Published online May 15, 2016. doi: 10.4291/wjgp.v7.i2.235
Peer-review started: December 11, 2015
First decision: January 13, 2016
Revised: January 21, 2016
Accepted: March 1, 2016
Article in press: March 2, 2016
Published online: May 15, 2016
Processing time: 154 Days and 1.7 Hours
AIM: To analyze the lipid distribution in gastric mucosae.
METHODS: Imaging mass spectrometry (MS) is a useful tool to survey the distribution of biomolecules in surgical specimens. Here we used the imaging MS apparatus named iMScope to identify the dominant molecules present in the human gastric mucosa near the fundic glands. Five gastric specimens were subjected to iMScope analysis. These specimens were also analyzed by immunohistochemistry using MUC5AC, H(+)-K(+)-ATPaseβ Claudin18 antibodies.
RESULTS: Three major molecules with m/z 725.5, 780.5, and 782.5 detected in the gastric mucosa were identified as sphingomyelin (SM) (d18:1/16:0), phosphatidylcholine (PC) (16:0/18:2), and PC (16:0/18:1), respectively, through MS/MS analyses. Using immunohistological staining, SM (d18:1/16:0) signals were mainly co-localized with the foveolar epithelium marker MUC5AC. In contrast, PC (16:0/18:2) signals were observed in the region testing positive for the fundic gland marker H(+)-K(+)-ATPaseβ. PC (16:0/18:1) signals were uniformly distributed throughout the mucosa.
CONCLUSION: Our basic data will contribute to the studies of lipid species in physical and pathological conditions of the human stomach.
Core tip: Imaging mass spectrometry (MS) is a useful tool to survey the distribution of biomolecules in surgical specimens. Here we used the imaging MS apparatus named iMScope to identify the dominant molecules present in the human gastric mucosa near the fundic glands. Three major molecules with m/z 725.5, 780.5, and 782.5 detected in the gastric mucosa were identified as sphingomyelin (d18:1/16:0), phosphatidylcholine (PC) (16:0/18:2), and PC (16:0/18:1), respectively.
- Citation: Kurabe N, Igarashi H, Ohnishi I, Tajima S, Inoue Y, Takahashi Y, Setou M, Sugimura H. Visualization of sphingolipids and phospholipids in the fundic gland mucosa of human stomach using imaging mass spectrometry. World J Gastrointest Pathophysiol 2016; 7(2): 235-241
- URL: https://www.wjgnet.com/2150-5330/full/v7/i2/235.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v7.i2.235
The wall of the stomach is composed of mucosa, submucosa, muscularis propria, and subserosa[1]. Except for the mucosa and proper glands, the structures of these layers are the same throughout the gastrointestinal tract. The mucosa of the stomach contains two structurally different layers: A superficial layer with foveolae and a deep layer with coiled glands. The lamina propria exists beneath the foveolar epithelium and harbors the proper gastric glands. The gastric mucosa possesses the ability to protect itself from numerous internal and external stimuli. Various intrinsic factors and systems, such as acid, mucus, bicarbonate, prostaglandins, biotin, blood flow, and the self-renewal of the epithelium as well as extrinsic infections, contribute to this defense mechanism. Loss of gastric mucosa causes gastric ulceration, erosion, or gastritis.
Imaging mass spectrometry (MS) is a recently developed modality that combines microscopy and MS[2-6]. Using this technique, the spatial distribution and molecular profiling of the analytes can be assessed simultaneously in a non-targeted manner. In fact, some lipids and proteins can be identified solely through imaging MS[7-9]. Because antibodies against lipids are difficult to generate, imaging MS is the most suitable option for the study of the lipid “metabolome”. Shimadzu Co. (Shimadzu, Kyoto, Japan) has developed a novel application for imaging MS named iMScope[10]. Because of its higher resolution compared with other imaging MS apparatuses, it enables us to visualize the localization of many lipids at one time. Using iMScope, we have already demonstrated the exact spatial distribution of lung surfactant and also discovered a specific phosphatidylcholine that is a potential biomarker in colorectal cancer tissue[11,12].
In this study, to investigate the molecular profile of human gastric mucosa in detail, iMScope was used to analyze the lipid distribution in the human gastric mucosa near the fundic glands. We identified, for the first time, the exact localization of lipids, including phospholipids and sphingolipid, in the human gastric mucosa near the fundic glands.
Five gastric samples were retrieved from the archives of Hamamatsu University Hospital. Non-disease portions (fundic gland area) of gastric tissues obtained from gastric surgical specimens were snap-frozen in liquid nitrogen and stored at -80 °C. The tissue blocks were put in the cryostat (CM1950; Leica, Microsystems, Wetzlar, Germany) at -20 °C for 30 min. The tissue blocks were then sectioned to a thickness of 8 μm at -20 °C. Then, the tissue sections were subjected to hematoxylin and eosin (HE) staining. The adjacent sections were mounted on indium-tin-oxide (ITO)-coated glass slides (Bruker Daltonics, Billerica, MA, United States) for imaging MS and on MAS coated glass slides for immunohistochemistry. The tissue sections on the ITO-coated glass slides were then kept at room temperature. Next, 2,5-dihydroxybenzoic acid (DHB; Bruker Daltonics) was deposited on the sections using a deposition apparatus[11].
An iMScope (Shimadzu) instrument, which consists of an atmospheric pressure matrix-assisted laser desorption/ionization system equipped with a quadrupole ion trap-time of flight analyzer, was used to obtain the imaging MS data[10]. The sample was scanned with a focused laser (a diode-pumped 355-nm Nd:YAG laser) to acquire the mass spectrum of each spot with a laser shot number of 200 per pixel and a 1000 Hz frequency. The reflection mode was applied to each measurement. The mass range was set to m/z 700-900 with a scan pitch of 7.5 μm (for 20 × magnification) or a 20 μm (for 2.5 × magnification) pixel size. The BioMap software (freeware: http://www.maldi-msi.org) graphical interface was used to visualize the ion images[13]. For each spectrum, baseline subtraction, smoothing, normalization to the total ion current, and recalibration were conducted using ClinProTools 2.2 software (Bruker Daltonics)[12]. The total ion currents were the sum of all spectrum intensities. The spectra processing parameters were as follows: Baseline correction [Top Hat algorithm (minimal baseline width set to 10%), resolution (500 ppm), and smoothing (Savitzky Golay, 5 cycles with a 2 m/z width)]. Recalibration was performed to reduce mass shifts. Peak picking was also performed based on the overall average spectrum for the whole mass range (signal to noise threshold of 5). The treated data was the average spectrum of input data sets. MS/MS analyses were performed to assign the molecular species using QSTAR Elite (Applied Biosystems, Foster City, CA, United States)[12]. The MS/MS spectral data were then verified using the LIPID MAPS database (http://lipidmaps.org).
Tissue preparation and immunohistochemical procedures were performed as previously described[14,15]. The 5 μm-thick sections were treated with 0.3% hydrogen peroxide to inactivate endogenous peroxidase activity. To identify the structure of the gastric mucosa, antibodies against MUC5AC (1:50, clone CLH2; Novocastra Laboratories, United Kingdom), claudin-18 (1:200, clone 5G7F2; proteintech, IL, United States), and H(+)-K(+)-ATPaseβ (1:1600, clone 2G11; Abcam, United Kingdom) were used to indicate the foveolar epithelium, fundic glands and foveolar epithelium, and fundic glands, respectively. For antigen retrieval, the slides were heated at 96 °C for 30 min in Tris-HCl-EDTA (TE) buffer (pH 9.0), followed by incubation at room temperature for 30 min. The sections were then incubated with a peroxidase-conjugated secondary antibody (Histofine Simple Stain MAX PO; Nichirei, Japan) at room temperature for 30 min. Next, the sections were treated with diaminobenzidine (DAB) substrate-chromogen solution (DAKO Cytomation; Carpinteria, CA, United States), followed by counterstaining with 0.1% hematoxylin. Images of these sections were obtained using Keyence BZ-9000 (Keyence, Tokyo, Japan). The stained sections were histologically evaluated by experienced pathologists[11,12].
