Published online Feb 15, 2016. doi: 10.4291/wjgp.v7.i1.171
Peer-review started: July 17, 2015
First decision: August 31, 2015
Revised: November 17, 2015
Accepted: December 16, 2015
Article in press: December 18, 2015
Published online: February 15, 2016
Processing time: 200 Days and 19.2 Hours
AIM: To investigate the efficacy and safety of mineral water with a high content of hydrogen carbonate in patients with heartburn.
METHODS: This open, single-center, single-arm clinical pilot study enrolled 50 patients, 18-64 years old, who had been suffering from heartburn at least twice a week for at least 3 mo before entering the study. Pharmacological treatment of heartburn was not permitted, and patients with severe organic diseases were excluded. After a run-in period of one week, the participants received 1.5 L of the test water for the following 6 wk; 300 mL with meals t.i.d., the remainder to be drunk throughout the day. During the trial, there were five visits at the study center (screening, baseline, two interim visits and the final visit). The efficacy endpoints included incidence and duration of heartburn episodes per week by patient’s self-assessment (heartburn diary) as well as changes in symptom severity as per symptom specific questionnaires [Reflux Disease Questionnaire (RDQ); Quality of Life in Reflux and Dyspepsia (QOLRAD); Gastrointestinal Quality of Life Index] and overall health-related quality of life per SF-12 (12-question short form) at each visit. At the end of the study, patients and investigators independently rated the overall efficacy of the test water on a 4-point Likert scale. Safety was assessed by evaluation of adverse events (AEs), vital signs (heart rate, blood pressure) and laboratory parameters. Changes from initial to final examinations were assessed by the non-parametric Wilcoxon test; categorical variables were compared using the χ2 test, and for more than 5 categories, by the U-test.
RESULTS: Twenty-eight participants were men, 22 women. The mean age of the patients in the full analysis set/intention-to treat population (FAS/ITT) was 40.6 years. Forty-two participants completed the study according to the study protocol and formed the per-protocol set (PP population); 48 participants drank the water at least once as requested and were analyzed as ITT population. The occurrence of heartburn was statistically significantly reduced at wk 6 in both the ITT and the PP populations. At wk 6, the mean number of heartburn episodes/week decreased by 5.1 episodes (P < 0.001) and the mean duration of heartburn symptoms by 19 min (ITT) (P = 0.002). The frequency of heartburn symptoms was reduced in 89.6% of the patients (P < 0.001), and the duration of symptoms in 79.2% of patients (ITT) (P < 0.001). All dimensions of the RDQ (heartburn, regurgitation, gastro-esophageal reflux disease symptoms, dyspepsia) showed a significant improvement at 6 wk. Likewise, disease-specific quality of life improved significantly (QOLRAD, GIQLI). Overall, 89.4% of patients rated the efficacy of the test water as “good” or “very good”, as did the investigators for 91.5% of the patients. There were no serious AEs. After 6 wk, systolic and diastolic blood pressure values decreased slightly but significantly [-3.5 and -3.0 mmHg, respectively (P = 0.008 and P = 0,002)]. Ninety-six percent of patients and investigators for the same percentage of patients rated the tolerability of the water as “good” or “very good”.
CONCLUSION: The data demonstrate effectiveness of a hydrogen carbonate-rich mineral water in alleviating heartburn frequency and severity, thereby improving quality of life. The water has excellent tolerability.
Core tip: This open, single-center, single-arm clinical study investigated the efficacy and safety of mineral water with a high content of hydrogen carbonate in patients with heartburn. After 6 wk, the occurrence of heartburn was statistically significantly reduced both in the intention-to-treat (48 patients) and the per-protocol populations (42 patients). All dimensions of the Reflux Disease Questionnaire (heartburn, regurgitation, gastroesophageal reflux disease symptoms, dyspepsia) showed significant improvement at week 6. Likewise, disease-specific quality of life improved significantly, and there was a slight but significant decrease in blood pressure. The tolerability was rated good or very good by 96% of patients and investigators.
- Citation: Beer AM, Uebelhack R, Pohl U. Efficacy and tolerability of hydrogen carbonate-rich water for heartburn. World J Gastrointest Pathophysiol 2016; 7(1): 171-180
- URL: https://www.wjgnet.com/2150-5330/full/v7/i1/171.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v7.i1.171
Gastroesophageal reflux disease (GERD) with insufficiency of the lower esophageal sphincter can significantly affect a person’s quality of life, even if endoscopy does not demonstrate any pathological findings [non-erosive reflux disease (NERD); early stage of GERD]. Symptoms include burning retrosternal and/or epigastric pains (“heartburn”) and range from belching/regurgitation of food remains to an unpleasant, salty/soapy taste in the mouth to the point of vomiting.
Heartburn is one of the most frequent gastrointestinal symptoms affecting patients. According to a current review, the range of GERD (heartburn and/or regurgitation on at least 1 d/wk) prevalence estimates was 8.8%-25.9% in Europe, 18.1%-27.8% in North America, but only 2.5%-7.8% in East Asia[1].
