Published online Nov 15, 2015. doi: 10.4291/wjgp.v6.i4.90
Peer-review started: May 27, 2015
First decision: July 3, 2015
Revised: July 27, 2015
Accepted: September 7, 2015
Article in press: September 8, 2015
Published online: November 15, 2015
Processing time: 174 Days and 22.2 Hours
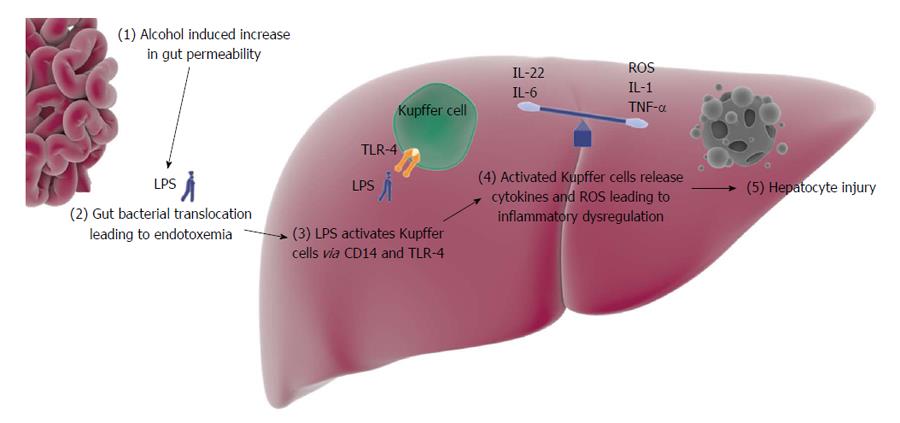
Kupffer cells play a central role in the pathogenesis of alcoholic hepatitis (AH). It is believed that alcohol increases the gut permeability that results in raised levels of serum endotoxins containing lipopolysaccharides (LPS). LPS binds to LPS-binding proteins and presents it to a membrane glycoprotein called CD14, which then activates Kupffer cells via a receptor called toll-like receptor 4. This endotoxin mediated activation of Kupffer cells plays an important role in the inflammatory process resulting in alcoholic hepatitis. There is no effective treatment for AH, although notable progress has been made over the last decade in understanding the underlying mechanism of alcoholic hepatitis. We specifically review the current research on the role of Kupffer cells in the pathogenesis of AH and the treatment strategies. We suggest that the imbalance between the pro-inflammatory and the anti-inflammatory process as well as the increased production of reactive oxygen species eventually lead to hepatocyte injury, the final event of alcoholic hepatitis.
Core tip: In this editorial we provide critical comments on the pivotal role of Kupffer cells on the development of alcoholic hepatitis with a focus on the pro-inflammatory as well as the anti-inflammatory pathways. We propose that the anti-inflammatory pathway should be further explored as a potential alternative for novel treatment strategies. This editorial is significant as it provides a platform for the future basic and clinical research in elucidating the pathogenesis and developing the management strategies of this common clinical pathology - alcoholic hepatitis.
- Citation: Suraweera DB, Weeratunga AN, Hu RW, Pandol SJ, Hu R. Alcoholic hepatitis: The pivotal role of Kupffer cells. World J Gastrointest Pathophysiol 2015; 6(4): 90-98
- URL: https://www.wjgnet.com/2150-5330/full/v6/i4/90.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v6.i4.90
Alcoholic hepatitis (AH) is defined as an acute hepatic inflammatory response to excess alcohol ingestion. It is estimated that 56809 hospital admissions in 2007 in the United States had a primary diagnosis of AH, 0.71% of all admissions[1]. In addition, hospitalization for AH is a leading cause of healthcare utilization[1]. In spite of such high costs and mortality, there has been little progress in the treatment strategies over the past 20 years. Histologically, alcoholic hepatitis is characterized by hepatocellular necrosis and immune cell infiltration around damaged hepatocytes[2]. This inflammatory and immune response leads to further hepatic injury and acute liver failure. Thus understanding this inflammatory cascade is vital to understanding alcoholic hepatitis and developing a treatment strategy. Currently there are only two pharmacologic treatments of AH: Corticosteroids and pentoxifylline. However these treatments are limited in their effectiveness and severe cases of AH still carry a short term mortality of 30%-50%[3]. Hepatic macrophages, called Kupffer cells, have been found to play a central role in hepatic inflammation[4]. Therefore, we will focus on providing a concise review of the role of Kupffer cells in AH, current treatments to disrupt this inflammatory pathway and potential basic and clinical research directions.
Kupffer cells are macrophages found in the liver. They were first identified by Kupffer[5] in 1876. Monocytes in the blood stream migrate into the liver and differentiate into Kupffer cells[6]. Kupffer cells makeup about 15% of all cells in the liver and comprise 50% of the total population of macrophages in the body[7]. They function to clear foreign matter from the portal circulation and in animal models have been shown to clear about 80%-90% of all particulate injected[8]. The particulate include immune complexes, bacterial components, endotoxins and collagen fragments. Kupffer cells can kill ingested organisms using oxygen dependent and independent mechanisms[9]. Studies in Kupffer cell depleted mice have shown that Kupffer cells play a critical role in neutrophil recruitment and granulomatous formation in the liver[10]. Kupffer cells are activated by endotoxins (Figure 1). Endotoxins are composed of the lipopolysaccharides (LPS) component of Gram-negative bacterial cell walls. LPS-binding proteins (LBPs), produced by hepatocytes, bind and present LPS to CD14, a membrane glycoprotein[9]. CD14 in turn activates Kupffer cells via a membrane complex that includes a pathogen recognition receptor called toll-like receptor 4 (TLR-4). Activated Kupffer cells release interleukin (IL)-1B, tumor necrosis factor (TNF)-α, IL-6, IL-8, macrophage chemotactic protein-1 and regulated normal T cell expressed and secreted. These cytokines, mainly TNF-α, then bind to hepatocyte receptors leading to tissue damage via oxidative stress and apoptosis[11].
Gut bacterial translocation likely plays a key role in AH. In a healthy individual, only a small quotient of gut bacterial endotoxin gets translocated into the portal blood. Alcohol ingestion has been shown to increase this endotoxin translocation[12]. Alteration of gut microflora and increased gut permeability are the driving forces behind this process. Experimentally induced bacterial overgrowth in rats has been shown to lead to increased bacterial translocation and subsequent liver injury[13]. Furthermore, evidence suggests that alcohol can alter gut microflora[14]. Jejunal aspirates of chronic alcohol abuse patients have shown increased aerobic and anaerobic bacteria[15,16]. The pathophysiology of bacterial overgrowth in chronic alcoholic patients is not clearly identified. Possible etiologies include impaired bile flow, reduced gastrointestinal motility and increased gastric pH[14,17-19]. In addition to bacterial overgrowth, alcohol can lead to intestinal dysbiosis. Animal studies have shown an increased predominance of Gram-negative bacteria in alcohol fed subjects[20,21]. Mice with antibiotic induced eradication of gut flora had decreased alcohol induced liver injury as compared to mice with intact gut flora when exposed to ethanol[22]. Similar results were found in mice that were fed with lactobacillus[23]. Intestinal decontamination with rifaximin has also shown increased liver hemodynamics and decreased incidence of hepatic encephalopathy in patients with alcoholic liver disease (ALD)[24,25]. The second component of alcohol induced endotoxemia is increased gut permeability. Alcohol is metabolized into acetaldehyde, which has been shown to open tight junctions and increase gut epithelium permeability[26,27]. Several studies have suggested the association between endotoxins and alcoholic liver injury. It was found that endotoxin levels in mice directly correlated with the severity of alcoholic liver injury[28]. Rats that had LPS administered in addition to alcohol were also shown to have worse liver injury than those exposed to ethanol alone[29]. In humans, endotoxin levels have been shown to be measurably higher in acute and chronic alcohol use[30].
Several lines of evidence suggest that Kupffer cells play an important role as inflammatory mediators in the setting of alcoholic hepatitis. TLR-4 defective rats exposed to ethanol were shown to have markedly less steatosis, inflammation, and necrosis as compared to wild-type rats[31]. Furthermore ethanol increased TNF-α in wild-type rats but failed to do so in the TLR-4 mutant rats[31]. In LBP and CD14 knockout mice, alcohol induced liver injury was also significantly reduced[31-33]. Mice in whom Kupffer cells were chemically destroyed had no alcohol induced liver injury[34]. Activated human Kupffer cells express CD163, a hemoglobin-haptoglobin scavenger surface receptor[35]. Although the function of CD163 is unknown, it has been used as a marker for macrophage activation. Studies have shown that CD163 is in fact not only elevated in ALD, but that the plasma concentration of CD163 also predicts mortality in acute liver failure[36]. In addition CD163 has been shown to be a predictor of clinical decompensation in the setting of liver cirrhosis, an independent prognostic indicator for variceal bleeds and a marker of portal hypertension[37-39]. It is important to note that a recent study comparing levels of CD163 in AH, chronic cirrhosis and healthy patients found that CD163 concentrations were 30% higher in AH patients than in chronic cirrhotic patients and 10 times higher as compared to healthy individuals[40]. Therefore, CD163 could serve as a diagnostic marker of alcoholic hepatitis as well as a potential prognosticator for patients with alcoholic hepatitis.
Kupffer cell-mediated products have been extensively studied to further characterize their association in AH. TNF-α has been identified as a key mediator in AH. Serum TNF-α have been found to correlate with endotoxemia and development of inflammation and fibrosis in patients with AH. It can even be used as a biomarker for fibrosis[41,42]. Studies have confirmed that monocytes from patients with alcoholic hepatitis had greater levels of TNF-α than healthy subjects[43]. Furthermore, analysis of liver biopsies in patients with AH have shown increased staining for TNF-α, IL-1 and IL-6[44]. Kupffer cells can also contribute to liver injury via oxidant stress. Kupffer cells in animals fed with alcohol produce free radicals. This is further supported by studies showing nicotinamide adenine dinucleotide phosphate oxidase knocked out mice demonstrated to have decreased liver necrosis and inflammation in addition to decreased nuclear factor-kappa B and TNF-α[45].
In addition to the resident Kupffer cell-mediated hepatic injury, recruited macrophages have also been shown to play a part in liver injury[46]. Murine models have shown that there is an increased accumulation of infiltrating monocytes in the setting of liver injury[47]. Recruitment of these monocytes is highly dependent on the chemokines CCL1 and CCL2. Of note, one of the major sources of CCL2 is hepatic stellate cells, which in turn are activated by the TLR-4 ligands. Mice lacking CCL2 have been shown to incur less liver injury[48]. Furthermore mice lacking CCR8, a receptor for CCL1, were also shown to be more protected from liver injury[49]. Infiltrating monocytes have been divided into two groups depending on surface protein expression, Ly6Chi and Ly6Clow. Ly6Chi monocytes exhibit a pro-inflammatory phenotype while Ly6Clow monocytes exhibit an anti-inflammatory phenotype. Mice fed with ethanol had a shift towards more Ly6Chi monocytes, resulting in significantly increased liver injury[50]. There is still much to be learned about the role and function of infiltrating monocytes in liver injury.
Kupffer cells have been shown to play central roles in other causes of liver injury such as nonalcoholic steatohepatitis (NASH) and viral hepatitis that are often also present in AH patients. Using a murine model of NASH, several studies have shown that sequential depletion of Kupffer cells reduced the incidence of steatosis[51-53]. Furthermore, targeted knockdown of TNF-α also decreased the incidence of NASH development[51,54]. Current understanding of the role of Kupffer cells in viral hepatitis is limited. Identification of a specific pathogenesis has been difficult due to similar characteristics of recruited macrophages and resident Kupffer cells. A recent study suggests that Kupffer cell interaction with hepatitis B surface antigen leads to pro-inflammatory cytokine production, which may contribute to liver pathology[55]. Studies have shown increased numbers of Kupffer cells during hepatitis C viral (HCV) infection[56]. Incubation of HCV E2 envelop protein with human liver cells resulted in Kupffer cell binding in a CD81-dependent manner[57]. In addition HCV core and NS3 stimulate human CD14+ Kupffer cells and monocyte derived macrophages to produce IL-1β, IL-6 and TNF-α[58,59]. It is likely that Kupffer cell activation contributes to the progression of liver disease in viral hepatitis. Increased numbers of Kupffer cells have been found in regions of liver fibrosis in the setting of chronic viral hepatitis[60]. Viral hepatitis has also been shown to induce Kupffer cells to release cytotoxic molecules that kill not only infected hepatocytes but also non-infected cells[61,62]. It is likely that Kupffer cells are involved in the pathogenesis of many types of liver pathologies and it may be the case that their activation is multifactorial in patients with AH as well as other hepatic comorbidities.
AH is an acute process and most patients will recover with nutritional support and abstinence from alcohol. However severe AH carries a high mortality rate: 35% at 28 d without effective treatment[63]. These high mortality rates are predominantly due to a lack of effective treatment for severe AH. Multiple clinical trials for treatment of alcoholic hepatitis have been published (Table 1). The American Association for the Study of Liver Diseases (AASLD) guidelines for management of AH currently stratifies the management depending on severity. Low risk patients are managed conservatively with nutrition, supportive care and close monitoring. High-risk individuals, defined as those with a Maddrey’s discriminant function greater than or equal to 32 or a model for end-stage liver disease score greater than or equal to 18, may benefit from pharmacological intervention with either prednisolone or pentoxifylline. Corticosteroids have been extensively studied with mixed results[63-67]. This is likely due to the fact that study design, severity of AH and exclusions criteria vary greatly between studies. One meta-analysis showed survival rates of 80% at 28 d with corticosteroids vs 66% in the control group in patients with severe AH[63]. Corticosteroids presumably improved outcomes by decreasing pro-inflammatory cytokines. Pentoxifylline is a nonselective phosphodiesterase inhibitor that increases intracellular concentration of adenosine 3’, 5’-cyclic monophosphate, which in turn inhibits the expression of pro-inflammatory cytokines[68]. AASLD recommends pentoxifylline as an alternative to corticosteroids when the use of steroids is contraindicated or in the setting of early renal failure. According to one randomized, double-blinded, placebo controlled trial, patients treated with pentoxifylline had a survival benefit (24.5% mortality vs 46.1% in the placebo group)[69]. Although multiple clinical trials have shown some benefit of treatment with steroids or pentoxifylline, a recent well designed, multicenter, double-blinded, randomized trial found no statistically significant mortality benefit in treatment with either pentoxifylline or prednisolone[70]. The study involved 1053 patients who were randomized to four arms: A group that received a pentoxifylline-matched placebo and a prednisolone-matched placebo, a group that received prednisolone and a pentoxifylline-matched placebo, a group that received pentoxifylline and a prednisolone-matched placebo, or a group that received both prednisolone and pentoxifylline. The prednisolone group was the only group associated with an initial reduction in 28-d mortality. However at 90 d and at 1 year there were no significant differences between the groups. There is no doubt that this well designed study certainly questions the currently established treatments of AH.
| Study | Topic | Methods | Findings |
| Prednisolone or pentoxifylline | |||
| Theodossi et al[99] | PRED vs placebo | Randomized control | No difference in mortality |
| Ramond et al[100] | PRED vs placebo | Double-blinded, randomized control | Improved mortality with PRED |
| Akriviadis et al[69] | PTX vs placebo | Double-blinded, randomized control | Improved mortality with PTX |
| Sidhu et al[101] | PTX vs placebo | Randomized control | Improved mortality with PTX |
| De et al[102] | PTX vs PRED | Double-blinded, randomized control | Reduced mortality with PTX |
| Park et al[103] | PTX vs PRED | Randomized control | Reduced mortality with PRED |
| Mathurin et al[104] | PRED vs PRED + PTX | Multicenter, double-blinded, randomized control | No difference in mortality |
| De et al[105] | PTX vs PTX + PRED | Double-blinded, randomized control | No difference in mortality |
| Thursz et al[70] | PTX vs PRED vs placebo | Multicenter, double-blinded, randomized control | No difference in mortality |
| N-acetylcysteine | |||
| Moreno et al[106] | NAC vs placebo | Multicenter, single-blinded, randomized control | No difference in mortality |
| Cytokine inhibitors | |||
| Naveau et al[72] | Infliximab vs placebo | Double-blinded, randomized control | Increased mortality with infliximab |
| Boetticher et al[71] | Etancercept vs placebo | Multicenter, single-blinded, randomized control | Increased mortality with etancercept |
While cytokine inhibitors have great potential in theory, trials with both infliximab and etanercept have resulted in increased mortality, primarily due to infection[71,72]. Liver transplantation is another treatment option in ALD. Most transplant centers require at least 6-months of abstinence[73,74]. This allows for disease regression in patients with recent alcohol use, time for proper counseling and demonstrates patients’ ability to abstain from alcohol. One meta-analysis comparing alcohol use in post-transplant patients showed no difference in the proportion of patients that used alcohol when comparing ALD to non-ALD patients, although ALD patients were more likely to drink excessively[75]. Risk of alcohol recurrence in ALD transplant patients continues to be an area of debate. In summary, treatment options for AH are limited with even the standard of care now being questioned, emphasizing the urgent need for effective and novel treatment strategies.
Identification of new therapeutic targets has been hampered by a lack of appropriate animal models. Current animal models do not develop severe liver injury as humans do. One possible area of future investigations would be the modulation of the LPS pathway. A recent study evaluating the effects of milk osteopontin on gut permeability found that milk osteopontin preserved gut architecture and prevented inflammation in ethanol fed mice[76]. Milk osteopontin has also been shown to directly bind to LPS and prevent Kupffer cell activation thereby disrupting the subsequent pro-inflammatory cascade[77]. Another study used probiotics to alter gut flora and TLR4 antagonists, which have been proposed for treatment of ALD[78].
Genetic factors leading to the predisposition for liver disease is another promising area of exploration in recent years. A number of studies have shown an association between variations in the PNPLA3 gene and liver fat content as well as plasma aspartate aminotransferase[79-82]. Furthermore two groups have independently found associations between the PNPLA3 single-nucleotide polymorphism rs738409 and ALD populations in Mexico and Germany[83,84]. During the last decade, a prominent area of research had been the inhibition of pro-inflammatory cytokines. However blocking TNF-α had led to unacceptable complications. More targeted inhibition using dexamethasone conjugates targeting the CD163 receptor on macrophages have shown some success in rats[85,86]. Yet another unique way of managing inflammation in AH patients is apheresis. A recent case series and literature review of 35 cases concluded that leukocytapheresisand granulocytapheresiswere effective in controlling leukocytosis as wells as inflammatory cytokines[87].
In contrast to pro-inflammatory cytokines, Kupffer cells also produce anti-inflammatory or hepato-protective cytokines, such as IL-6 and IL-22[88] (Figure 1). Activated Kupffer cells release IL-6, which then stimulates signal transducer and activator of transcription 3 (STAT3) leading to increased expression of genes that are anti-apoptotic, anti-oxidative, and promote mitochondrial DNA repair[89,90]. Studies have shown that IL-6 deficient mice are in fact more susceptible to hepatic steatosis, cellular apoptosis and mitochondrial DNA damage when exposed to ethanol[89-91]. Furthermore STAT3 knockout mice have been shown to have greater degree of hepatic steatosis as compared to wild-type mice[92]. Ethanol induced liver injury was alleviated by treatment with IL-6[93]. IL-22 is another hepato-protective cytokine that has been found to ameliorate hepatocellular damage in fatty liver as well as acute and chronic alcoholic liver injury[94-97]. It is believed that both IL-6 and IL-22 share the same pathway, STAT3 mediated hepatoprotection[96].
Another potentially important observation relevant to alcoholic hepatitis is a recently reported finding that the administration of lactate reduced inflammation and organ injury in mice with an immune mediated hepatitis[98]. Lactate interacted with the specific receptor G protein-coupled receptor 81 (GPR 81) to reduce inflammation and injury. Further, lactate and GPR 81 prevented LPS-induced macrophage activation (Kuppfer cells) suggesting that the beneficial effects were mediated by the effects of lactate on activated macrophages. These results suggest that hepatic injury due to macrophage activation may be treated by ligands including lactate that interact with GPR 81.
AH is a major cause of morbidity and mortality worldwide. The underlying mechanisms are poorly understood, which has resulted in a lack of specific treatments. The absence of animal models further hampered the progress in elucidating the molecular mechanisms which may provide scientific evidence for designing more targeted treatment strategies. Given the inconsistent results of currently available treatment strategies, which mainly target the pro-inflammatory process, we speculate that it is also important to recognize the potential effort of targeting the anti-inflammatory pathway or targeting both the anti and the pro-inflammatory pathways simultaneously. With the recognition of the anti-inflammatory process mediated by Kupffer cells, it may be the prime time for a well-designed clinical trial to target the unique anti-inflammatory pathway. This may lead to the development of novel effective treatment strategies for this common clinical entity.
P- Reviewer: Baffy G, Morini S, Paydas S S- Editor: Tian YL L- Editor: A E- Editor: Liu SQ
| 1. | Liangpunsakul S. Clinical characteristics and mortality of hospitalized alcoholic hepatitis patients in the United States. J Clin Gastroenterol. 2011;45:714-719. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 98] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 2. | Lefkowitch JH. Morphology of alcoholic liver disease. Clin Liver Dis. 2005;9:37-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 139] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 3. | O’Shea RS, Dasarathy S, McCullough AJ. Alcoholic liver disease. Hepatology. 2010;51:307-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 837] [Cited by in RCA: 852] [Article Influence: 56.8] [Reference Citation Analysis (2)] |
| 4. | Chedid A, Arain S, Snyder A, Mathurin P, Capron F, Naveau S. The immunology of fibrogenesis in alcoholic liver disease. Arch Pathol Lab Med. 2004;128:1230-1238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Kupffer C. Ueber Sternzellen der Leber. Archiv fmikrosk Anat. 1876;12:353-358. [RCA] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 117] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | van Furth R, Cohn ZA, Hirsch JG, Humphrey JH, Spector WG, Langevoort HL. The mononuclear phagocyte system: a new classification of macrophages, monocytes, and their precursor cells. Bull World Health Organ. 1972;46:845-852. [PubMed] |
| 7. | Wisse E. Kupffer cell reactions in rat liver under various conditions as observed in the electron microscope. J Ultrastruct Res. 1974;46:499-520. [PubMed] |
| 8. | Benacerraf B, Sebestyen MM, Schlossman S. A quantitative study of the kinetics of blood clearance of P32-labelled Escherichia coli and Staphylococci by the reticuloendothelial system. J Exp Med. 1959;110:27-48. [PubMed] |
| 9. | Gao B, Seki E, Brenner DA, Friedman S, Cohen JI, Nagy L, Szabo G, Zakhari S. Innate immunity in alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol. 2011;300:G516-G525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 184] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 10. | Ebe Y, Hasegawa G, Takatsuka H, Umezu H, Mitsuyama M, Arakawa M, Mukaida N, Naito M. The role of Kupffer cells and regulation of neutrophil migration into the liver by macrophage inflammatory protein-2 in primary listeriosis in mice. Pathol Int. 1999;49:519-532. [PubMed] |
| 11. | Bradham CA, Plümpe J, Manns MP, Brenner DA, Trautwein C. Mechanisms of hepatic toxicity. I. TNF-induced liver injury. Am J Physiol. 1998;275:G387-G392. [PubMed] |
| 12. | Fujimoto M, Uemura M, Nakatani Y, Tsujita S, Hoppo K, Tamagawa T, Kitano H, Kikukawa M, Ann T, Ishii Y. Plasma endotoxin and serum cytokine levels in patients with alcoholic hepatitis: relation to severity of liver disturbance. Alcohol Clin Exp Res. 2000;24:48S-54S. [PubMed] |
| 13. | Lichtman SN, Sartor RB, Keku J, Schwab JH. Hepatic inflammation in rats with experimental small intestinal bacterial overgrowth. Gastroenterology. 1990;98:414-423. [PubMed] |
| 14. | Bode C, Bode JC. Effect of alcohol consumption on the gut. Best Pract Res Clin Gastroenterol. 2003;17:575-592. [PubMed] |
| 15. | Casafont Morencos F, de las Heras Castaño G, Martín Ramos L, López Arias MJ, Ledesma F, Pons Romero F. Small bowel bacterial overgrowth in patients with alcoholic cirrhosis. Dig Dis Sci. 1996;41:552-556. [PubMed] |
| 16. | Bode JC, Bode C, Heidelbach R, Dürr HK, Martini GA. Jejunal microflora in patients with chronic alcohol abuse. Hepatogastroenterology. 1984;31:30-34. [PubMed] |
| 17. | Inagaki T, Moschetta A, Lee YK, Peng L, Zhao G, Downes M, Yu RT, Shelton JM, Richardson JA, Repa JJ. Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proc Natl Acad Sci USA. 2006;103:3920-3925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 744] [Cited by in RCA: 894] [Article Influence: 47.1] [Reference Citation Analysis (0)] |
| 18. | Bauer TM, Steinbrückner B, Brinkmann FE, Ditzen AK, Schwacha H, Aponte JJ, Pelz K, Kist M, Blum HE. Small intestinal bacterial overgrowth in patients with cirrhosis: prevalence and relation with spontaneous bacterial peritonitis. Am J Gastroenterol. 2001;96:2962-2967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 181] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 19. | Shindo K, Machida M, Miyakawa K, Fukumura M. A syndrome of cirrhosis, achlorhydria, small intestinal bacterial overgrowth, and fat malabsorption. Am J Gastroenterol. 1993;88:2084-2091. [PubMed] |
| 20. | Mutlu E, Keshavarzian A, Engen P, Forsyth CB, Sikaroodi M, Gillevet P. Intestinal dysbiosis: a possible mechanism of alcohol-induced endotoxemia and alcoholic steatohepatitis in rats. Alcohol Clin Exp Res. 2009;33:1836-1846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 295] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 21. | Yan AW, Fouts DE, Brandl J, Stärkel P, Torralba M, Schott E, Tsukamoto H, Nelson KE, Brenner DA, Schnabl B. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology. 2011;53:96-105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 668] [Cited by in RCA: 638] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 22. | Adachi Y, Moore LE, Bradford BU, Gao W, Thurman RG. Antibiotics prevent liver injury in rats following long-term exposure to ethanol. Gastroenterology. 1995;108:218-224. [PubMed] |
| 23. | Nanji AA, Khettry U, Sadrzadeh SM. Lactobacillus feeding reduces endotoxemia and severity of experimental alcoholic liver (disease). Proc Soc Exp Biol Med. 1994;205:243-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 303] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 24. | Bass NM, Mullen KD, Sanyal A, Poordad F, Neff G, Leevy CB, Sigal S, Sheikh MY, Beavers K, Frederick T. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362:1071-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 868] [Cited by in RCA: 868] [Article Influence: 57.9] [Reference Citation Analysis (0)] |
| 25. | Vlachogiannakos J, Saveriadis AS, Viazis N, Theodoropoulos I, Foudoulis K, Manolakopoulos S, Raptis S, Karamanolis DG. Intestinal decontamination improves liver haemodynamics in patients with alcohol-related decompensated cirrhosis. Aliment Pharmacol Ther. 2009;29:992-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 138] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 26. | Ma TY, Nguyen D, Bui V, Nguyen H, Hoa N. Ethanol modulation of intestinal epithelial tight junction barrier. Am J Physiol. 1999;276:G965-G974. [PubMed] |
| 27. | Rao R. Endotoxemia and gut barrier dysfunction in alcoholic liver disease. Hepatology. 2009;50:638-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 375] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 28. | Nanji AA, Khettry U, Sadrzadeh SM, Yamanaka T. Severity of liver injury in experimental alcoholic liver disease. Correlation with plasma endotoxin, prostaglandin E2, leukotriene B4, and thromboxane B2. Am J Pathol. 1993;142:367-373. [PubMed] |
| 29. | Mathurin P, Deng QG, Keshavarzian A, Choudhary S, Holmes EW, Tsukamoto H. Exacerbation of alcoholic liver injury by enteral endotoxin in rats. Hepatology. 2000;32:1008-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 230] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 30. | Parlesak A, Schäfer C, Schütz T, Bode JC, Bode C. Increased intestinal permeability to macromolecules and endotoxemia in patients with chronic alcohol abuse in different stages of alcohol-induced liver disease. J Hepatol. 2000;32:742-747. [PubMed] |
| 31. | Uesugi T, Froh M, Arteel GE, Bradford BU, Thurman RG. Toll-like receptor 4 is involved in the mechanism of early alcohol-induced liver injury in mice. Hepatology. 2001;34:101-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 395] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 32. | Yin M, Bradford BU, Wheeler MD, Uesugi T, Froh M, Goyert SM, Thurman RG. Reduced early alcohol-induced liver injury in CD14-deficient mice. J Immunol. 2001;166:4737-4742. [PubMed] |
| 33. | Uesugi T, Froh M, Arteel GE, Bradford BU, Wheeler MD, Gäbele E, Isayama F, Thurman RG. Role of lipopolysaccharide-binding protein in early alcohol-induced liver injury in mice. J Immunol. 2002;168:2963-2969. [PubMed] |
| 34. | Adachi Y, Bradford BU, Gao W, Bojes HK, Thurman RG. Inactivation of Kupffer cells prevents early alcohol-induced liver injury. Hepatology. 1994;20:453-460. [PubMed] |
| 35. | Møller HJ. Soluble CD163. Scand J Clin Lab Invest. 2012;72:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 291] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 36. | Møller HJ, Grønbaek H, Schiødt FV, Holland-Fischer P, Schilsky M, Munoz S, Hassanein T, Lee WM. Soluble CD163 from activated macrophages predicts mortality in acute liver failure. J Hepatol. 2007;47:671-676. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 120] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 37. | Grønbaek H, Sandahl TD, Mortensen C, Vilstrup H, Møller HJ, Møller S. Soluble CD163, a marker of Kupffer cell activation, is related to portal hypertension in patients with liver cirrhosis. Aliment Pharmacol Ther. 2012;36:173-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 121] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 38. | Rode A, Nicoll A, Møller HJ, Lim L, Angus PW, Kronborg I, Arachchi N, Gorelik A, Liew D, Kazankov K. Hepatic macrophage activation predicts clinical decompensation in chronic liver disease. Gut. 2013;62:1231-1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 39. | Waidmann O, Brunner F, Herrmann E, Zeuzem S, Piiper A, Kronenberger B. Macrophage activation is a prognostic parameter for variceal bleeding and overall survival in patients with liver cirrhosis. J Hepatol. 2013;58:956-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 40. | Sandahl TD, Grønbaek H, Møller HJ, Støy S, Thomsen KL, Dige AK, Agnholt J, Hamilton-Dutoit S, Thiel S, Vilstrup H. Hepatic macrophage activation and the LPS pathway in patients with alcoholic hepatitis: a prospective cohort study. Am J Gastroenterol. 2014;109:1749-1756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 41. | Kliaritskaia IL, Stilidi EI. [Pathogenetic importance of proinflammatory cytokines in the formation and progression of fibrosis in alcoholic hepatitis]. Eksp Klin Gastroenterol. 2013;13-20. [PubMed] |
| 42. | Hanck C, Rossol S, Böcker U, Tokus M, Singer MV. Presence of plasma endotoxin is correlated with tumour necrosis factor receptor levels and disease activity in alcoholic cirrhosis. Alcohol Alcohol. 1998;33:606-608. [PubMed] |
| 43. | McClain CJ, Barve S, Barve S, Deaciuc I, Hill DB. Tumor necrosis factor and alcoholic liver disease. Alcohol Clin Exp Res. 1998;22:248S-252S. [PubMed] |
| 44. | McClain CJ, Barve S, Deaciuc I, Kugelmas M, Hill D. Cytokines in alcoholic liver disease. Semin Liver Dis. 1999;19:205-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 291] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 46. | Szabo G, Mandrekar P, Dolganiuc A. Innate immune response and hepatic inflammation. Semin Liver Dis. 2007;27:339-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 146] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 47. | Karlmark KR, Weiskirchen R, Zimmermann HW, Gassler N, Ginhoux F, Weber C, Merad M, Luedde T, Trautwein C, Tacke F. Hepatic recruitment of the inflammatory Gr1+ monocyte subset upon liver injury promotes hepatic fibrosis. Hepatology. 2009;50:261-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 640] [Cited by in RCA: 623] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 48. | Galastri S, Zamara E, Milani S, Novo E, Provenzano A, Delogu W, Vizzutti F, Sutti S, Locatelli I, Navari N. Lack of CC chemokine ligand 2 differentially affects inflammation and fibrosis according to the genetic background in a murine model of steatohepatitis. Clin Sci (Lond). 2012;123:459-471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 49. | Heymann F, Hammerich L, Storch D, Bartneck M, Huss S, Rüsseler V, Gassler N, Lira SA, Luedde T, Trautwein C. Hepatic macrophage migration and differentiation critical for liver fibrosis is mediated by the chemokine receptor C-C motif chemokine receptor 8 in mice. Hepatology. 2012;55:898-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 147] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 50. | Wang M, You Q, Lor K, Chen F, Gao B, Ju C. Chronic alcohol ingestion modulates hepatic macrophage populations and functions in mice. J Leukoc Biol. 2014;96:657-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 125] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 51. | Tosello-Trampont AC, Landes SG, Nguyen V, Novobrantseva TI, Hahn YS. Kuppfer cells trigger nonalcoholic steatohepatitis development in diet-induced mouse model through tumor necrosis factor-α production. J Biol Chem. 2012;287:40161-40172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 350] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 52. | Huang W, Metlakunta A, Dedousis N, Zhang P, Sipula I, Dube JJ, Scott DK, O’Doherty RM. Depletion of liver Kupffer cells prevents the development of diet-induced hepatic steatosis and insulin resistance. Diabetes. 2010;59:347-357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 365] [Cited by in RCA: 420] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 53. | Lanthier N, Molendi-Coste O, Cani PD, van Rooijen N, Horsmans Y, Leclercq IA. Kupffer cell depletion prevents but has no therapeutic effect on metabolic and inflammatory changes induced by a high-fat diet. FASEB J. 2011;25:4301-4311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 54. | Tomita K, Tamiya G, Ando S, Ohsumi K, Chiyo T, Mizutani A, Kitamura N, Toda K, Kaneko T, Horie Y. Tumour necrosis factor alpha signalling through activation of Kupffer cells plays an essential role in liver fibrosis of non-alcoholic steatohepatitis in mice. Gut. 2006;55:415-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 352] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 55. | Boltjes A, van Montfoort N, Biesta PJ, Op den Brouw ML, Kwekkeboom J, van der Laan LJ, Janssen HL, Boonstra A, Woltman AM. Kupffer cells interact with hepatitis B surface antigen in vivo and in vitro, leading to proinflammatory cytokine production and natural killer cell function. J Infect Dis. 2015;211:1268-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 56. | Khakoo SI, Soni PN, Savage K, Brown D, Dhillon AP, Poulter LW, Dusheiko GM. Lymphocyte and macrophage phenotypes in chronic hepatitis C infection. Correlation with disease activity. Am J Pathol. 1997;150:963-970. [PubMed] |
| 57. | Petracca R, Falugi F, Galli G, Norais N, Rosa D, Campagnoli S, Burgio V, Di Stasio E, Giardina B, Houghton M. Structure-function analysis of hepatitis C virus envelope-CD81 binding. J Virol. 2000;74:4824-4830. [PubMed] |
| 58. | Tu Z, Pierce RH, Kurtis J, Kuroki Y, Crispe IN, Orloff MS. Hepatitis C virus core protein subverts the antiviral activities of human Kupffer cells. Gastroenterology. 2010;138:305-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 59. | Chang S, Dolganiuc A, Szabo G. Toll-like receptors 1 and 6 are involved in TLR2-mediated macrophage activation by hepatitis C virus core and NS3 proteins. J Leukoc Biol. 2007;82:479-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 163] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 60. | Sandler NG, Koh C, Roque A, Eccleston JL, Siegel RB, Demino M, Kleiner DE, Deeks SG, Liang TJ, Heller T. Host response to translocated microbial products predicts outcomes of patients with HBV or HCV infection. Gastroenterology. 2011;141:1220-1230, 1230.e1-3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 250] [Cited by in RCA: 252] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 61. | Tordjmann T, Soulie A, Guettier C, Schmidt M, Berthou C, Beaugrand M, Sasportes M. Perforin and granzyme B lytic protein expression during chronic viral and autoimmune hepatitis. Liver. 1998;18:391-397. [PubMed] |
| 62. | Kolios G, Valatas V, Kouroumalis E. Role of Kupffer cells in the pathogenesis of liver disease. World J Gastroenterol. 2006;12:7413-7420. [PubMed] |
| 63. | Mathurin P, O’Grady J, Carithers RL, Phillips M, Louvet A, Mendenhall CL, Ramond MJ, Naveau S, Maddrey WC, Morgan TR. Corticosteroids improve short-term survival in patients with severe alcoholic hepatitis: meta-analysis of individual patient data. Gut. 2011;60:255-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 305] [Article Influence: 21.8] [Reference Citation Analysis (1)] |
| 64. | Christensen E, Gluud C. Glucocorticoids are ineffective in alcoholic hepatitis: a meta-analysis adjusting for confounding variables. Gut. 1995;37:113-118. [PubMed] |
| 65. | Daures JP, Peray P, Bories P, Blanc P, Yousfi A, Michel H, Gremy F. [Corticoid therapy in the treatment of acute alcoholic hepatitis. Results of a meta-analysis]. Gastroenterol Clin Biol. 1991;15:223-228. [PubMed] |
| 66. | Imperiale TF, McCullough AJ. Do corticosteroids reduce mortality from alcoholic hepatitis? A meta-analysis of the randomized trials. Ann Intern Med. 1990;113:299-307. [PubMed] |
| 67. | Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572-1585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1244] [Cited by in RCA: 1495] [Article Influence: 106.8] [Reference Citation Analysis (0)] |
| 68. | Strieter RM, Remick DG, Ward PA, Spengler RN, Lynch JP, Larrick J, Kunkel SL. Cellular and molecular regulation of tumor necrosis factor-alpha production by pentoxifylline. Biochem Biophys Res Commun. 1988;155:1230-1236. [PubMed] |
| 69. | Akriviadis E, Botla R, Briggs W, Han S, Reynolds T, Shakil O. Pentoxifylline improves short-term survival in severe acute alcoholic hepatitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000;119:1637-1648. [PubMed] |
| 70. | Thursz MR, Richardson P, Allison M, Austin A, Bowers M, Day CP, Downs N, Gleeson D, MacGilchrist A, Grant A. Prednisolone or pentoxifylline for alcoholic hepatitis. N Engl J Med. 2015;372:1619-1628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 526] [Cited by in RCA: 556] [Article Influence: 55.6] [Reference Citation Analysis (1)] |
| 71. | Boetticher NC, Peine CJ, Kwo P, Abrams GA, Patel T, Aqel B, Boardman L, Gores GJ, Harmsen WS, McClain CJ. A randomized, double-blinded, placebo-controlled multicenter trial of etanercept in the treatment of alcoholic hepatitis. Gastroenterology. 2008;135:1953-1960. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 265] [Cited by in RCA: 255] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 72. | Naveau S, Chollet-Martin S, Dharancy S, Mathurin P, Jouet P, Piquet MA, Davion T, Oberti F, Broët P, Emilie D. A double-blind randomized controlled trial of infliximab associated with prednisolone in acute alcoholic hepatitis. Hepatology. 2004;39:1390-1397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 322] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 73. | Beresford TP, Everson GT. Liver transplantation for alcoholic liver disease: bias, beliefs, 6-month rule, and relapse--but where are the data? Liver Transpl. 2000;6:777-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 74. | Consensus conference: Indications for Liver Transplantation, January 19 and 20, 2005, Lyon-Palais Des Congrès: text of recommendations (long version). Liver Transpl. 2006;12:998-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 75] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 75. | Bravata DM, Olkin I, Barnato AE, Keeffe EB, Owens DK. Employment and alcohol use after liver transplantation for alcoholic and nonalcoholic liver disease: a systematic review. Liver Transpl. 2001;7:191-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 64] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 76. | Ge X, Lu Y, Leung TM, Sørensen ES, Nieto N. Milk osteopontin, a nutritional approach to prevent alcohol-induced liver injury. Am J Physiol Gastrointest Liver Physiol. 2013;304:G929-G939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 77. | Ge X, Leung TM, Arriazu E, Lu Y, Urtasun R, Christensen B, Fiel MI, Mochida S, Sørensen ES, Nieto N. Osteopontin binding to lipopolysaccharide lowers tumor necrosis factor-α and prevents early alcohol-induced liver injury in mice. Hepatology. 2014;59:1600-1616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 78. | Mencin A, Kluwe J, Schwabe RF. Toll-like receptors as targets in chronic liver diseases. Gut. 2009;58:704-720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 273] [Cited by in RCA: 268] [Article Influence: 16.8] [Reference Citation Analysis (1)] |
| 79. | Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, Boerwinkle E, Cohen JC, Hobbs HH. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461-1465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2701] [Cited by in RCA: 2596] [Article Influence: 152.7] [Reference Citation Analysis (0)] |
| 80. | Yuan X, Waterworth D, Perry JR, Lim N, Song K, Chambers JC, Zhang W, Vollenweider P, Stirnadel H, Johnson T. Population-based genome-wide association studies reveal six loci influencing plasma levels of liver enzymes. Am J Hum Genet. 2008;83:520-528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 360] [Cited by in RCA: 360] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 81. | Kotronen A, Johansson LE, Johansson LM, Roos C, Westerbacka J, Hamsten A, Bergholm R, Arkkila P, Arola J, Kiviluoto T. A common variant in PNPLA3, which encodes adiponutrin, is associated with liver fat content in humans. Diabetologia. 2009;52:1056-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 247] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 82. | Kollerits B, Coassin S, Beckmann ND, Teumer A, Kiechl S, Döring A, Kavousi M, Hunt SC, Lamina C, Paulweber B. Genetic evidence for a role of adiponutrin in the metabolism of apolipoprotein B-containing lipoproteins. Hum Mol Genet. 2009;18:4669-4676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 83. | Tian C, Stokowski RP, Kershenobich D, Ballinger DG, Hinds DA. Variant in PNPLA3 is associated with alcoholic liver disease. Nat Genet. 2010;42:21-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 343] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 84. | Stickel F, Buch S, Lau K, Meyer zu Schwabedissen H, Berg T, Ridinger M, Rietschel M, Schafmayer C, Braun F, Hinrichsen H. Genetic variation in the PNPLA3 gene is associated with alcoholic liver injury in caucasians. Hepatology. 2011;53:86-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 217] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 85. | Graversen JH, Svendsen P, Dagnæs-Hansen F, Dal J, Anton G, Etzerodt A, Petersen MD, Christensen PA, Møller HJ, Moestrup SK. Targeting the hemoglobin scavenger receptor CD163 in macrophages highly increases the anti-inflammatory potency of dexamethasone. Mol Ther. 2012;20:1550-1558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 111] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 86. | Granfeldt A, Hvas CL, Graversen JH, Christensen PA, Petersen MD, Anton G, Svendsen P, Sølling C, Etzerodt A, Tønnesen E. Targeting dexamethasone to macrophages in a porcine endotoxemic model. Crit Care Med. 2013;41:e309-e318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 87. | Kamimura K, Imai M, Sakamaki A, Mori S, Kobayashi M, Mizuno K, Takeuchi M, Suda T, Nomoto M, Aoyagi Y. Granulocytapheresis for the treatment of severe alcoholic hepatitis: a case series and literature review. Dig Dis Sci. 2014;59:482-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 88. | Miller AM, Horiguchi N, Jeong WI, Radaeva S, Gao B. Molecular mechanisms of alcoholic liver disease: innate immunity and cytokines. Alcohol Clin Exp Res. 2011;35:787-793. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 145] [Article Influence: 10.4] [Reference Citation Analysis (2)] |
| 89. | Hong F, Kim WH, Tian Z, Jaruga B, Ishac E, Shen X, Gao B. Elevated interleukin-6 during ethanol consumption acts as a potential endogenous protective cytokine against ethanol-induced apoptosis in the liver: involvement of induction of Bcl-2 and Bcl-x(L) proteins. Oncogene. 2002;21:32-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 143] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 90. | Zhang X, Tachibana S, Wang H, Hisada M, Williams GM, Gao B, Sun Z. Interleukin-6 is an important mediator for mitochondrial DNA repair after alcoholic liver injury in mice. Hepatology. 2010;52:2137-2147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 91. | El-Assal O, Hong F, Kim WH, Radaeva S, Gao B. IL-6-deficient mice are susceptible to ethanol-induced hepatic steatosis: IL-6 protects against ethanol-induced oxidative stress and mitochondrial permeability transition in the liver. Cell Mol Immunol. 2004;1:205-211. [PubMed] |
| 92. | Horiguchi N, Wang L, Mukhopadhyay P, Park O, Jeong WI, Lafdil F, Osei-Hyiaman D, Moh A, Fu XY, Pacher P. Cell type-dependent pro- and anti-inflammatory role of signal transducer and activator of transcription 3 in alcoholic liver injury. Gastroenterology. 2008;134:1148-1158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 150] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 93. | Hong F, Radaeva S, Pan HN, Tian Z, Veech R, Gao B. Interleukin 6 alleviates hepatic steatosis and ischemia/reperfusion injury in mice with fatty liver disease. Hepatology. 2004;40:933-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 159] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 94. | Yang L, Zhang Y, Wang L, Fan F, Zhu L, Li Z, Ruan X, Huang H, Wang Z, Huang Z. Amelioration of high fat diet induced liver lipogenesis and hepatic steatosis by interleukin-22. J Hepatol. 2010;53:339-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 133] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 95. | Xing WW, Zou MJ, Liu S, Xu T, Wang JX, Xu DG. Interleukin-22 protects against acute alcohol-induced hepatotoxicity in mice. Biosci Biotechnol Biochem. 2011;75:1290-1294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 96. | Ki SH, Park O, Zheng M, Morales-Ibanez O, Kolls JK, Bataller R, Gao B. Interleukin-22 treatment ameliorates alcoholic liver injury in a murine model of chronic-binge ethanol feeding: role of signal transducer and activator of transcription 3. Hepatology. 2010;52:1291-1300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 364] [Cited by in RCA: 367] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 97. | Aujla SJ, Kolls JK. IL-22: a critical mediator in mucosal host defense. J Mol Med (Berl). 2009;87:451-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 131] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 98. | Hoque R, Farooq A, Ghani A, Gorelick F, Mehal WZ. Lactate reduces liver and pancreatic injury in Toll-like receptor- and inflammasome-mediated inflammation via GPR81-mediated suppression of innate immunity. Gastroenterology. 2014;146:1763-1774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 391] [Article Influence: 35.5] [Reference Citation Analysis (1)] |
| 99. | Theodossi A, Eddleston AL, Williams R. Controlled trial of methylprednisolone therapy in severe acute alcoholic hepatitis. Gut. 1982;23:75-79. [PubMed] |
| 100. | Ramond MJ, Poynard T, Rueff B, Mathurin P, Théodore C, Chaput JC, Benhamou JP. A randomized trial of prednisolone in patients with severe alcoholic hepatitis. N Engl J Med. 1992;326:507-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 258] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 101. | Sidhu SS, Goyal O, Singla M, Bhatia KL, Chhina RS, Sood A. Pentoxifylline in severe alcoholic hepatitis: a prospective, randomised trial. J Assoc Physicians India. 2012;60:20-22. [PubMed] |
| 102. | De BK, Gangopadhyay S, Dutta D, Baksi SD, Pani A, Ghosh P. Pentoxifylline versus prednisolone for severe alcoholic hepatitis: a randomized controlled trial. World J Gastroenterol. 2009;15:1613-1619. [PubMed] |
| 103. | Park SH, Kim DJ, Kim YS, Yim HJ, Tak WY, Lee HJ, Sohn JH, Yoon KT, Kim IH, Kim HS. Pentoxifylline vs. corticosteroid to treat severe alcoholic hepatitis: a randomised, non-inferiority, open trial. J Hepatol. 2014;61:792-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 104. | Mathurin P, Louvet A, Duhamel A, Nahon P, Carbonell N, Boursier J, Anty R, Diaz E, Thabut D, Moirand R. Prednisolone with vs without pentoxifylline and survival of patients with severe alcoholic hepatitis: a randomized clinical trial. JAMA. 2013;310:1033-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 146] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 105. | De B, Mandal S, Sau D, Mani S, Chatterjee S, Mondal S, Bhattacharya K, Sil K, Bhattacharya R. Pentoxifylline Plus Prednisolone versus Pentoxifylline Only for Severe Alcoholic Hepatitis: A Randomized Controlled Clinical Trial. Ann Med Health Sci Res. 2014;4:810-816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 106. | Moreno C, Langlet P, Hittelet A, Lasser L, Degré D, Evrard S, Colle I, Lemmers A, Devière J, Le Moine O. Enteral nutrition with or without N-acetylcysteine in the treatment of severe acute alcoholic hepatitis: a randomized multicenter controlled trial. J Hepatol. 2010;53:1117-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |