Published online Oct 15, 2012. doi: 10.4291/wjgp.v3.i5.92
Revised: November 20, 2012
Accepted: December 6, 2012
Published online: October 15, 2012
AIM: To evaluate the role of tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) in cirrhotic patients who have hepatic and renal impairment with spontaneous bacterial peritonitis (SBP).
METHODS: We prospectively studied 120 cirrhotic patients with SBP and 80 cirrhotic patients with sterile ascitic fluid. They included 144 males and 56 females with ages ranging between 34 and 62 years. The diagnosis of cirrhosis was established by clinical and laboratory criteria that did not require histological confirmation. The severity of underlying liver disease was evaluated using Pugh’s modification of Child’s criteria (Child-Pugh scores). Ascitic fluid was sent to the laboratory for cell count, culture, sensitivity testing, and measurement of chemical elements (i.e., albumin, glucose). Specimens were inoculated into aerobic and anaerobic blood culture bottles. Serum and ascitic fluid were also collected in sterile tubes at study entry (before the initiation of antibiotic treatment) and 48 h later. Assays for TNF-α and IL-6 in the serum and ascitic fluid were performed with an immunoenzymometric assay using manufacture’s instructions.
RESULTS: Cytokine levels in serum and ascitic fluid were significantly higher in the patients with SBP. (plasma TNF-α: 135.35 ng/mL ± 11.21 ng/mL vs 92.86 ng/mL ± 17.56 ng/mL, P < 0.001; plasma IL-6: 32.30 pg/mL ± 7.07 pg/mL vs 12.11 pg/mL ± 6.53 pg/mL, P < 0.001; ascitic fluid TNF-α: 647.54 ± 107.11 ng/mL vs 238.43 ng/mL ± 65.42 ng/mL, P < 0.001); ascitic fluid IL-6: 132.84 ng/mL ± 34.13 vs 40.41 ± 12.85 pg/mL, P < 0.001). About 48 (40%) cirrhotic patients with SBP developed renal and hepatic impairment and showed significantly higher plasma and ascitic fluid cytokine levels at diagnosis of infection. [(plasma TNF-α: 176.58 ± 17.84 vs 135.35 ± 11.21 ng/mL) (P < 0.001) and (IL-6: 57.83 ± 7.85 vs 32.30 ± 7.07 pg/mL) (P < 0.001); ascitic fluid TNF-α: 958.39 ± 135.72 vs 647.54 ± 107.11 ng/mL, (P < 0.001), ascitic fluid IL-6: 654.74 ± 97.43 vs 132.84 ± 34.13 pg/mL, (P < 0.001)]. Twenty nine patients (60.4%) with SBP and renal impairment died whereas, only four patients (5.55%) with SBP but without renal impairment died from gastrointestinal hemorrhage (P < 0.0005).
CONCLUSION: It appears that TNF-α production may enhance liver cell injury and lead to renal impairment. This correlated well with the poor prognosis and significantly increased mortality associated with SBP in cirrhotic patients.
- Citation: Suliman MA, Khalil FM, Alkindi SS, Pathare AV, Almadhani AA, Soliman NA. Tumor necrosis factor-α and interleukin-6 in cirrhotic patients with spontaneous bacterial peritonitis. World J Gastrointest Pathophysiol 2012; 3(5): 92-98
- URL: https://www.wjgnet.com/2150-5330/full/v3/i5/92.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v3.i5.92
Spontaneous bacterial peritonitis (SBP) is a severe complication in cirrhotic patients with ascites[1]. It is defined as infection of a previously sterile ascitic fluid without any apparent intra-abdominal source of infection[2].
Although the pathogenesis of SBP is not completely understood, it is generally accepted that it involves three major steps: passage of bacteria from the intestinal lumen to the systemic circulation, bacteremia secondary to the impairment of the reticuloendothelial system and infection of ascites due to defective bactericidal activity of ascitic fluid[3]. Gut motility alterations, along with bacterial overgrowth and changes in intestinal permeability, probably play a role in bacterial translocation. Bacterial translocation is further associated with colonization of mesenteric lymph nodes, bacteremia and finally, seeding of bacteria into the ascitic fluid[4,5].