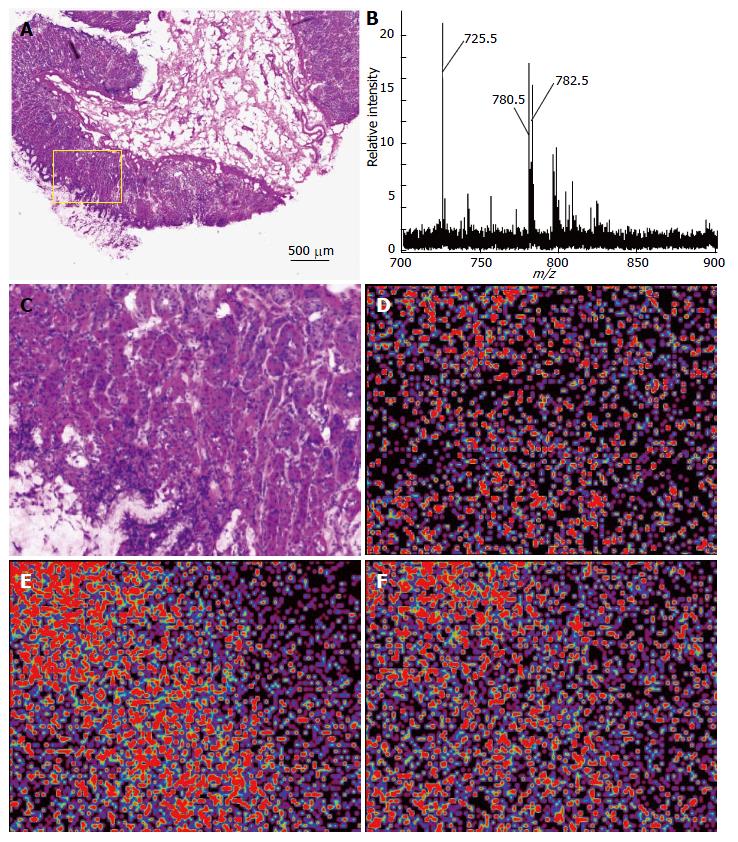
We used the imaging MS modality called iMScope to analyze the spatial distribution of lipids in the gastric mucosae from five individuals. The gastric mucosal region (Figure 1A; inset) was subjected to imaging MS analysis. Table 1 presents the list of ions obtained in the five gastric mucosae using imaging MS analysis. A representative mass spectrum obtained from the gastric mucosa near the fundic gland is shown in Figure 1B. Three major peaks were observed (m/z 725.5, 780.5, and 782.5) among these ions. The most intense peak was the ion at m/z 725.5. We subsequently used BioMap software to image the spatial distribution of these ions. Figure 1C presents the region of interest (ROI) of the gastric mucosa used to perform imaging MS. The strong signals from these ions were observed in the mucosal region of the gastric wall (Figure 1D for m/z 725.5, E for m/z 780.5, and F for m/z 782.5).
| m/z | Averaged intensity |
| 723.4 | 5.02 ± 1.75 |
| 725.5 | 29.09 ± 13.48 |
| 741.4 | 8.24 ± 5.30 |
| 756.5 | 7.11 ± 2.70 |
| 772.4 | 5.99 ± 3.03 |
| 780.5 | 22.16 ± 23.27 |
| 781.5 | 10.69 ± 9.95 |
| 782.5 | 22.55 ± 16.04 |
| 796.5 | 12.69 ± 8.95 |
| 798.5 | 14.90 ± 9.58 |
| 804.5 | 8.09 ± 4.87 |
| 806.5 | 6.55 ± 3.02 |
| 808.5 | 9.61 ± 6.41 |
| 820.5 | 5.92 ± 2.40 |
| 824.5 | 8.26 ± 4.54 |
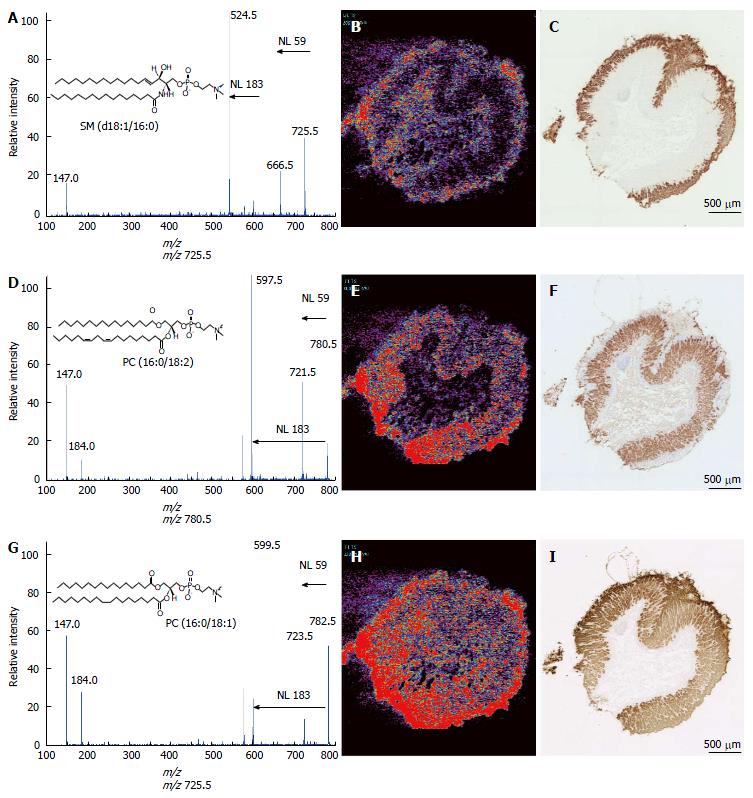
MS/MS analyses were performed to assign these ion species. Figure 2A presents the MS/MS spectrum obtained for the ion at m/z 725.5. This spectral pattern was identical to the one previously reported by Sudano et al[16]. Thus, this ion was shown to be sphingomyelin (SM) [SM (d18:1/16:0) + Na]+. The ions at m/z 780.5 and 782.5 were identified as phosphatidylcholoine (PC) [PC (16:0/18:2) + Na]+ and [PC (16:0/18:1) + Na]+, respectively, because of the neutral losses of 59 Da and 183 Da (Figure 2D and G).
To specify the spatial distribution patterns of these lipids more precisely, we compared the ion images with the staining of three gastric mucosal markers. Figure 2B, E, and H are the low-power field ion images of these lipids, and Figure 2C, F, and I are the immunohistological staining patterns of the gastric mucosal markers MUC5AC[17], H(+)-K(+)-ATPaseβ[18] and claudin18[19], respectively. MUC5AC staining was specific for the surface region of the mucosa. H(+)-K(+)-ATPaseβ is a fundic gland marker. Claudin18 is expressed throughout the mucosa. The ion at m/z 725.5 was present in the surface of the gastric mucosa, which corresponded to the area of MUC5AC staining. The ion at m/z 780.5 was highly expressed in the bottom of the mucosa, which contains the fundic glands. The ion at m/z 782.5 was uniformly spread in the mucosa, similar to the area of claudin18 staining.
Lipids are important functional molecules in the human body. Phospholipids, which are constituents of plasma membrane, have recently been recognized to have important roles in cellular systems. For example, PC (16:0/16:0) plays an important role as a surfactant in the reduction of surface tension in the lung[20,21]. PC (16:0/18:1) has been shown to be a physiological PPARα ligand, regulating lipid metabolism and glucose homeostasis[22]. Moreover, PC (16:0/20:4) and PC (16:0/18:2) are crucial for the inactivation of Akt kinase[23]. Sphingolipids are also involved in cellular functions such as the cell cycle, apoptosis, senescence, and inflammation[24-26]. In this study, we identified three highly expressed lipid molecules, SM (d18:1/16:0), PC (16:0/18:2) and PC (16:0/18:1), in gastric mucosae (Figures 1 and 2). SM (d18:1/16:0) was mainly localized to the foveolar epithelium of the gastric mucosa (Figure 2B and C). The foveolar epithelium secretes mucus and bicarbonate ions to prevent the damaging effects by pepsin and acid. Because SM molecules are mainly distributed in the plasma membrane, they may cooperate with mucus and bicarbonate ions to protect the mucosal surface. PC (16:0/18:2) co-localized with the fundic gland marker H(+)-K(+)-ATPaseβ. Intriguingly, this observation may be related to the knowledge that Akt phosphorylation is suppressed in fundic glands under ordinary conditions (Figure 2E and F). Considering that Akt phosphorylation may increase the risk of various cancers, including gastric cancer[27,28], the presence of PC (16:0/18:2) may be involved in the sustainability of the gastric mucosa, including the prevention of malignant transformation of gastric mucosae. The role of PC (16:0/18:1) in the gastric mucosa is unknown. This PC species is an endogenous PPARα ligand, leading to the activation of target genes such as Acox1 and Cpt1a; this pathway lowers triglycerides and raises HDL. However, PPARα itself is not expressed in the stomach[29]; it is abundant in the liver. Thus PC (16:0/18:1) in gastric mucosae may have a function other than as a PPARα ligand (Figure 2H and I).
In conclusion, this study, for the first time, clarified the lipids localized in the human gastric mucosa near the fundic glands. Because we have just reached this level of the modality, in terms of resolution and the ability to identify molecules, the information available on human tissue is currently limited. Our results will be the basis for further investigations of phosphatidylcholine and sphingomyelin species in physical and pathological conditions of the human stomach and will help the precise understanding of the nature of lipid function in the stomach.
Because antibodies against lipids are difficult to generate, more innovative methodologies are needed in lipid research field to analyze human disease. The authors developed the imaging mass spectrometry (MS) apparatus “iMScope” to visualize the lipid distribution in the pathological specimen and applied this technique to the measurement of gastric mucosae.
iMScope can irradiate using a thinner laser than other imaging MS modalities, which enables the finest ion image of lipids in the world.
To the best of the authors’ knowledge, this is the first time that lipid images of gastric mucosae were obtained.
Because the authors showed functional lipid images in gastric mucosae, these lipid distributions may reflect the significant role of lipids in the homeostasis of gastric mucosae.
Imaging MS is a novel technique that enables us to visualize many biomolecules at one time. The apparatus of imaging MS is composed of a microscope and a mass spectrometer. In the microscopic part, the authors can determine the region of interest (ROI) within the specimen sample and then scan this ROI with the laser. Ions from the evaporated vapors are transferred to the mass spectrometric part, where their mass spectra are obtained. The scanned data are then visualized along a two-dimensional axis.
This report combines the imaging MS with immunohistochemistry to show the lipid spatial distribution on gastric mucosae. Imaging MS is shown to be a useful tool to survey the distribution of biomolecules in the pathological samples. This report firstly applied the iMSope to locate the lipids including both phospholipids and sphingolipid in gastric mucosa, which is helpful to better understand the lipid’s function in stomach.
P- Reviewer: Liu DL S- Editor: Qi Y L- Editor: A E- Editor: Lu YJ
| 1. | David AO. Stomach. 4th ed. Mills SE, editor. Philadelphia: Lippincott Williams Wilkins 2012; 633-646. |
| 2. | McDonnell LA, Heeren RM. Imaging mass spectrometry. Mass Spectrom Rev. 2007;26:606-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 869] [Cited by in RCA: 761] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 3. | Chaurand P, Sanders ME, Jensen RA, Caprioli RM. Proteomics in diagnostic pathology: profiling and imaging proteins directly in tissue sections. Am J Pathol. 2004;165:1057-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 186] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 4. | Cornett DS, Reyzer ML, Chaurand P, Caprioli RM. MALDI imaging mass spectrometry: molecular snapshots of biochemical systems. Nat Methods. 2007;4:828-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 655] [Cited by in RCA: 664] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 5. | Seeley EH, Caprioli RM. Molecular imaging of proteins in tissues by mass spectrometry. Proc Natl Acad Sci USA. 2008;105:18126-18131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 232] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 6. | Kakimoto Y, Tsuruyama T, Yamamoto T, Furuta M, Kotani H, Ozeki M, Yoshizawa A, Haga H, Tamaki K. Novel in situ pretreatment method for significantly enhancing the signal in MALDI-TOF MS of formalin-fixed paraffin-embedded tissue sections. PLoS One. 2012;7:e41607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Cazares LH, Troyer D, Mendrinos S, Lance RA, Nyalwidhe JO, Beydoun HA, Clements MA, Drake RR, Semmes OJ. Imaging mass spectrometry of a specific fragment of mitogen-activated protein kinase/extracellular signal-regulated kinase kinase kinase 2 discriminates cancer from uninvolved prostate tissue. Clin Cancer Res. 2009;15:5541-5551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 150] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 8. | Morita Y, Ikegami K, Goto-Inoue N, Hayasaka T, Zaima N, Tanaka H, Uehara T, Setoguchi T, Sakaguchi T, Igarashi H. Imaging mass spectrometry of gastric carcinoma in formalin-fixed paraffin-embedded tissue microarray. Cancer Sci. 2010;101:267-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 83] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 9. | Liu Y, Chen Y, Momin A, Shaner R, Wang E, Bowen NJ, Matyunina LV, Walker LD, McDonald JF, Sullards MC. Elevation of sulfatides in ovarian cancer: an integrated transcriptomic and lipidomic analysis including tissue-imaging mass spectrometry. Mol Cancer. 2010;9:186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 99] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 10. | Harada T, Yuba-Kubo A, Sugiura Y, Zaima N, Hayasaka T, Goto-Inoue N, Wakui M, Suematsu M, Takeshita K, Ogawa K. Visualization of volatile substances in different organelles with an atmospheric-pressure mass microscope. Anal Chem. 2009;81:9153-9157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 101] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 11. | Kurabe N, Hayasaka T, Igarashi H, Mori H, Sekihara K, Tao H, Yamada H, Kahyo T, Onishi I, Tsukui H. Visualization of phosphatidylcholine (16: 0/16: 0) in type II alveolar epithelial cells in the human lung using imaging mass spectrometry. Pathol Int. 2013;63:195-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Kurabe N, Hayasaka T, Ogawa M, Masaki N, Ide Y, Waki M, Nakamura T, Kurachi K, Kahyo T, Shinmura K. Accumulated phosphatidylcholine (16: 0/16: 1) in human colorectal cancer; possible involvement of LPCAT4. Cancer Sci. 2013;104:1295-1302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 102] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 13. | Shimma S, Sugiura Y, Hayasaka T, Zaima N, Matsumoto M, Setou M. Mass imaging and identification of biomolecules with MALDI-QIT-TOF-based system. Anal Chem. 2008;80:878-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 126] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 14. | Sugimura H, Mori H, Nagura K, Kiyose S, Tao H, Isozaki M, Igarashi H, Shinmura K, Hasegawa A, Kitayama Y. Fluorescence in situ hybridization analysis with a tissue microarray: ‘FISH and chips’ analysis of pathology archives. Pathol Int. 2010;60:543-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Igarashi H, Sugimura H, Maruyama K, Kitayama Y, Ohta I, Suzuki M, Tanaka M, Dobashi Y, Kino I. Alteration of immunoreactivity by hydrated autoclaving, microwave treatment, and simple heating of paraffin-embedded tissue sections. APMIS. 1994;102:295-307. [PubMed] |
| 16. | Sudano MJ, Santos VG, Tata A, Ferreira CR, Paschoal DM, Machado R, Buratini J, Eberlin MN, Landim-Alvarenga FD. Phosphatidylcholine and sphingomyelin profiles vary in Bos taurus indicus and Bos taurus taurus in vitro- and in vivo-produced blastocysts. Biol Reprod. 2012;87:130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 94] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 17. | Reis CA, David L, Nielsen PA, Clausen H, Mirgorodskaya K, Roepstorff P, Sobrinho-Simões M. Immunohistochemical study of MUC5AC expression in human gastric carcinomas using a novel monoclonal antibody. Int J Cancer. 1997;74:112-121. [PubMed] |
| 18. | Chow DC, Forte JG. Characterization of the beta-subunit of the H(+)-K(+)-ATPase using an inhibitory monoclonal antibody. Am J Physiol. 1993;265:C1562-C1570. [PubMed] |
| 19. | Niimi T, Nagashima K, Ward JM, Minoo P, Zimonjic DB, Popescu NC, Kimura S. claudin-18, a novel downstream target gene for the T/EBP/NKX2.1 homeodomain transcription factor, encodes lung- and stomach-specific isoforms through alternative splicing. Mol Cell Biol. 2001;21:7380-7390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 215] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 20. | Bernhard W, Haagsman HP, Tschernig T, Poets CF, Postle AD, van Eijk ME, von der Hardt H. Conductive airway surfactant: surface-tension function, biochemical composition, and possible alveolar origin. Am J Respir Cell Mol Biol. 1997;17:41-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 90] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 21. | Lang CJ, Postle AD, Orgeig S, Possmayer F, Bernhard W, Panda AK, Jürgens KD, Milsom WK, Nag K, Daniels CB. Dipalmitoylphosphatidylcholine is not the major surfactant phospholipid species in all mammals. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1426-R1439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 75] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Chakravarthy MV, Lodhi IJ, Yin L, Malapaka RR, Xu HE, Turk J, Semenkovich CF. Identification of a physiologically relevant endogenous ligand for PPARalpha in liver. Cell. 2009;138:476-488. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 471] [Cited by in RCA: 424] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 23. | Koeberle A, Shindou H, Koeberle SC, Laufer SA, Shimizu T, Werz O. Arachidonoyl-phosphatidylcholine oscillates during the cell cycle and counteracts proliferation by suppressing Akt membrane binding. Proc Natl Acad Sci USA. 2013;110:2546-2551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 24. | Smith ER, Merrill AH, Obeid LM, Hannun YA. Effects of sphingosine and other sphingolipids on protein kinase C. Methods Enzymol. 2000;312:361-373. [PubMed] |
| 25. | Venable ME, Lee JY, Smyth MJ, Bielawska A, Obeid LM. Role of ceramide in cellular senescence. J Biol Chem. 1995;270:30701-30708. [PubMed] |
| 26. | Hla T. Physiological and pathological actions of sphingosine 1-phosphate. Semin Cell Dev Biol. 2004;15:513-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 321] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 27. | Cinti C, Vindigni C, Zamparelli A, La Sala D, Epistolato MC, Marrelli D, Cevenini G, Tosi P. Activated Akt as an indicator of prognosis in gastric cancer. Virchows Arch. 2008;453:449-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Murakami D, Tsujitani S, Osaki T, Saito H, Katano K, Tatebe S, Ikeguchi M. Expression of phosphorylated Akt (pAkt) in gastric carcinoma predicts prognosis and efficacy of chemotherapy. Gastric Cancer. 2007;10:45-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 29. | Braissant O, Foufelle F, Scotto C, Dauça M, Wahli W. Differential expression of peroxisome proliferator-activated receptors (PPARs): tissue distribution of PPAR-alpha, -beta, and -gamma in the adult rat. Endocrinology. 1996;137:354-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1162] [Cited by in RCA: 1345] [Article Influence: 46.4] [Reference Citation Analysis (0)] |