Published online Nov 15, 2015. doi: 10.4291/wjgp.v6.i4.124
Peer-review started: April 21, 2015
First decision: May 18, 2015
Revised: July 7, 2015
Accepted: August 30, 2015
Article in press: September 7, 2015
Published online: November 15, 2015
Processing time: 210 Days and 15.6 Hours
Central nervous system (CNS) complications or manifestations of inflammatory bowel disease deserve particular attention because symptomatic conditions can require early diagnosis and treatment, whereas unexplained manifestations might be linked with pathogenic mechanisms. This review focuses on both symptomatic and asymptomatic brain lesions detectable on imaging studies, as well as their frequency and potential mechanisms. A direct causal relationship between inflammatory bowel disease (IBD) and asymptomatic structural brain changes has not been demonstrated, but several possible explanations, including vasculitis, thromboembolism and malnutrition, have been proposed. IBD is associated with a tendency for thromboembolisms; therefore, cerebrovascular thromboembolism represents the most frequent and grave CNS complication. Vasculitis, demyelinating conditions and CNS infections are among the other CNS manifestations of the disease. Biological agents also represent a risk factor, particularly for demyelination. Identification of the nature and potential mechanisms of brain lesions detectable on imaging studies would shed further light on the disease process and could improve patient care through early diagnosis and treatment.
Core tip: Central nervous system complications or manifestations of inflammatory bowel disease deserve particular attention because symptomatic conditions can require early diagnosis and treatment, whereas unexplained manifestations might be linked to pathogenic mechanisms. This review focuses on both symptomatic and asymptomatic brain lesions detectable on imaging studies, as well as their frequency and potential mechanisms. A direct causal relationship between inflammatory bowel disease and asymptomatic structural brain changes has not been demonstrated, but several possible explanations, including vasculitis, thromboembolism and malnutrition, have been proposed. Identification of the nature and potential mechanisms of brain lesions on imaging studies would improve patient care through early diagnosis and treatment.
- Citation: Dolapcioglu C, Dolapcioglu H. Structural brain lesions in inflammatory bowel disease. World J Gastrointest Pathophysiol 2015; 6(4): 124-130
- URL: https://www.wjgnet.com/2150-5330/full/v6/i4/124.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v6.i4.124
Inflammatory bowel diseases (IBDs), namely ulcerative colitis (UC) and Crohn’s disease (CD), are chronic, debilitating conditions with their onset at relatively young ages. CD is a transmural disease of gastrointestinal mucosa, and it has the potential to affect the entire gastrointestinal tract; in contrast, UC is not a transmural disease, and it affects the colon[1,2]. Both have relapsing and remitting courses. It is estimated that the total (UC plus CD) prevalence of IBD is approximately 0.4% in the Western populations[3].
Because IBD can involve body parts other than the gastrointestinal tract, it can be regarded as a systemic disease. Involvement of the skin, eyes, joints, liver, biliary tract, kidneys, and bone, as well as hematological, and neurological involvement, can occur, preceding, accompanying or following gastrointestinal symptoms. These extraintestinal manifestations are more common in CD patients, although a substantial number of IBD patients can develop these conditions[4-6].
Neurological manifestations are relatively rare but are of clinical importance, particularly in terms of the need for timely diagnosis and management. Several mechanisms, including thromboembolisms, immunologic abnormalities, drug side effects, malabsorption, and infections, have been suggested as pathogeneses[7]. Most central neurological manifestations of IBD can be detected by brain imaging studies because they cause structural alterations of neural structures to some extent. However, lesions of unknown clinical relevance have also been detected in these patients, with significantly higher prevalence than in the normal population.
This review focuses in particular on structural brain lesions with positive findings on imaging studies, including symptomatic conditions and asymptomatic situations with brain lesions as well (Table 1).
| Asymptomatic structural changes |
| Symptomatic structural changes |
| Cerebrovascular lesions |
| Demyelinating conditions |
| Cerebral vasculitis |
| CNS infections |
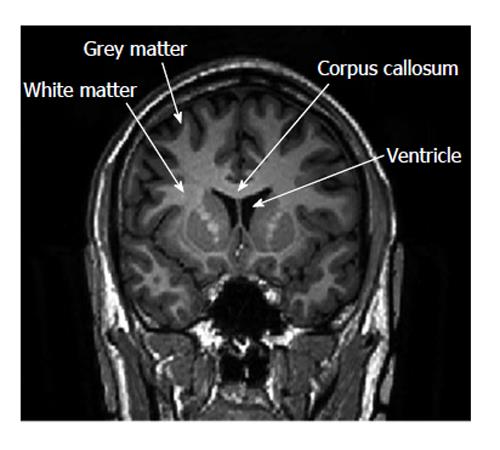
To date, a small number of studies have examined the presence of white matter lesions and other structural alterations on imaging studies in patients with IBD. These lesions were asymptomatic with potential associations with IBD, and whether these structural changes represent a unique extraintestinal manifestation of the disease remains unclear. Figure 1 depicts white matter and gray matter (GM) on an magnetic resonance imaging (MRI) scan.
Initial reports examining the associations between IBD and asymptomatic brain lesions were conflicting[8,9]. In a study by Geissler et al[8], 72 patients with IBD (48 cases of CD and 24 of UC) and 50 healthy age-matched controls underwent magnetic resonance imaging with gadolinium-enhanced studies. In that series, hyperintense focal white-matter lesions of 2-8 mm in diameter were found in 42% and 46% of patients with CD and UC, respectively, whereas such lesions were only present in 16% of healthy controls, resulting in relative risks of 2.6 for CD (95%CI: 1.3-5.3) and 2.9 for UC (95%CI: 1.3-6.2). A longer duration of disease and older age were associated with an increased tendency for the lesions, and none of the patients had neurological symptoms. In contrast to the study by Geissler et al[8], Hart et al[9] did not find a significantly increased frequency of asymptomatic brain white matter lesions on the MRIs of IBD patients (n = 40), compared to a control group consisting of 40 age- and sex-matched patients admitted for tension-type headache (12.5% vs 5%, P = 0.43). Although the relatively small sample size of the latter study might have prevented the differences from attaining statistical significance, the authors emphasized that such asymptomatic lesions had previously been reported consistently in healthy subjects[10], and they expressed concerns about the clinical relevance of these findings for patients with IBD. However, it should be emphasized that both reports dated from two decades ago. In contrast, a recent study with a relatively small sample size also could not find an increased rate of white matter lesions among patients with IBD, compared to healthy controls[11]. In that study, the frequencies of white matter lesions and other brainstem parenchymal lesions were similar, but among the subjects with white matter lesions, the number of lesions was significantly higher in IBD patients.
Two recent studies compared the frequency of white matter lesions between IBD patients and normal subjects using advanced magnetic resonance imaging techniques and equipment[12,13]. Chen et al[12] found a very high prevalence of hyperintense white matter lesions in patients with CD, compared to age-matched controls (75% vs 34%, P < 0.001). Their study had a relatively large sample size (54 Crohn’s patients and 100 age-matched controls). Similarly, Zikou et al[13] found a significantly increased frequency of white matter lesions among patients with IBD (Crohn’s and UC), compared to controls (66% vs 45%, P < 0.05). Both studies used fluid-attenuated inversion recovery images to evaluate white matter hyperintensities.
The development of advanced MRI techniques has allowed for better examination of brain structures, including estimation of volume differences and the evaluation of microstructural integrity. Voxel-based morphometry is a technique used to compare the regional brain volumes of subjects, and it uses MRI data. Several studies using this technique have found a decreased GM volume among IBD patients, compared to controls. Agostini et al[14], in a study of CD patients, found decreased GM volume in parts of the frontal gyrus and in the anterior midcingulate cortex. In addition, negative correlations between disease duration and GM volume in several brain regions were found in the former study. In contrast, a recent study by the same lead author did not identify any decrease in GM volume in UC patients, compared to controls[15]. Zikou et al[13], however, found more diffuse GM volume decreases in a sample of IBD patients, consisting of subjects with CD and UC, involving the right and left fusiform gyri, the right and left temporal inferior gyri, the right precentral gyrus, the right superior motor area, the right middle frontal gyrus and the left superior parietal gyrus.
Diffusion tensor imaging (DTI) is an MRI technique that measures molecular diffusion. It is valuable for the identification of axonal injury and can be useful in differentiating between primary and Wallerian degeneration[16]. To date, only a single study has examined the brains of IBD patients using DTI techniques and compared them with age-matched healthy controls[13]. That study found decreased white matter axial diffusivity in the right corticospinal tract and the right superior longitudinal fasciculus in IBD patients (Crohn’s and UC) compared to controls, indicating a possible degree of change in neural structures[17-21].
Several possible explanations have been proposed for the increased prevalence of white matter lesions, the decreased GM volume, and the decreased diffusivity in major tracks among patients with IBD. White matter lesions on brain MRI examinations have been associated with many conditions, including migraine headaches, hypertension, diabetes, celiac disease, and cerebrovascular disease, as well as being found in healthy subjects[10,22-26]. The increased frequency of white matter hyperintensities in IBD patients might be due to central nervous system (CNS) vasculitis, likely secondary to coagulation and vessel obstruction[13]. IBD is known to be associated with thromboembolism and hypercoagulability[27-29]. Another possible explanation is malabsorption because such lesions were previously described in celiac disease[26].
One explanation for decreased GM volume might be excito-toxicity due to chronic pain, which can result in neural atrophy or loss[14,30]. Structural changes of varying types have been observed in pain-related brain regions in other chronic pain syndromes[31-36], with GM decreases in the frontal and cingulate cortices being the most common anomalies[30]. Another possible mechanism might be increased inflammatory cytokines, resulting in astrocyte and oligodendrocyte apoptosis, decreased neurogenesis, and increased oxidative stress, thus leading to GM volume loss[37,38]. Similarly, cerebral small vessel vasculitis and the neurotoxic effects of cytokines could be responsible for the decreased axial diffusivity in major tracts[13].
Although a direct causal relationship between IBD and asymptomatic structural brain changes cannot be established, the available findings and their implications deserve further investigation, particularly in the context of the brain functional changes observed in patients with IBD[39].
Patients with IBD have an increased tendency for thrombotic events so that IBD could be considered a prothrombotic condition that increases the risk of cerebral arterial and venous thrombosis[40-44]. A recent meta-analysis found a modestly increased risk of cerebrovascular accidents among patients with IBD (OR = 1.18)[45]. The increased risk was more prominent among female patients and young patients. Venous thrombosis has also been seen in these patients with remarkable frequency[44,46-49]. Cerebrovascular events have been documented in up to 4% of IBD patients[50]. These cases are of particular clinical importance when the consequences of these conditions and the young age of the patient population are considered.
A number of pathological conditions have been linked to IBD in attempts to propose a mechanism for the increased tendency for thromboembolic events, including abnormalities of platelets and coagulation factors, genetic mutations, vitamin B6 deficiency due to hypercatabolism and malabsorption, antiphospholipid antibodies, hyperhomocystinemia, dehydration, and immobilization[4,7,40,49,51-58]. However, to date, the exact mechanism has not been fully understood.
Cerebral arterial thromboembolism can present with headache, paresis, seizures or dysphagia, and it can result in high mortality and morbidity[51]. Large infarcts involving both the anterior and posterior circulation and lacunar infarcts have been reported in IBD[59-63]. In addition, UC has been associated with thrombotic thrombocytopenic purpura and small and large cerebral artery thrombosis risks[64-66]. Infarcts associated with IBD can be identified on computerized tomography and magnetic resonance imaging.
Cerebral venous thrombosis and sinus thrombosis seem to be more frequent in patients with UC than in CD patients[67]. Most often, the superior sagittal sinus and lateral sinuses are involved; however, thrombosis of the cortical venous sinuses has also been reported[68]. Young and male patients seem to be at greater risk[50,69,70]. The most common presenting symptom is headache, usually followed by neurological impairment, and cerebral infarction can develop due to extension of thrombus[71]. A combination of magnetic resonance imaging and magnetic resonance venography could identify venous occlusion[71]. The radiological characteristics, as well as the clinical course and prognosis of IBD-related cerebral venous thrombosis, seem to be similar to those in cases not related to IBD[44,72,73].
Demyelinating conditions have been reported in the setting of IBD, in association or not with biological treatments.
Multiple sclerosis (MS) or MS-like conditions have long been reported in patients with IBD[74-77]. Such a relationship was first reported by Rang et al[74]. A retrospective study found an increased incidence of demyelinating disease among patients with IBD, particularly in UC[77]. Nevertheless, MS has been reported in both UC and CD[78,79]. The findings of a recent meta-analysis supported a relationship between MS and IBD[80]. Both the development of an MS-like syndrome in the setting of IBD and the development of IBD in MS patients have been reported[81-83]. Because the diagnostic criteria for MS have evolved over time, some lesions found in previous studies might not actually be MS; rather, they could represent an MS-like syndrome, which might potentially be linked to IBD. The mechanism of these relationships has not been fully explained, but a role for impairments in functional T-cell subsets has been proposed[4,57].
Anti-tumor necrosis factor (TNF)-alpha drugs and anti-alpha4 integrin drugs (such as natalizumab), which are biological agents used in the treatment of IBD, can have adverse neurological effects[84,85]. Progressive multifocal leukoencephalopathy is the gravest complication, particularly when associated with natalizumab therapy, although few cases have been reported in association with TNF-alpha drugs[85]. Reactivation of John Cunningham virus (JCV) is responsible for the development of PML, and it is associated with visual defects, mental impairment, confusion and personality changes, followed by motor weakness[57]. MRI is helpful in the diagnosis, showing white matter lesions with typical T1 and T2 signals[86]. Diagnosis can be confirmed by polymerase chain reaction for JCV DNA. Despite treatment, PML has a high mortality rate of 60%[87]. In addition, development/exacerbation of MS or demyelination has been reported in association with anti-TNF-alpha therapy[88-90].
Cerebral vasculitis has been reported in patients with UC in a number of studies[91-96]. In addition, a case of cerebral vasculitis was reported in association with CD[97]. Mostly immune-mediated mechanisms have been proposed for the development of vasculitis in UC. Magnetic resonance imaging is abnormal and shows hyperintense lesions[95,96], and magnetic resonance angiography can aid in diagnosis[97]. CNS vasculitis has also been reported in association with anti-TNF therapy[57]. The major symptoms of cerebral vasculitis are stroke, headache and encephalopathy. Other symptoms include seizures, cranial nerve palsies or myelopathies[98]. Cerebral vasculitis mimicking migraine with aura was reported in a case of CD, and the authors stated that migraine with aura can be the only finding in cerebral vasculitis[99]. Cerebral vasculitis resulting in stroke has been rarely reported in UC[61]. Cerebral vasculitis presented with right paresis and unbalanced gait in a 35-year-old woman with UC[93]. Another UC case was complicated by convulsions and was diagnosed as cerebral vasculitis on magnetic resonance imaging[96].
Anti-TNF agents can suppress the immune system to such an extent that opportunistic infections develop, including of the CNS, in IBD patients. These patients present with meningeal signs, seizures, symptoms resembling stroke, and encephalopathy[100]; abnormal MRI findings and/or mass lesions are found on imaging studies. Among these opportunistic infections are fungal infections, cerebral tuberculosis, Epstein-Barr virus infection, nocardiosis, toxoplasmosis, herpes simplex virus infection, meningococcal infection, Campylobacter fetus infections, and listeria infections[57]. In a severe case of CD with ileocecal involvement, opportunistic meningitis with varicella zoster was reported after adalimumab and prednisone treatment[101]. In a patient with CD, meningococcal meningoencephalitis was reported after certolizumab pegol treatment[102].
P- Reviewer: Lakatos PL, Swaminath A S- Editor: Ma YJ L- Editor: A E- Editor: Liu SQ
| 1. | Baumgart DC, Sandborn WJ. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet. 2007;369:1641-1657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1247] [Cited by in RCA: 1353] [Article Influence: 75.2] [Reference Citation Analysis (0)] |
| 2. | Baumgart DC, Carding SR. Inflammatory bowel disease: cause and immunobiology. Lancet. 2007;369:1627-1640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1299] [Cited by in RCA: 1506] [Article Influence: 83.7] [Reference Citation Analysis (2)] |
| 3. | Watts DA, Satsangi J. The genetic jigsaw of inflammatory bowel disease. Gut. 2002;50 Suppl 3:III31-III36. [PubMed] |
| 4. | Morís G. Inflammatory bowel disease: an increased risk factor for neurologic complications. World J Gastroenterol. 2014;20:1228-1237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 67] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 5. | Veloso FT. Extraintestinal manifestations of inflammatory bowel disease: do they influence treatment and outcome? World J Gastroenterol. 2011;17:2702-2707. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 68] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 6. | Bernstein CN, Blanchard JF, Rawsthorne P, Yu N. The prevalence of extraintestinal diseases in inflammatory bowel disease: a population-based study. Am J Gastroenterol. 2001;96:1116-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 509] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 7. | Zois CD, Katsanos KH, Kosmidou M, Tsianos EV. Neurologic manifestations in inflammatory bowel diseases: current knowledge and novel insights. J Crohns Colitis. 2010;4:115-124. [PubMed] |
| 8. | Geissler A, Andus T, Roth M, Kullmann F, Caesar I, Held P, Gross V, Feuerbach S, Schölmerich J. Focal white-matter lesions in brain of patients with inflammatory bowel disease. Lancet. 1995;345:897-898. [PubMed] |
| 9. | Hart PE, Gould SR, MacSweeney JE, Clifton A, Schon F. Brain white-matter lesions in inflammatory bowel disease. Lancet. 1998;351:1558. [PubMed] |
| 10. | Horikoshi T, Yagi S, Fukamachi A. Incidental high-intensity foci in white matter on T2-weighted magnetic resonance imaging. Frequency and clinical significance in symptom-free adults. Neuroradiology. 1993;35:151-155. [PubMed] |
| 11. | Dolapcioglu C, Guleryuzlu Y, Uygur-Bayramicli O, Ahishali E, Dabak R. Asymptomatic brain lesions on cranial magnetic resonance imaging in inflammatory bowel disease. Gut Liver. 2013;7:169-174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 12. | Chen M, Lee G, Kwong LN, Lamont S, Chaves C. Cerebral white matter lesions in patients with Crohn’s disease. J Neuroimaging. 2012;22:38-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Zikou AK, Kosmidou M, Astrakas LG, Tzarouchi LC, Tsianos E, Argyropoulou MI. Brain involvement in patients with inflammatory bowel disease: a voxel-based morphometry and diffusion tensor imaging study. Eur Radiol. 2014;24:2499-2506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 14. | Agostini A, Benuzzi F, Filippini N, Bertani A, Scarcelli A, Farinelli V, Marchetta C, Calabrese C, Rizzello F, Gionchetti P. New insights into the brain involvement in patients with Crohn’s disease: a voxel-based morphometry study. Neurogastroenterol Motil. 2013;25:147-e82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 94] [Article Influence: 7.8] [Reference Citation Analysis (1)] |
| 15. | Agostini A, Campieri M, Bertani A, Scarcelli A, Ballotta D, Calabrese C, Rizzello F, Gionchetti P, Nichelli P, Benuzzi F. Absence of change in the gray matter volume of patients with ulcerative colitis in remission: a voxel based morphometry study. Biopsychosoc Med. 2015;9:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Pierpaoli C, Barnett A, Pajevic S, Chen R, Penix LR, Virta A, Basser P. Water diffusion changes in Wallerian degeneration and their dependence on white matter architecture. Neuroimage. 2001;13:1174-1185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 702] [Cited by in RCA: 722] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 17. | Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15:435-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3340] [Cited by in RCA: 3399] [Article Influence: 147.8] [Reference Citation Analysis (0)] |
| 18. | Zivadinov R, Shucard JL, Hussein S, Durfee J, Cox JL, Bergsland N, Dwyer MG, Benedict RH, Ambrus J, Shucard DW. Multimodal imaging in systemic lupus erythematosus patients with diffuse neuropsychiatric involvement. Lupus. 2013;22:675-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 19. | Pievani M, Paternicò D, Benussi L, Binetti G, Orlandini A, Cobelli M, Magnaldi S, Ghidoni R, Frisoni GB. Pattern of structural and functional brain abnormalities in asymptomatic granulin mutation carriers. Alzheimers Dement. 2014;10:S354-S363.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 20. | Padilla N, Junqué C, Figueras F, Sanz-Cortes M, Bargalló N, Arranz A, Donaire A, Figueras J, Gratacos E. Differential vulnerability of gray matter and white matter to intrauterine growth restriction in preterm infants at 12 months corrected age. Brain Res. 2014;1545:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 21. | Adamson C, Yuan W, Babcock L, Leach JL, Seal ML, Holland SK, Wade SL. Diffusion tensor imaging detects white matter abnormalities and associated cognitive deficits in chronic adolescent TBI. Brain Inj. 2013;27:454-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Bryan RN, Manolio TA, Schertz LD, Jungreis C, Poirier VC, Elster AD, Kronmal RA. A method for using MR to evaluate the effects of cardiovascular disease on the brain: the cardiovascular health study. AJNR Am J Neuroradiol. 1994;15:1625-1633. [PubMed] |
| 23. | Meguro K, Yamaguchi T, Hishinuma T, Miyazawa H, Ono S, Yamada K, Matsuzawa T. Periventricular hyperintensity on magnetic resonance imaging correlated with brain ageing and atrophy. Neuroradiology. 1993;35:125-129. [PubMed] |
| 24. | Swartz RH, Kern RZ. Migraine is associated with magnetic resonance imaging white matter abnormalities: a meta-analysis. Arch Neurol. 2004;61:1366-1368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 211] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 25. | Pavese N, Canapicchi R, Nuti A, Bibbiani F, Lucetti C, Collavoli P, Bonuccelli U. White matter MRI hyperintensities in a hundred and twenty-nine consecutive migraine patients. Cephalalgia. 1994;14:342-345. [PubMed] |
| 26. | Kieslich M, Errázuriz G, Posselt HG, Moeller-Hartmann W, Zanella F, Boehles H. Brain white-matter lesions in celiac disease: a prospective study of 75 diet-treated patients. Pediatrics. 2001;108:E21. [PubMed] |
| 27. | Novacek G, Miehsler W, Kapiotis S, Katzenschlager R, Speiser W, Vogelsang H. Thromboembolism and resistance to activated protein C in patients with inflammatory bowel disease. Am J Gastroenterol. 1999;94:685-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Lam A, Borda IT, Inwood MJ, Thomson S. Coagulation studies in ulcerative colitis and Crohn’s disease. Gastroenterology. 1975;68:245-251. [PubMed] |
| 29. | Twig G, Zandman-Goddard G, Szyper-Kravitz M, Shoenfeld Y. Systemic thromboembolism in inflammatory bowel disease: mechanisms and clinical applications. Ann N Y Acad Sci. 2005;1051:166-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 62] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 30. | May A. Chronic pain may change the structure of the brain. Pain. 2008;137:7-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 469] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 31. | Kuchinad A, Schweinhardt P, Seminowicz DA, Wood PB, Chizh BA, Bushnell MC. Accelerated brain gray matter loss in fibromyalgia patients: premature aging of the brain? J Neurosci. 2007;27:4004-4007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 424] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 32. | Apkarian AV, Sosa Y, Sonty S, Levy RM, Harden RN, Parrish TB, Gitelman DR. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci. 2004;24:10410-10415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 970] [Cited by in RCA: 989] [Article Influence: 49.5] [Reference Citation Analysis (0)] |
| 33. | Schmidt-Wilcke T, Leinisch E, Straube A, Kämpfe N, Draganski B, Diener HC, Bogdahn U, May A. Gray matter decrease in patients with chronic tension type headache. Neurology. 2005;65:1483-1486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 304] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 34. | Blankstein U, Chen J, Diamant NE, Davis KD. Altered brain structure in irritable bowel syndrome: potential contributions of pre-existing and disease-driven factors. Gastroenterology. 2010;138:1783-1789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 200] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 35. | Seminowicz DA, Labus JS, Bueller JA, Tillisch K, Naliboff BD, Bushnell MC, Mayer EA. Regional gray matter density changes in brains of patients with irritable bowel syndrome. Gastroenterology. 2010;139:48-57.e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 240] [Cited by in RCA: 232] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 36. | Schweinhardt P, Kuchinad A, Pukall CF, Bushnell MC. Increased gray matter density in young women with chronic vulvar pain. Pain. 2008;140:411-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 143] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 37. | Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732-741. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2981] [Cited by in RCA: 2786] [Article Influence: 174.1] [Reference Citation Analysis (0)] |
| 38. | Buntinx M, Moreels M, Vandenabeele F, Lambrichts I, Raus J, Steels P, Stinissen P, Ameloot M. Cytokine-induced cell death in human oligodendroglial cell lines: I. Synergistic effects of IFN-gamma and TNF-alpha on apoptosis. J Neurosci Res. 2004;76:834-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 104] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 39. | Agostini A, Filippini N, Cevolani D, Agati R, Leoni C, Tambasco R, Calabrese C, Rizzello F, Gionchetti P, Ercolani M. Brain functional changes in patients with ulcerative colitis: a functional magnetic resonance imaging study on emotional processing. Inflamm Bowel Dis. 2011;17:1769-1777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 40. | Canero A, Parmeggiani D, Avenia N, Atelli PF, Goffredi L, Peltrini R, Madonna I, Ambrosino P, Apperti S, Apperti M. Thromboembolic tendency (TE) in IBD (Inflammatory bowel disease) patients. Ann Ital Chir. 2012;83:313-317. [PubMed] |
| 41. | Kappelman MD, Horvath-Puho E, Sandler RS, Rubin DT, Ullman TA, Pedersen L, Baron JA, Sørensen HT. Thromboembolic risk among Danish children and adults with inflammatory bowel diseases: a population-based nationwide study. Gut. 2011;60:937-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 242] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 42. | Barclay AR, Keightley JM, Horrocks I, Garrick V, McGrogan P, Russell RK. Cerebral thromboembolic events in pediatric patients with inflammatory bowel disease. Inflamm Bowel Dis. 2010;16:677-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 43. | Benavente L, Morís G. Neurologic disorders associated with inflammatory bowel disease. Eur J Neurol. 2011;18:138-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 44. | Cognat E, Crassard I, Denier C, Vahedi K, Bousser MG. Cerebral venous thrombosis in inflammatory bowel diseases: eight cases and literature review. Int J Stroke. 2011;6:487-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 45. | Singh S, Singh H, Loftus EV, Pardi DS. Risk of cerebrovascular accidents and ischemic heart disease in patients with inflammatory bowel disease: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2014;12:382-93.e1: quiz e22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 213] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 46. | Nudelman RJ, Rosen DG, Rouah E, Verstovsek G. Cerebral sinus thrombosis: a fatal neurological complication of ulcerative colitis. Patholog Res Int. 2010;2010:132754. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 47. | Casella G, Spreafico C, Costantini M, Pagni F, Cortelezzi CC, Baldini V, DeLodovici ML, Bono G. Cerebral sinus thrombosis in ulcerative colitis. Inflamm Bowel Dis. 2011;17:2214-2216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 48. | Katsanos AH, Katsanos KH, Kosmidou M, Giannopoulos S, Kyritsis AP, Tsianos EV. Cerebral sinus venous thrombosis in inflammatory bowel diseases. QJM. 2013;106:401-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 49. | Ferro JM, Oliveira S. Neurologic manifestations of gastrointestinal and liver diseases. Curr Neurol Neurosci Rep. 2014;14:487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 50. | Miehsler W, Reinisch W, Valic E, Osterode W, Tillinger W, Feichtenschlager T, Grisar J, Machold K, Scholz S, Vogelsang H. Is inflammatory bowel disease an independent and disease specific risk factor for thromboembolism? Gut. 2004;53:542-548. [PubMed] |
| 51. | Saibeni S, Spina L, Vecchi M. Exploring the relationships between inflammatory response and coagulation cascade in inflammatory bowel disease. Eur Rev Med Pharmacol Sci. 2004;8:205-208. [PubMed] |
| 52. | Dolapcioglu C, Soylu A, Kendir T, Ince AT, Dolapcioglu H, Purisa S, Bolukbas C, Sokmen HM, Dalay R, Ovunc O. Coagulation parameters in inflammatory bowel disease. Int J Clin Exp Med. 2014;7:1442-1448. [PubMed] |
| 53. | Tsiolakidou G, Koutroubakis IE. Thrombosis and inflammatory bowel disease-the role of genetic risk factors. World J Gastroenterol. 2008;14:4440-4444. [PubMed] |
| 54. | Chamouard P, Grunebaum L, Wiesel ML, Freyssinet JM, Duclos B, Cazenave JP, Baumann R. Prevalence and significance of anticardiolipin antibodies in Crohn’s disease. Dig Dis Sci. 1994;39:1501-1504. [PubMed] |
| 55. | Souto JC, Borrell M, Fontcuberta J, Roca M. Antiphospholipid antibodies in inflammatory bowel disease. Dig Dis Sci. 1995;40:1524-1525. [PubMed] |
| 56. | Saibeni S, Cattaneo M, Vecchi M, Zighetti ML, Lecchi A, Lombardi R, Meucci G, Spina L, de Franchis R. Low vitamin B(6) plasma levels, a risk factor for thrombosis, in inflammatory bowel disease: role of inflammation and correlation with acute phase reactants. Am J Gastroenterol. 2003;98:112-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 130] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 57. | Casella G, Tontini GE, Bassotti G, Pastorelli L, Villanacci V, Spina L, Baldini V, Vecchi M. Neurological disorders and inflammatory bowel diseases. World J Gastroenterol. 2014;20:8764-8782. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 58. | Danese S, Papa A, Saibeni S, Repici A, Malesci A, Vecchi M. Inflammation and coagulation in inflammatory bowel disease: The clot thickens. Am J Gastroenterol. 2007;102:174-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 282] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 59. | Lossos A, Stener I. Cerebrovascular disease in inflammatory bowel disease. Uncommon causes of stroke. Cambridge: Cambridge University Press 2001; 161-168. |
| 60. | Keene DL, Matzinger MA, Jacob PJ, Humphreys P. Cerebral vascular events associated with ulcerative colitis in children. Pediatr Neurol. 2001;24:238-243. [PubMed] |
| 62. | Schneiderman JH, Sharpe JA, Sutton DM. Cerebral and retinal vascular complications of inflammatory bowel disease. Ann Neurol. 1979;5:331-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 91] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 63. | Fukuhara T, Tsuchida S, Kinugasa K, Ohmoto T. A case of pontine lacunar infarction with ulcerative colitis. Clin Neurol Neurosurg. 1993;95:159-162. [PubMed] |
| 64. | Scheid R, Hegenbart U, Ballaschke O, Von Cramon DY. Major stroke in thrombotic-thrombocytopenic purpura (Moschcowitz syndrome). Cerebrovasc Dis. 2004;18:83-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 65. | Baron BW, Jeon HR, Glunz C, Peterson A, Cohen R, Hanauer S, Rubin D, Hart J, Baron JM. First two patients with ulcerative colitis who developed classical thrombotic thrombocytopenic purpura successfully treated with medical therapy and plasma exchange. J Clin Apher. 2002;17:204-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 66. | Hisada T, Miyamae Y, Mizuide M, Shibusawa N, Iida T, Masuo T, Okada S, Sagawa T, Ishizuka T, Kusano M. Acute thrombocytopenia associated with preexisting ulcerative colitis successfully treated with colectomy. Intern Med. 2006;45:87-91. [PubMed] |
| 67. | Spina L, Saibeni S, Battaglioli T, Peyvandi F, de Franchis R, Vecchi M. Thrombosis in inflammatory bowel diseases: role of inherited thrombophilia. Am J Gastroenterol. 2005;100:2036-2041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 61] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 68. | Saadatnia M, Fatehi F, Basiri K, Mousavi SA, Mehr GK. Cerebral venous sinus thrombosis risk factors. Int J Stroke. 2009;4:111-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 69. | Diakou M, Kostadima V, Giannopoulos S, Zikou AK, Argyropoulou MI, Kyritsis AP. Cerebral venous thrombosis in an adolescent with ulcerative colitis. Brain Dev. 2011;33:49-51. [PubMed] |
| 70. | Benjilali L, Aidi S, El Mansouri H, Benabdejlil M, Jiddane M, El Alaoui Faris M. Cerebral thrombosis complicating Crohn’s disease: two cases. J Stroke Cerebrovasc Dis. 2011;20:565-569. [PubMed] |
| 71. | Stam J. Thrombosis of the cerebral veins and sinuses. N Engl J Med. 2005;352:1791-1798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 959] [Cited by in RCA: 875] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 72. | Ferro JM, Canhão P, Stam J, Bousser MG, Barinagarrementeria F. Prognosis of cerebral vein and dural sinus thrombosis: results of the International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT). Stroke. 2004;35:664-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1336] [Cited by in RCA: 1412] [Article Influence: 67.2] [Reference Citation Analysis (0)] |
| 73. | Rodríguez S, Calleja S, Morís G. Cluster-like headache heralding cerebral venous thrombosis. Cephalalgia. 2008;28:906-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 74. | Rang EH, Brooke BN, Hermon-Taylor J. Association of ulcerative colitis with multiple sclerosis. Lancet. 1982;2:555. [PubMed] |
| 75. | Pandian JD, Pawar G, Singh GS, Abraham R. Multiple sclerosis in a patient with chronic ulcerative colitis. Neurol India. 2004;52:282-283. [PubMed] |
| 76. | Bernstein CN, Wajda A, Blanchard JF. The clustering of other chronic inflammatory diseases in inflammatory bowel disease: a population-based study. Gastroenterology. 2005;129:827-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 415] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 77. | Gupta G, Gelfand JM, Lewis JD. Increased risk for demyelinating diseases in patients with inflammatory bowel disease. Gastroenterology. 2005;129:819-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 199] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 78. | Scheid R, Teich N. Neurologic manifestations of ulcerative colitis. Eur J Neurol. 2007;14:483-493. [PubMed] |
| 79. | Purrmann J, Arendt G, Cleveland S, Borchard F, Fürst W, Gemsa R, Bertrams J, Hengels KJ. Association of Crohn’s disease and multiple sclerosis. Is there a common background? J Clin Gastroenterol. 1992;14:43-46. [PubMed] |
| 80. | Dobson R, Giovannoni G. Autoimmune disease in people with multiple sclerosis and their relatives: a systematic review and meta-analysis. J Neurol. 2013;260:1272-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 81. | Buccino GP, Corrente G, Visintini D. Crohn’s disease and multiple sclerosis: a single case report. Ital J Neurol Sci. 1994;15:303-306. [PubMed] |
| 82. | Kitchin LI, Knobler RL, Friedman LS. Crohn’s disease in a patient with multiple sclerosis. J Clin Gastroenterol. 1991;13:331-334. [PubMed] |
| 83. | Beaugerie L, Lamy P, Ganne N, Carbonnel F, Le Quintrec Y, Cosnes J, Gendre JP. [Morbid associations in Crohn’s disease. Study of a series of 832 patients]. Presse Med. 1997;26:892-894. [PubMed] |
| 84. | Deepak P, Stobaugh DJ, Ehrenpreis ED. Infectious complications of TNF-α inhibitor monotherapy versus combination therapy with immunomodulators in inflammatory bowel disease: analysis of the Food and Drug Administration Adverse Event Reporting System. J Gastrointestin Liver Dis. 2013;22:269-276. [PubMed] |
| 85. | Van Assche G, Van Ranst M, Sciot R, Dubois B, Vermeire S, Noman M, Verbeeck J, Geboes K, Robberecht W, Rutgeerts P. Progressive multifocal leukoencephalopathy after natalizumab therapy for Crohn’s disease. N Engl J Med. 2005;353:362-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 813] [Cited by in RCA: 763] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 86. | Yousry TA, Major EO, Ryschkewitsch C, Fahle G, Fischer S, Hou J, Curfman B, Miszkiel K, Mueller-Lenke N, Sanchez E. Evaluation of patients treated with natalizumab for progressive multifocal leukoencephalopathy. N Engl J Med. 2006;354:924-933. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 614] [Cited by in RCA: 537] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 87. | Singh S, Kumar N, Loftus EV, Kane SV. Neurologic complications in patients with inflammatory bowel disease: increasing relevance in the era of biologics. Inflamm Bowel Dis. 2013;19:864-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 88. | Lozeron P, Denier C, Lacroix C, Adams D. Long-term course of demyelinating neuropathies occurring during tumor necrosis factor-alpha-blocker therapy. Arch Neurol. 2009;66:490-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 141] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 89. | Pfueller CF, Seipelt E, Zipp F, Paul F. Multiple sclerosis following etanercept treatment for ankylosing spondylitis. Scand J Rheumatol. 2008;37:397-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 90. | Lees CW, Ali AI, Thompson AI, Ho GT, Forsythe RO, Marquez L, Cochrane CJ, Aitken S, Fennell J, Rogers P. The safety profile of anti-tumour necrosis factor therapy in inflammatory bowel disease in clinical practice: analysis of 620 patient-years follow-up. Aliment Pharmacol Ther. 2009;29:286-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 100] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 91. | Nelson J, Barron MM, Riggs JE, Gutmann L, Schochet SS. Cerebral vasculitis and ulcerative colitis. Neurology. 1986;36:719-721. [PubMed] |
| 92. | Carmona MA, Jaume Anselmi F, Ramírez Rivera J. Cerebral thrombosis and vasculitis: an uncommon complication of ulcerative colitis. Bol Asoc Med P R. 2000;92:9-11. [PubMed] |
| 93. | Pandian JD, Henderson RD, O’Sullivan JD, Rajah T. Cerebral vasculitis in ulcerative colitis. Arch Neurol. 2006;63:780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 94. | Friol-Vercelletto M, Mussini JM, Bricout JH, Magne C. [Ulcero-hemorrhagic rectocolitis. Possible manifestation, angiitis of the central nervous system]. Presse Med. 1984;13:1218. [PubMed] |
| 95. | Druschky A, Heckmann JG, Druschky K, Huk WJ, Erbguth F, Neundörfer B. Severe neurological complications of ulcerative colitis. J Clin Neurosci. 2002;9:84-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 96. | Masaki T, Muto T, Shinozaki M, Kuroda T. Unusual cerebral complication associated with ulcerative colitis. J Gastroenterol. 1997;32:251-254. [PubMed] |
| 97. | Schluter A, Krasnianski M, Krivokuca M, Spielmann RP, Neudecker S, Hirsch W. Magnetic resonance angiography in a patient with Crohn’s disease associated cerebral vasculitis. Clin Neurol Neurosurg. 2004;106:110-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 98. | Berlit P. Diagnosis and treatment of cerebral vasculitis. Ther Adv Neurol Disord. 2010;3:29-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 108] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 99. | Holzer K, Esposito L, Stimmer H, Hemmer B, Poppert H. Cerebral vasculitis mimicking migraine with aura in a patient with Crohn’s disease. Acta Neurol Belg. 2009;109:44-48. [PubMed] |
| 100. | Viget N, Vernier-Massouille G, Salmon-Ceron D, Yazdanpanah Y, Colombel JF. Opportunistic infections in patients with inflammatory bowel disease: prevention and diagnosis. Gut. 2008;57:549-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 131] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 101. | Ma C, Walters B, Fedorak RN. Varicella zoster meningitis complicating combined anti-tumor necrosis factor and corticosteroid therapy in Crohn’s disease. World J Gastroenterol. 2013;19:3347-3351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 102. | Majumder S, Kumar A. Meningococcal meningoencephalitis after certolizumab pegol treatment in a patient with Crohn’s disease. J Crohns Colitis. 2013;7:e19. [PubMed] |