The pathogenesis of the symptoms of NERD has not yet been finally clarified. The direct acidic effect is probably an essential factor, but possibly not the only one. Under discussion are the increased sensitivity of intra-esophageal pain receptors, increased diffusion of gastric acid into the esophageal epithelium and up-regulation of the peripheral pain receptors with resultant central sensitization of spinal neurons[2].
The treatment of NERD or GERD focuses on the neutralization of gastric acid using a multistage approach. The first stage involves lifestyle changes such as weight reduction and abstinence from certain foods or beverages. If mild symptoms persist in spite of these measures, calcium, magnesium or aluminum antacids are used to neutralize gastric acid. In a subsequent step, histamine-2 receptor blockers (ranitidine, famotidine) or proton-pump inhibitors (PPIs) (omeprazole, panto-prazole) are utilized[3].
An alternative that also meets patients’ increasing desire for complementary- medicine approaches is the administration of hydrogen carbonate-rich mineral water (minimum content of hydrogen carbonate 1300 mg/L). As part of a buffer reaction, the hydrogen carbonate anion binds the protons of the gastric acid in a similar way to pharmacological antacids (H+ + HCO3-→ H2O + CO2). Additionally, a dilution effect is achieved by the volume of liquid in which the HCO3- anions are dissolved. Various waters containing hydrogen carbonate have displayed positive study results in functional dyspepsia[4-6].
Moreover, hydrogen carbonate-rich mineral water strengthens the natural protective mechanisms of the gastric mucosa with an improvement in mucosal secretion and blood flow. Finally, an influence of hydrogen carbonate on the homoeostasis of enterohormones has been discussed, whereby secretin and cholecystokinin secretion would be promoted and gastrin secretion inhibited; the overall equilibrium would be shifted to-wards acid inhibition[7].
Within this context, an open pilot clinical study on the efficacy and tolerability of a mineral water containing hydrogen carbonate was conducted in patients with mild heartburn symptoms. The aim was to test whether a mineral water with a high content of hydrogen carbonate leads to an improvement in the symptoms and the concomitant quality of life.
A total of 50 patients aged 18 to 64 years were included in the open, single-arm, single-center clinical study over a time period of 7 mo. Before inclusion, the patients were extensively informed by the investigator of the benefits and risks of the trial and also asked to read the comprehensive patient information. In case of patient’s decision to participate in the study, both the patient and the investigator signed the informed consent.
For at least 3 mo before the start of the study, the participants had to have had heartburn symptoms that had not been treated with medication, involving at least two episodes a week according to information provided by the patient. As a further requirement, the participants should be drinking at least 1.3 L of fluids (water, tea, soft drinks) a day, to ensure a corresponding fluid intake.
During the one-week run-in phase, the patients documented their episodes of heartburn and drinking habits in a daily diary. For further participation in the clinical trial, the patients had to have heartburn at least twice a week and consume at least 1.3 L of fluids (water, tea, soft drinks) per day.
Patients with the following conditions were excluded: Serious organic diseases of the heart or gastrointestinal tract, history of surgical interventions of the esophagus, stomach and small intestine, irritable bowel syndrome, endocrinological diseases, serious renal insufficiency, a tendency towards formation of calcium or “infection” kidney stones, E. coli urinary tract infections, iron-deficiency anemia, persistent vomiting or a family history of gastrointestinal tumors. Further exclusion criteria were: Medication for acid blockade (intake of antacids and histamine-2 receptor blockers in the 2 d before and during the study, intake of PPIs in the 14 d before and during the study), Helicobacter eradication drugs (in the 3 mo before and during the study), acetylsalicylic acid and non-steroidal anti-inflammatory drugs during the study, expected non-compliance and pregnancy and breastfeeding.
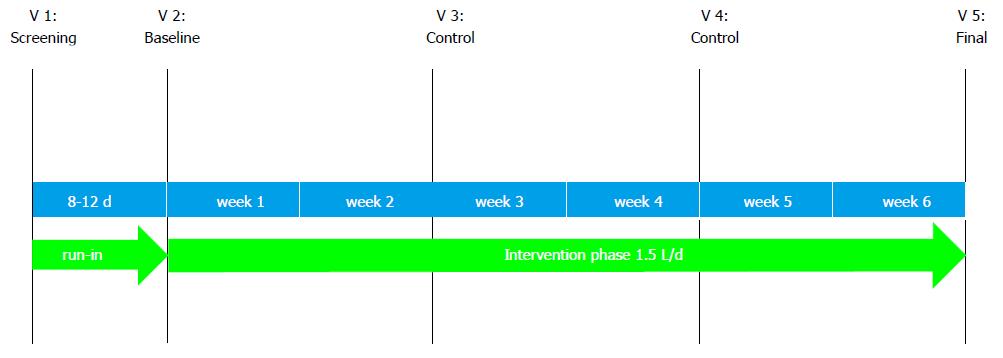
A schematic of the study conduct can be seen in Figure 1. The duration of the study was 7 wk per patient. During this time, there were five visits to the study center: (1) visit 1 (V1, screening): Providing the patients with information on the aim and course of the study, written patient consent, taking the patient’s history and recording drug intake, a physical examination involving measurement of heart rate and blood pressure, taking blood and urine samples, performing a pregnancy test where appropriate, issue of patient diary; (2) visit 2 (V2, 8 to 12 d after V1, baseline): Initial examination with documentation of new or changed concomitant diseases/concomitant medication, return and examination of the patient diary with regard to symptoms and fluid intake, measurement of blood pressure and heart rate, completion of the Reflux Disease Questionnaire (RDQ), Quality of Life in Reflux and Dyspepsia (QOLRAD), GIQLI and SF-12 questionnaires, issue of patient diary; (3) visit 3 and 4 (V3, V4, control visits 14 ± 3 d and 28 ± 5 d after V2): Documentation of new or changed concomitant medication, documentation of any adverse events (AEs), measurement of blood pressure and heart rate, completion of the questionnaires, return, examination and issue of patient diary; and (4) visit 5 (V5, final visit, 42 ± 5 d after V2): Documentation of new or changed concomitant medication, documentation of any AEs, measurement of blood pressure and heart rate, blood and urine samples, pregnancy test where appropriate, completion of the questionnaires, return and examination of patient diary, overall assessment of the efficacy and tolerability by investigator and patient, final examination, return of the test water after the visit.
The study protocol was approved by the relevant ethics committee [State Office of Health and Social Affairs Berlin (Landesamt für Gesundheit und Soziales Berlin)] and the competent authority [Federal Institute for Drugs and Medical Devices (Bundesinstitut für Arzneimittel und Medizinprodukte, Bonn)]. Implementation of the study was in line with the principles of the World Medical Association (Declaration of Helsinki), the guidelines for Good Clinical Practice [CPMP/ICH/135/95; Topic E6 (R1)], the German Medicinal Products Act (Arzneimittelgesetz) and the Ordinance on Good Clinical Practice.
A mineral water with a high hydrogen carbonate content was examined (Table 1).
| Cations | Content(mg/L) | Anions | Content(mg/L) |
| Lithium | 0.14 | Fluoride | 0.15 |
| Sodium | 121.0 | Chloride | 39.0 |
| Potassium | 10.2 | Bromide | 0.11 |
| Rubidium | 0.01 | Iodide | 0.019 |
| Magnesium | 109.4 | Nitrate | 8.28 |
| Calcium | 331.0 | Sulphate | 34.8 |
| Strontium | 2.54 | Hydrogen phosphate | 0.1 |
| Barium | 0.055 | Hydrogen carbonate | 1775.0 |
| Chromium | 0.005 | Undissociated substances | |
| Manganese | 0.03 | Metasilicic acid | 37.2 |
| Iron | 0.068 | Metaboric acid | 1.1 |
| Nickel | 0.002 | Metatitanic acid | 0.002 |
| Copper | 0.002 | Gaseous substances | |
| Silver | 0.002 | Free dissolved carbon dioxide | 3500 |
| Zinc | 0.007 | ||
| Aluminium | 0.004 | ||
| Lead | 0.005 |
The mineral water is currently approved in Germany for use in supporting gastrointestinal function, improving calcium and magnesium supply, and supportive treatment of chronic urinary tract infections.
The study was registered in the EU Clinical Trials Register under the EudraCT No. 2013-001584-22.
For a period of 6 wk (± 5 d), all the participants had to drink 1.5 L of the test water daily, divided into three times 300 mL with the main meals, the rest being drunk in several portions during the course of the day.
Efficacy: Frequency and duration of heartburn episodes based on entries in the patient diaries, therapeutic course and subjective perception of general health using validated German versions of the RDQ, QOLRAD, GIQLI and SF-12 questionnaires, each time comparing visit 2 with visits 3, 4 and 5 (after 2, 4 and 6 wk); overall assessment by patients and investigators.
Tolerability: AEs/effects during the study according to information provided by the patients, changes in heart rate and blood pressure and changes in laboratory values in a comparison between visit 2 and visit 5; overall assessment by patients and investigators.
Heartburn diary: Here, the patients recorded each occurrence of heartburn and stated whether they had taken an agent against it as an exceptional measure. The number and duration of the heartburn episodes and the need for rescue medication were established on the basis of this information. The patient diary was kept throughout the study.
Drink diary: This questionnaire records daily what and how much the patients drank, and additionally, after visit 2, the daily intake of the investigational product. The drink diary was kept throughout the study.
RDQ: This questionnaire records the symptoms of the upper gastrointestinal tract[8], and comprises 12 questions in four dimensions (heartburn, regurgitation, GERD, dyspepsia) regarding the frequency and severity of the symptoms during the past week in each instance. The symptoms include a burning feeling and pains behind the sternum and in the middle of the upper abdomen, an acidic taste in the mouth and an unpleasant belching of the stomach contents. The frequency of symptoms is recorded on a 5-point scale (“did not occur at all” to “daily”), and the severity of symptoms on a 6-point scale (“did not occur at all” to “strong”). The questionnaire was used at visit 2, visit 3, visit 4 and visit 5.
QOLRAD[9]: This questionnaire comprises 25 questions in five dimensions (emotional stress, sleep disturbances, problems with eating and drinking, physical and social function, vitality) during the past week in each instance. The frequency and severity of symptoms are recorded on a seven-point scale in each instance (“constant” to “never” or “very strong” to “not at all”). The questionnaire was used at visit 2, visit 3, visit 4 and visit 5.
GIQLI[10]: This questionnaire evaluates the quality of life of patients with diseases of the gastrointestinal tract. It comprises 36 questions on symptoms of the disease, psychological wellbeing and physical and social function, mainly during the past 2 wk in each instance. There is also a question to determine the impact of the treatment. The answers are on a five-point scale. The questionnaire was used at visit 2, visit 3, visit 4 and visit 5.
The Short Form-12 Health Survey Questionnaire[11]: This questionnaire is a short form of the SF-36 Health Survey Questionnaire on health-related quality of life. With its 12 questions in eight dimensions it covers the subjective perception of health (physical functioning, physical role functioning, bodily pain, general health perceptions, vitality, social role functioning, emotional role functioning and mental health) during the past week in each instance (in the version used). The questionnaire was used at visit 2, visit 3, visit 4 and visit 5.
Overall subjective assessment: The efficacy of the investigational product was assessed by investigator and patients on a four-point Likert scale using the rating points “very good”, “good”, “moderate” and “poor”.
AEs: According to information provided by the patient, the investigator recorded the nature, duration and degree of severity of adverse events. Furthermore, the causality between the test water and an adverse event was assessed as being “definite”, “probable”, “possible”, “improbable”, “none” or “not assessable”.
Laboratory parameters: Fasting blood samples with - at visit 1 and visit 5 - determination of hemoglobin, hematocrit, erythrocytes, thrombocytes, leukocytes, alanine transaminase, aspartate aminotransferase, gamma-glutamyl transferase, alkaline phosphatase, bilirubin, creatinine, urea, uric acid and - only at visit 1 - of glycated hemoglobin and thyroid-stimulating hormone. Blood pressure and heart rate at every visit.
Overall assessment of tolerability: At visit 5, the tolerability of the investigational product was subjectively rated by the investigators and the patients using a four-point Likert scale with the rating points “very good”, “good”, “moderate”, and “poor”.
Compliance: Was checked on the basis of the unused test water returned at the end of the study. Deviation in the used quantity of water by 25% or more or total duration of use by over 5 d, was considered non-compliance.
Statistical evaluation was performed regarding the absolute and relative changes in the assessed parameters in the comparison between visit V2 and visit V5. All parameters for the examination of efficacy and tolerance as well as further parameters relevant to the study were examined and descriptively evaluated using the methods for exploratory data analysis. Metric data (continuous measurement data) were collected, and evaluation comprised descriptive statistical parameters (number of cases, average, standard deviation, median, extremes and quartiles). For ordinal or nominal data, the frequency distributions were evaluated.
The examination of changes between the initial, control and final examinations was performed using the non-parametric Wilcoxon test. For comparison of proportional values the Chi-Square test was used, and for comparison of more than five categories, the U-test. Parametric procedures were also used for the interpretation of quantitative results.
A type 1 error (alpha error) of 5.0% (two-way) or 2.5% (one-way) and a power of 80% were assumed. Ninety-five percent confidence intervals were deter-mined. All P-values from statistical tests are to be understood as being exclusively exploratory. SPSS®, Version 22 for Windows™ was used for the evaluation.
The investigational product was assigned to a total of 50 patients. The following groups can be distinguished for the study evaluation: (1) The FAS/ITT population (Full Analysis Set; Intent-to-treat population) included all patients who had drunk the test water at least once and for whom efficacy data were available: n = 48; (2) The VCAS/PP population (Valid Case Analysis Set; Per-protocol population) included those patients who had drunk the test water in accordance with the study plan and for whom there were no major protocol violations: n = 42; and (3) The safety population for examination of tolerability included all patients who had used the investigational product at least once and for whom safety data were available: n = 49.
A total of 28 males and 22 females were included. The mean age of the patients was 40.6 ± 11.5 in the FAS population. The ethnicity of all patients was “Caucasian”.
The most common concomitant diseases were those of the gastrointestinal tract (54 items), followed by “surgical or other procedures” (39 items). The most common concomitant medications stated were substances from the group “sexual hormones and their inhibitors” (7 items), “substances with influence on the renin-angiotensin system” and “substances with influence on lipid metabolism” (2 items each).
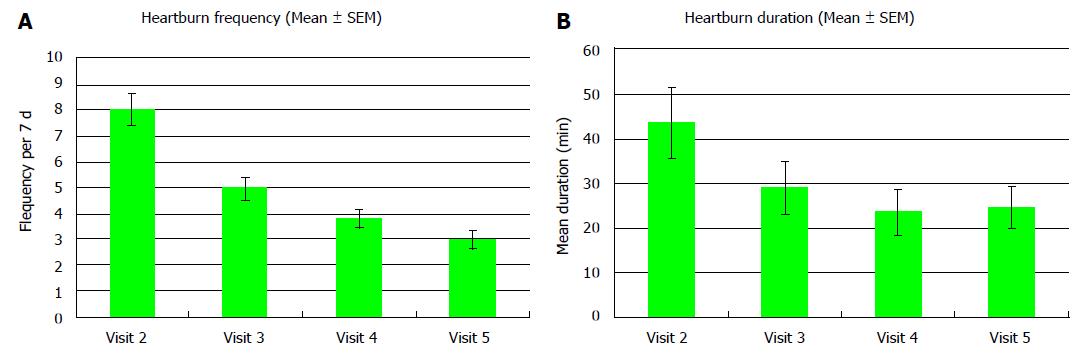
After 6 wk, the mean number of heartburn episodes per week in the FAS population had decreased by 5.1 ± 4.8 at visit 5 compared to 8.1 at visit 2 (P < 0.001) (Figure 2A). The qualitative change in the frequency of heartburn per week from visit 2 to visit 5 was statistically significant in both populations (FAS, VCAS) evaluated (P < 0.001). In the FAS population, after 6 wk of treatment, 43 patients reported less frequent heartburn episodes (89.6%), in one patient the frequency remained unchanged (2.1%) and in 4 patients more episodes were recorded (8.3%) (Table 2) (P < 0.001).
| Heartburn (n = 48) | V2mean (SD)median | V5mean (SD)median | V2-V5mean (SD)median | P value | Proportion of patients displaying a decrease/an increase | P value |
| Frequency | 8.06 (4.43) | 3.00 (2.29) | 5.06 (4.81) | < 0.001 | 89.6%/8.3% | < 0.001 |
| 8.0 | 2.5 | 5.0 | ||||
| Duration (min) | 43.7 (55.9) | 24.7 (32.2) | 19.0 (40.7) | 0.002 | 79.2%/20.8% | < 0.001 |
| 24.5 | 12.7 | 4.2 |
After 6 wk, the mean duration of heartburn per week had decreased significantly by 19 min at visit 5 compared to 43.7 min at visit 2 (P < 0.001) (Figure 2B). The duration of heartburn decreased in 38 patients of the FAS population (79.2%) (P < 0.001) (Table 2).
Changes were observed for the following dimensions:
Heartburn: The mean heartburn score decreased by 3.6 ± 3.7 points (from 5.40 at visit 2 to 1.83 at visit 5). In the FAS population, the frequency of heartburn decreased in 63.8% of the patients. In 70.2% of the patients, the intensity of the heartburn decreased from visit 2 to visit 5. These differences were statistically significant.
Regurgitation: The mean regurgitation score decreased by 5.0 ± 4.8 points (from 7.83 at visit 2 to 2.80 at visit 5). In the FAS population, 76.1% of the patients experienced less frequent regurgitation. In 71.7% of the patients, the intensity of the regurgitation decreased from visit 2 to visit 5. These differences were statistically significant.
GERD: The term designates both the disease itself and one of the dimensions in the RDQ questionnaire. The mean GERD score decreased by 8.7 ± 7.0 points (from 13.35 at visit 2 to 4.67 at visit 5). In the FAS population, 91.3% of the patients experienced GERD symptoms less frequently. In 87.0% of the patients, the intensity of the symptoms decreased from visit 2 to visit 5. These differences were statistically significant.
Dyspepsia: The mean dyspepsia score decreased by 3.5 ± 4.2 points (from 5.82 at visit 2 to 2.29 at visit 5). In the FAS population, 66.7% of the patients experienced dyspeptic symptoms less frequently. In 62.2% of the patients, the intensity of symptoms decreased from visit 2 to visit 5. These differences were statistically significant.
Changes were observed for the following dimensions:
Emotional stress: The mean score in the FAS population significantly (i.e., improved) by 0.8 ± 1.1 points (from 5.76 at visit 2 to 6.59 at visit 5).
Sleep quality: The mean score in the FAS population increased significantly by 0.7 ± 1.0 points (from 5.76 at visit 2 to 6.48 at visit 5).
Problems with eating and drinking: The mean score in the FAS population increased significantly by 1.1 ± 1.2 points (from 5.26 at visit 2 to 6.36 at visit 5).
Physical/social functioning: The mean score in the FAS population increased significantly by 0.6 ± 0.8 points (from 6.14 at visit 2 to 6.77 at visit 5).
Vitality: The mean score in the FAS population increased significantly by 0.8 ± 1.1 points (from 5.38 at visit 2 to 6.21 at visit 5).
The total score in the FAS population increased significantly by 16.6 ± 16.2 points (from 110.5 at visit 2 to 127.0 at visit 5). Changes were observed for the following dimensions:
Symptoms: The mean score in the FAS population increased significantly by 10.9 ± 9.4 points (from 55.9 at visit 2 to 66.7 at visit 5).
Emotions: The mean score in the FAS population increased significantly by 2.2 ± 3.1 points (from 15.3 at visit 2 to 17.5 at visit 5).
Physical functioning: The mean score in the FAS population increased significantly by 2.6 ± 4.9 points (from 21.4 at visit 2 to 23.9 at visit 5).
Social function: The mean score in the FAS population increased significantly by 1.0 ± 2.2 points (from 14.1 at visit 2 to 15.1 at visit 5).
In the assessment per SF-12 questionnaire, statistically significant improvements regarding the following parameters took place between visit 2 and visit 5: (1) General state of health, quantitative (P < 0.001); (2) Being able to do only certain things (P = 0.044); (3) Pain during everyday activities (P < 0.001); and (4) Calm and relaxed (P < 0.002); Full of energy (P < 0.003).
At the end of the study, 89.4% of the patients assessed the efficacy as being “good” or “very good”. For 91.5% of the patients, the investigators assessed the efficacy as being “good” or “very good”. Statistically significant differences between the patients’ assessments and those of the investigators were not observed (P = 1.00).
In the course of the clinical trial, a total of two adverse events were documented by the investigators, namely moderate headache in 2 patients (4.0%). Neither of them was assessed by the investigators as being linked to the investigational product. The patients treated the headaches themselves with paracetamol and/or ibuprofen. No adverse effects were observed.
During the course of the study, the systolic blood pressure decreased by 3.5 mmHg (P = 0.008) and the diastolic blood pressure by 3.0 mmHg (P = 0.002). The heart rate also decreased from 71/min (visit 2) to 68.8/min (visit 5) (P = 0.012).
There were no relevant changes in the examined laboratory parameters.
Ninety-five point eight percent of the patients assessed the tolerability of the test water as being “very good” or “good”, and for 95.7% of the patients, investigators assessed the patients’ tolerability on the Likert scale as being “very good” or “good”.
Our 6-wk intervention, with a daily consumption of 1.5 L of a hydrogen carbonate-rich mineral water, clearly reduced the symptoms in patients with heartburn and improved their quality of life. The frequency of heartburn episodes continuously decreased in the course of the study, while the duration of the episodes increased slightly at the final visit in comparison with the second control visit. One possible explanation could be that the duration of the episode is a very subjective parameter, and documentation by the patients may be somewhat less accurate with respect to the assessment of minor differences, as observed between visit 4 and visit 5.
The water was well tolerated, and no adverse effects were observed.
The questionnaires used to assess the subjective symptoms (RDQ) and quality of life (QOLRAD and GILQI) show an improvement of the symptoms and the symptom-associated limitations in quality of life.
Similar results have been reported by Gunasekaran et al[12], who studied the effect of GERD on health-related quality of life in 134 adolescent subjects. Using QOLRAD, the authors found a negative effect on the symptoms of GERD at baseline. After 8 wk of omeprazole administration, the values in all five dimensions improved significantly and to a clinically relevant extent, similar to - or even more pronounced than in - adults. In the present study with the hydrogen carbonate-rich mineral water, the symptom reduction was, in all dimensions, of clinical relevance (effect size ≥ 0.5) and in a similar range as reported in the open-label study with the proton pump inhibitor.
Interestingly, PPI treatment showed success in certain patients with typical reflux symptoms who could not be diagnosed with GERD via pH-impedance monitoring[13]. Similarly, such patients may also be responders to hydrogen carbonate-rich mineral water treatment - or possibly even to a placebo treatment.
Chen et al[13] used the GIQLI to compare the quality of life in 25 patients undergoing either laparoscopic or open cholecystectomy. They concluded that this questionnaire could be used as a good measure of the quality of life in patients before and after the intervention. The reported improvement in quality of life related to the gastrointestinal tract is comparable to that observed in the present study, both for the total score and for individual dimensions. This example from the literature demonstrates that it is possible to compare the success of different treatments by comparing GIQLI results.
“Lifestyle changes” are often recommended as part of the overall treatment concept, e.g., small low-fat meals, which should not be eaten immediately before going to bed, weight reduction, an elevated upper-body position at night and abstinence from “triggers” such as coffee, chocolate, nicotine and alcohol[14]. However, natural mineral waters are rarely mentioned as part of this concept, even though more and more people are trying to alleviate their symptoms using natural remedies before seeking medical attention.
Nevertheless, the positive influence of hydrogen carbonate-rich water on heartburn and gastrointestinal symptoms in general has already been demonstrated several times. An Italian group administered 250 mL of a calcium- and hydrogen carbonate-rich mineral water (Ca2+ 486.6 mg/L; HCO3- 1750.7 mg/L) to patients with gastroesophageal reflux as demonstrated by pH metry. The group then established clear and lasting pH increases in the esophagus and stomach, with levels differing significantly from those in the control subjects who had been given tap water. The patients also reported a subjective improvement in their heartburn following administration of the water containing calcium hydrogen carbonate[4].
For a period of 30 d, Bertoni et al[5] administered water with high mineral content (hydrogen carbonate content 683 mg/L, 1.5 L/d) to a total of 18 patients with dyspeptic symptoms within the past 3 mo. The frequency and severity of the symptoms were assessed at the beginning of the study and after 30 d on a five-point Likert scale (0: Symptoms never occur; 4: Symptoms occur daily/are very marked). After the 30-d test phase, there was a significant improvement in the severity and frequency of dyspeptic symptoms, including epigastric pain and heartburn[5].
Another multicentric study examined the efficacy of a mineral-water cure in 1667 patients with functional dyspepsia. This involved an initial cycle during which the patients received 2 L of a hydrogen carbonate-rich mineral water daily for 21 d. One year later, 996 participants were re-examined and underwent a second treatment cycle. At the beginning of the study and after one year, each patient completed a questionnaire on symptoms, lifestyle and utilization of medicinal services. At the beginning of the study as well as after the two drinking cure periods, the gastrointestinal symptoms were assessed, and in 60 patients the secretion of gastric acid was also determined at these times. In all patients, a significant decrease in the frequency of symptoms was established at the end of the first and second drinking cure cycles. After the drinking cures, the secretion of gastric acid was also reduced in 87.5% of the patients who were examined in this regard[6].
In an animal model, it was demonstrated that water containing hydrogen carbonate (683 mg/L) can prevent alcohol-induced damage to the gastric mucosa, even with extremely high amounts of alcohol (23 mL of 100% ethanol/kg), although the water was only effective if administered 30 min before the alcohol load. With longer intervals, it was not possible to prevent the toxic effect[15].
Furthermore, back in 2001, Böhmer et al[7] reported in an overview that functional dyspeptic symptoms, including acid reflux, are improved by the appropriate waters. One postulated principle of action is that the HCO3- anions have a direct buffer effect on the protons of the gastric acid. Purely arithmetically speaking, a hydrogen carbonate-rich mineral water can deliver a similar buffer capacity to that achieved by over-the-counter antacids based on calcium carbonate/magnesium carbonate: 1.7 g hydrogen carbonate has a buffer capacity of 27.5 mEq, compared to 15.5 mEq for one antacid tablet.
Further advantages of mineral waters include the improved supply of fluids as well as the higher level of safety, which virtually excludes intoxication. Also, the cations dissolved in the mineral water in addition to the HCO3- (in the test water examined mainly Ca2+ and Mg2+), have good bioavailability and contribute to supplying the organism with minerals. Additionally, since it is frequently stated that heartburn occurs postprandially, it appears that intake at mealtimes is favorable.
Even when heartburn symptoms are not attributable to a serious disease, they affect the quality of life of the concerned person - sometimes considerably. According to a British study of 924 patients with reflux symptoms, 50% complained of limitations both in their everyday working life and during leisure activities[16]. Among 108 clinical patients with GERD, 22.3% had regular sleep disturbances, 27.8% had problems with eating and drinking, and 11.2% had disorders that hindered professional activity[17].
Accordingly, the improvement in quality of life plays a considerable role in treatment. Treatment with PPIs, for example, reduced the symptoms’ effect on quality of life in patients who responded to it. However, only 186 out of 482 patients treated with PPIs reported that their efficacy was complete or good[16].
The present study comprises a number of limitations. The number of patients examined was relatively small, and there was no control group. Because of the open design, the participants knew that they were drinking water that might possibly reduce their symptoms - a placebo effect might have influenced the subjective assessments used for the evaluation of the defined endpoints, both in the diaries and in the questionnaires. Apart from the blood pressure measurement, which showed a significant reduction in both systolic and diastolic blood pressure, and the assessment of laboratory parameters, no further objective assessments, e.g., pH measurements, were carried out.
Most of the remaining study conditions were not controlled, either. While the participants were not supposed to change their other eating habits, this was not checked within the study. Therefore, an influence of food on the results of the study cannot be excluded.
Additionally, the nature of the patient recruitment probably led to selection bias, since participants with a particular interest in health topics were included, as it was apparent from the advertising material that the test substance was a natural product.
A hydrogen carbonate-rich mineral water can qualitatively and quantitatively reduce heartburn symptoms and improve the subjective wellbeing of patients affected. Further studies of a randomized, placebo-controlled and blinded design should follow this open pilot study, which was mainly carried out to generate hypotheses for later confirmatory trials.
The authors thank Margret I. Moré for critically reading and formatting the manuscript.
This open, single-center, single-arm clinical study investigated the efficacy and safety of mineral water with a high content of hydrogen carbonate in patients with heartburn. Previous studies have also demonstrated the positive influence of hydrogen carbonate-rich water on heartburn and gastrointestinal symptoms.
Hydrogen carbonate-rich water as effective heartburn remedy deserves more scientific attention.
This study applies a whole range of different test instruments to measure and evaluate the effects of the mineral water on heartburn. Therefore it is an excellent standard for further studies on heartburn or gastroesophageal reflux disease (GERD). It is also a prerequisite for a larger randomized controlled study.
Hydrogen carbonate-rich water should become a standard treatment for any kind of mild heartburn problems in addition to life-style changes. It may be just as efficient or even more efficient than antacids, and may even offer advantages in more severe heartburn cases generally treated with proton pump inhibitors or histamine-2 receptor blockers. This remains to be tested.
GERD: Gastroesophageal reflux disease; NERD: Non-erosive reflux disease, early stage of GERD. Both include symptoms of heartburn.
This is a well-designed and written paper that aimed to explore the efficacy of hydrogen carbonate-rich water in patients with heartburn showing a very nice reduction in heartburn perception and the duration of heartburn per week. The statistical analysis has been well-conducted.
P- Reviewer: de Bortoli N S- Editor: Kong JX L- Editor: A E- Editor: Jiao XK
| 1. | El-Serag HB, Sweet S, Winchester CC, Dent J. Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2014;63:871-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1057] [Cited by in RCA: 1265] [Article Influence: 115.0] [Reference Citation Analysis (2)] |
| 2. | Altomare A, Guarino MP, Cocca S, Emerenziani S, Cicala M. Gastroesophageal reflux disease: Update on inflammation and symptom perception. World J Gastroenterol. 2013;19:6523-6528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 70] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 3. | Lacy BE, Talley NJ, Locke GR, Bouras EP, DiBaise JK, El-Serag HB, Abraham BP, Howden CW, Moayyedi P, Prather C. Review article: current treatment options and management of functional dyspepsia. Aliment Pharmacol Ther. 2012;36:3-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 168] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 4. | Grassi M, Fraioli A, Pappalardo G, Messina B, Belardinelli L, Guadalaxara A. Alkalizing activity of a calcium-bicarbonate-containing water, evaluated for pH, in patients with gastroesophageal reflux. Clin Ter. 1993;143:131-136. [PubMed] |
| 5. | Bertoni M, Olivieri F, manghetti M, Boccolini E, Bellomini MG, Blandizzi C, Bonino F, Del Tacca M. Effects of a bicarbonate-alkaline mineral water on gastric functions and functional dyspepsia: a preclinical and clinical study. Pharmacol Res. 2002;46:525-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Gasbarrini G, Candelli M, Graziosetto RG, Coccheri S, Di Iorio F, Nappi G. Evaluation of thermal water in patients with functional dyspepsia and irritable bowel syndrome accompanying constipation. World J Gastroenterol. 2006;12:2556-2562. [PubMed] |
| 7. | Böhmer H, Resch K, Waldow R. Dyspepsie: Hydrogencarbonathaltige Heilwässer unterstützen die Therapie mit Antazida. Natura Med. 2001;16:28-33. |
| 8. | Nocon M, Kulig M, Leodolter A, Malfertheiner P, Willich SN. Validation of the Reflux Disease Questionnaire for a German population. Eur J Gastroenterol Hepatol. 2005;17:229-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Kulich KR, Malfertheiner P, Madisch A, Labenz J, Bayerdörffer E, Miehlke S, Carlsson J, Wiklund IK. Psychometric validation of the German translation of the Gastrointestinal Symptom Rating Scale (GSRS) and Quality of Life in Reflux and Dyspepsia (QOLRAD) questionnaire in patients with reflux disease. Health Qual Life Outcomes. 2003;1:62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Eypasch E, Williams JI, Wood-Dauphinee S, Ure BM, Schmülling C, Neugebauer E, Troidl H. Gastrointestinal Quality of Life Index: development, validation and application of a new instrument. Br J Surg. 1995;82:216-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 852] [Cited by in RCA: 885] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 11. | Gandek B, Ware JE, Aaronson NK, Apolone G, Bjorner JB, Brazier JE, Bullinger M, Kaasa S, Leplege A, Prieto L. Cross-validation of item selection and scoring for the SF-12 Health Survey in nine countries: results from the IQOLA Project. International Quality of Life Assessment. J Clin Epidemiol. 1998;51:1171-1178. [PubMed] |
| 12. | Gunasekaran T, Tolia V, Colletti RB, Gold BD, Traxler B, Illueca M, Crawley JA. Effects of esomeprazole treatment for gastroesophageal reflux disease on quality of life in 12- to 17-year-old adolescents: an international health outcomes study. BMC Gastroenterol. 2009;9:84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Chen L, Tao SF, Xu Y, Fang F, Peng SY. Patients’ quality of life after laparoscopic or open cholecystectomy. J Zhejiang Univ Sci B. 2005;6:678-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Oliver K, Davies G. Heartburn: influence of diet and lifestyle. Nutr Food Sci. 2007;38:548-554. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Nassini R, Andrè E, Gazzieri D, De Siena G, Zanasi A, Geppetti P, Materazzi S. A bicarbonate-alkaline mineral water protects from ethanol-induced hemorrhagic gastric lesions in mice. Biol Pharm Bull. 2010;33:1319-1323. [PubMed] |
| 16. | Jones R, Liker HR, Ducrotté P. Relationship between symptoms, subjective well-being and medication use in gastro-oesophageal reflux disease. Int J Clin Pract. 2007;61:1301-1307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Lee SW, Lien HC, Lee TY, Yang SS, Yeh HJ, Chang CS. Heartburn and regurgitation have different impacts on life quality of patients with gastroesophageal reflux disease. World J Gastroenterol. 2014;20:12277-12282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |