Published online Nov 15, 2015. doi: 10.4291/wjgp.v6.i4.169
Peer-review started: June 26, 2015
First decision: August 16, 2015
Revised: August 28, 2015
Accepted: October 12, 2015
Article in press: October 13, 2015
Published online: November 15, 2015
Processing time: 144 Days and 18.9 Hours
The impact of antibiotics on the human gut microbiota is a significant concern. Antibiotic-associated diarrhea has been on the rise for the past few decades with the increasing usage of antibiotics. Clostridium difficile infections (CDI) have become one of the most prominent types of infectious diarrheal disease, with dramatically increased incidence in both the hospital and community setting worldwide. Studies show that variability in the innate host response may in part impact upon CDI severity in patients. That being said, CDI is a disease that shows the most prominent links to alterations to the gut microbiota, in both cause and treatment. With recurrence rates still relatively high, it is important to explore alternative therapies to CDI. Fecal microbiota transplantation (FMT) and other types of bacteriotherapy have become exciting avenues of treatment for CDI. Recent clinical trials have generated excitement for the use of FMT as a therapeutic option for CDI; however, the exact components of the human gut microbiota needed for protection against CDI have remained elusive. Additional investigations on the effects of antibiotics on the human gut microbiota and subsequent CDI will help reduce the socioeconomic burden of CDI and potentially lead to new therapeutic modalities.
Core tip: Emergent literature demonstrates the critical role of the human microbiota in the susceptibility to Clostridium difficile (C. difficile) infection (CDI). Microbial communities may exert effects on the metabolic composition within the GI tract that influence CDI pathogenesis (e.g., bile salt metabolism). The identification of protective and susceptible human gut microbiomes would enable the development of screening tools to identify at-risk patients. Ultimately, the rational design of probiotic cocktails could assist in attenuating C. difficile transmission in hospital or community settings. Prevention of CDI would lead to decreased morbidity and mortality, as well as reduction of hospitalization time and health care costs associated with treatment.
-
Citation: Schenck LP, Beck PL, MacDonald JA. Gastrointestinal dysbiosis and the use of fecal microbial transplantation in
Clostridium difficile infection. World J Gastrointest Pathophysiol 2015; 6(4): 169-180 - URL: https://www.wjgnet.com/2150-5330/full/v6/i4/169.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v6.i4.169
The discovery and application of antibiotics in the early 20th century was one of the most influential breakthroughs in medical history. It has led to the treatment of many diseases and improved survival rates of serious wounds and surgeries. However, the antibiotics used to treat these infections are not always specifically targeted towards the disease-causing bacteria, as broad-spectrum antibiotics can target other bacteria, including the commensal bacteria in the human intestinal tract, known as the human gut microbiota (HGM). Furthermore, calls from various health agencies and ministries have been made for improved antibiotic stewardship, as over-prescription of antibiotics has led to escalations in antibiotic-resistant bacteria[1-4], including Clostridium difficile (C. difficile)[5]. While the increases in antibiotic resistance cannot be over looked, the indirect impact of antibiotics on the HGM is a growing problem. Antibiotic-associated diarrhea (AAD) has been on the rise for the past few decades with the increasing usage of antibiotics. C. difficile infection (CDI) has become one of the most prominent types of AAD with increasing rates in both the hospital and community setting worldwide[6-8]. This increased burden on both the patient population and healthcare costs has become an alarming predicament. Investigations into the effects of antibiotics on the HGM and subsequent CDI will help reduce this burden and potentially lead to new therapeutics to treat this emergent epidemic.
The human gastrointestinal tract houses one of the most dynamic bacterial communities on the planet, with hundreds of species and thousands of strains competing for nutrients while producing by-products that can be beneficial to the host. Additionally, the HGM plays a key role in host defense, providing a protective and competitive layer to resist growth of different pathogens. However, dysbiosis and overgrowth of microbiota has been seen in several different diseases, including inflammatory bowel disease (IBD)[9] and irritable bowel syndrome[10], allergy and asthma[11], tumorigenesis[12], nonalcoholic fatty liver disease[13], cardiovascular disease[14], autism[15,16], and obesity[17,18]. It is clear that proper maintenance and turnover of the microbiota is essential for proper health.
The HGM plays a large role in health and disease, yet until recently much of its composition and function remained unknown[19]. The HGM has been shown to play important roles in early life development, including vitamin and nutrient absorption, stimulation of intestinal angiogenesis, protection from pathogens and immune development[20]. This complex ecosystem is rapidly responding to the harsh environment within the gastrointestinal tract, including nutrient and pH fluctuations, niche competition with other bacteria, and antimicrobial peptides being excreted by the host[21]. While specific bacterial pathogens are well known to cause discrete disease, overall bacterial dysbiosis has been linked to many chronic diseases that are prevalent in society[16]. The mutualism exhibited by the HGM and its human host is paralleled by few other host-microbe interactions. Ultimately, knowledge of the HGM could be utilized to analyze disease status and therapeutic responses.
The study of the gut microbiota has been ongoing for almost a century. The earliest studies isolated animals into a germ-free state, allowing development without any influence from bacteria or their products. In answer to a challenge originally issued by Louis Pasteur, scientists at the University of Notre Dame were able to deliver guinea pigs by caesarean section and house them in germ-free containment. These guinea pigs were then bred under germ-free conditions, to provide axenic animals[22]. The hope was for the animals to be used in identifying bacterial roles in normal physiology, as well as their role in proper immune defense against bacteria, as antibiotic resistance was already a rising threat.
Bacteria from the HGM are difficult to isolate in culture, making it challenging to determine the overall microbial community. The study of the HGM has expanded in the last decade due to advances in culture-independent methods of monitoring microbial diversity. Most techniques target the 16S ribosomal RNA (16S rRNA) gene, as it contains conserved and variable regions that can be used to distinguish different bacterial species[23]. The 16S rRNA gene is approximately 1500 nucleotide base pairs in length, and contains nine conserved regions and nine variable regions (V1-V9)[23]. These variable regions have been targeted for bacterial group and species identification via different techniques. Basic techniques that began the culture-independent revolution include denaturing gradient gel electrophoresis (DGGE) and terminal restriction fragment length polymorphism (TRFLP).
Introduced in 1993, DGGE provided a snapshot of overall microbial diversity within a sample. The 16S rRNA gene is amplified in a polymerase chain reaction (PCR) and subjected to separation in polyacrylamide containing a denaturing gradient of urea and formamide[24]. This allowed DNA fragments to be separated based on melting temperature, fragment length and guanine-cytosine content, resulting in a unique band pattern. These band patterns can be compared to prepared bacterial standards, or bands can be excised and analyzed by sequencing[24]. However, multiple bacterial taxa could occupy one band in a gel such that underestimation of bacterial diversity frequently occurred[25].
TRFLP, first described in 1997, involves PCR amplification of the 16S rRNA gene using a fluorescently-labelled forward primer[26]. This reaction is followed by restriction digestion targeted towards a conserved sequence, which produces terminal-restriction fragments (TRFs). Fluorescent primers allow for quantification of the TRFs, giving an overall and relative abundance. Identification to the phyla or deeper levels was possible by comparing the TRFs to an in silico library, which was generated from the Ribosomal Database project[27].
The strengths of DGGE and TRFLP in the early 2000’s included relatively high throughput combined with lower costs in an age of expensive genomic sequencing technology. Both methods were able to provide a fingerprint of the microbial community within a fecal sample and enabled inter-sample comparisons. However, both were also associated with relatively poor resolution of the HGM community members, sometimes failing to identify bacteria beyond the phyla level. With the development of new and more cost-effective genomic sequencing [i.e., next-generation sequencing (NGS) methods[28]], the method is now at the forefront of HGM research. While sequencing of the HGM has become for readily available, the bioinformatic analysis still provides challenges for the facile interpretation of datasets. Several computing programs and pipelines have been assembled, primarily through the Human Microbiome Project, which aid in the analysis[29]. “Quantitative insights into microbial ecology”, or QIIME, has been the most prominent pipeline developed thus far, as it allows the input of thousands of sequences and navigates through sequence alignment to known databases and downstream community analysis[30]. With the development of rapid and feasible sequencing, as well as improved bioinformatic output, the study of the HGM has shifted to culture-independent dominated processes. However, the cultivation of bacteria is still considered the gold standard, and microbial DNA in samples does not indicate whether the sample came from an active or dead bacteria[31]. Nonetheless, the NGS technologies have allowed for new studies to examine the HGM in health and disease.
C. difficile is an anaerobic, spore-forming bacterium that was originally identified as a natural part of the infant microbiota[32]. However, research since the 1970’s has linked C. difficile colonization as the primary cause of antibiotic-associated and nosocomial diarrhea in the adult and elderly populations[33,34]. CDI symptoms vary amongst patients, ranging from mild to severe diarrhea (> 15 bowel movements per day), with severe cases resulting in toxic megacolon or death[35]. The CDI mortality rate has been increasing over the last decade, due to the development of hypervirulent and antibiotic-resistant strains[36,37]. C. difficile transmission has become a major problem in hospitals across the developed world, as C. difficile spores are highly resistant to normal cleaning agents, including alcohol-based hand washes.
C. difficile has risen to prominence internationally, with several large breakouts within hospitals causing the death of many patients. In 2003, an outbreak of the hypervirulent C. difficile, strain North American pulsed-field gel electrophoresis type-1 (NAP1), in Quebec hospitals caused the death of nearly 2000 people[38]. Recently, it was also demonstrated that the spread of the NAP1 strain worldwide originated from this Quebec breakout[37]. In the United States and Europe, hospital-associated CDI is estimated to incur annual healthcare costs over $4 billion[36]. Furthermore, there is increasing incidence in developing countries in Asia, although not from the NAP1 strain[7]. There are several risk factors with high association with CDI, including recent antibiotic exposure, age (> 65 years), recent hospitalization, and proton pump inhibitor use[35]. Antibiotic exposure shows the highest correlation, with odds ratios ranging from 1.31-1.87[39]. Certain antibiotics also demonstrate higher correlations, such as clindamycin[39]; indeed, this finding correlates with the history of the disease since CDI was originally referred to as clindamycin-associated pseudomembranous colitis[33]. There is also an increased threat from community-acquired CDI (CA-CDI), which is less studied compared to hospital-acquired CDI (HA-CDI), and the corresponding incidents of CA-CDI have increased approximately 5-fold over the last decade as well[40]. CA-CDI is normally defined as an outpatient presenting with C. difficile toxin-positive stool, or an inpatient presenting less than 2 d after admission. A recent study suggested that the risk factors in these patients were different compared to HA-CDI, as 36% of patients did not have recent antibiotic exposure[41]. Another investigation determined that advanced age is also not a factor, as CA-CDI patients were significantly younger compared to HA-CDI[40]. An interesting area of study is the source of C. difficile within the community, as hospitals are the normal “reservoir” Recent studies have revealed relatively common contamination of retail prepared foods, including meat, seafood and vegetables, with C. difficile spores[42-45]. While CA-CDI risk factors and sources differ from HA-CDI, the increasing rates in both are alarming.
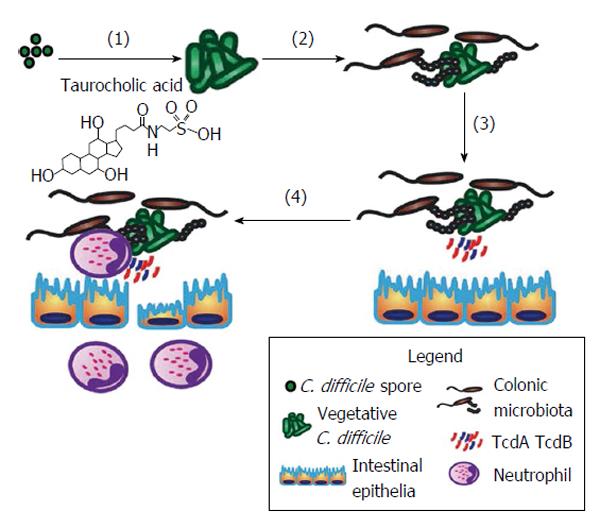
C. difficile, as a pathogen, has an interesting lifecycle that ensures its success. Although anaerobic, C. difficile can survive for months on aerobic surfaces (e.g., hospital walls, doors, surgical tools, cell phones, etc.) in spore form. The spore structure contains several layers, including an exosporium, coat, cortex, membrane, and a DNA-containing core[46]. This makes the spores resistant to alcohol-based cleaning agents used commonly in hospitals. C. difficile pathogenesis involves germination of spores into vegetative cells, colonization within the gut microbiota, productions of toxins which lead to toxin-induced intestinal damage and inflammation (Figure 1). When ingested, the multiple layers of the spore help protect it from stomach acids and digestive enzymes. Spore germination occurs upon interaction with the appropriate germinants within the intestinal tract, which include taurocholic acid, a taurine-conjugated bile acid, and glycine[47,48]. The receptor for taurocholic acid on a C. difficile spore, CspC, was only recently discovered[49], and researchers demonstrated that mutations to this protein changed germination dynamics. Indeed, mutated CspC led to decreased mortality in the hamster model of CDI. Germination results in the upregulation of several genes, and the entry into the vegetative state of the C. difficile lifecycle. This involves the breakdown of the spore cortex, core expansion and water uptake into the core, allowing an increase in enzymatic activity[50]. This complex process has not been well studied in C. difficile but has in related species[51]. Germination normally occurs within the cecum and colon as other factors in the small intestine, such as high concentration of chenodeoxycholic acid, act to suppress wide-scale germination[48].
Subsequently, C. difficile initiates the activation of the pathogenicity locus (PaLoc) in the genome. The PaLoc, approximately 19.6 kb in size, is composed of 5 genes, tcdA, tcdB, tcdC, tcdR, and tcdE, which are responsible for the production of two large clostridial toxins, A (TcdA) and B (TcdB)[52,53]. The gene tcdR, which is found upstream of the toxin genes, is a positive regulator of gene expression whereas tcdC is a negative regulator. A pore-forming holin is encoded by tcdE, which allows the release of TcdA and TcdB. Literature has suggested that hypervirulent NAP1 C. difficile released more toxin due to decreased expression of tcdC; however, this has come into question due to recent evidence that demonstrates no change in toxin production[54]. Another gene regulator is CodY, which binds and represses tcdR and thus inhibits toxin production, when essential nutrients are not available[55].
Importantly, CDI-induced colitis only occurs when either of TcdA or TcdB is present[56]. Indeed, patients with pseudomembranous colitis are C. difficile toxin-positive > 96% of the time[57]. TcdA was originally believed to be the only toxin necessary for virulence until the discovery of TcdA-/TcdB+C. difficile strain in major breakouts worldwide[58]; however, TcdA+/TcdB- strains are equally likely to cause disease[59]. Recent studies in animals have attempted to delineate the importance of each toxin in vivo; new animal model to study host response to intrarectal instillation of C. difficile toxins revealed that TcdA was important for the majority of the damage, whereas TcdB alone caused no damage to the mouse colon but could potentiate the effects of TcdA[60]. Interestingly, individuals infected with the same C. difficile strain can respond very differently. While the mechanism has not been elucidated, it has been linked to development of antibodies against TcdA and/or TcdB[61,62]. However, this theory has come into question due to the increasing recurrence rates, with recent literature demonstrating that asymptomatic carriers and diseased patients having similar antibody loads towards the toxins[63]. Differences in microbiota between patients with a single case vs recurrent CDI have been suggested to play a role in patient susceptibility and variability[64]. Different animal models have been employed to determine important bacterial and host factors involved in CDI.
C. difficile toxins induce epithelial injury, barrier dysfunction and activation of the mucosal immune system[52]. The classic endoscopic and histological feature of CDI is pseudo-membranous colitis, characterized by severe inflammation (neutrophilic/monocytic), ulceration and pseudomembranes[33]. CDI has a rapid onset and previous exposure does not confer protection. There is marked variability in disease severity in patients with similar risk factors who are infected with the same C. difficile strain. Some of the variability in responses might, in part, be due to altered innate immune pathways. For example, the nucleotide-binding domain leucine-rich repeat family of genes contains a number of intracellular innate immune receptors that respond to a variety of microbial and non-microbial danger signals. Indeed, TcdA and TcdB can trigger pro-inflammatory interleukin-1B release by activating an intracellular inflammasome[65]. A number of studies also support the activation of humoral immune responses to C. difficile toxin proteins during CDI (reviewed in[66]). Most healthy adults have detectable serum antibody titers to TcdA and TcdB (as well as non-toxin antigens) that may originate from transient environmental exposure to C. difficile from infancy[67]. The anti-toxin antibody responses (e.g., IgG) in relation to the clinical course of CDI, as well as disease recurrence, have been reported in a number of studies[62,68-71]. Immunity to TcdB may be important in the early stages of CDI; however, antigenic variation in TcdB suggests that acquired immunity may not provide cross-protection among different C. difficile strains[62]. Researchers are now examining the efficacy of protective immunity provided by vaccines against C. difficile.
The current therapeutic paradigm for CDI is the removal of the causative antibiotics (i.e., clindamycin) and treatment with vancomycin (complicated disease) or metronidazole (mild disease)[72]. Fidaxomicin is a therapeutic option for patients with recurrent CDI or a high risk of recurrence. Occasionally, when CDI progresses to toxic megacolon, colectomy is required. A recent study demonstrated that survival rates post-colectomy are low, identifying this as a last-resort in CDI treatment[73]. Oral metronidazole was the normal first-line treatment, where vancomycin is used in more severe cases or with metronidazole failure[74]. These guidelines were confirmed by a double-blind, randomized, placebo-controlled clinical trial, showing equal efficacy with vancomycin being more successful in severe patients[75]. However, recurrence of CDI, caused by relapse or re-infection, occurs in about 20%-35% of these patients[76]. Fidaxomicin is a recently introduced antibiotic that targets C. difficile more selectively and shows equal efficacy compared to vancomycin[77,78]. This is likely due to the fact that fidaxomicin has reduced effects on the commensal gut microbiota compared to vancomycin. Importantly, there was also decreased CDI recurrence rates in patients taking fidaxomicin compared to vancomycin, highlighting its role as a superior alternative antibiotic[77,78]. However, with recurrence rates still relatively high, it is important to explore alternative therapies to CDI. In this regard, fecal microbiota transplantation (FMT) and other types of bacteriotherapy have become exciting avenues for the treatment for CDI.
Identifying mechanisms by which the microbiota controls colonization by C. difficile and susceptibility to CDI, known as colonization resistance, has been pushed to the forefront of research[79-83]. C. difficile pathogenesis requires spore germination, colonization, and toxin production, which lead to the host immune response. Hypotheses for colonization resistance mechanisms include inhibiting germination, limiting important nutrients for colonization, or stimulation of the host immune response. Germination of C. difficile spores into vegetative, toxin-producing cells can be inhibited via several pathways. As previously mentioned, taurocholate is a primary bile salt that is formed in the liver and is involved in initiating the germination of C. difficile spores[84]. There are a few mechanisms by which bacteria actively modify the structure of bile salts, rendering them unable to stimulate germination. Some bacteria produce bile salt hydrolases (BSH) that catalyze the deconjugation of the amino acid (taurine or glycine) from carbon-24 (C-24) of bile salts[85]. BSHs tend to be intracellular enzymes, though one species, Clostridium perfringens, produces extracellular BSH[86]. Bile hydrolysis is quite common for different gut bacteria and has been demonstrated in Clostridia, Bacteroides, Parabacteroides, Lactobacillus, Bifidobacterium, and Enterococcus[85,87-89]. BSHs are hypothesized to be beneficial to commensal bacteria by liberating nutrients (e.g., amino acids[90]), possibly giving a competitive advantage to BSH-producing bacteria. Additional studies have demonstrated that increasing BSH gene copies in Listeria result in increased survival in vivo, while decreasing BSH expression results in decreased bacterial growth[91].
Specific members of Eubacterium and Clostridia genera have the ability to epimerize bile acids at the C-7 position (conversion of 7α- to 7β-hydroxy), converting primary bile salts (cholate and chenodeoxycholate) into secondary bile acids (deoxycholic acid and lithocholic acid, respectively) via 7α-dehydroxysteroid dehydrogenases (7α-HSDH) and 7β-HSDH[92]. These enzymes exist solely in the large intestine of humans[85]. HSDHs also exist for the C-3 and C-12 positions on bile salts. Bacteria also produce dehydroxylation enzymes, which are important in the excretion of bile salts. Interestingly, these enzymes can only function on deconjugated bile acids, requiring the activity of BSHs first[93]. This requires the bacteria to either produce BSH, or live in close proximity to a microbe that does. Taurocholate is rarely available to allow C. difficile to germinate in a healthy gut given the constant catabolism of bile salts. However, recent studies have identified that exposure to antibiotics decreases the metabolism rate of primary bile salts. Naïve mouse cecal contents incubated with C. difficile spores are unable to permit germination of C. difficile in vitro[47]. However, intraperitoneal injections of clindamycin and subsequent cecal content incubation led to C. difficile germination. This was corroborated with increased primary bile salts and decreased secondary bile salts in the cecum[47]. Additionally, incubating taurocholate with isolated cecal microbiota from naïve mice resulted in breakdown of the majority of the primary bile salt, whereas clindamycin-treated mice completely lost their ability to metabolize taurocholate. This was recently corroborated with a metabolomic study that assessed bile in cecal contents of mice[94]. Most recently, analyses of the HGM of hospitalized CDI patients identified resistance-associated bacteria[80]. Clostridium scindens (C. scindens), a bile acid 7α-dehydroxylating intestinal bacterium, was associated with colonization resistance. Probiotic administration of C. scindens provided resistance to CDI in a secondary bile acid dependent fashion. Taken together, these studies demonstrate a potential link between microbiota function, bile metabolism and CDI susceptibility.
Pharmacologic agents that target the interaction between C. difficile spores and taurocholate have also been investigated as a potential therapeutic option[95]. A bile salt analog, cholate meta-benzene sulfonic acid (CamSA), was a strong inhibitor of C. difficile germination in vitro and in vivo. In this case, CamSA (50 mg/kg) was able to completely inhibit C. difficile germination in a mouse, resulting in no CDI pathology[95]. Interestingly, a bile salt sequestrant, cholestyramine, was previously used as an adjunct therapy with antibiotics for CDI[96]. The mechanism of action was considered to be binding of C. difficile toxins but could have also been associated with C. difficile spore germination. One case study also detailed a patient with recurring CDI who was cured after prolonged cholestyramine therapy, potentially due to decreased germination[97]. Clearly, alteration of C. difficile germination is able to play a protective role in CDI.
In recent years, the aim for developing treatment for CDI has been a narrow-spectrum antibiotic against C. difficile (e.g., fidaxomicin) and microbiota sparing[75-78]. However, treatment strategies that rely on antibiotics impose strong selection for resistance as well as the disruption of the normal microbiota. Members of the gut microbiota can also produce antimicrobial compounds, termed bacteriocins, which target a narrow range of bacterial species. Researchers in Ireland identified a C. difficile-targeting bacteriocin, Thuricin CD, produced by Bacillus thuringensis[98]. Thuricin CD was shown to be as effective as antibiotics in vitro for the elimination of C. difficile, while also having limited impact on the host microbiota. The group recently published that intrarectal instillation of Thuricin CD into mice was able to reduce shed C. difficile in the feces, though showed low bioavailability when orally gavaged[99]. Another contractile bacteriocin protein complex (R-type; diffocin) was engineered to kill specific C. difficile pathogens[81]. The diffocins (i.e., Avidocin-CDs) prevented colonization of NAP1-type C. difficile strains and limited their transmission. Avidocin-CDs administered in drinking water survived passage through the mouse gastrointestinal tract, did not detectably alter the mouse intestinal microbiota and did not disrupt natural colonization resistance to C. difficile.
A group at the University of Michigan identified that antibiotic-treated mice had reduced levels of Lachnospiraceae in their feces, which correlated with increased CDI severity[100]. Reeves et al[101] later demonstrated that, while germ-free mice were extremely susceptible to CDI, the addition of multiple Lachnospiraceae family members suppressed the growth of C. difficile by 20-fold, and decreased the toxin production by 25%. Only complete cecal microbiota transfer entirely inhibited CDI. This parallels with studies completed in the 1980’s, where Itoh et al[102] transferred several different species, but only a full fecal transfer conferred protection.
Different groups have looked at the ability of certain bacterial species to inhibit toxin production in vitro. Kolling et al[103] determined that Streptococcus thermophilus produced lactic acid, which was able to suppress the transcription of tcdA and release of TcdA in vitro. Furthermore, mice treated with Streptococcus thermophilus have less severe CDI with decreases in weight loss, tissue injury on pathology, diarrhea, detectable C. difficile bacteria and toxin levels. This study demonstrated the ability of bacteria and bacterial products to suppress C. difficile growth and toxin production.
A recent study by Lawley et al[104] identified a consortium of six bacterial species in mice that could treat a recurrent carrier-model of CDI. These species, Bacteroidetes sp. nov., Enterorhabdus sp. nov., Enterococcus hirae, Lactobacillus reuteri, Staphylococcus warneri, and Anaerostipes sp. nov., were able to reduce inflammatory parameters of CDI as well as fecal counts of C. difficile. Individually, these six species were unable to confer protection, and no metabolic pathways could be identified to rationalize the effectiveness of the treatment[104]. The addition of these species did lead to increased bacterial diversity within the mouse intestinal microbiota, which was suggested as the potential mechanism for restoring colonization resistance. However, these species are limited to mice and not seen in HGM, so its application to human CDI is not clear.
FMT has risen to prominence in the past few years as an exciting therapeutic approach for CDI, especially recurrent CDI. However, FMT actually has a very extensive history and has been successfully used to treat diarrhea for over 50 years. Four patients were treated with fecal enemas in 1958 to resolve pseudomembranous colitis, and they exhibited dramatic resolution over 24-48 h post-treatment[105]. This study was conducted before C. difficile was recognized as the primary cause of pseudomembranous colitis, but it has since been validated by other studies using fecal enema[106]. The current protocol for involves fairly intense screening followed by simple techniques[107,108]. First, the FMT recipient ceases antibiotics and an FMT donor is selected. A donor completes a questionnaire and is screened for different pathogens, including bacteria (C. difficile, Listeria monocytogenes, Vibrio cholera, Helicobacter pylori, Treponema pallidum), parasites (Giardia, Cryptosporidium), and viruses (rotavirus, hepatitis A/B/C, Creutzfeldt-Jakob, and human immunodeficiency virus)[109]. Donors can also be excluded if they have had recent antibiotics or tattoos, or a history of gastrointestinal disease. Stool is then collected and prepared for transplant (e.g., diluted and homogenized before filtration through gauze pads to remove large particulate matter), although a recent study has demonstrated that frozen/thawed stool works as well as fresh stool[110,111]. The fecal filtrate can then delivered by oral capsule, nasogastric tube, colonoscopy or rectal enema.
Many trials interrogating the efficacy of FMT for CDI have taken place worldwide over the past few years[112-116]. Two healthy donors were used for 27 patients in a recent trial at McMaster University (Hamilton, Canada), showing an overall cure rate of 93%[112]. In this study, failure of FMT was linked to lack of retention of enema. A smaller study from the University of Toronto (Toronto, Canada) examined the different microbiota profiles involved in successful vs failed FMT[113]. The study identified several groups of bacteria (reduction in Bacteroidetes, increase in Proteobacteria) that were important in CDI cases, but indicated that overall diversity was important for success of FMT. The first controlled trial of nasogastric/duodenal infusion of fecal filtrate was published with encouraging results[114]. The FMT arm demonstrated 81% success (13/16 patients) after a single infusion of donor feces, and two of the three failed patients were later successfully treated after infusion of feces from a different donor, for an overall success rate of 94%. This was significantly better than the 31% success (4/13 patients) for standard vancomycin treatment. In a multicenter retrospective series, the use of FMT was examined in immunocompromised patients with CDI that was recurrent, refractory, or severe[115]. The cure rate after a single FMT was 78%, and 89% after repeat FMT in 99 patients in various states of immunosuppression (e.g., human immunodeficiency virus/acquired immune deficiency syndrome, solid organ transplant, chemotherapy, immunosuppressive therapy for IBD). Some patients (14% of IBD patients) experienced disease flare post FMT. Importantly, there were no related infectious complications reported in these high-risk patients.
A systematic review in 2013 found that different forms of FMT had been used to treat 273 patients with confirmed CDI from 1946-2012[117] with an overall success rate of 89% for FMT in treating CDI. In this analysis, a higher FMT success was correlated with lower gastrointestinal delivery routes (colonoscopy and enema vs nasoduodenal), but that donor relationship with recipient was uncorrelated. A more recent systematic review completed in 2015 suggests that FMT was associated with symptom resolution of recurrent CDI but its role in primary and severe CDI was not established[72]. The report concludes that treatment strategies should be aligned with disease severity, history of prior CDI, and the patient’s risk of recurrence. The exact mechanism by which FMT works on CDI has remained elusive, though it has been suggested to act through the restoration of colonization resistance. Unfortunately, there are also significant drawbacks of FMT. The screening process for known bacterial and viral pathogens can be costly and accessibility to an appropriate donor in a timely manner may be challenging. Additionally, a recent case report of FMT treatment detailed a patient with CDI that resulted in flare of dormant ulcerative colitis[118]. This adverse event suggests that microbial species that are protective in some disease states may be deleterious in others. Furthermore, links between the HGM and extra-intestinal diseases, including type II diabetes, obesity and behavioural disorders, have suggested FMT could have long-term safety issues[119-121]. Therefore, a defined and refined bacterial cocktail would be beneficial for treating CDI and avoiding deleterious effects.
Other bacteriotherapy attempts have been made to replace donor stool as the means of conveyance for HGM. These methods generally involve the development of defined bacterial mixtures from laboratory bioreactors. Tvede and Rask-Madsen demonstrated that a mixture of ten different bacteria could be curative to five (out of five) CDI patients in 1989[122]. The investigators found that recurrent CDI patients had decreased Bacteroides spp. that recovered after bacteriotherapy. A more recent study, termed rePOOPulation, used a combination of 33 bacteria to successfully treat two CDI patients[123]. These bacteria were cultured from a stool-sample and grown in the laboratory, but study design allowed for the transfer of a reproducible, known quantity of bacteria. However, these studies have still not identified a key mechanism provided by FMT to cure CDI. Identifying how the microbiota interacts and resists colonization by C. difficile will result in rational design of probiotic prevention or treatment of CDI.
The excitement of FMT as a treatment for recurrent CDI is increasing. A recent commentary has suggested that FMT could potentially be used to treat all cases of CDI, including a patient’s first case[124]. While this suggestion is exciting, detailed safety profiling is still required before FMT is a widespread therapy[108,125,126]. That being said, CDI is a disease that shows the most prominent links to alterations to the HGM, in both cause and treatment. Studies investigating the effectiveness of FMT for CDI are leading to a better understanding of the roles of the microbial community in both host health and disease. Nonetheless, elucidating a specific combination of bacteria that can treat or prevent future CDI cases would be an impactful discovery for the advancement of bacteriotherapy as a viable treatment.
The authors apologize to all those researchers whose work could not be cited owing to space limitations. Justin A MacDonald is an Alberta Innovates Health Solutions (AIHS) Senior Scholar, and Paul L Beck is an AIHS Clinical Senior Scholar. L Patrick Schenck was funded by an AIHS Graduate Scholarship.
P- Reviewer: Hu S, Shimizu Y S- Editor: Gong XM L- Editor: A E- Editor: Liu SQ
| 1. | So AD, Gupta N, Cars O. Tackling antibiotic resistance. BMJ. 2010;340:c2071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Johnson AP. Surveillance of antibiotic resistance. Philos Trans R Soc Lond B Biol Sci. 2015;370:20140080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 3. | Blair JM, Webber MA, Baylay AJ, Ogbolu DO, Piddock LJ. Molecular mechanisms of antibiotic resistance. Nat Rev Microbiol. 2015;13:42-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2022] [Cited by in RCA: 2444] [Article Influence: 222.2] [Reference Citation Analysis (0)] |
| 4. | Woolhouse M, Ward M, van Bunnik B, Farrar J. Antimicrobial resistance in humans, livestock and the wider environment. Philos Trans R Soc Lond B Biol Sci. 2015;370:20140083. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 318] [Cited by in RCA: 382] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 5. | Amy J, Johanesen P, Lyras D. Extrachromosomal and integrated genetic elements in Clostridium difficile. Plasmid. 2015;80:97-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | O’Connor JR, Johnson S, Gerding DN. Clostridium difficile infection caused by the epidemic BI/NAP1/027 strain. Gastroenterology. 2009;136:1913-1924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 267] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 7. | Burke KE, Lamont JT. Clostridium difficile infection: a worldwide disease. Gut Liver. 2014;8:1-6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 201] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 8. | Lessa FC, Winston LG, McDonald LC. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372:2369-2370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 200] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 9. | Schippa S, Conte MP. Dysbiotic events in gut microbiota: impact on human health. Nutrients. 2014;6:5786-5805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 157] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 10. | Collins SM. A role for the gut microbiota in IBS. Nat Rev Gastroenterol Hepatol. 2014;11:497-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 261] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 11. | Fujimura KE, Lynch SV. Microbiota in allergy and asthma and the emerging relationship with the gut microbiome. Cell Host Microbe. 2015;17:592-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 283] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 12. | Schulz MD, Atay C, Heringer J, Romrig FK, Schwitalla S, Aydin B, Ziegler PK, Varga J, Reindl W, Pommerenke C. High-fat-diet-mediated dysbiosis promotes intestinal carcinogenesis independently of obesity. Nature. 2014;514:508-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 353] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 13. | van Best N, Jansen PL, Rensen SS. The gut microbiota of nonalcoholic fatty liver disease: current methods and their interpretation. Hepatol Int. 2015;9:406-415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Org E, Mehrabian M, Lusis AJ. Unraveling the environmental and genetic interactions in atherosclerosis: Central role of the gut microbiota. Atherosclerosis. 2015;241:387-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 15. | Toh MC, Allen-Vercoe E. The human gut microbiota with reference to autism spectrum disorder: considering the whole as more than a sum of its parts. Microb Ecol Health Dis. 2015;26:26309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Williams BL, Hornig M, Buie T, Bauman ML, Cho Paik M, Wick I, Bennett A, Jabado O, Hirschberg DL, Lipkin WI. Impaired carbohydrate digestion and transport and mucosal dysbiosis in the intestines of children with autism and gastrointestinal disturbances. PLoS One. 2011;6:e24585. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 310] [Cited by in RCA: 354] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 17. | Janssen AW, Kersten S. The role of the gut microbiota in metabolic health. FASEB J. 2015;29:3111-3123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 140] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 18. | Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, Griffin NW, Lombard V, Henrissat B, Bain JR. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341:1241214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2415] [Cited by in RCA: 2722] [Article Influence: 226.8] [Reference Citation Analysis (0)] |
| 19. | Ding T, Schloss PD. Dynamics and associations of microbial community types across the human body. Nature. 2014;509:357-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 551] [Cited by in RCA: 615] [Article Influence: 55.9] [Reference Citation Analysis (0)] |
| 20. | Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA. Diversity of the human intestinal microbial flora. Science. 2005;308:1635-1638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5700] [Cited by in RCA: 5587] [Article Influence: 279.4] [Reference Citation Analysis (2)] |
| 21. | Flint HJ, Duncan SH, Scott KP, Louis P. Interactions and competition within the microbial community of the human colon: links between diet and health. Environ Microbiol. 2007;9:1101-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 419] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 22. | Glimstedt G. The germfree animal as a research tool. Ann N Y Acad Sci. 1959;78:281-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 23. | Winker S, Woese CR. A definition of the domains Archaea, Bacteria and Eucarya in terms of small subunit ribosomal RNA characteristics. Syst Appl Microbiol. 1991;14:305-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 158] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 24. | Muyzer G, de Waal EC, Uitterlinden AG. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microbiol. 1993;59:695-700. [PubMed] |
| 25. | Muyzer G, Smalla K. Application of denaturing gradient gel electrophoresis (DGGE) and temperature gradient gel electrophoresis (TGGE) in microbial ecology. Antonie Van Leeuwenhoek. 1998;73:127-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1411] [Cited by in RCA: 1030] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 26. | Liu WT, Marsh TL, Cheng H, Forney LJ. Characterization of microbial diversity by determining terminal restriction fragment length polymorphisms of genes encoding 16S rRNA. Appl Environ Microbiol. 1997;63:4516-4522. [PubMed] |
| 27. | Shyu C, Soule T, Bent SJ, Foster JA, Forney LJ. MiCA: a web-based tool for the analysis of microbial communities based on terminal-restriction fragment length polymorphisms of 16S and 18S rRNA genes. Microb Ecol. 2007;53:562-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 144] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 28. | Mardis ER. A decade’s perspective on DNA sequencing technology. Nature. 2011;470:198-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 585] [Cited by in RCA: 507] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 29. | Peterson J, Garges S, Giovanni M, McInnes P, Wang L, Schloss JA, Bonazzi V, McEwen JE, Wetterstrand KA, Deal C. The NIH Human Microbiome Project. Genome Res. 2009;19:2317-2323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1410] [Cited by in RCA: 1387] [Article Influence: 86.7] [Reference Citation Analysis (0)] |
| 30. | Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335-336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29318] [Cited by in RCA: 23733] [Article Influence: 1582.2] [Reference Citation Analysis (0)] |
| 31. | Walker AW, Duncan SH, Louis P, Flint HJ. Phylogeny, culturing, and metagenomics of the human gut microbiota. Trends Microbiol. 2014;22:267-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 162] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 32. | Hall IC, O’Toole E. Intestinal flora in new-born infants with a description of a new pathogenic anaerobe Bacillus difficilis. Am J Dis Child. 1935;49:390-402. [RCA] [DOI] [Full Text] [Cited by in Crossref: 520] [Cited by in RCA: 517] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 33. | Tedesco FJ, Stanley RJ, Alpers DH. Diagnostic features of clindamycin-associated pseudomembranous colitis. N Engl J Med. 1974;290:841-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 54] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 34. | George RH, Symonds JM, Dimock F, Brown JD, Arabi Y, Shinagawa N, Keighley MR, Alexander-Williams J, Burdon DW. Identification of Clostridium difficile as a cause of pseudomembranous colitis. Br Med J. 1978;1:695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 286] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 35. | Potter VA, Aravinthan A. Identifying patients at risk of severe Clostridium difficile-associated disease. Br J Hosp Med (Lond). 2012;73:265-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 36. | Kuijper EJ, Coignard B, Tüll P. Emergence of Clostridium difficile-associated disease in North America and Europe. Clin Microbiol Infect. 2006;12 Suppl 6:2-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 617] [Cited by in RCA: 655] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 37. | He M, Miyajima F, Roberts P, Ellison L, Pickard DJ, Martin MJ, Connor TR, Harris SR, Fairley D, Bamford KB. Emergence and global spread of epidemic healthcare-associated Clostridium difficile. Nat Genet. 2013;45:109-113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 525] [Cited by in RCA: 572] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 38. | Loo VG, Poirier L, Miller MA, Oughton M, Libman MD, Michaud S, Bourgault AM, Nguyen T, Frenette C, Kelly M. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N Engl J Med. 2005;353:2442-2449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1563] [Cited by in RCA: 1522] [Article Influence: 76.1] [Reference Citation Analysis (0)] |
| 39. | Slimings C, Riley TV. Antibiotics and hospital-acquired Clostridium difficile infection: update of systematic review and meta-analysis. J Antimicrob Chemother. 2014;69:881-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 377] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 40. | Khanna S, Pardi DS, Aronson SL, Kammer PP, Orenstein R, St Sauver JL, Harmsen WS, Zinsmeister AR. The epidemiology of community-acquired Clostridium difficile infection: a population-based study. Am J Gastroenterol. 2012;107:89-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 480] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 41. | Chitnis AS, Holzbauer SM, Belflower RM, Winston LG, Bamberg WM, Lyons C, Farley MM, Dumyati GK, Wilson LE, Beldavs ZG. Epidemiology of community-associated Clostridium difficile infection, 2009 through 2011. JAMA Intern Med. 2013;173:1359-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 317] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 42. | Metcalf D, Avery BP, Janecko N, Matic N, Reid-Smith R, Weese JS. Clostridium difficile in seafood and fish. Anaerobe. 2011;17:85-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 43. | Metcalf DS, Costa MC, Dew WM, Weese JS. Clostridium difficile in vegetables, Canada. Lett Appl Microbiol. 2010;51:600-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 44. | Weese JS, Avery BP, Rousseau J, Reid-Smith RJ. Detection and enumeration of Clostridium difficile spores in retail beef and pork. Appl Environ Microbiol. 2009;75:5009-5011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 136] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 45. | Mooyottu S, Flock G, Kollanoor-Johny A, Upadhyaya I, Jayarao B, Venkitanarayanan K. Characterization of a multidrug resistant C. difficile meat isolate. Int J Food Microbiol. 2015;192:111-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 46. | Lawley TD, Croucher NJ, Yu L, Clare S, Sebaihia M, Goulding D, Pickard DJ, Parkhill J, Choudhary J, Dougan G. Proteomic and genomic characterization of highly infectious Clostridium difficile 630 spores. J Bacteriol. 2009;191:5377-5386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 176] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 47. | Giel JL, Sorg JA, Sonenshein AL, Zhu J. Metabolism of bile salts in mice influences spore germination in Clostridium difficile. PLoS One. 2010;5:e8740. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 156] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 48. | Howerton A, Ramirez N, Abel-Santos E. Mapping interactions between germinants and Clostridium difficile spores. J Bacteriol. 2011;193:274-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 49. | Francis MB, Allen CA, Shrestha R, Sorg JA. Bile acid recognition by the Clostridium difficile germinant receptor, CspC, is important for establishing infection. PLoS Pathog. 2013;9:e1003356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 216] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 50. | Setlow P. Spore germination. Curr Opin Microbiol. 2003;6:550-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 681] [Cited by in RCA: 656] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 51. | Paredes-Sabja D, Torres JA, Setlow P, Sarker MR. Clostridium perfringens spore germination: characterization of germinants and their receptors. J Bacteriol. 2008;190:1190-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 124] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 52. | Voth DE, Ballard JD. Clostridium difficile toxins: mechanism of action and role in disease. Clin Microbiol Rev. 2005;18:247-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 815] [Cited by in RCA: 845] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 53. | Metcalf DS, Weese JS. Binary toxin locus analysis in Clostridium difficile. J Med Microbiol. 2011;60:1137-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 54. | Cartman ST, Kelly ML, Heeg D, Heap JT, Minton NP. Precise manipulation of the Clostridium difficile chromosome reveals a lack of association between the tcdC genotype and toxin production. Appl Environ Microbiol. 2012;78:4683-4690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 180] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 55. | Dineen SS, Villapakkam AC, Nordman JT, Sonenshein AL. Repression of Clostridium difficile toxin gene expression by CodY. Mol Microbiol. 2007;66:206-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 189] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 56. | Kelly CP, LaMont JT. Clostridium difficile--more difficult than ever. N Engl J Med. 2008;359:1932-1940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1035] [Cited by in RCA: 1021] [Article Influence: 60.1] [Reference Citation Analysis (0)] |
| 57. | Bartlett JG. Clostridium difficile: history of its role as an enteric pathogen and the current state of knowledge about the organism. Clin Infect Dis. 1994;18 Suppl 4:S265-S272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 184] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 58. | Freeman J, Bauer MP, Baines SD, Corver J, Fawley WN, Goorhuis B, Kuijper EJ, Wilcox MH. The changing epidemiology of Clostridium difficile infections. Clin Microbiol Rev. 2010;23:529-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 645] [Cited by in RCA: 642] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 59. | Kuehne SA, Cartman ST, Heap JT, Kelly ML, Cockayne A, Minton NP. The role of toxin A and toxin B in Clostridium difficile infection. Nature. 2010;467:711-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 606] [Cited by in RCA: 646] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 60. | Hirota SA, Iablokov V, Tulk SE, Schenck LP, Becker H, Nguyen J, Al Bashir S, Dingle TC, Laing A, Liu J. Intrarectal instillation of Clostridium difficile toxin A triggers colonic inflammation and tissue damage: development of a novel and efficient mouse model of Clostridium difficile toxin exposure. Infect Immun. 2012;80:4474-4484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 61. | Kelly CP, Kyne L. The host immune response to Clostridium difficile. J Med Microbiol. 2011;60:1070-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 149] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 62. | Islam J, Taylor AL, Rao K, Huffnagle G, Young VB, Rajkumar C, Cohen J, Papatheodorou P, Aronoff DM, Llewelyn MJ. The role of the humoral immune response to Clostridium difficile toxins A and B in susceptibility to C. difficile infection: a case-control study. Anaerobe. 2014;27:82-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 63. | Sánchez-Hurtado K, Corretge M, Mutlu E, McIlhagger R, Starr JM, Poxton IR. Systemic antibody response to Clostridium difficile in colonized patients with and without symptoms and matched controls. J Med Microbiol. 2008;57:717-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 64. | Johnson S. Recurrent Clostridium difficile infection: causality and therapeutic approaches. Int J Antimicrob Agents. 2009;33 Suppl 1:S33-S36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 65. | Ng J, Hirota SA, Gross O, Li Y, Ulke-Lemee A, Potentier MS, Schenck LP, Vilaysane A, Seamone ME, Feng H. Clostridium difficile toxin-induced inflammation and intestinal injury are mediated by the inflammasome. Gastroenterology. 2010;139:542-52, 552.e1-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 187] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 66. | Zhao S, Ghose-Paul C, Zhang K, Tzipori S, Sun X. Immune-based treatment and prevention of Clostridium difficile infection. Hum Vaccin Immunother. 2014;10:3522-3530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 67. | Viscidi R, Laughon BE, Yolken R, Bo-Linn P, Moench T, Ryder RW, Bartlett JG. Serum antibody response to toxins A and B of Clostridium difficile. J Infect Dis. 1983;148:93-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 142] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 68. | Winckler J. [Fixation of enzymes in freeze-dried tissue with gases]. Acta Histochem Suppl. 1976;16:259-264. [PubMed] |
| 69. | Kyne L, Warny M, Qamar A, Kelly CP. Asymptomatic carriage of Clostridium difficile and serum levels of IgG antibody against toxin A. N Engl J Med. 2000;342:390-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 735] [Cited by in RCA: 724] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 70. | Kyne L, Warny M, Qamar A, Kelly CP. Association between antibody response to toxin A and protection against recurrent Clostridium difficile diarrhoea. Lancet. 2001;357:189-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 626] [Cited by in RCA: 609] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 71. | Leav BA, Blair B, Leney M, Knauber M, Reilly C, Lowy I, Gerding DN, Kelly CP, Katchar K, Baxter R. Serum anti-toxin B antibody correlates with protection from recurrent Clostridium difficile infection (CDI). Vaccine. 2010;28:965-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 194] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 72. | Bagdasarian N, Rao K, Malani PN. Diagnosis and treatment of Clostridium difficile in adults: a systematic review. JAMA. 2015;313:398-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 345] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 73. | Dallas KB, Condren A, Divino CM. Life after colectomy for fulminant Clostridium difficile colitis: a 7-year follow up study. Am J Surg. 2014;207:533-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 74. | ASHP therapeutic position statement on the preferential use of metronidazole for the treatment of Clostridium difficile-associated disease. Am J Health Syst Pharm. 1998;55:1407-1411. [PubMed] |
| 75. | Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45:302-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 945] [Cited by in RCA: 938] [Article Influence: 52.1] [Reference Citation Analysis (0)] |
| 76. | Kuipers EJ, Surawicz CM. Clostridium difficile infection. Lancet. 2008;371:1486-1488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 77. | Mullane KM, Miller MA, Weiss K, Lentnek A, Golan Y, Sears PS, Shue YK, Louie TJ, Gorbach SL. Efficacy of fidaxomicin versus vancomycin as therapy for Clostridium difficile infection in individuals taking concomitant antibiotics for other concurrent infections. Clin Infect Dis. 2011;53:440-447. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 172] [Cited by in RCA: 180] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 78. | Louie TJ, Miller MA, Mullane KM, Weiss K, Lentnek A, Golan Y, Gorbach S, Sears P, Shue YK. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011;364:422-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1140] [Cited by in RCA: 1181] [Article Influence: 84.4] [Reference Citation Analysis (0)] |
| 79. | Lewis BB, Buffie CG, Carter RA, Leiner I, Toussaint NC, Miller LC, Gobourne A, Ling L, Pamer EG. Loss of Microbiota-Mediated Colonization Resistance to Clostridium difficile Infection With Oral Vancomycin Compared With Metronidazole. J Infect Dis. 2015;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 155] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 80. | Buffie CG, Bucci V, Stein RR, McKenney PT, Ling L, Gobourne A, No D, Liu H, Kinnebrew M, Viale A. Precision microbiome reconstitution restores bile acid mediated resistance to Clostridium difficile. Nature. 2015;517:205-208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1172] [Cited by in RCA: 1351] [Article Influence: 122.8] [Reference Citation Analysis (0)] |
| 81. | Gebhart D, Lok S, Clare S, Tomas M, Stares M, Scholl D, Donskey CJ, Lawley TD, Govoni GR. A modified R-type bacteriocin specifically targeting Clostridium difficile prevents colonization of mice without affecting gut microbiota diversity. MBio. 2015;6:pii: e02368-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 97] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 82. | Britton RA, Young VB. Role of the intestinal microbiota in resistance to colonization by Clostridium difficile. Gastroenterology. 2014;146:1547-1553. [PubMed] |
| 83. | Jump RL, Polinkovsky A, Hurless K, Sitzlar B, Eckart K, Tomas M, Deshpande A, Nerandzic MM, Donskey CJ. Metabolomics analysis identifies intestinal microbiota-derived biomarkers of colonization resistance in clindamycin-treated mice. PLoS One. 2014;9:e101267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 84. | Burns DA, Heap JT, Minton NP. Clostridium difficile spore germination: an update. Res Microbiol. 2010;161:730-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 85. | Begley M, Gahan CG, Hill C. The interaction between bacteria and bile. FEMS Microbiol Rev. 2005;29:625-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1071] [Cited by in RCA: 1222] [Article Influence: 61.1] [Reference Citation Analysis (0)] |
| 86. | Kishinaka M, Umeda A, Kuroki S. High concentrations of conjugated bile acids inhibit bacterial growth of Clostridium perfringens and induce its extracellular cholylglycine hydrolase. Steroids. 1994;59:485-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 87. | Takamine F, Imamura T. 7 beta-dehydroxylation of 3,7-dihydroxy bile acids by a Eubacterium species strain C-25 and stimulation of 7 beta-dehydroxylation by Bacteroides distasonis strain K-5. Microbiol Immunol. 1985;29:1247-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 88. | Bateup JM, McConnell MA, Jenkinson HF, Tannock GW. Comparison of Lactobacillus strains with respect to bile salt hydrolase activity, colonization of the gastrointestinal tract, and growth rate of the murine host. Appl Environ Microbiol. 1995;61:1147-1149. [PubMed] |
| 89. | Breton YL, Mazé A, Hartke A, Lemarinier S, Auffray Y, Rincé A. Isolation and characterization of bile salts-sensitive mutants of Enterococcus faecalis. Curr Microbiol. 2002;45:434-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 90. | Van Eldere J, Celis P, De Pauw G, Lesaffre E, Eyssen H. Tauroconjugation of cholic acid stimulates 7 alpha-dehydroxylation by fecal bacteria. Appl Environ Microbiol. 1996;62:656-661. [PubMed] |
| 91. | Dussurget O, Cabanes D, Dehoux P, Lecuit M, Buchrieser C, Glaser P, Cossart P. Listeria monocytogenes bile salt hydrolase is a PrfA-regulated virulence factor involved in the intestinal and hepatic phases of listeriosis. Mol Microbiol. 2002;45:1095-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 259] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 92. | Sutherland JD, Williams CN. Bile acid induction of 7 alpha- and 7 beta-hydroxysteroid dehydrogenases in Clostridium limosum. J Lipid Res. 1985;26:344-350. [PubMed] |
| 93. | Stellwag EJ, Hylemon PB. 7alpha-Dehydroxylation of cholic acid and chenodeoxycholic acid by Clostridium leptum. J Lipid Res. 1979;20:325-333. [PubMed] |
| 94. | Theriot CM, Koenigsknecht MJ, Carlson PE, Hatton GE, Nelson AM, Li B, Huffnagle GB, Z Li J, Young VB. Antibiotic-induced shifts in the mouse gut microbiome and metabolome increase susceptibility to Clostridium difficile infection. Nat Commun. 2014;5:3114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 635] [Cited by in RCA: 721] [Article Influence: 72.1] [Reference Citation Analysis (0)] |
| 95. | Howerton A, Patra M, Abel-Santos E. A new strategy for the prevention of Clostridium difficile infection. J Infect Dis. 2013;207:1498-1504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 96. | Chang TW, Onderdonk AB, Bartlett JG. Anion-exchange resins in antibiotic-associated colitis. Lancet. 1978;2:258-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 97. | Moncino MD, Falletta JM. Multiple relapses of Clostridium difficile-associated diarrhea in a cancer patient. Successful control with long-term cholestyramine therapy. Am J Pediatr Hematol Oncol. 1992;14:361-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 30] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 98. | Rea MC, Sit CS, Clayton E, O’Connor PM, Whittal RM, Zheng J, Vederas JC, Ross RP, Hill C. Thuricin CD, a posttranslationally modified bacteriocin with a narrow spectrum of activity against Clostridium difficile. Proc Natl Acad Sci USA. 2010;107:9352-9357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 392] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 99. | Rea MC, Alemayehu D, Casey PG, O’Connor PM, Lawlor PG, Walsh M, Shanahan F, Kiely B, Ross RP, Hill C. Bioavailability of the anti-clostridial bacteriocin thuricin CD in gastrointestinal tract. Microbiology. 2014;160:439-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 100. | Reeves AE, Theriot CM, Bergin IL, Huffnagle GB, Schloss PD, Young VB. The interplay between microbiome dynamics and pathogen dynamics in a murine model of Clostridium difficile Infection. Gut Microbes. 2011;2:145-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 224] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 101. | Reeves AE, Koenigsknecht MJ, Bergin IL, Young VB. Suppression of Clostridium difficile in the gastrointestinal tracts of germfree mice inoculated with a murine isolate from the family Lachnospiraceae. Infect Immun. 2012;80:3786-3794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 230] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 102. | Itoh K, Lee WK, Kawamura H, Mitsuoka T, Magaribuchi T. Intestinal bacteria antagonistic to Clostridium difficile in mice. Lab Anim. 1987;21:20-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 103. | Kolling GL, Wu M, Warren CA, Durmaz E, Klaenhammer TR, Timko MP, Guerrant RL. Lactic acid production by Streptococcus thermophilus alters Clostridium difficile infection and in vitro Toxin A production. Gut Microbes. 2012;3:523-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 104. | Lawley TD, Clare S, Walker AW, Stares MD, Connor TR, Raisen C, Goulding D, Rad R, Schreiber F, Brandt C. Targeted restoration of the intestinal microbiota with a simple, defined bacteriotherapy resolves relapsing Clostridium difficile disease in mice. PLoS Pathog. 2012;8:e1002995. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 417] [Cited by in RCA: 425] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 105. | Eiseman B, Silen W, Bascom GS, Kauvar AJ. Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery. 1958;44:854-859. [PubMed] |
| 106. | Schwan A, Sjölin S, Trottestam U, Aronsson B. Relapsing clostridium difficile enterocolitis cured by rectal infusion of homologous faeces. Lancet. 1983;2:845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 137] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 107. | Bakken JS, Borody T, Brandt LJ, Brill JV, Demarco DC, Franzos MA, Kelly C, Khoruts A, Louie T, Martinelli LP. Treating Clostridium difficile infection with fecal microbiota transplantation. Clin Gastroenterol Hepatol. 2011;9:1044-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 650] [Cited by in RCA: 683] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 108. | Kelly CR, Kahn S, Kashyap P, Laine L, Rubin D, Atreja A, Moore T, Wu G. Update on Fecal Microbiota Transplantation 2015: Indications, Methodologies, Mechanisms, and Outlook. Gastroenterology. 2015;149:223-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 423] [Article Influence: 42.3] [Reference Citation Analysis (1)] |
| 109. | Paramsothy S, Borody TJ, Lin E, Finlayson S, Walsh AJ, Samuel D, van den Bogaerde J, Leong RW, Connor S, Ng W. Donor Recruitment for Fecal Microbiota Transplantation. Inflamm Bowel Dis. 2015;21:1600-1606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 110] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 110. | Hamilton MJ, Weingarden AR, Sadowsky MJ, Khoruts A. Standardized frozen preparation for transplantation of fecal microbiota for recurrent Clostridium difficile infection. Am J Gastroenterol. 2012;107:761-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 544] [Article Influence: 41.8] [Reference Citation Analysis (2)] |
| 111. | Costello SP, Conlon MA, Vuaran MS, Roberts-Thomson IC, Andrews JM. Faecal microbiota transplant for recurrent Clostridium difficile infection using long-term frozen stool is effective: clinical efficacy and bacterial viability data. Aliment Pharmacol Ther. 2015;42:1011-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 111] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 112. | Kassam Z, Hundal R, Marshall JK, Lee CH. Fecal transplant via retention enema for refractory or recurrent Clostridium difficile infection. Arch Intern Med. 2012;172:191-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 113. | Shahinas D, Silverman M, Sittler T, Chiu C, Kim P, Allen-Vercoe E, Weese S, Wong A, Low DE, Pillai DR. Toward an understanding of changes in diversity associated with fecal microbiome transplantation based on 16S rRNA gene deep sequencing. MBio. 2012;3:pii: e00338-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 134] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 114. | van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, de Vos WM, Visser CE, Kuijper EJ, Bartelsman JF, Tijssen JG. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368:407-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2582] [Cited by in RCA: 2677] [Article Influence: 223.1] [Reference Citation Analysis (0)] |
| 115. | Kelly CR, Ihunnah C, Fischer M, Khoruts A, Surawicz C, Afzali A, Aroniadis O, Barto A, Borody T, Giovanelli A. Fecal microbiota transplant for treatment of Clostridium difficile infection in immunocompromised patients. Am J Gastroenterol. 2014;109:1065-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 431] [Cited by in RCA: 501] [Article Influence: 45.5] [Reference Citation Analysis (1)] |
| 116. | Aroniadis OC, Brandt LJ, Greenberg A, Borody T, Kelly CR, Mellow M, Surawicz C, Cagle L, Neshatian L, Stollman N. Long-term Follow-up Study of Fecal Microbiota Transplantation for Severe and/or Complicated Clostridium difficile Infection: A Multicenter Experience. J Clin Gastroenterol. 2015;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 117. | Kassam Z, Lee CH, Yuan Y, Hunt RH. Fecal microbiota transplantation for Clostridium difficile infection: systematic review and meta-analysis. Am J Gastroenterol. 2013;108:500-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 617] [Cited by in RCA: 659] [Article Influence: 54.9] [Reference Citation Analysis (0)] |
| 118. | De Leon LM, Watson JB, Kelly CR. Transient flare of ulcerative colitis after fecal microbiota transplantation for recurrent Clostridium difficile infection. Clin Gastroenterol Hepatol. 2013;11:1036-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 158] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 119. | Kassam Z, Lee CH, Yuan Y, Hunt RH. Navigating long-term safety in fecal microbiota transplantation. Am J Gastroenterol. 2013;108:1538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 120. | Collins SM, Kassam Z, Bercik P. The adoptive transfer of behavioral phenotype via the intestinal microbiota: experimental evidence and clinical implications. Curr Opin Microbiol. 2013;16:240-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 140] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 121. | Alang N, Kelly CR. Weight gain after fecal microbiota transplantation. Open Forum Infect Dis. 2015;2:ofv004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 270] [Cited by in RCA: 302] [Article Influence: 30.2] [Reference Citation Analysis (1)] |
| 122. | Tvede M, Rask-Madsen J. Bacteriotherapy for chronic relapsing Clostridium difficile diarrhoea in six patients. Lancet. 1989;1:1156-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 330] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 123. | Petrof EO, Gloor GB, Vanner SJ, Weese SJ, Carter D, Daigneault MC, Brown EM, Schroeter K, Allen-Vercoe E. Stool substitute transplant therapy for the eradication of Clostridium difficile infection: ‘RePOOPulating’ the gut. Microbiome. 2013;1:3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 534] [Cited by in RCA: 523] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 124. | Borody TJ, Peattie D, Kapur A. Could fecal microbiota transplantation cure all Clostridium difficile infections? Future Microbiol. 2014;9:1-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 125. | Vandenplas Y, Pierard D, De Greef E. Fecal Microbiota Transplantation: Just a Fancy Trend? J Pediatr Gastroenterol Nutr. 2015;61:4-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 126. | Smith MB, Kelly C, Alm EJ. Policy: How to regulate faecal transplants. Nature. 2014;506:290-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 101] [Article Influence: 9.2] [Reference Citation Analysis (0)] |