inhibition of diacylglycerol acyltransferase-1 (DGAT1) and insights into postprandial gut peptide secretion” describes studies that evaluate the effects of loss of DGAT1 function either pharmacologically or genetically on the incretin response. We demonstrate a synergistic effect on the incretin response with the combination of a DGAT1 inhibitor and sitagliptin, a dipeptidyl peptidase-IV inhibitor. Additional studies performed address the molecular mechanism by with pharmacological inhibition of DGAT1 results in increased gut peptide secretion. These data provide insight into the role of DGAT1 in the intestinal hormone release and its potential as a drug target for the treatment of type 2 diabetes.
- Citation: Maciejewski BS, Manion TB, Steppan CM. Pharmacological inhibition of diacylglycerol acyltransferase-1 and insights into postprandial gut peptide secretion. World J Gastrointest Pathophysiol 2017; 8(4): 161-175
- URL: https://www.wjgnet.com/2150-5330/full/v8/i4/161.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v8.i4.161
Metabolic abnormalities are major risk factors for cardiovascular disease, obesity and type 2 diabetes mellitus (T2DM)[1]. Impaired glucose uptake, glycogen synthesis and glucose oxidation can result from abnormal amounts of triglyceride (TG) in the blood and non-adipose tissues leading to potential insulin resistance[2-6]. Gut hormones are known to play an important role in glucose and lipid absorption. These gut peptides include glucagon like peptide-1 (GLP-1), peptide tyrosine-tyrosine (PYY) (both produced by L cells) and glucose dependent insulinotropic polypeptide (GIP) (produced by K cells). These peptides are secreted as a complex nutrient sensing system to control glucose homeostasis[7]. An impaired incretin effect in diabetic patients is most likely responsible for defective insulin release[7] as levels of GLP-1 and GIP can be reduced as much as 54% in T2DM patients[8]. GLP-1 is responsible for conveying glucose competence to beta-cells[9] and up-regulates genes involved in insulin secretion such as glucokinase and GLUT2[10,11]. GLP-1 mimetics like exenatide-4 and enhancers like dipeptidyl peptidase-IV (DPP-IV) inhibitors have also shown promise in elevating incretin levels to promote insulin release, decrease fasting glucose levels and further influence weight loss[12-16]. Furthermore, levels of GLP-1 and GIP can be augmented by lipid delivered to the intestinal lumen and can also be further altered by the degree of fatty acid (FA) saturation delivered[17-19]. The data supports the notion that endogenous lipids and their enzymatic modifiers potentially play an important role in the release of incretin hormones from the gut.
Acyl-CoA:diacylglycerol acyltransferase-1 (DGAT1) is one of the key enzymes responsible for the breakdown and reassembly of hydrolyzed lipid products 2-monoacylglycerol (2-MG) and FA. Diacylglycerol (DG) produced from acyl coenzyme A by A:monoacylglycerol acyltransferase (MGAT) is esterified by DGAT1 to resynthesize TG for repackaging and entry into systemic circulation[20,21]. The systemic deletion of DGAT1 as well as its acute pharmacological inhibition has been shown to elicit a blunted post absorptive lipid profile[22-27] with promise in modulating postprandial incretin levels[28-30]. DGAT1 deficient mice are phenotypically lean, resistant to diet-induced obesity and hepatic steatosis, exhibit increased insulin and leptin sensitivity, have a reduced tissue TG concentration and delayed chylomicron release with lipid treatment and high fat diet[28,31-35]. In the enterocytes DGAT1 inhibition modulates lipid absorption and alters FA/TG circulation resulting in increased levels of GLP-1[29,30]. Intestinal DGAT1 deletion during a lipid challenge in mice has also exhibited increased levels of GLP-1, PYY and decreased GIP[36]. Furthermore, pharmacological inhibition of DGAT1 has been shown to alter the temporal and spatial pattern of lipid absorption in the small intestine as well as alter the type of lipids released into circulation[37] which could change the pattern of enteroendocrine cell secretion of gut hormones in the small intestine. While the evidence regarding the importance of the DGAT1 enzymatic downstream effect on incretin release is growing, the mechanism of action still remains elusive.
One possible regulator of gut hormone release are G-protein coupled receptors such as GPR119. GPR119 is expressed predominantly in pancreatic beta-cells and intestinal L cells and are known to stimulate GLP-1 release[38]. This receptor may promote glycemic control via the incretin effect as well as insulin regulation[39]. Agonists to this G-protein have been shown to reduce food intake and decrease body weight in rodents[40] while GPR119 deficient mice have reduced fasting plasma GLP-1 and impaired glucose tolerance[39]. Furthermore, increased FA after meal ingestion such as oleoylethanolamides are known activators of GPR119[39,41,42]. The monoacylglycerol species 2-oleoyl glycerol, an activator of the GPR119 receptor and diacylgycerol precursor, was presented to elevate plasma GLP-1 and GIP levels in humans after oral administration[43]. This evidence provides a potential role of GPR119 using certain FA signals to promote gut hormone and insulin release, but further investigation is needed to understand this mechanism.
In the current study, we evaluated the impact of pharmacological inhibition of DGAT1 on the incretin effect during a lipid challenge. We used different genetic rodent models and pharmacological interventions including DGAT1 deficient mice, GPR119 deficient mice, DPP-IV inhibitor Sitaglpitin (Merck) and pancreatic lipase inhibitor Orlistat (Roche) in combination with PF-04620110, a pyrimidooxazepinone, a competitive DGAT1 inhibitor with a Ki of 94 nmol/L in mice[27,29] to study incretin release into systemic circulation. These data further confirm the important role DGAT1 plays in postprandial incretin hormone release and provides insight as to molecular mechanism by which this occurs.
The discovery of PF-04620110 has been reported previously[27] and is a potent and selective small molecule inhibitor of DGAT1 with 100-fold selectivity vs human DGAT2, ACAT1, AWAT1, AWAT2, MGAT2, MGAT3 and mouse MGAT1. Briefly, the ability of PF-04620110 to inhibit recombinant human (38 nmol/L), rat (94 nmol/L) and mouse (64 nmol/L) DGAT1 enzymatic activity was determined by measuring the incorporation of [3H]n-decanoyl Coenzyme A into DG to form TG. Additionally, in a cell-based assay in intestinal derived HT-29 cells, PF-04620110 (IC50 approximately 39 nmol/L) inhibits the incorporation of 3H-glycerol into TG[27].
C57BL/6J male mice (5-6 wk of age) (Jackson Laboratories), B6.129S4-Dgat1tm1Far male mice (DGAT1 knockout mice, 5-6 wk of age) (Jackson Laboratories) and GPR119 male mice (GPR119 knockout mice, 10-12 wk of age) (Charles River) were allowed ad libitum access to water and normal chow (5001, Purina) on a 6am-6pm light/dark cycle. All procedures were approved by the Institutional Animal Care and Use Committee and all animals received humane treatment according to the criteria stated by the National Academy of Sciences National Research Council (NRC) publication 86-23, 1985.
Mice were randomized according to body weight on the day of experimentation with 5-8 mice per group per timepoint. The mice were fasted for three hours prior to a single oral dose of vehicle (0.5% methylcellulose) or PF-04620110 at 10, 1, 0.1 or 0.01 mg/kg. Thirty minutes after PF-04620110 dosing, the animals were administered 5 mL/kg corn oil (Sigma) by oral gavage. Sitagliptin (Merck) and Orlistat (Roche) were administered one hour prior to the corn oil bolus at 10 mg/kg and 25 mg/kg respectively. Blood was obtained in EDTA/aprotinin/DPP-IV inhibitor treated tubes via cardiac puncture just prior (t = 0) to corn oil administration and at 1, 2, 4, 6, 8 and 10 h post corn oil administration. Plasma samples were collected for incretin analysis.
Solid phase extraction was used to clean up plasma samples for incretin analysis. In brief, EDTA/DPP-IV/aprotinin treated plasma and carbon stripped serum were ran through Oasis HLB LP (60 mg) extraction plates (Waters). Samples were washed 3 times through the extraction plate with 100:1 water/trifluoroacetic acid mixture. A (60/40/1) mixture of acetonitrile/water/trifluoroacetic acid was used for the final elution and collection. The samples were placed on an Argonaut SDE Dry-Dual apparatus under 50 L/min N2 pressure at 40 °C and allowed to dry overnight. The samples where then reconstituted in 300 μL of assay buffer and processed according to protocol for active GLP-1 (EGLP-35K, Linco), total amide GLP-1 (K110FAC-1, Meso Scale Discovery), PYY and GIP (RGT-88K-02, Milliplex map gut hormone panel immunoassay, Millipore).
Analysis of incretin levels involved using a Student’s t-test (Microsoft Excel) or ANOVA (GraphPad Prism) to compare treated vs control groups at each time point. Group comparisons were conducted at the 5% significance level.
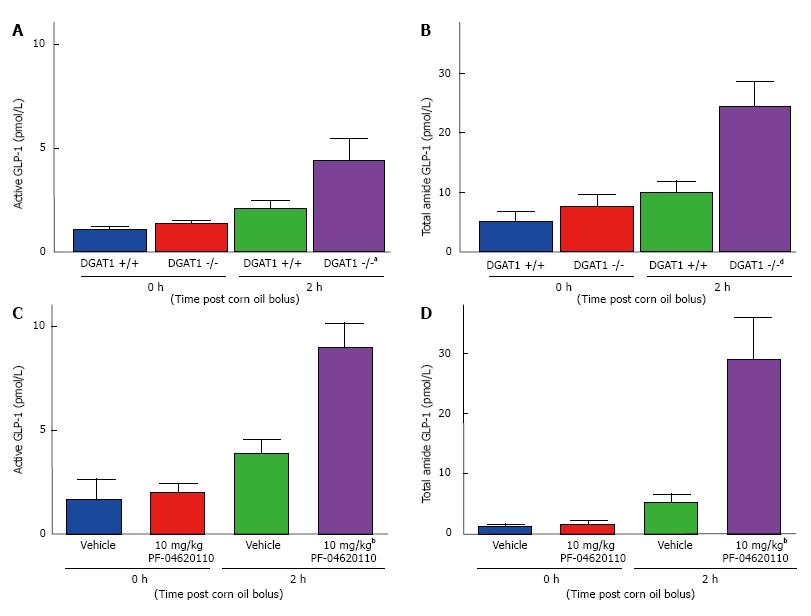
To understand the role of DGAT1 on the incretin effect, fasted DGAT1 deficient mice and wildtype C57/BL6J mice were challenged with a corn oil bolus and plasma levels of total and active GLP-1 were measured for 2 h post lipid administration. Levels of both active and total GLP-1 in the plasma were increased 1.9-fold in DGAT1 wildtype mice from 1.13 pmol/L to 2.14 pmol/L for active GLP-1 and 5.11 pmol/L to 9.94 pmol/L for total GLP-1 (Figure 1A and B). A statistically significant increase was detected in DGAT1 knockout mice of 3.1-fold from 1.41 pmol/L to 4.49 pmol/L for active GLP-1 and 7.23 pmol/L to 24.24 pmol/L for total GLP-1 (Figure 1A and B). We then utilized a selective and potent DGAT1 inhibitor, PF-04620110 to compare pharmacological inhibition to genetic loss of function. Levels of active and total GLP-1 in the plasma were increased 2.3-fold (active GLP-1) from 1.71 pmol/L to 3.96 pmol/L and 4.1-fold from 1.36 pmol/L to 5.48 pmol/L (total GLP-1) in vehicle treated wildtype mice 2 h post corn oil bolus. This response was further augmented as evidence by 4.5-fold increase in active GLP-1 from 2.03 pmol/L to 9.08 pmol/L and 17.2-fold increase in total GLP-1 from 1.71 pmol/L to 29.35 pmol/L with DGAT1 inhibitor PF-04620110 treated mice 2 h post corn oil bolus (Figure 1C and D). Acute pharmacological inhibition of DGAT1 via PF-04620110 administration resulted in a greater increase of active and total GLP-1 as compared to DGAT1 deficient mice, 1.4 and 14.1 fold, respectively in the context of postprandial lipid challenge.
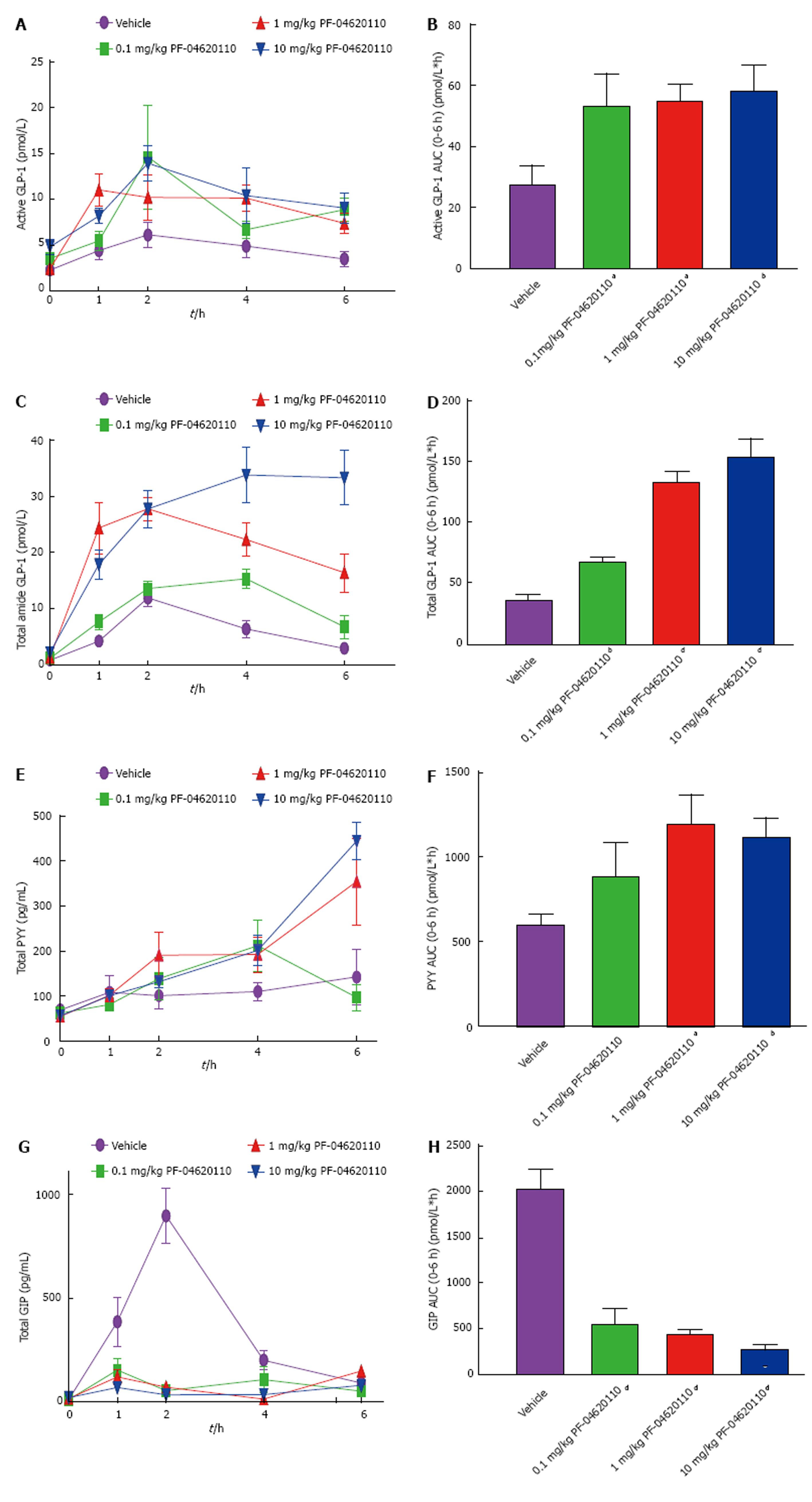
We then explored if pharmacological inhibition of DGAT1 is dose responsively altering gut hormones over a 6-h period. We monitored the levels of active and total GLP-1, total PYY and total GIP in the plasma over time following an oral gavage of corn oil. Levels of active GLP-1 AUC were significantly increased almost 2-fold higher in all drug groups of over the 6-h period with peak levels seen at 2 h, the highest level of lipid excursion during this challenge (Figure 2A and B)[37]. Levels of total GLP-1 were increased with increasing dose of PF-04620110 over time with a maximum 4.3-fold increase in AUC at 10 mg/kg of PF-04620110 compared to vehicle (Figure 2C and D). Levels of total PYY were also increased with increasing dose of PF-04620110 over time with a highest significant difference compared to vehicle at 1 and 10 mg/kg doses (Figure 2E and F). Interestingly, levels of GIP were significantly blunted with all drug doses over the 6-h postprandial excursion compared to vehicle (Figure 2G and H).
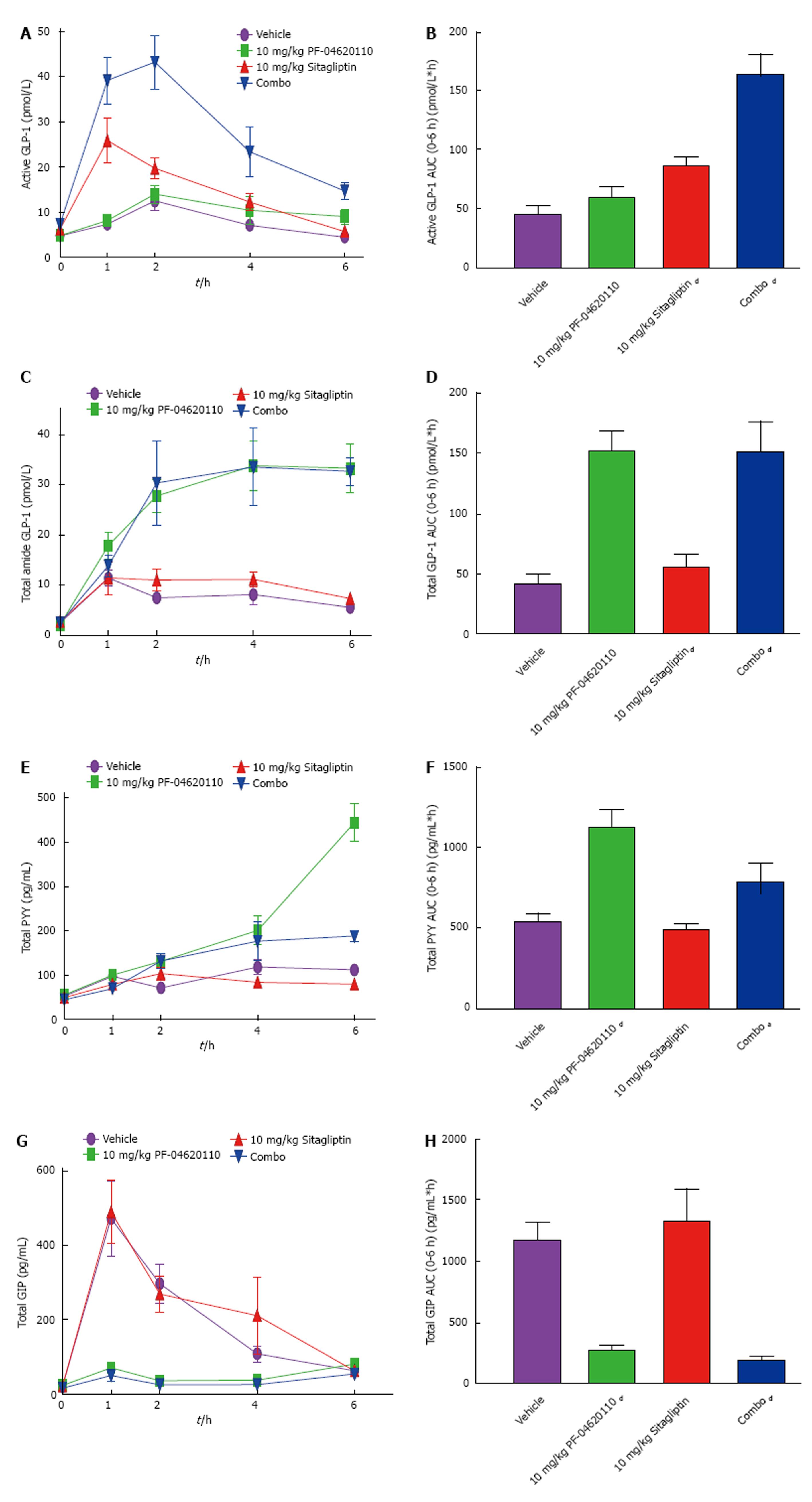
Next, we hypothesized that administration of a DGAT1 inhibitor which is stimulating increased GLP-1 and PYY secretion in combination a DPP-IV inhibitor which is blocking the clearance pathways of those gut hormones would result in synergy on plasma gut hormone levels. To test this hypothesis, we monitored the levels of active and total GLP-1, total PYY and total GIP in the plasma over a 6-h period following an oral gavage corn oil following the administration of either vehicle, PF-04620110, Sitagliptin or a fixed dose combination of PF-04620110 and Sitagliptin.
Levels of active GLP-1 AUC were modestly increased with singular administration of 10 mg/kg PF-04620110 1.3-fold 6 h AUC relative to vehicle treated mice from 18.48 pmol/L*h to 36.93 pmol/L*h and increased significantly with Sitagliptin 1.9-fold at 10 mg/kg (Figure 3A and B). The fixed dose combination resulted in 6 h AUC of 136.6 pmol/L*h or a 3.7-fold increase (Figure 3A and B). Levels of total GLP-1 AUC were significantly and identically increased over the 6-h excursion by 3.5-fold in both 10 mg/kg dose of PF-04620110 and combined administration of PF-04620110 and Sitagliptin compared to vehicle (Figure 3C and D). Levels of total GLP-1 were not changed with Sitagliptin therapy alone. Levels of total PYY AUC were significantly increased with PF-04620110 by 2-fold and combined administration of PF-04620110 and Sitagliptin by 1.5-fold over time compared to vehicle (Figure 3E and F) with no changes in Sitagliptin alone compared to vehicle treatment. Levels of GIP AUC were significantly blunted with PF-04620110 by 4.1-fold and combined administration of PF-04620110 and Sitagliptin 6-fold over time compared to vehicle (Figure 2E and F) and no changes in Sitagliptin alone compared to vehicle treatment. Synergistically, inhibition of DGAT1 and inhibition of DPP-IV enhance postprandial levels of active GLP-1.
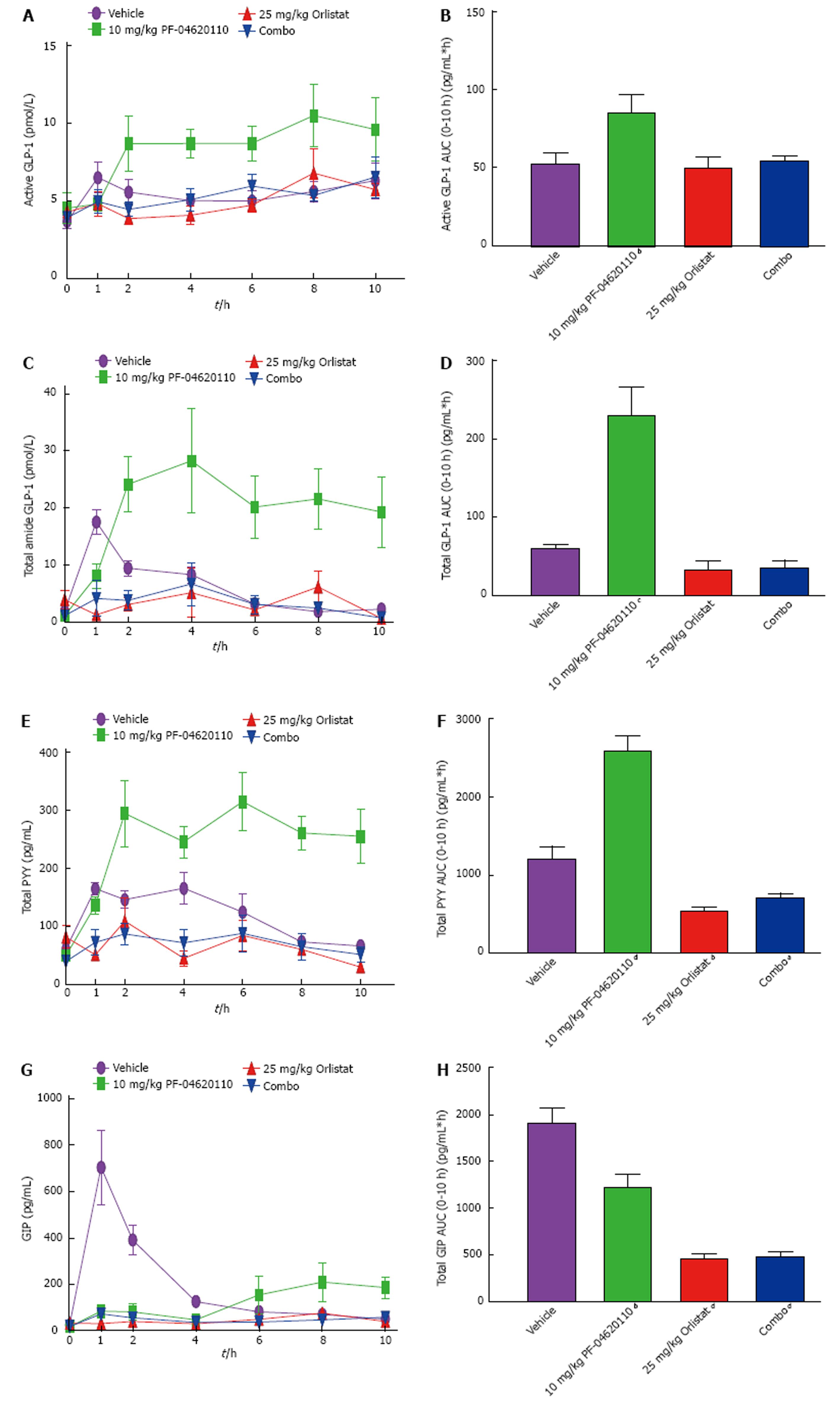
To gain mechanistic insight as to how DGAT1 inhibition is eliciting an enhanced incretin response, we tested if dietary lipid absorption is required. Therefore, we examined the postprandial incretin effect while inhibiting the absorption of fats from the diet using a pancreatic lipase inhibitor. Orlistat was administered to mice treated with and without PF-04620110 and monitored over a 10-h period with corn oil administration. Levels of active GLP-1 AUC were significantly increased over the 10 h excursion by 1.6-fold with PF-04620110 treatment alone, but no change in active GLP-1 AUC levels with Orlistat or the combination of Orlistat and PF-04620110 compared to vehicle (Figure 4A and B). Similar effects were seen with total GLP-1 AUC and PYY AUC levels (Figure 4D-F). Levels of total GLP-1 AUC were increased 3.8-fold and PYY AUC levels increased 2.1-fold by PF-04620110 compared to vehicle. Total GLP-1 AUC decreased 1.8-fold with Orlistat and 1.6-fold with Orlistat/PF-04620110 combination treatment (Figure 4C and D). Total PYY AUC significantly decreased 2.2-fold with Orlistat treatment and 1.7-fold combined Orlistat/PF-04620110 treatment compared to vehicle (Figure 4E and F). Total GIP AUC levels were significantly decreased 1.6-fold by PF-04620110, 4.2-fold by Orlistat and 3.9-fold by combination of PF-04620110 and Orlistat compared to vehicle (Figure 4G and H). The combination of this data suggests that inhibiting pancreatic lipase blocks the effects of DGAT1 inhibition on postprandial incretin levels.
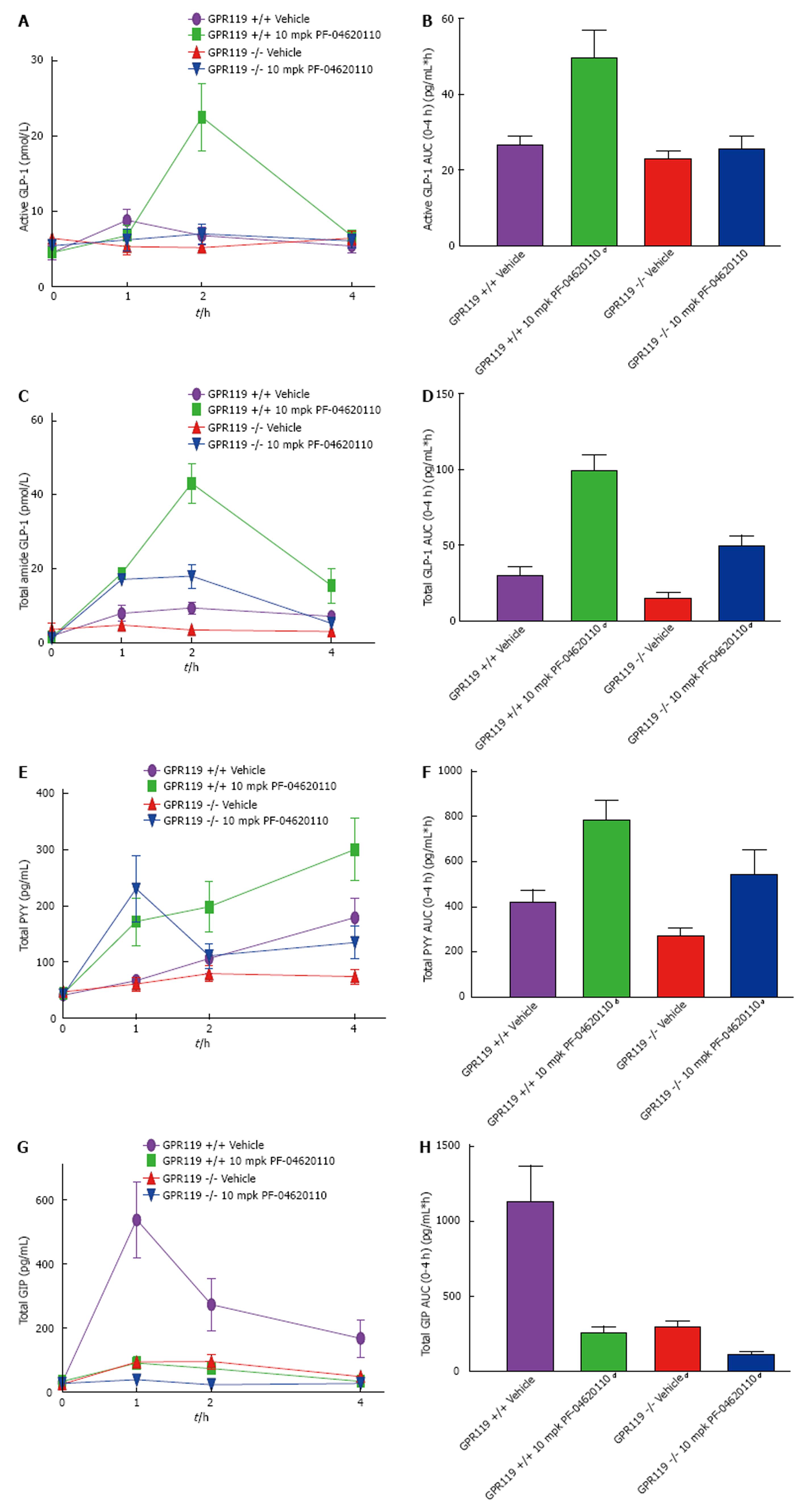
To examine the incretin effect with whole body knockout of GPR119, GPR119 knockout and wildtype mice were administered with vehicle or PF-04620110 and monitored over a 4-h period during a lipid challenge. Levels of active GLP-1 AUC were significantly increased as expected over the 4-h excursion by 1.9-fold with PF-04620110 treatment in wildtype mice, but no change in active GLP-1 AUC levels was observed in vehicle treated knockout mice and PF-04620110 treated knockout mice compared to wildtype vehicle treated mice (Figure 5A and B). Total GLP-1 AUC levels were increased 3.4-fold in wildtype mice treated with PF-04620110, decreased 2-fold in vehicle treated knockout mice and significantly increased 1.2-fold in PF-04620110 treated knockout mice compared to vehicle treated wildtype mice (Figure 5C and D). Similarly, levels of PYY AUC were increased 1.8-fold in PF-04620110 treated wildtype mice, slightly decreased by 1.5-fold in vehicle treated knockout mice and significantly increased by 1.3-fold in PF-04620110 treated knockout mice compared to vehicle treated wildtype mice (Figure 5E and F). Notably, increased levels of total GLP-1 and PYY in GPR119 knockout mice treated with PF-04620110 were lower than wildtype mice treated with PF-04620110. Total GIP AUC levels were significantly decreased 4.5-fold by PF-04620110 treated wildtype mice, 3.8-fold by vehicle treated knockout mice and 9.9-fold by PF-04620110 treated knockout mice compared to vehicle treated wildtype (Figure 5G and H). These data indicate that the altered incretin response with PF-04620110 inhibition is partially inhibited by knocking out GPR119.
In this study, we evaluated the role of DGAT1 in the secretion of gut derived hormones during a post-prandial lipid challenge. Previously, we have demonstrated that pharmacological inhibition of DGAT1 using PF-04620110 inhibits TG and vitamin A absorption in mice as well as delay gastric emptying, increase fecal fat and temporally shift triglyceride absorption downstream in the small intestine[37]. These observations led us to further investigate how DGAT1 inhibition impacts the release of gut derived hormones. DGAT1 deficient mice have been previously demonstrated to have increased postprandial levels of circulating GLP-1 and our DGAT1 knockout mouse data is consistent with these studies[44]. Interestingly, DGAT1 inhibition with PF-04620110 when compared to DGAT1 deficient mice results in a further elevation of GLP-1 and PYY plasma levels while attenuating GIP. The increase in GLP-1 and PYY occurs in a dose dependent manner. To elevate circulating levels even further, we tested the combined effect of DPP-IV (Sitagliptin) and DGAT1 (PF-04620110) inhibition for synergistic effects in mice following administration of inhibitors. Sitagliptin alone increases active GLP-1 but in combination prolongs the circulating active GLP-1 elevated by PF-04620110.
Due to our previous observations of the role of DGAT1 in the temporal and spatial absorption of dietary lipids, we speculated if the “signal” generated by DGAT1 inhibition to enhance gut hormone secretion was generated in the lumen of the gut or intracellularly within the enterocytes. DGAT1 is expressed on enterocytes whereas incretins are secreted from enteroendocrine L and K cells. To gain some insight, we used a pancreatic lipase inhibitor which blocks the luminal absorption of dietary lipids. Coadministration of Orlistat completely blunts the ability of PF-04620110 to elevate GLP-1 and PYY levels demonstrating a requirement of luminal TG degradation and lipid absorption. Thereby, these data suggest DGAT1 inhibition enhances the incretin response from the lumen and breakdown of lipids in the lumen are upstream of the effects of DGAT1 inhibition. We then hypothesize the mechanism by which DGAT1 inhibition effects incretin release is by increasing luminal long chain fatty acids and 2-MAG which could signal via GPR119 on enteroendocrine cells. To understand if GPR119, whose ligand, 2-monoacylglycerol is presumably being elevated via DGAT1 inhibition[45], is playing a role in the ability of PF-04620110 to elevate gut hormones, we utilized GPR119 deficient mice. GPR119 deficient mice were treated with PF-04620110 and subjected to a TG tolerance test. GLP-1, PYY and GIP were all reduced postprandially and drastically reduced the PF-04620110 profile of GLP-1, PYY and GIP. The decrease in GLP-1 AUC demonstrated in GPR119 knockout mice in either vehicle or PF-04620110 treated mice is 50% of GPR119 wildtype mice, suggestive that effects of DGAT1 inhibition on gut hormone secretion are partially dependent on GPR119. We speculate that additional long chain fatty acid/2-MAG GPCRs are also activated by DGAT1 inhibition. Collectively, these data suggest DGAT1 inhibition requires upstream TG hydrolysis and is partially mediated by altering GPR119.
The augmented plasma GLP-1 and attenuation of GIP observed with genetic deficiency and pharmacological inhibition of DGAT1 in rodents is in agreement with previously reported studies[28,29,44-46]. It has been hypothesized that the delay in gastric emptying and temporal shift in intestinal lipid absorption in these rodent models can contribute to increased GLP-1 and attenuation of GIP[47]. The reduction in GIP secretion via DGAT1 inhibition is potentially affecting K cell signaling by increasing lipid transit bypassing the proximal portion of the intestine. Apical L cell stimulation via GLP-1 and PYY release would also be enhanced with increased gut transit as more lipids ends up further down the GI tract. The PF-04620110 mediated increase in PYY is consistent with other studies as well as being increased in DGAT1 deficient mice, but the mechanism of action is not well understood[44,45]. PYY secreting L cells are located predominantly in the lower portion of the gut (ilium and colon)[48]. Since it is clear inhibiting the resynthesis of TG through DGAT1 inhibition is causing an increase in PYY concurrent to GLP-1 release, it is most likely occurring through an ancillary pathway.
The incretin response is altered via the lipid load as well as the degree of FA saturation presented to the K and L cell population in the intestine[17]. The dose responsiveness of the enhanced gut hormone response following DGAT1 inhibition suggests the “signal” is potentially the generation of ligands for fatty acid GPCRs. The exact molecular mechanism of how inhibition of DGAT1 in the enterocytes results in impacts on enteroendocrine cells within the intestine remains to be fully understood. While DGAT1 plays a major role in endogenous TG synthesis, it does not rule out other enzyme contributions as residual TG synthesis is present in DGAT1 deficient mice and other enzymes like DGAT2 could compensate for the absence of DGAT1[49]. One limitation to these experiments includes measuring these gut hormones only 6 h post corn oil administration in mice. It is possible that the GIP signal returns at later time points when the lipid load reaches the distal sections of the intestine. This has been observed in DGAT1 inhibitor treated canines where GIP signaling returned after 4 h[45].
The prolonged incretin signal produced by the combination of DPP-IV inhibitor Sitagliptin and PF-0462 0110 is in agreement with a published study using a different DGAT1 inhibitor[45]. In humans, the DPP-IV inhibitor Vildagliptin singularly increased active GLP-1 and GIP circulation while decreasing PYY, glucose and increasing insulin in the presence of intraduodenal fat infusion[50]. While we see similar effects with Sitagliptin alone in our mouse study, the addition of PF-04620110 is able to further enhance active GLP-1 indicating the dose combination could be beneficial in a clinical setting. Increased PYY circulation seen with combination of PF-04620110 and Sitagliptin compared to vehicle could also potentially have long term effects of suppressed appetite and weight loss, but further studies are needed to understand this combined compound effect. The notion that a fixed dose combination therapy of a DPP-IV and DGAT1 inhibitors would provide additional therapeutic benefit than either moiety alone is supported by these studies.
Combined oral dosing of the pancreatic lipase inhibitor Orlistat and PF-04620110 results in a blocking of GLP-1, PYY and GIP release into circulation. This provides further evidence that the DGAT1 inhibitor induced gut hormone response is being mediated by a lipid or lipid derived signal emanating from within the enterocyte. Fatty acids being used as gut peptide signaling have been studied previously with the use of Orlistat to block the initial breakdown of fat in the intestinal lumen[51,52]. This combined dosing also seems to be blocking all other methods of TG synthesis in the enterocytes since there is complete gut peptide inhibition, giving us more confidence that lipids or their derivatives are the likely secretagogue signal.
Increasing FFA in L cell rich areas of the intestine could signal G-protein coupled receptors (GPCR) with GLP-1 and PYY secretagogues capabilities. To this end, we investigated the administration of PF-04620110 to mice deficient in GPR119, a GPCR present in both beta-cells and intestinal L cells which are activated by FFA including 2-MAG[39]. Incretin levels and PYY were drastically decreased in these GPR119 deficient mice even in the presence of PF-04620110. This suggests that lacking GPR119 cannot be rescued by PF-04620110 and the effects of DGAT1 inhibition on gut hormone secretion are partially dependent on GPR119 and potentially could be acting as a GPR119 inhibitor by limiting lipid exposure to enteroendocrine cells. It is hypothesized that GPR119 may promote glycemic control via various incretin mechanisms and is known to be co-expressed in GLP-1 producing L cells[39]. GPR119 agonists in vivo have been shown to increase GLP-1 and GIP as well as reverse these effects in GPR119 deficient mice[39]. Furthermore, other GPCRs have the capability of using FA signals via incretin response to induce insulin secretion including GPR120 and GPR40[41,42]. It is possible that the lipid signals created by DGAT1 are affecting other GPCRs indicated by the slight increase in GLP-1 and PYY seen in the GPR119 knockout mice with PF-04620110 administration. Future studies exploring the combined effects of GPR119 agonists and DGAT1 inhibitors on gut hormone secretion might result in an additive response.
The importance of gut hormones and their role in glucose and lipid absorption only become more significant with the ongoing development of novel gut hormone altering pharmaceuticals. Incretin mimetics and incretin enhancers have become staples in the battle against T2DM and continue to offer hope towards controlling hyperglycemia and insulin secretion with varying degrees of side effects. Understanding the mechanism behind what alters the secretion of gut hormones could help to determine if combinatorial therapy could be beneficial or detrimental in controlling side effects or further increase the benefits seen with current dosing regimens. Pharmacological inhibition of DGAT1 can postprandially increase incretin hormones and PYY. Furthermore, combined dosing of a DPP-IV inhibitor with DGAT1 inhibition can synergistically extend the benefits seen with DGAT1 inhibition. Collectively these data provides new insights for the impact of pharmacological DGAT1 inhibition and future long term studies in T2DM patients.
Incretin hormones such as glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic peptide (GIP) are released from the enteroendocrine cells into the circulation in response to dietary nutrition. Peptide tyrosine-tyrosine is also a gut released hormone involved in energy homeostasis. Impaired incretin effect in type 2 diabetics could in part be responsible for defective insulin release, beta-cell failure and often have alterations in fat metabolism, in particular elevated plasma fatty acids and triglycerides. Acyl-CoA:diacylglycerol acyltransferase 1 (DGAT1), catalyzes the final step in triacylglycerol formation. In the intestine, DGAT1 is the predominate enzyme in the re-esterification of dietary TG and its inhibition or deletion in rodents has increased levels of GLP-1 in circulation. While the evidence regarding the importance of the DGAT1 enzymatic downstream effect on incretin release is growing, the mechanism of action still remains elusive.
Previous intestinal studies have hinted towards FA signaling molecules and their involvement in incretin hormone release, but the mechanisms behind this release remains not fully understood.
This study is the first to evaluate how DGAT1 inhibition manipulates lipid signaling in the gut and its involvement in manipulating G-protein coupled receptor signaling such as GPR119 in enteroendocrine cells to alter the incretin effect.
Examining oral lipid absorption in mice has been a proven way to relate to human intestinal physiology and the ability to delay lipid absorption with DGAT1 inhibitors is a way to study how lipid signals in the gut effect incretin hormone release. Utilizing genetic ablation mouse models and different drug combination therapies to study intestinal absorption can provide us more insight into the mechanism by which inhibition of DGAT1 enhances intestinal hormone release and its relevance to humans.
Type 2 diabetes mellitus is a complex disease caused by a variety of issues that ultimately leads to insulin resistance and uncontrolled levels of glucose in the blood. The incretin effect is a complex nutrient sensing system consequential of food ingestion put in place to control glucose homeostasis and ultimately leads to insulin secretion. This effect is made up of gut hormones GLP-1 and GIP produced predominantly by enteroendocrine cells of the gut lumen.
The experimental work presented in the manuscript is well designed and of good quality, data are presented in the logical manner.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Akiba Y, Efanov AM S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Mooradian AD. Dyslipidemia in type 2 diabetes mellitus. Nat Clin Pract Endocrinol Metab. 2009;5:150-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 541] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 2. | Thorburn AW, Gumbiner B, Bulacan F, Brechtel G, Henry RR. Multiple defects in muscle glycogen synthase activity contribute to reduced glycogen synthesis in non-insulin dependent diabetes mellitus. J Clin Invest. 1991;87:489-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 99] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 3. | Cline GW, Petersen KF, Krssak M, Shen J, Hundal RS, Trajanoski Z, Inzucchi S, Dresner A, Rothman DL, Shulman GI. Impaired glucose transport as a cause of decreased insulin-stimulated muscle glycogen synthesis in type 2 diabetes. N Engl J Med. 1999;341:240-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 457] [Cited by in RCA: 424] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 4. | Shafrir E, Raz I. Diabetes: mellitus or lipidus? Diabetologia. 2003;46:433-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Tomkin GH. Targets for intervention in dyslipidemia in diabetes. Diabetes Care. 2008;31 Suppl 2:S241-S248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest. 2000;106:171-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1893] [Cited by in RCA: 1870] [Article Influence: 74.8] [Reference Citation Analysis (0)] |
| 7. | Holst JJ, Gromada J. Role of incretin hormones in the regulation of insulin secretion in diabetic and nondiabetic humans. Am J Physiol Endocrinol Metab. 2004;287:E199-E206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 409] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 8. | McKennon SA, Campbell RK. The physiology of incretin hormones and the basis for DPP-4 inhibitors. Diabetes Educ. 2007;33:55-56, 60-62, 65-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Cornu M, Modi H, Kawamori D, Kulkarni RN, Joffraud M, Thorens B. Glucagon-like peptide-1 increases beta-cell glucose competence and proliferation by translational induction of insulin-like growth factor-1 receptor expression. J Biol Chem. 2010;285:10538-10545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 10. | Lam NT, Kieffer TJ. The multifaceted potential of glucagon-like peptide-1 as a therapeutic agent. Minerva Endocrinol. 2002;27:79-93. [PubMed] |
| 11. | Buteau J, Roduit R, Susini S, Prentki M. Glucagon-like peptide-1 promotes DNA synthesis, activates phosphatidylinositol 3-kinase and increases transcription factor pancreatic and duodenal homeobox gene 1 (PDX-1) DNA binding activity in beta (INS-1)-cells. Diabetologia. 1999;42:856-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 288] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 12. | Rolin B, Larsen MO, Gotfredsen CF, Deacon CF, Carr RD, Wilken M, Knudsen LB. The long-acting GLP-1 derivative NN2211 ameliorates glycemia and increases beta-cell mass in diabetic mice. Am J Physiol Endocrinol Metab. 2002;283:E745-E752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 213] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 13. | Iltz JL, Baker DE, Setter SM, Keith Campbell R. Exenatide: an incretin mimetic for the treatment of type 2 diabetes mellitus. Clin Ther. 2006;28:652-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 14. | Ahrén B, Hughes TE. Inhibition of dipeptidyl peptidase-4 augments insulin secretion in response to exogenously administered glucagon-like peptide-1, glucose-dependent insulinotropic polypeptide, pituitary adenylate cyclase-activating polypeptide, and gastrin-releasing peptide in mice. Endocrinology. 2005;146:2055-2059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 77] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 15. | Xu G, Stoffers DA, Habener JF, Bonner-Weir S. Exendin-4 stimulates both beta-cell replication and neogenesis, resulting in increased beta-cell mass and improved glucose tolerance in diabetic rats. Diabetes. 1999;48:2270-2276. [PubMed] |
| 16. | Brandt I, Joossens J, Chen X, Maes MB, Scharpé S, De Meester I, Lambeir AM. Inhibition of dipeptidyl-peptidase IV catalyzed peptide truncation by Vildagliptin ((2S)-{[(3-hydroxyadamantan-1-yl)amino]acetyl}-pyrrolidine-2-carbonitrile). Biochem Pharmacol. 2005;70:134-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 93] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 17. | Diakogiannaki E, Gribble FM, Reimann F. Nutrient detection by incretin hormone secreting cells. Physiol Behav. 2012;106:387-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 18. | Rocca AS, Brubaker PL. Stereospecific effects of fatty acids on proglucagon-derived peptide secretion in fetal rat intestinal cultures. Endocrinology. 1995;136:5593-5599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 69] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Thomsen C, Storm H, Holst JJ, Hermansen K. Differential effects of saturated and monounsaturated fats on postprandial lipemia and glucagon-like peptide 1 responses in patients with type 2 diabetes. Am J Clin Nutr. 2003;77:605-611. [PubMed] |
| 20. | Cheng D, Iqbal J, Devenny J, Chu CH, Chen L, Dong J, Seethala R, Keim WJ, Azzara AV, Lawrence RM. Acylation of acylglycerols by acyl coenzyme A:diacylglycerol acyltransferase 1 (DGAT1). Functional importance of DGAT1 in the intestinal fat absorption. J Biol Chem. 2008;283:29802-29811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 21. | Yen CL, Stone SJ, Koliwad S, Harris C, Farese RV Jr. Thematic review series: glycerolipids. DGAT enzymes and triacylglycerol biosynthesis. J Lipid Res. 2008;49:2283-2301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 890] [Cited by in RCA: 818] [Article Influence: 48.1] [Reference Citation Analysis (0)] |
| 22. | King AJ, Segreti JA, Larson KJ, Souers AJ, Kym PR, Reilly RM, Collins CA, Voorbach MJ, Zhao G, Mittelstadt SW. In vivo efficacy of acyl CoA: diacylglycerol acyltransferase (DGAT) 1 inhibition in rodent models of postprandial hyperlipidemia. Eur J Pharmacol. 2010;637:155-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Buhman KK, Smith SJ, Stone SJ, Repa JJ, Wong JS, Knapp FF Jr, Burri BJ, Hamilton RL, Abumrad NA, Farese RV Jr. DGAT1 is not essential for intestinal triacylglycerol absorption or chylomicron synthesis. J Biol Chem. 2002;277:25474-25479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 186] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 24. | Lee B, Fast AM, Zhu J, Cheng JX, Buhman KK. Intestine-specific expression of acyl CoA:diacylglycerol acyltransferase 1 reverses resistance to diet-induced hepatic steatosis and obesity in Dgat1-/- mice. J Lipid Res. 2010;51:1770-1780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 25. | Zhao G, Souers AJ, Voorbach M, Falls HD, Droz B, Brodjian S, Lau YY, Iyengar RR, Gao J, Judd AS. Validation of diacyl glycerolacyltransferase I as a novel target for the treatment of obesity and dyslipidemia using a potent and selective small molecule inhibitor. J Med Chem. 2008;51:380-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 116] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 26. | Birch AM, Birtles S, Buckett LK, Kemmitt PD, Smith GJ, Smith TJ, Turnbull AV, Wang SJ. Discovery of a potent, selective, and orally efficacious pyrimidinooxazinyl bicyclooctaneacetic acid diacylglycerol acyltransferase-1 inhibitor. J Med Chem. 2009;52:1558-1568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 27. | Dow RL, Li JC, Pence MP, Gibbs EM, LaPerle JL, Litchfield J, Piotrowski DW, Munchhof MJ, Manion TB, Zavadoski WJ. Discovery of PF-04620110, a Potent, Selective, and Orally Bioavailable Inhibitor of DGAT-1. ACS Med Chem Lett. 2011;2:407-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 28. | Ables GP, Yang KJ, Vogel S, Hernandez-Ono A, Yu S, Yuen JJ, Birtles S, Buckett LK, Turnbull AV, Goldberg IJ. Intestinal DGAT1 deficiency reduces postprandial triglyceride and retinyl ester excursions by inhibiting chylomicron secretion and delaying gastric emptying. J Lipid Res. 2012;53:2364-2379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 29. | Enayetallah AE, Ziemek D, Leininger MT, Randhawa R, Yang J, Manion TB, Mather DE, Zavadoski WJ, Kuhn M, Treadway JL. Modeling the mechanism of action of a DGAT1 inhibitor using a causal reasoning platform. PLoS One. 2011;6:e27009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 30. | Perregaux DG, Treadway JL, Steppan CM, Dow R, Gibbs EM, Zavadoski W, Mather D, Wisiniewski J, Boyer S, Joshi J. Pharmacological inhibition of DGAT-1 acutely reduces food intake and modulates incretin secretion in rats. Keystone Symposia, Triglycerides and Triglyceride Rich Particles in Health and Diseases. 2010;79. |
| 31. | Chen HC, Farese RV Jr. DGAT and triglyceride synthesis: a new target for obesity treatment? Trends Cardiovasc Med. 2000;10:188-192. [PubMed] |
| 32. | Wang SJ, Cornick C, O’Dowd J, Cawthorne MA, Arch JR. Improved glucose tolerance in acyl CoA:diacylglycerol acyltransferase 1-null mice is dependent on diet. Lipids Health Dis. 2007;6:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 33. | Chen HC, Farese RV Jr. Inhibition of triglyceride synthesis as a treatment strategy for obesity: lessons from DGAT1-deficient mice. Arterioscler Thromb Vasc Biol. 2005;25:482-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 121] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 34. | Chen HC, Ladha Z, Smith SJ, Farese RV Jr. Analysis of energy expenditure at different ambient temperatures in mice lacking DGAT1. Am J Physiol Endocrinol Metab. 2003;284:E213-E218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 35. | Smith SJ, Cases S, Jensen DR, Chen HC, Sande E, Tow B, Sanan DA, Raber J, Eckel RH, Farese RV Jr. Obesity resistance and multiple mechanisms of triglyceride synthesis in mice lacking Dgat. Nat Genet. 2000;25:87-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 688] [Cited by in RCA: 662] [Article Influence: 26.5] [Reference Citation Analysis (1)] |
| 36. | Liu J, Gorski JN, Gold SJ, Chen D, Chen S, Forrest G, Itoh Y, Marsh DJ, McLaren DG, Shen Z. Pharmacological inhibition of diacylglycerol acyltransferase 1 reduces body weight and modulates gut peptide release--potential insight into mechanism of action. Obesity (Silver Spring). 2013;21:1406-1415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 37. | Maciejewski BS, LaPerle JL, Chen D, Ghosh A, Zavadoski WJ, McDonald TS, Manion TB, Mather D, Patterson TA, Hanna M. Pharmacological inhibition to examine the role of DGAT1 in dietary lipid absorption in rodents and humans. Am J Physiol Gastrointest Liver Physiol. 2013;304:G958-G969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 38. | Lan H, Lin HV, Wang CF, Wright MJ, Xu S, Kang L, Juhl K, Hedrick JA, Kowalski TJ. Agonists at GPR119 mediate secretion of GLP-1 from mouse enteroendocrine cells through glucose-independent pathways. Br J Pharmacol. 2012;165:2799-2807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 39. | Chu ZL, Carroll C, Alfonso J, Gutierrez V, He H, Lucman A, Pedraza M, Mondala H, Gao H, Bagnol D. A role for intestinal endocrine cell-expressed g protein-coupled receptor 119 in glycemic control by enhancing glucagon-like Peptide-1 and glucose-dependent insulinotropic Peptide release. Endocrinology. 2008;149:2038-2047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 318] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 40. | Overton HA, Babbs AJ, Doel SM, Fyfe MC, Gardner LS, Griffin G, Jackson HC, Procter MJ, Rasamison CM, Tang-Christensen M. Deorphanization of a G protein-coupled receptor for oleoylethanolamide and its use in the discovery of small-molecule hypophagic agents. Cell Metab. 2006;3:167-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 505] [Cited by in RCA: 526] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 41. | Hirasawa A, Tsumaya K, Awaji T, Katsuma S, Adachi T, Yamada M, Sugimoto Y, Miyazaki S, Tsujimoto G. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat Med. 2005;11:90-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1108] [Cited by in RCA: 1171] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 42. | Edfalk S, Steneberg P, Edlund H. Gpr40 is expressed in enteroendocrine cells and mediates free fatty acid stimulation of incretin secretion. Diabetes. 2008;57:2280-2287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 450] [Cited by in RCA: 471] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 43. | Hansen KB, Rosenkilde MM, Knop FK, Wellner N, Diep TA, Rehfeld JF, Andersen UB, Holst JJ, Hansen HS. 2-Oleoyl glycerol is a GPR119 agonist and signals GLP-1 release in humans. J Clin Endocrinol Metab. 2011;96:E1409-E1417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 214] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 44. | Okawa M, Fujii K, Ohbuchi K, Okumoto M, Aragane K, Sato H, Tamai Y, Seo T, Itoh Y, Yoshimoto R. Role of MGAT2 and DGAT1 in the release of gut peptides after triglyceride ingestion. Biochem Biophys Res Commun. 2009;390:377-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 45. | Lin HV, Chen D, Shen Z, Zhu L, Ouyang X, Vongs A, Kan Y, Levorse JM, Kowalik EJ Jr, Szeto DM, Yao X, Xiao J, Chen S, Liu J, Garcia-Calvo M, Shin MK, Pinto S. Diacylglycerol acyltransferase-1 (DGAT1) inhibition perturbs postprandial gut hormone release. PLoS One. 2013;8:e54480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 46. | Liu J, McLaren DG, Chen D, Kan Y, Stout SJ, Shen X, Murphy BA, Forrest G, Karanam B, Sonatore L. Potential mechanism of enhanced postprandial glucagon-like peptide-1 release following treatment with a diacylglycerol acyltransferase 1 inhibitor. Pharmacol Res Perspect. 2015;3:e00193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 47. | Cho YM, Kieffer TJ. K-cells and glucose-dependent insulinotropic polypeptide in health and disease. Vitam Horm. 2010;84:111-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 48. | Taylor IL. Distribution and release of peptide YY in dog measured by specific radioimmunoassay. Gastroenterology. 1985;88:731-737. [PubMed] |
| 49. | Li C, Li L, Lian J, Watts R, Nelson R, Goodwin B, Lehner R. Roles of Acyl-CoA:Diacylglycerol Acyltransferases 1 and 2 in Triacylglycerol Synthesis and Secretion in Primary Hepatocytes. Arterioscler Thromb Vasc Biol. 2015;35:1080-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 50. | Heruc GA, Horowitz M, Deacon CF, Feinle-Bisset C, Rayner CK, Luscombe-Marsh N, Little TJ. Effects of dipeptidyl peptidase IV inhibition on glycemic, gut hormone, triglyceride, energy expenditure, and energy intake responses to fat in healthy males. Am J Physiol Endocrinol Metab. 2014;307:E830-E837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 51. | Ellrichmann M, Kapelle M, Ritter PR, Holst JJ, Herzig KH, Schmidt WE, Schmitz F, Meier JJ. Orlistat inhibition of intestinal lipase acutely increases appetite and attenuates postprandial glucagon-like peptide-1-(7-36)-amide-1, cholecystokinin, and peptide YY concentrations. J Clin Endocrinol Metab. 2008;93:3995-3998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 52. | McLaughlin J. Long-chain fatty acid sensing in the gastrointestinal tract. Biochem Soc Trans. 2007;35:1199-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |