Published online Feb 15, 2016. doi: 10.4291/wjgp.v7.i1.131
Peer-review started: June 28, 2015
First decision: August 26, 2015
Revised: October 17, 2015
Accepted: November 10, 2015
Article in press: November 11, 2015
Published online: February 15, 2016
Processing time: 220 Days and 1.9 Hours
Barrett’s esophagus (BE), a premalignant condition to Barrett’s adenocarcinoma (BAC), is closely associated with chronic inflammation due to gastro-esophageal reflux. Caudal type homeobox 2 (CDX2), a representative marker of BE, is increased during the metaplastic and neoplastic transformation of BE. Nitric oxide (NO) has been proposed to be a crucial mediator of Barrett’s carcinogenesis. We previously demonstrated that CDX2 might be induced directly under stimulation of large amounts of NO generated around the gastro-esophageal junction (GEJ) by activating epithelial growth factor receptor in a ligand-independent manner. Thus, we reviewed recent developments on the role of NO in Barrett’s carcinogenesis. Notably, recent studies have reported that microbial communities in the distal esophagus are significantly different among groups with a normal esophagus, reflux esophagitis, BE or BAC, despite there being no difference in the bacterial quantity. Considering that microorganism components can be one of the major sources of large amounts of NO, these studies suggest that the bacterial composition in the distal esophagus might play an important role in regulating NO production during the carcinogenic process. Controlling an inflammatory reaction due to gastro-esophageal reflux or bacterial composition around the GEJ might help prevent the progression of Barrett’s carcinogenesis by inhibiting NO production.
Core tip: Barrett’s carcinogenesis is closely associated with chronic inflammation caused by gastro-esophageal reflux of gastric acid, bile acid and intraluminal microorganisms. Caudal type homeobox 2 (CDX2) is a crucial biomarker of Barrett’s esophagus. We previously demonstrated that a high amount of nitric oxide (NO) at the gastro-esophageal junction (GEJ) might directly induce CDX2 through phosphorylation of epidermal growth factor receptor (EGFR) in a ligand-independent manner. Together with a possible effect of anti-EGFR-targeting drugs in inhibiting Barrett’s adenocarcinoma, future research for controlling NO production around the GEJ might provide a new insight for developing a management strategy of Barrett’s carcinogenesis.
- Citation: Kusaka G, Uno K, Iijima K, Shimosegawa T. Role of nitric oxide in the pathogenesis of Barrett’s-associated carcinogenesis. World J Gastrointest Pathophysiol 2016; 7(1): 131-137
- URL: https://www.wjgnet.com/2150-5330/full/v7/i1/131.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v7.i1.131
Barrett’s esophagus (BE) is defined as the premalignant condition which squamous epithelium is replaced by columnar epithelium, a well-known precursor of Barrett’s adenocarcinoma (BAC)[1-3]. Because the incidence of Barrett’s-associated neoplasm has increased sharply, it is important to elucidate its risk factors during the surveillance period. Many studies on the origin of Barrett’s mucosa have proposed several theories, such as the differentiation of the stem cells in esophageal squamous tissues and a phenotypic switch and formation of metaplastic epithelium by gastro-esophageal refluxant[4-6].
Caudal type homeobox 2 (CDX2) is a member of the homeobox family of genes involved in the embryonic development of the alimentary tract and in the differentiation, proliferation, adhesion and apoptosis of the intestinal epithelium[7-9]. It is well-known that CDX2 is induced during the metaplastic and neoplastic transformation of BE, while it is not expressed in normal esophageal squamous epithelium and non-metaplastic gastric epithelium[8-11]. Thus, it has been reported that an immune-stain of CDX2 could become a useful biomarker of Barrett’s-associated neoplasms[12-14]. Previous studies have revealed that reactive oxygen species (ROS) produced by the stimulation of acid, bile salts or major noxious agents in refluxed gastric juice might take a critical role for the induction of CDX2 during the sequential process of the metaplastic and neoplastic transformation of BE[7,11,15-28]. However, this mechanism is not fully understood.
Nitric oxide (NO) is a crucial regulatory mediator which has cytotoxic and cytostatic effects, depending on the environment[16,26]. A large amount of NO production is involved in cytotoxic processes, such as apoptosis, angiogenesis and DNA damage during cancer development[19]. In fact, it was reported that NO might work as a principle mediator in the induction of CDX2 during Barrett’s carcinogenesis[7] and that reactive nitrogen oxide species might contribute to the progression from BE to BAC because of genotoxicity, such as DNA damage, strand breakage or modification[21,29,30].
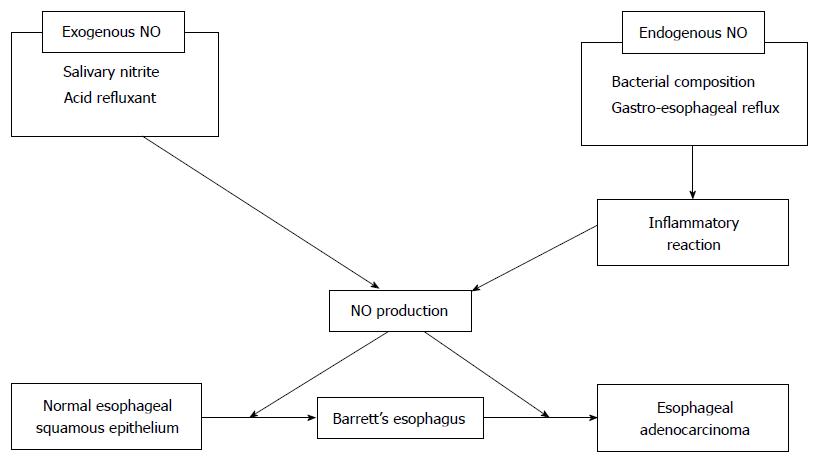
From the point of view of NO generation, NO is classified into endogenous and exogenous[27] (Figure 1). Endogenous NO is derived from L-arginine by a family of related enzymes, which includes three different isoforms of NO synthase (NOS)[27,31]. Endothelial NOS (eNOS) in endothelial cells and a neuronal NOS (nNOS) in neuronal cells were reported to be physiological mediators of NO production at low concentrations[27]. Inducible NO (iNOS) is an inflammation-induced enzyme, which can mediate harmful production of high amounts of NO[27,32]. The induction of iNOS might be critically involved in Barrett’s carcinogenesis because its expression was shown to be gradually overexpressed during the sequential development toward BAC, but not in normal esophageal/gastric mucosa[19,27,32].
Exogenous NO is generated within the upper-gastrointestinal tract lumen from the reduction of salivary nitrite to NO in a reaction with acid refluxant[33-35]. After ingesting nitrate-containing food, human salivary nitrite concentrations increase to 2-5 mmol/L, which maintained for a few hours by entero-salivary recirculation of the dietary nitrate[35,36]. Swallowed saliva is the main source of nitrate. The gastric juice containing the acidity and ascorbic acid content might convert the salivary nitrite to NO at the gastro-esophageal junction (GEJ)[21,37]. Iijima et al[21] investigated the serum and saliva nitrate concentrations within the upper gastrointestinal tract lumen in healthy volunteers of Helicobacter pylori-negative under basal conditions and after nitrate ingestion using custom-made sensors. After ingesting 2 mmol of potassium nitrate, the nitrate concentration of the serum, salivary and intra-lumin increased from 30 μmol/L to 95 μmol/L, from 36 μmol/L to 252 μmol/L, and from 4.7 μmol/L to 23.2 μmol/L, respectively. The highest concentrations of NO within the upper gastrointestinal tract lumen were observed at the site of the neutral pH of esophageal fell to the acidic pH of gastric. This suggested that the luminal generation of NO from nitrate ingestion through salivary nitrite was maximal at the GEJ. Thus, the site of generation of luminal NO would move to the oral side of the distal esophagus in patients with gastro-esophageal reflux[38].
Additionally, luminal NO with a high concentration due to both exogenous and endogenous NO production around the GEJ is rapidly diffused across epithelial membranes, resulting in damage to the tissues surrounding tissues the GEJ[29,30,39-41]. Such a large amount of NO also causes the production of peroxynitrite, a representative genotoxic mediator, and can contribute to the development of Barrett’s carcinogenesis via a rapid reaction with superoxide in an inflammatory/tumor microenvironment[20,21,26,27,37]. Of the plasma membranous receptors of the esophageal tissue, we previously demonstrated that during Barrett’s carcinogenesis, a large amount of NO might directly activate epithelial growth factor receptor (EGFR)[42], a 170-kDa glycoprotein which is a member of the receptor tyrosine kinase family and is involved in cell proliferation, differentiation, angiogenesis, invasion and tumor progression[1,3,43-46]. Previous studies demonstrated that overexpression of EGFR was observed during histological progression in BE tissue, as well as during tumor infiltration and metastasis[1,45]. Although it was previously indicated that ROS might indirectly enhance the CDX2 expression by the exposure of bile acid through EGFR activation in ligand-dependent and -independent manners[3,8,15,46], we investigated the direct role of NO through activation of EGFR during Barrett’s carcinogenesis[42]. When we compared immune-staining of EGFR, CDX2 and nitrotyrosine, a marker of intra-cellular protein damaged by peroxynitrite, in normal human esophageal squamous epithelium specimens with those in BE specimens, we revealed the stronger expression of EGFR, CDX2 and nitrotyrosine in BE compared with those in normal human esophageal squamous epithelium with a positive correlation with BE progression. Second, we also investigated the effect of peroxynitrite, NOC7 which is NO donor and SIN-1 which is stimulant of peroxynitrite on the expression of phosphorylated EGFR and CDX2 by western blotting and reverse transcription-polymerase chain reaction. This showed that the phosphorylated EGFR and CDX2 were induced in dose- and time-dependent manners after expose to peroxynitrite, NOC7 and SIN-1. The expression of phosphorylated EGFR and CDX2 induced after expose to peroxynitrite, NOC7 and SIN-1 was diminished by EGFR-specific inhibitors AG1428 and EGFR-siRNA. Additionally, the induction of phosphorylated EGFR and CDX2 after exposure to NOC7 was diminished by carboxy-PTIO which is the NO scavenger in a dose-dependent manner. Taken together, these results suggest that the expression of CDX2 might be induced directly by the effect of extrinsic NO through the activation of EGFR in the progression of BE. Accordingly, these results indicate that the stimulation of high concentration of NO on the normal esophageal epithelium might directly induce the expression of CDX2 through EGFR activation and result in the progression of BE[7,8,27].
Notably, EGFR2 (HER2) is regarded as a useful target in controlling Barrett’s cancer development, despite the uncertain treatment effect of HER2-targetting anti-cancer medications[47,48]. While researchers reported that the clinical trials targeting HER2 in the treatment of BAC were result it was not promising, another study showed that targeting erbB-2 in HER2-positive patients with adenocarcinoma at GEJ had better outcomes[49,50]. Gefitinib is another small molecule tyrosine kinase inhibitor (TKI) and the most common HER2-targetting anti-cancer medication that has been used to treat several malignancies. It is a representative oral TKI and inhibits EGF-induced phosphorylation and downstream signaling pathways. Janmaat et al[51] reported a phase II study of 36 patients who had failed first-line treatment for advanced esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC) and were administered gefitinib at 500 mg/d. In this report, gefitinib had modest activity as a second-line treatment with only 2.8% of the patients with a partial response (PR), 27.8% with stable disease and 47.8% with disease progression. The progression-free survival time and the median overall survival time were 59 d and 164 d, respectively. Javle et al[52] reported a phase I/II study of 6 patients who had locally advanced unresectable EAC. This study used a combination of gefitinib and chemoradiotherapy. The protocol were composed of administration of gefitinib orally at 250 mg/d for 1 year, oxaliplatin intravenously at 85 or 100 mg/m2 on days 1, 15, 29, and preoperative radiotherapy. They reported that this combination study was tolerable, but had limited efficacy. The median overall survival time and the disease-free survival time were 10.8 and 8.4 mo, respectively. Ilson et al[53] reported a phase II study of the treatment effect of Erlotinib, another oral TKI, in 30 patients who had failed first-line treatment for ESCC and EAC. The patients had a 6.7% PR and 10.3 mo of median overall survival. These results supported an important role for EGFR in Barrett’s cancer development. Targeted inhibition of EGFR activity and inhibition of EGFR activity by controlling NO production around the GEJ might be a new clue to improve the management of Barrett’s-associated neoplasm.
Several studies have reported that the bacterial composition in the distal esophagus might play an important role in the carcinogenic process. Human microbiota is estimated to be composed of approximately 1014 bacterial cells. The relationship the bacteria and host can be commensal and pathogenic[54]. Human microbiota in the gastrointestinal tract are essential for human survival, aiding digestion, assisting in the synthesis of vitamins and maintaining a normal physiological environment[54,55]. The host provides these microbiota safe housing and nutrition in return for the benefits from the microbes. An imbalance in this relationship may cause chronic inflammation and inflammation-associated malignancies.
Previous report mentioned that the normal distal esophagus have 6 phyla and 140 species of bacterial compositions[56]. The impairment of the mucosal barrier in the distal esophagus by reflux of gastric juice might disrupt the microenvironmental homeostasis, resulting in progression of Barrett’s-associated neoplasm with remarkable changes in esophageal bacterial biota[56-58]. In fact, it was reported that the characteristics of bacterial communities differed between groups with normal esophagus, reflux esophagitis (RE), BE or BAC, despite there being no significant differences in the amount of bacteria[55,58].
By investigating the composition of bacteria in the distal esophagus of 3 groups (group with normal esophagus, group with RE and group with BE) from endoscopic biopsy specimens using a histological examination and DNA extraction, Liu et al[55] reported that Fusobacterium was found not in the group with a normal esophagus, but in the group with RE and BE, and that the most common bacterium was Streptococcus in the groups with normal esophagus and RE and Veillonella in the group with BE. However, the total amount of the detected bacterial DNA was not different between the groups[55]. Narikiyo et al[59] investigated the bacteria in esophageal carcinomatous tissues, the surrounding non-cancerous tissues and saliva from healthy people to determine the correlation between microorganisms and inflammation/carcinogenesis of the esophagus. They showed that Treponema denticola and Streptococcus anginosus (S. anginosus) were detected in the saliva from patients with BAC. Both of these bacteria were in low numbers in the samples from healthy people. They also reported that S. angiosus and Streptoccus mitis infections induced inflammatory cytokines, including IL-8 and GROα, and suggested these bacteria might play a significant role in Barrett’s carcinogenesis through chronic inflammation. Yang et al[54] reported a dramatic alteration of the esophageal microbiome, such as Gram-positive bacteria were decreased and Gram-negative bacteria were increased in patients with esophagitis and BE. In fact, Gram-negative bacteria have lipopolysaccharides (LPS) in their outer membrane, which can promote the induction of pro-inflammatory cytokines, including IL-1β, IL-8, COX-2 and iNOS, by activating Toll-like receptor 4 and its downstream cascade, which are related to proliferation, differentiation and migration[60,61]. In addition, LPS may also cause the relaxation of the lower esophageal sphincter by activating iNOS and delaying gastric employing by increasing the intra-gastric pressure through COX-2 activation[62,63]. This suggests that the alteration of Gram-negative bacteria in bacterial composition in the distal esophagus might contribute to the development of inflammation-associated Barrett’s carcinogenesis. Thus, interventions with the microorganism in the distal esophagus provided novel insights for new preventive/treatment strategy of BE.
Many studies have contributed to a better understanding of the role of NO in Barrett’s carcinogenesis. There may be three ways to control NO production in Barrett’s mucosa, such as: (1) diminishing acidity of the gastro-esophageal refluxant by using a proton-pump inhibitor[64]; (2) medical intervention of intramucosal microorganisms; and (3) medical inhibition of iNOS activity. Recent studies have indicated a significant role for esophageal microbiology in Barrett’s carcinogenesis because of the differences in the bacterial communities between groups with normal esophagus, RE, BE or BAC[55,58]. Therefore, it is likely that the eradication of these bacteria by antibiotics or probiotics might be effective in reducing Barrett’s cancer risk, although limited data of suitable antibiotics combinations for the microbiome have been reported. Yang et al[54] reported that Gram-negative bacteria were increased in the patients with esophagitis and BE. LPS from Gram-negative bacteria can up-regulate gene expression of inflammatory cytokines, including COX-2 and iNOS, by activating the NF-κB pathway[54]. Several studies have suggested that NF-κB inhibitors, including nonsteroidal anti-inflammatory drugs (NSAIDs), sulfasalazine and immunosuppressive agents, could be effective in reducing Barrett’s inflammation. Therefore, they were regarded as a potential medication for preventing Barrett’s carcinogenesis[61]. Moreover, Hansel et al[62] demonstrated that the prodrug L-N6-(1-iminoethyl)lysine 5-tetrazole amide, an iNOS inhibitor, reduced the levels of NO in exhaled breath in healthy volunteers and patients with mild asthma without remarkable side-effects. These results suggest the therapeutic potential of iNOS inhibitors to reduce the risk of Barrett’s cancer. Additionally, we previously demonstrated that the interaction between iNOS and COX-2 activities might play a crucial role in LPS-induced inflammation in a rat-model[65]. Therefore, controlling COX-2 activity can also regulate iNOS-derived NO production in an inflammation reaction. Therefore, COX-2 inhibitors might have therapeutic potential in controlling the risk of neoplastic progression in patients with BE. Moreover, Calatayud et al[66] reported that the delay of gastric employing with an increase of the intra-gastric pressure from LPS stimulation could be blocked by down-regulating COX-2 activity using the selective COX-2 inhibitor NS398. In fact, in a meta-analysis of anti-cancerous effects of NSAIDs and aspirin for 1813 patients with EAC, Corley et al[67] suggested that the preventive role of COX-2 inhibitors in Barrett’s carcinogenesis, although their clinical relevance against the development of Barrett’s -associated neoplasm was unproven[67-69]. Accordingly, controlling enzymatic activity of iNOS and microorganisms in Barrett’s tumor microenvironment may provide important insight into Barrett’s carcinogenesis.
Barrett’s carcinogenesis was associated with chronic inflammation as a consequence of gastro-esophageal reflux and microbiota in the gastrointestinal tract. This review focused on the role of NO in controlling inflammation during the Barrett’s carcinogenic process. The control of NO production in chronic inflammation caused by gastro-esophageal reflux and microbiota might contribute to prevent Barrett’s cancer development. Further studies are required to assess the therapeutic potential of controlling NO production.
P- Reviewer: Miheller P, Shimoyama S S- Editor: Ji FF L- Editor: A E- Editor: Jiao XK
| 1. | Cronin J, McAdam E, Danikas A, Tselepis C, Griffiths P, Baxter J, Thomas L, Manson J, Jenkins G. Epidermal growth factor receptor (EGFR) is overexpressed in high-grade dysplasia and adenocarcinoma of the esophagus and may represent a biomarker of histological progression in Barrett’s esophagus (BE). Am J Gastroenterol. 2011;106:46-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 2. | Shaheen N, Ransohoff DF. Gastroesophageal reflux, Barrett esophagus, and esophageal cancer: clinical applications. JAMA. 2002;287:1982-1986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 57] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Li Y, Wo JM, Ray MB, Jones W, Su RR, Ellis S, Martin RC. Cyclooxygenase-2 and epithelial growth factor receptor up-regulation during progression of Barrett’s esophagus to adenocarcinoma. World J Gastroenterol. 2006;12:928-934. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Zhang HY, Spechler SJ, Souza RF. Esophageal adenocarcinoma arising in Barrett esophagus. Cancer Lett. 2009;275:170-177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Abdel-Latif MM, Duggan S, Reynolds JV, Kelleher D. Inflammation and esophageal carcinogenesis. Curr Opin Pharmacol. 2009;9:396-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 91] [Article Influence: 5.7] [Reference Citation Analysis (1)] |
| 6. | Souza RF, Krishnan K, Spechler SJ. Acid, bile, and CDX: the ABCs of making Barrett’s metaplasia. Am J Physiol Gastrointest Liver Physiol. 2008;295:G211-G218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 135] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 7. | Vaninetti N, Williams L, Geldenhuys L, Porter GA, Guernsey DL, Casson AG. Regulation of CDX2 expression in esophageal adenocarcinoma. Mol Carcinog. 2009;48:965-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Rahman FB, Kadowaki Y, Ishihara S, Tobita H, Imaoka H, Fukuhara H, Aziz MM, Furuta K, Amano Y, Kinoshita Y. Fibroblast-derived HB-EGF promotes Cdx2 expression in esophageal squamous cells. Lab Invest. 2010;90:1033-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Takahashi O, Hamada J, Abe M, Hata S, Asano T, Takahashi Y, Tada M, Miyamoto M, Kondo S, Moriuchi T. Dysregulated expression of HOX and ParaHOX genes in human esophageal squamous cell carcinoma. Oncol Rep. 2007;17:753-760. [PubMed] |
| 10. | McColl KE. When saliva meets acid: chemical warfare at the oesophagogastric junction. Gut. 2005;54:1-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 70] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Burnat G, Rau T, Elshimi E, Hahn EG, Konturek PC. Bile acids induce overexpression of homeobox gene CDX-2 and vascular endothelial growth factor (VEGF) in human Barrett’s esophageal mucosa and adenocarcinoma cell line. Scand J Gastroenterol. 2007;42:1460-1465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Johnson DR, Abdelbaqui M, Tahmasbi M, Mayer Z, Lee HW, Malafa MP, Coppola D. CDX2 protein expression compared to alcian blue staining in the evaluation of esophageal intestinal metaplasia. World J Gastroenterol. 2015;21:2770-2776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Freund JN, Domon-Dell C, Kedinger M, Duluc I. The Cdx-1 and Cdx-2 homeobox genes in the intestine. Biochem Cell Biol. 1998;76:957-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 66] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Silberg DG, Swain GP, Suh ER, Traber PG. Cdx1 and cdx2 expression during intestinal development. Gastroenterology. 2000;119:961-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 461] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 15. | Avissar NE, Toia L, Hu Y, Watson TJ, Jones C, Raymond DP, Matousek A, Peters JH. Bile acid alone, or in combination with acid, induces CDX2 expression through activation of the epidermal growth factor receptor (EGFR). J Gastrointest Surg. 2009;13:212-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Clemons NJ, McColl KE, Fitzgerald RC. Nitric oxide and acid induce double-strand DNA breaks in Barrett’s esophagus carcinogenesis via distinct mechanisms. Gastroenterology. 2007;133:1198-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Feagins LA, Zhang HY, Zhang X, Hormi-Carver K, Thomas T, Terada LS, Spechler SJ, Souza RF. Mechanisms of oxidant production in esophageal squamous cell and Barrett’s cell lines. Am J Physiol Gastrointest Liver Physiol. 2008;294:G411-G417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Rantanen TK, Räsänen JV, Sihvo EI, Ahotupa MO, Färkkilä MA, Salo JA. The impact of antireflux surgery on oxidative stress of esophageal mucosa caused by gastroesophageal reflux disease: 4-yr follow-up study. Am J Gastroenterol. 2006;101:222-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Clemons NJ, Shannon NB, Abeyratne LR, Walker CE, Saadi A, O’Donovan ML, Lao-Sirieix PP, Fitzgerald RC. Nitric oxide-mediated invasion in Barrett’s high-grade dysplasia and adenocarcinoma. Carcinogenesis. 2010;31:1669-1675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Iijima K, Grant J, McElroy K, Fyfe V, Preston T, McColl KE. Novel mechanism of nitrosative stress from dietary nitrate with relevance to gastro-oesophageal junction cancers. Carcinogenesis. 2003;24:1951-1960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 52] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Iijima K, Henry E, Moriya A, Wirz A, Kelman AW, McColl KE. Dietary nitrate generates potentially mutagenic concentrations of nitric oxide at the gastroesophageal junction. Gastroenterology. 2002;122:1248-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 105] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 22. | Jenkins GJ, D’Souza FR, Suzen SH, Eltahir ZS, James SA, Parry JM, Griffiths PA, Baxter JN. Deoxycholic acid at neutral and acid pH, is genotoxic to oesophageal cells through the induction of ROS: The potential role of anti-oxidants in Barrett’s oesophagus. Carcinogenesis. 2007;28:136-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 84] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 23. | Dvorak K, Payne CM, Chavarria M, Ramsey L, Dvorakova B, Bernstein H, Holubec H, Sampliner RE, Guy N, Condon A. Bile acids in combination with low pH induce oxidative stress and oxidative DNA damage: relevance to the pathogenesis of Barrett’s oesophagus. Gut. 2007;56:763-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 190] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 24. | Jenkins GJ, Cronin J, Alhamdani A, Rawat N, D’Souza F, Thomas T, Eltahir Z, Griffiths AP, Baxter JN. The bile acid deoxycholic acid has a non-linear dose response for DNA damage and possibly NF-kappaB activation in oesophageal cells, with a mechanism of action involving ROS. Mutagenesis. 2008;23:399-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 25. | Morelli MP, Cascone T, Troiani T, De Vita F, Orditura M, Laus G, Eckhardt SG, Pepe S, Tortora G, Ciardiello F. Sequence-dependent antiproliferative effects of cytotoxic drugs and epidermal growth factor receptor inhibitors. Ann Oncol. 2005;16 Suppl 4:iv61-iv68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 88] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 26. | Vaninetti NM, Geldenhuys L, Porter GA, Risch H, Hainaut P, Guernsey DL, Casson AG. Inducible nitric oxide synthase, nitrotyrosine and p53 mutations in the molecular pathogenesis of Barrett’s esophagus and esophageal adenocarcinoma. Mol Carcinog. 2008;47:275-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 27. | Bae JD, Jung KH, Ahn WS, Bae SH, Jang TJ. Expression of inducible nitric oxide synthase is increased in rat Barrett’s esophagus induced by duodenal contents reflux. J Korean Med Sci. 2005;20:56-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Marchetti M, Caliot E, Pringault E. Chronic acid exposure leads to activation of the cdx2 intestinal homeobox gene in a long-term culture of mouse esophageal keratinocytes. J Cell Sci. 2003;116:1429-1436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 75] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 29. | Ara N, Iijima K, Asanuma K, Yoshitake J, Ohara S, Shimosegawa T, Yoshimura T. Disruption of gastric barrier function by luminal nitrosative stress: a potential chemical insult to the human gastro-oesophageal junction. Gut. 2008;57:306-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Ito H, Iijima K, Ara N, Asanuma K, Endo H, Asano N, Koike T, Abe Y, Imatani A, Shimosegawa T. Reactive nitrogen oxide species induce dilatation of the intercellular space of rat esophagus. Scand J Gastroenterol. 2010;45:282-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 31. | Ruano MJ, Hernández-Hernando S, Jiménez A, Estrada C, Villalobo A. Nitric oxide-induced epidermal growth factor-dependent phosphorylations in A431 tumour cells. Eur J Biochem. 2003;270:1828-1837. [PubMed] |
| 32. | Wilson KT, Fu S, Ramanujam KS, Meltzer SJ. Increased expression of inducible nitric oxide synthase and cyclooxygenase-2 in Barrett’s esophagus and associated adenocarcinomas. Cancer Res. 1998;58:2929-2934. [PubMed] |
| 33. | Benjamin N, O’Driscoll F, Dougall H, Duncan C, Smith L, Golden M, McKenzie H. Stomach NO synthesis. Nature. 1994;368:502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 466] [Cited by in RCA: 458] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 34. | Lundberg JO, Weitzberg E, Lundberg JM, Alving K. Intragastric nitric oxide production in humans: measurements in expelled air. Gut. 1994;35:1543-1546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 455] [Cited by in RCA: 451] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 35. | McKnight GM, Smith LM, Drummond RS, Duncan CW, Golden M, Benjamin N. Chemical synthesis of nitric oxide in the stomach from dietary nitrate in humans. Gut. 1997;40:211-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 223] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 36. | Pannala AS, Mani AR, Spencer JP, Skinner V, Bruckdorfer KR, Moore KP, Rice-Evans CA. The effect of dietary nitrate on salivary, plasma, and urinary nitrate metabolism in humans. Free Radic Biol Med. 2003;34:576-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 196] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 37. | Mukaida H, Toi M, Hirai T, Yamashita Y, Toge T. Clinical significance of the expression of epidermal growth factor and its receptor in esophageal cancer. Cancer. 1991;68:142-148. [PubMed] |
| 38. | Suzuki H, Iijima K, Scobie G, Fyfe V, McColl KE. Nitrate and nitrosative chemistry within Barrett’s oesophagus during acid reflux. Gut. 2005;54:1527-1535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 39. | Ishiyama F, Iijima K, Asanuma K, Ara N, Yoshitake J, Abe Y, Koike T, Imatani A, Ohara S, Shimosegawa T. Exogenous luminal nitric oxide exacerbates esophagus tissue damage in a reflux esophagitis model of rats. Scand J Gastroenterol. 2009;44:527-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 40. | Endo H, Iijima K, Asanuma K, Ara N, Ito H, Asano N, Uno K, Koike T, Imatani A, Shimosegawa T. Exogenous luminal nitric oxide exposure accelerates columnar transformation of rat esophagus. Int J Cancer. 2010;127:2009-2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 41. | Asanuma K, Iijima K, Sugata H, Ohara S, Shimosegawa T, Yoshimura T. Diffusion of cytotoxic concentrations of nitric oxide generated luminally at the gastro-oesophageal junction of rats. Gut. 2005;54:1072-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 42. | Kusaka G, Uno K, Iijima K, Endo H, Asano N, Koike T, Imatani A, Shimosegawa T. The role of nitric oxide in the induction of caudal-type homeobox 2 through epidermal growth factor receptor in the development of Barrett’s esophagus. Scand J Gastroenterol. 2012;47:1148-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 43. | Lee HC, An S, Lee H, Woo SH, Jin HO, Seo SK, Choe TB, Yoo DH, Lee SJ, Hong YJ. Activation of epidermal growth factor receptor and its downstream signaling pathway by nitric oxide in response to ionizing radiation. Mol Cancer Res. 2008;6:996-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 44. | Andl CD, Mizushima T, Nakagawa H, Oyama K, Harada H, Chruma K, Herlyn M, Rustgi AK. Epidermal growth factor receptor mediates increased cell proliferation, migration, and aggregation in esophageal keratinocytes in vitro and in vivo. J Biol Chem. 2003;278:1824-1830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 205] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 45. | Hanawa M, Suzuki S, Dobashi Y, Yamane T, Kono K, Enomoto N, Ooi A. EGFR protein overexpression and gene amplification in squamous cell carcinomas of the esophagus. Int J Cancer. 2006;118:1173-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 232] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 46. | Goldman A, Chen HD, Roesly HB, Hill KA, Tome ME, Dvorak B, Bernstein H, Dvorak K. Characterization of squamous esophageal cells resistant to bile acids at acidic pH: implication for Barrett’s esophagus pathogenesis. Am J Physiol Gastrointest Liver Physiol. 2011;300:G292-G302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 47. | Gowryshankar A, Nagaraja V, Eslick GD. HER2 status in Barrett’s esophagus & amp; esophageal cancer: a meta analysis. J Gastrointest Oncol. 2014;5:25-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 48. | Xu Y, Sheng L, Mao W. Role of epidermal growth factor receptor tyrosine kinase inhibitors in the treatment of esophageal carcinoma and the suggested mechanisms of action. Oncol Lett. 2013;5:19-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 49. | Clemons NJ, Phillips WA, Lord RV. Signaling pathways in the molecular pathogenesis of adenocarcinomas of the esophagus and gastroesophageal junction. Cancer Biol Ther. 2013;14:782-795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 50. | Rubenstein JH, Dahlkemper A, Kao JY, Zhang M, Morgenstern H, McMahon L, Inadomi JM. A pilot study of the association of low plasma adiponectin and Barrett’s esophagus. Am J Gastroenterol. 2008;103:1358-1364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 51. | Janmaat ML, Gallegos-Ruiz MI, Rodriguez JA, Meijer GA, Vervenne WL, Richel DJ, Van Groeningen C, Giaccone G. Predictive factors for outcome in a phase II study of gefitinib in second-line treatment of advanced esophageal cancer patients. J Clin Oncol. 2006;24:1612-1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 164] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 52. | Javle M, Pande A, Iyer R, Yang G, LeVea C, Wilding G, Black J, Nava H, Nwogu C. Pilot study of gefitinib, oxaliplatin, and radiotherapy for esophageal adenocarcinoma: tissue effect predicts clinical response. Am J Clin Oncol. 2008;31:329-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 53. | Ilson DH, Kelsen D, Shah M, Schwartz G, Levine DA, Boyd J, Capanu M, Miron B, Klimstra D. A phase 2 trial of erlotinib in patients with previously treated squamous cell and adenocarcinoma of the esophagus. Cancer. 2011;117:1409-1414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 75] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 54. | Yang L, Francois F, Pei Z. Molecular pathways: pathogenesis and clinical implications of microbiome alteration in esophagitis and Barrett esophagus. Clin Cancer Res. 2012;18:2138-2144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 135] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 55. | Liu N, Ando T, Ishiguro K, Maeda O, Watanabe O, Funasaka K, Nakamura M, Miyahara R, Ohmiya N, Goto H. Characterization of bacterial biota in the distal esophagus of Japanese patients with reflux esophagitis and Barrett’s esophagus. BMC Infect Dis. 2013;13:130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 56. | Pei Z, Bini EJ, Yang L, Zhou M, Francois F, Blaser MJ. Bacterial biota in the human distal esophagus. Proc Natl Acad Sci USA. 2004;101:4250-4255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 337] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 57. | Yang L, Chaudhary N, Baghdadi J, Pei Z. Microbiome in reflux disorders and esophageal adenocarcinoma. Cancer J. 2014;20:207-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 58. | Blackett KL, Siddhi SS, Cleary S, Steed H, Miller MH, Macfarlane S, Macfarlane GT, Dillon JF. Oesophageal bacterial biofilm changes in gastro-oesophageal reflux disease, Barrett’s and oesophageal carcinoma: association or causality? Aliment Pharmacol Ther. 2013;37:1084-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 157] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 59. | Narikiyo M, Tanabe C, Yamada Y, Igaki H, Tachimori Y, Kato H, Muto M, Montesano R, Sakamoto H, Nakajima Y. Frequent and preferential infection of Treponema denticola, Streptococcus mitis, and Streptococcus anginosus in esophageal cancers. Cancer Sci. 2004;95:569-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 136] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 60. | Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E, Ben-Neriah Y. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1879] [Cited by in RCA: 2014] [Article Influence: 95.9] [Reference Citation Analysis (0)] |
| 61. | Yamamoto Y, Gaynor RB. Therapeutic potential of inhibition of the NF-kappaB pathway in the treatment of inflammation and cancer. J Clin Invest. 2001;107:135-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1137] [Cited by in RCA: 1166] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 62. | Hansel TT, Kharitonov SA, Donnelly LE, Erin EM, Currie MG, Moore WM, Manning PT, Recker DP, Barnes PJ. A selective inhibitor of inducible nitric oxide synthase inhibits exhaled breath nitric oxide in healthy volunteers and asthmatics. FASEB J. 2003;17:1298-1300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 144] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 63. | Buchholz BM, Chanthaphavong RS, Bauer AJ. Nonhemopoietic cell TLR4 signaling is critical in causing early lipopolysaccharide-induced ileus. J Immunol. 2009;183:6744-6753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 64. | Islami F, Kamangar F, Boffetta P. Use of proton pump inhibitors and risk of progression of Barrett’s esophagus to neoplastic lesions. Am J Gastroenterol. 2009;104:2646-2648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 65. | Uno K, Iuchi Y, Fujii J, Sugata H, Iijima K, Kato K, Shimosegawa T, Yoshimura T. In vivo study on cross talk between inducible nitric-oxide synthase and cyclooxygenase in rat gastric mucosa: effect of cyclooxygenase activity on nitric oxide production. J Pharmacol Exp Ther. 2004;309:995-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 66. | Calatayud S, García-Zaragozá E, Hernández C, Quintana E, Felipo V, Esplugues JV, Barrachina MD. Downregulation of nNOS and synthesis of PGs associated with endotoxin-induced delay in gastric emptying. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1360-G1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 67. | Corley DA, Kerlikowske K, Verma R, Buffler P. Protective association of aspirin/NSAIDs and esophageal cancer: a systematic review and meta-analysis. Gastroenterology. 2003;124:47-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 379] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 68. | Heath EI, Canto MI, Piantadosi S, Montgomery E, Weinstein WM, Herman JG, Dannenberg AJ, Yang VW, Shar AO, Hawk E. Secondary chemoprevention of Barrett’s esophagus with celecoxib: results of a randomized trial. J Natl Cancer Inst. 2007;99:545-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 133] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 69. | Gatenby PA, Ramus JR, Caygill CP, Winslet MC, Watson A. Aspirin is not chemoprotective for Barrett’s adenocarcinoma of the oesophagus in multicentre cohort. Eur J Cancer Prev. 2009;18:381-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |