Published online Feb 15, 2015. doi: 10.4291/wjgp.v6.i1.23
Peer-review started: May 21, 2014
First decision: June 27, 2014
Revised: November 18, 2014
Accepted: January 18, 2015
Article in press: January 18, 2015
Published online: February 15, 2015
Processing time: 266 Days and 6.5 Hours
AIM: To elucidate the effect of a proton pump inhibitor (PPI, rabeparazole) on oesophageal bile reflux in oesophagitis after total gastrectomy.
METHODS: Twenty-one 8-week-old male Wistar rats were studied. They were performed oesophagoduodenostomy of total gastrectomy to induce oesophageal reflux of biliary and pancreatic juice. Five rats were performed the sham operation (Sham). On post-operative day 7, they were treated with saline (Control) (n = 8) or PPI (rabeprazole, 30 mg/kg per day, ip ) (n = 8) for 2 wk. On post-operative 21, all rats were sacrificed and each oesophagus was evaluated histologically. Oesophageal injury was evaluated by macroscopic and microscopic findings as well as the expression of cyclooxygenase-2 (COX2). We measured bile acid in the oesophageal lumen and the common bile duct.
RESULTS: At 3 wk after surgery, a histological study analysis revealed an increase in the thickness of the epithelium, elongation of the lamina propria and basal cell hyperplasia in the oesophageal mucosa. The macroscopic ulcer score and microscopic ulcer length of the control group were significantly higher compared to those of the rabeprazole- treated group. The expression of COX2 was significantly increased according to the immunostaining in the control group compared to rabeprazole- treated group. Although there was no difference between the control and PPI groups in the total bile acid in the common bile duct, the bile acid activity in the oesophageal lumen was significantly decreased in the rabeprazole- treated group due to augmentation of the duodenal motor complex.
CONCLUSION: With this model, rabeprazole is good effect for reflux esophagitis after total gastrectomy from bile reflux. Bile acid is an important factor in the mucosal lesion induced by duodenal reflux.
Core tip: To elucidate the effect of proton pump inhibitor (PPI, rabeparazole) on reflux oesophagitis. Sixteen 8-week-old male Wistar rats were studied. They were performed oesophagoduodenostomy of total gastrectomy to induce oesophageal reflux of biliary and pancreatic juice. Five rats were performed the sham operation (Sham). On post-operative day 7, they were treated with saline (Control) (n = 8) or PPI (rabeprazole, 30 mg/kg per day, ip) (n = 8) for 2 wk. On post-operative 21, all rats were sacrificed and each oesophagus was evaluated histologically. Oesophageal injury was evaluated by macroscopic and microscopic findings as well as the expression of cyclooxygenase-2 (COX2). We measured bile acid in the oesophageal lumen and the common bile duct. The macroscopic ulcer score and microscopic ulcer length of the control group were significantly higher compared to those of the rabeprazole- treated group. The expression of COX2 was significantly increased according to the immunostaining in the control group compared to rabeprazole- treated group. Although there was no difference between the control and PPI groups in the total bile acid in the common bile duct, the bile acid activity in the oesophageal lumen was significantly decreased in the rabeprazole- treated group due to augmentation of the duodenal motor complex. With this model, rabeprazole is good effect for reflux oesophagitis after total gastrectomy from bile reflux.
- Citation: Hashimoto N. Rabeprazole is effective for bile reflux oesophagitis after total gastrectomy in a rat model. World J Gastrointest Pathophysiol 2015; 6(1): 23-28
- URL: https://www.wjgnet.com/2150-5330/full/v6/i1/23.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v6.i1.23
Reflux of duodenal contents contributes to the development of oesophageal mucosal lesion[1] Oesophagitis after total gastrectomy has been associated with the reflux of duodenal content (biliary and pancreatic juice) into the oesophagus.
Camostat mesilate[2] is commonly used in medical therapy for reflux oesophagitis after total gastrectomy. However, camostat mesilate therapy alone may not result in complete recovery of reflux oesophagitis after total gastrectomy.
More than 10% of patients are reported to have relapses of oesophagitis, even if camostat mesilate is used for maintenance therapy. Proton pump inhibitors (PPIs) are considered the medical therapy for reflux oesophagitis. Although medications such as PPIs are thought to be efficacious in the treatment of reflux oesophagitis, the mechanism of the curative effect of such drugs remains unclear.
This study was to elucidate the effect of a PPI (rabeprazole, Eisai, Tokyo, Japan) on reflux oesophagitis.
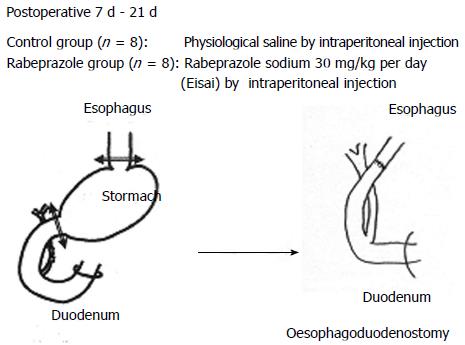
Eight week old male Wistar rats weighing 200-250 g were used in this study. The animal care and use committee of Kinki University prospectively approved all procedures.
The rats were permitted to acclimate for 2 wk before surgery. Prior to surgery, the animals were fasted for 24 h. An oesophago-duodenal anastomosis was performed under general anaesthesia (pentobarbital 50 mg/kg body wt intraperitoneal injection) through an upper midline incision. The gastroesophageal junction was ligated and the distal oesophagus was transected 2 mm above the ligature. Moreover, the gastroduodenal junction was also ligated, and the proximal duodenum was transected 3 mm distal to the pylorus. A total gastrectomy was performed with the removal of the entire stomach, and end-to-end anastomosis of the oesophagus and duodenum. In the sham group, five rats underwent a sham operation, with a midline laparotomy alone without further surgical intervention.
Postoperatively, the rats were allowed to drink water after 6 h and were fed the following day. Feeding with a commercial chow (Oriental Co Ltd) was resumed on day 2.
Seven days postoperatively, 16 operated rats with reflux oesophagitis were allocated into two groups, a control group (n = 8) that was treated with intraperitoneal injection of vehicle (physiological saline )/per day for 2 wk and a PPI group (n = 8) that was given rabeprazole sodium (Eisai, Tokyo,Japan) by intraperitoneal injection at a dose of 30 mg/kg per day for 2 wk. The Sham group (n = 5) was given regular rat chow to obtain normal control tissue. Fujisaki et al[3] reported that the subcutaneous injection of rabeprazole at doses of 1-30 mg/kg to rats was effective for reducing reflux esophagitis induced by ligation of both the boundary regions between the forestomach and the glandular portion as well as between the pylorus and the duodenum in Sprague-Dawley rats. Rabeprazole, at a dose of 30 mg/kg, inhibited reflux oesophagitis that had been induced by 10 h of ligation. In the present study, we selected a sufficient dose (30 mg/kg per day) of rabeprazole against rat oesophagitis (Figure 1).
Rats were evaluated at 21 d postoperatively under general anaesthesia. All the oesophagi were cut longitudinally; they were fixed in 10% buffered formalin. The formalin-fixed oesophagus was Swiss-rolled, processed and embedded in paraffin. Five-micron sections were mounted onto glass slides and were used for the pathological and immunohistochemical analysis.
A person who was blinded to the treatment scored the macroscopic ulcer lesions as follows: normal glistening mucosal appearance (score 0), oedematous mucosa with focal haemorrhagic spots (score 1); multiple erosions with haematins attached (score 2); linear ulcerations with yellowish exudates (score 3) or haemorrhagic coalesced ulcerations (score 4).
The entire area of damage was collected and fixed in 10% formalin for the histological evaluation. The degree of epithelial loss was measured by micrometre as the ulcer length.
COX2: Localisation of COX2 protein was determined by immunohistochemical staining using specific antibodies. The DAKO EnVision system (Dako Cytomation Japan Co. Ltd., Kyoto, Japan) was used with autoclave acceleration. After blocking with endogenous peroxidase, deparaffinized sections covered with a protein block and serum-free media (Dako) were incubated overnight at 4 °C with individual primary antibodies, including antimouse COX2 (1:50, mouse monoclonal; BD Transduction Laboratories, San Jose, Calif). Sections were treated with a secondary biotinylated antibody (Dako). 3,3’-diaminobenzidine tetrahydrochloride was used as the chromogen, and the sections were counterstained with haematoxylin.
The oesophagus was removed and lavaged with 0.5 mL of saline. The saline used for the lavage was centrifuged at 1500 g at 4 °C for 5 min. The supernatant was frozen and stored.
I cut down the common bile duct and insert PE 10 polyethylene tube to common bile duct to collect bile juice. The total bile acid concentration was measured with an ENZa BILE kit (Daiichi Chemical, Tokyo).
Data are expressed as the mean ± SD of each group. The Mann-Whitney U test was used to compare each group. Differences were considered significant when the P value was < 0.05.
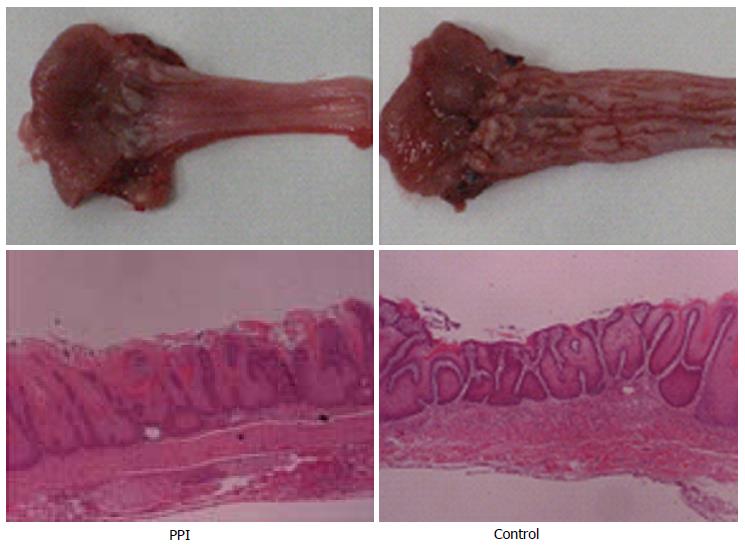
In control rats, the oesophageal wall showed shortening and dilatation compared to PPI group and was covered with whitish nodular patches. However, the gross appearance of the oesophagus from the PPI group, showed only scattered erosions or mild haemorrhage spots along the oesophagus. The ulcer score was significantly (P < 0.05) decreased by treatment with a PPI (score 1: 2, score 2: 3, score 3: 3 and score 4: 0) compared with control (score 1: 0 score 2: 0 score 3: 1, and score 4: 7) (Figure 2).
The control group had evident thickening of the epithelium, elongation of the lamina papillae, and basal cell hyperplasia in the oesophageal mucosa. Histological examination revealed much more severe oesophagitis in the control group compared to the PPI group. The microscopic ulcer length was significantly (P < 0.05) increased in the control group (8 ± 1 mm) compared to the PPI group (5 ± 1 mm) (Figure 2).
Total bile acid in the oesophageal lumen (μmol/L)
The control group (175 ± 50) was significantly higher compared to the sham operated rats (35 ± 5) in the total bile acid in the oesophageal lumen. The treatment with a PPI (45 ± 5) significantly (P < 0.05) inhibited the increase in the total bile acid activity in the oesophageal lumen.
There is no difference between the control group (26.5 ± 5.1) and the PPI group (22.9 ± 3.5). However, the control and PPI groups have significantly (P < 0.05) higher levels than the sham group (18.2 ± 2.4).
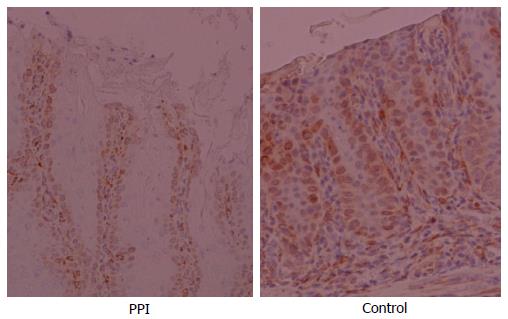
COX2 was not detected in the normal oesophagus. However, COX2 was over- expressed in the oesophageal mucosal lesion causing chronic duodenal esophageal reflux. The expression of COX2 was significantly (P < 0.05) increased according to immunostaining in the control group compared to the PPI group (Figure 3).
It is well known that bile reflux plays an important role in the etiology of reflux esophagitis. In patients with gastroesophageal reflux disease, the concentration of bile acids in the oesophageal refluxate correlates with the degree of oesophageal mucosal injury[4]. In my experimental animals, the duodenoesophageal anastomosis led to reflux oesophagitis[5]. Bile acids is harmful and induce mucosal injury[6].
Helsingen[7] performed total gastrectomy and oesophagoduodenostomy on rats, examined the oesophageal mucosa from postoperative 4 d to 4 mo, and reported that the mucosal epithelium was destroyed and inflammation occurred inside the lamina muscularis mucosae relatively early. In our experiment on rats that underwent total gastrectomy and oesophagoduodenostomy, erosion and ulceration of the oesophagus were noted 2 wk after the operation. Hyperplasia, ulceration and inflammation (polymorphonuclear and lymphocytic infiltration) of the mucosal epithelium were the histologic features of reflux oesophagitis in rats, which were same feature of Helsingen’s data.
This study provides concrete evidence that bile acid induces oesophageal mucosal lesions in rats by oesophagoduodenal reflux and that treatment with RPZ, a PPI, is decreasing histological findings of reflux oesophagitis as well as decreasing the expression of COX2 in affected oesophageal mucosa compared to saline treatment.
The most striking finding in the present study was that RPZ, PPI, attenuated oesophageal mucosal injury in duodenoesophageal reflux. Bile acid plays a significant role in the etiology of reflux esophagitis previously. Bile acid is one of the important factors of duodenal fluid. We observed over-expression of COX2 in inflamed oesophageal mucosa in an oesophagoduodenal anastomosis. However, we could not detect COX2 in normal oesophageal mucosa. Bile acid is harmful to the oesophagus, causing reflux esophagitis.
The precise mechanisms by which bile acid causes oesophageal injury remain unclear. Bile acids induce COX2 by both transcriptional and post-transcriptional mechanisms[8,9]. Protein kinase C was important factor for the bile acid-mediating the induction of COX2. Bile acids can also stimulate PI-3K activity[10]; this finding suggests that PI-3K could be involved in mediating the induction of COX2. We found that 2 inhibitors of PI-3K activity blocked the induction of COX2 by bile acids. ERK1/2MAPK is downstream of PI-3K and is important role of the regulation of COX2[11]. In fact, ERK1/2MAPK is involved in regulating both the transcription of COX2 and stability of COX2 mRNA. Treatment with bile acid induced ERK1/2 activity, and inhibiting the activation of ERK1/2 blocked the induction of COX2 by bile acid. These datas suggest that the bile acid-mediated induction of COX2 involves a signaling cascade that consists of PKC, PI-3K and ERK1/2MAPK.
Data on how RPZ reduces the degree of bile reflux are scarce. Champion et al[12] found a reduction in the percentage time of bilirubin absorbance > 0.14 from 32.8% to 4.7% with 40 mg of omeprazole daily in nine patients (3 GERD and 6 Barrett’s oesophagus). Administering the same dose of omeprazole to 11BO patients, Marshall et al observed a decrease in the oesophageal bilirubin exposure from a median of 28.9% to 2.4%[13].
There are two possible explanations for the reduction of DGER with acid suppressant therapy. First, PPIs generally reduce gastric secretion by approximately 40%, decreasing the volume of refluxate[14]. Second, PPIs have been shown to augment the antral and duodenal phase III migrating motor complex in healthy individuals, accelerating antroduodenal passage of gastric contents, which should reduce duodenogastric reflux[15]. These findings show that PPIs can reduce the reflux of bile acids into the oesophagus. In our total gastrectomy model, gastric secretion was not affected by PPI. There was no difference between the control (26.5 ± 5.1 mmol/L) and PPI groups (22.9 ± 3.5 mmol/L) in bile acid concentration from the common bile duct. PPIs do not inhibit the secretion of bile acid from the common bile duct. Therefore, we speculate that PPIs accelerate the duodenal phase III migrating motor complex, accelerating the duodenal passage of duodenal contents (bile acids), which should reduce duodenoesophageal reflux. Therefore, the bile acid activity in the oesophageal lumen was significantly decreased in the rabeprazole- treated group due to augmentation of the duodenal motor complex.
Recent studies suggest that PPIs have effects beyond acid suppression. Actually PPI reduced IL-8 in the oesophageal mucosa of GERD patients[16]. Additionally, histological improvement may also implicate the cytoprotective properties of rabeprazole against bile induced oesophageal damage[17].
This evidence suggests that PPIs not only inhibit acid secretion but also reduce inflammation in the oesophageal mucosa. In our study described above, rabeprazole was good effect for reflux oesophagitis after total gastrectomy due to bile reflux.
In conclusion, we have demonstrated, with our model, that rabeprazole is good effect for reflux esophagitis after total gastrectomy from bile reflux. Bile acid is an important factor in the mucosal lesion induced by duodenal reflux.
Bile acid into the oesophagus has a role in the etiology of oesophageal lesions.
In this study, Rabeprazole is good effect for reflux esophagitis after total gastrectomy from bile reflux. Bile acid is an important factor in the mucosal lesion induced by duodenal reflux.
Rabeprazole is good effect for reflux oesophagitis.
Hematoxylin eosin, cyclooxygenase-2 (COX2) and proton pump inhibitor (PPI).
In the present study, the authors examined the effect of PPI on esophageal bile reflux in esophagitis after total gastrectomy in rat’s experimental model. They revealed that the macroscopic and microscopic reflux esophagitis were significantly reduced by rabeprazole treatment, and the COX2 expression was also markedly inhibited by rabeprazole treatment. Further, the bile acid activity in the esophageal lumen was significantly decreased by rabeprazole treatment. This study was well designed and the results were interesting.
P- Reviewer: Osawa S S- Editor: Song XX L- Editor: A E- Editor: Wu HL
| 1. | Kauer WK, Peters JH, DeMeester TR, Ireland AP, Bremner CG, Hagen JA. Mixed reflux of gastric and duodenal juices is more harmful to the esophagus than gastric juice alone. The need for surgical therapy re-emphasized. Ann Surg. 1995;222:525-531; discussion 531-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Tamura Y, Hirado M, Okamura K, Minato Y, Fujii S. Synthetic inhibitors of trypsin, plasmin, kallikrein, thrombin, C1r-, and C1 esterase. Biochim Biophys Acta. 1977;484:417-422. [PubMed] |
| 3. | Fujisaki H, Oketani K, Hirota K. Effects of Rabeprazole Sodium and Famotidine on Reflux Esophagitis in Rats. JNRC. 2003;52:752-760. |
| 4. | Nehra D, Howell P, Williams CP, Pye JK, Beynon J. Toxic bile acids in gastro-oesophageal reflux disease: influence of gastric acidity. Gut. 1999;44:598-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 299] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 5. | Goldstein SR, Yang GY, Curtis SK, Reuhl KR, Liu BC, Mirvish SS, Newmark HL, Yang CS. Development of esophageal metaplasia and adenocarcinoma in a rat surgical model without the use of a carcinogen. Carcinogenesis. 1997;18:2265-2270. [PubMed] |
| 6. | Kivilaakso E, Fromm D, Silen W. Effect of bile salts and related compounds on isolated esophageal mucosa. Surgery. 1980;87:280-285. [PubMed] |
| 7. | Helsingen N. Oesophageal lesions following total gastrectomy in rats. I. Development and nature. Acta Chir Scand. 1960;118:202-216. [PubMed] |
| 8. | Galli J, Cammarota G, Calò L, Agostino S, D’Ugo D, Cianci R, Almadori G. The role of acid and alkaline reflux in laryngeal squamous cell carcinoma. Laryngoscope. 2002;112:1861-1865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 73] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 9. | Galli J, Calò L, Agostino S, Cadoni G, Sergi B, Cianci R, Cammarota G. Bile reflux as possible risk factor in laryngopharyngeal inflammatory and neoplastic lesions. Acta Otorhinolaryngol Ital. 2003;23:377-382. [PubMed] |
| 10. | Narisawa T, Magadia NE, Weisburger JH, Wynder EL. Promoting effect of bile acids on colon carcinogenesis after intrarectal instillation of N-methyl-N’-nitro-N-nitrosoguanidine in rats. J Natl Cancer Inst. 1974;53:1093-1097. [PubMed] |
| 11. | Zhang F, Subbaramaiah K, Altorki N, Dannenberg AJ. Dihydroxy bile acids activate the transcription of cyclooxygenase-2. J Biol Chem. 1998;273:2424-2428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 144] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 12. | Champion G, Richter JE, Vaezi MF, Singh S, Alexander R. Duodenogastroesophageal reflux: relationship to pH and importance in Barrett’s esophagus. Gastroenterology. 1994;107:747-754. [PubMed] [DOI] [Full Text] |
| 13. | Marshall RE, Anggiansah A, Manifold DK, Owen WA, Owen WJ. Effect of omeprazole 20 mg twice daily on duodenogastric and gastro-oesophageal bile reflux in Barrett’s oesophagus. Gut. 1998;43:603-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 77] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Lind T, Cederberg C, Ekenved G, Haglund U, Olbe L. Effect of omeprazole--a gastric proton pump inhibitor--on pentagastrin stimulated acid secretion in man. Gut. 1983;24:270-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 397] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 15. | Vinter-Jensen L, Kraglund K, Pedersen SA. A double-blind placebo-controlled trial of omeprazole on characteristics of the migrating motor complex in healthy volunteers. Aliment Pharmacol Ther. 1989;3:615-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 16. | Yoshida N, Yoshikawa T. Defense mechanism of the esophageal mucosa and esophageal inflammation. J Gastroenterol. 2003;38 Suppl 15:31-34. [PubMed] |
| 17. | Miner PB. Review article: physiologic and clinical effects of proton pump inhibitors on non-acidic and acidic gastro-oesophageal reflux. Aliment Pharmacol Ther. 2006;23 Suppl 1:25-32. [PubMed] |