The sepsis syndrome in SBP, unfortunately causes release of multiple endogenous mediators that are responsible for the inflammatory response[6], in particular, tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6). Although the aim of this response is to counter the infection, it may be associated with adverse hemodynamic and metabolic consequences[1]. This could in turn impact on liver and renal functions, as well as survival.
This study was thus designed to assess the role of TNF-α and IL-6 in cirrhotic patients who have hepatic and renal impairment with SBP. The study further documents that TNF-α and IL-6 levels are higher in SBP patients. We have also shown that these cytokine levels are highest in patients who have renal impairment and are associated with increased mortality and poor prognosis.
We prospectively studied 200 patients admitted to Benha University Hospital from 2006 to 2008, who had cirrhosis with ascites and were suspected to have SBP. They included 144 (72%) males and 56 (28%) females with ages ranging between 34 and 62 years. The study was initiated after approval by the institutional review board and informed consent was obtained from all the study patients. The diagnosis of cirrhosis was established by clinical and laboratory criteria that did not require histological confirmation. The severity of underlying liver disease was evaluated using Pugh's modification of Child's criteria (Child-Pugh scores). The patients were divided into two groups. Group I consisted of 120 patients with cirrhosis and ascites, complicated by SBP. Group II comprised of 80 patients with cirrhosis and ascites, with sterile ascitic fluid (SAF) not complicated by SBP. Group I was further subclassified according to renal impairment into group Ia (n = 48) as patients with renal impairment (SBP-RI) and group Ib (n = 72) as patients without renal impairment. The diagnosis of SBP was established by the presence of: polymorphonuclear cell count (PMN) higher than 250 cells/mm of ascitic fluid, positive ascitic fluid culture, and absence of findings suggesting secondary peritonitis[5]. Paracentesis was performed on the first day of hospitalization for all patients when clinical manifestations suggested SBP. Paracentesis was repeated 48 h later, if SPB was diagnosed. The existence of a gut perforation or an intra-abdominal source of infection was excluded in all cases. Likewise, tuberculosis, pancreatitis, peritoneal carcinomatosis, and hemorrhagic ascites were also excluded on the basis of appropriate studies on the ascitic fluid. Patients who had received antibiotic therapy during the week before admission were also excluded. Following SBP diagnosis, treatment with third-generation cephalosporin was initiated and the dosage was adjusted throughout the treatment period according to kidney function. SBP resolution was considered, when all signs of infection had disappeared and the PMN count in ascitic fluid had decreased to a level of < 250/mm3. Ascitic fluid was cultured using conventional culture techniques.
Specimens were inoculated into aerobic and anaerobic blood culture bottles. Ascitic fluid was sent to the laboratory for cell count, culture, sensitivity testing, and measurement of albumin, and glucose. Serum and ascitic fluid were collected in sterile tubes at study entry (before the initiation of antibiotic treatment) and 48 h later. Assays for TNF-α and IL-6 in the serum and ascitic fluid were performed with an immunoenzymometric assay. Results are expressed as the mean and standard error of the mean. A P value < 0.05 was considered statistically significant. All statistical analysis was performed using SPSS software (ver.15).
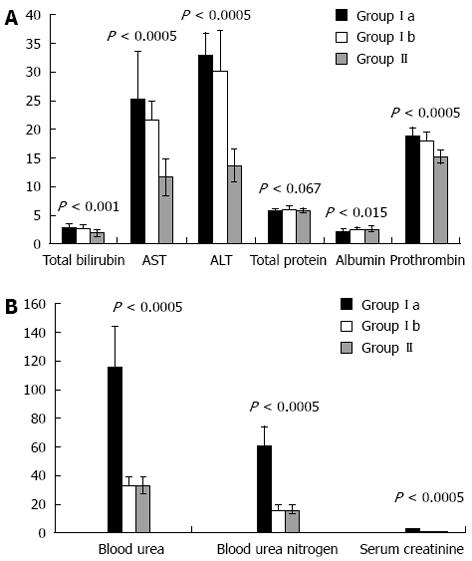
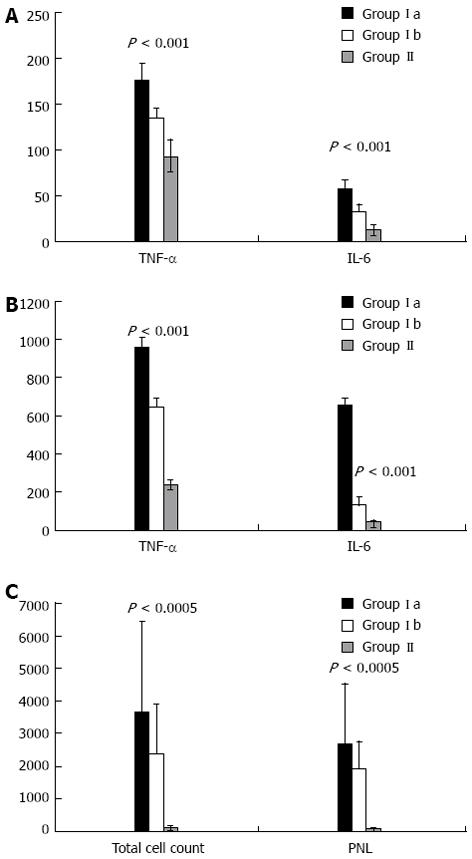
Comparison of groups Ia, Ib and II with regards to liver and renal function tests are shown in Figure 1A and B, and with regards to serum TNF-α and IL-6, ascitic fluid TNF-α and IL-6 and total cell count and ascitic fluid PNL are shown in Figure 2.
All patients in both groups had ascites with shrunken cirrhotic liver and lower limb edema. About 36 (30%) patients of the SBP group reported a previous incidence of SBP, whereas, 48 (40%) patients had a history of gastrointestinal tract bleeding with injection sclerotherapy. In the SBP group, ascitic fluid cultures were positive in 65 (54.16%) patients. The Child-Pugh scores were significantly higher in the SBP group than in the SAF group (P < 0.001). Forty eight patients who had SBP (40%) developed renal impairment (SBP-RI). These patients showed lower mean arterial pressure during admission. In the remaining 72 (60%) patients of the SBP group, serum creatinine and blood urea nitrogen following infection resolution were similar to those observed at diagnosis.
SBP resolved in 91 cases (75.83%). Twenty nine patients (60.42%) with SBP and renal impairment died whereas 19 patients (39.58%) recovered from the episode. Furthermore, only four patients (5.55%) of SBP without renal impairment (n = 72) died from gastrointestinal hemorrhage (P < 0.0005). Additionally, two patients (2.5%) with SAF also died from gastrointestinal hemorrhage.
Levels of TNF-αand IL-6 in serum: Cirrhotic patients with SBP showed significantly higher plasma levels of cytokines than cirrhotic patients without SBP (TNF-α: 135.35 ± 11.21 vs 92.86 ± 17.56, P < 0.001; IL-6: 32.30 ± 7.07 vs 12.11 ± 6.53, P < 0.001, Figuer 2A).
Levels of TNF-αand IL-6 in ascitic fluid: Cirrhotic patients with SBP showed significantly higher ascitic fluid levels of cytokines than cirrhotic patients without SBP (TNF-α: 647.54 ± 107.11 vs 238.43 ± 65.42, P < 0.001; IL-6: 132.84 ± 34.13 vs 40.41 ± 12.85, P < 0.001, Figuer 2B).
Pearson’s Correlation “r” with statistical significance “P” between serum TNF-and IL-6 with biochemical parameters of liver and renal function are shown in Table 1. There was a positive correlation between serum and ascitic fluid levels of TNF-α and IL-6 in patients with SBP. These differences in serum and ascitic fluid concentrations of TNF-α and IL-6 between cirrhotic patients with and without SBP were particularly striking, despite the fact that liver disease severity was similar in both groups, as estimated by the Child-Pugh score. Furthermore, the ascitic fluid level of cytokines was higher in comparison with the serum level.
| Tumor necrosis factor-α | Interleukin -6 | |||
| r | P-value | r | P-value | |
| Albumin | -0.547 | < 0.002 | -0.578 | < 0.001 |
| Prothrombin time | 0.593 | < 0.001 | 0.616 | < 0.0005 |
| Total bilirubin | 0.184 | 0.331 | 0.221 | 0.24 |
| Aspartate aminotransferase | 0.764 | < 0.0005 | 0.788 | < 0.0005 |
| Alanine transaminase | 0.787 | < 0.0005 | 0.802 | < 0.0005 |
| Blood urea nitrogen | 0.764 | < 0.0005 | 0.756 | < 0.0005 |
| Blood urea | 0.757 | < 0.0005 | 0.741 | < 0.0005 |
| Serum creatinine | 0.810 | < 0.0005 | 0.815 | < 0.0005 |
A significant correlation was also observed between TNF-α and IL-6 levels in ascitic fluid and PMN cell count in ascitic fluid (r = 5.80, P < 0.001). Patients with culture-positive SBP presented with significantly higher plasma and ascitic fluid levels of TNF-α and IL-6 than patients with culture-negative SBP. The PMN cell count in ascites was also significantly higher in the former group of patients (P < 0.001).
The plasma and ascitic fluid levels of TNF-α and IL-6 declined in patients with SBP after treatment and the changes in their levels were statistically significant (P < 0.01, data not shown). There was also a significant reduction of ascitic fluid PMN concentration after treatment (P < 0.0001, data not shown).
About 48 patients (40%) who developed SBP-RI showed a significantly higher plasma and ascitic fluid levels of cytokines at diagnosis of the infection than patients who did not (P < 0.001). Furthermore, patients with SBP-RI also had a significantly higher Child-Pugh score, ascitic fluid PMN cell count and higher rate of culture-positive SBP. Twenty nine of the 48 patients (60%) with SBP-RI died during hospitalization, whereas, this occurred in only 4 of the 72 (5.5%) patients without SBP-RI (P < 0.0001). Multivariate analysis disclosed that renal failure at the time of SBP diagnosis was the only independent predictor of hospital mortality (P < 0.001).
There were significant positive correlations between serum and ascitic fluid for both TNF-α and IL-6 with alanine aminotransaminase (ALT) (P < 0.001), aspartate aminotransferase (AST) (P < 0.001), and prothrombin time (P < 0.001). Furthermore, although there was a positive correlation between cytokine levels and serum bilirubin, it did not reach statistical significance. In comparison, there was a significant negative correlation between serum and ascitic fluid for both TNF-α and IL-6 with serum albumin (P < 0.01).
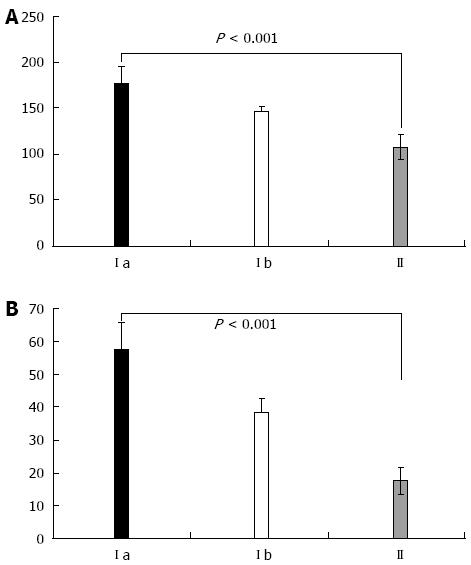
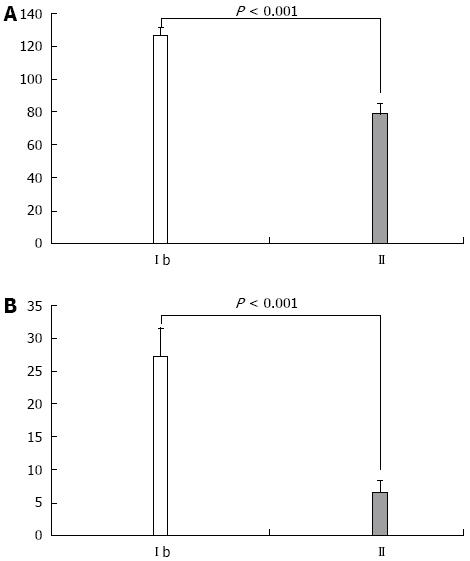
When comparing cytokine levels in serum and ascitic fluid for patients with the same Child class in the study groups, both TNF-α and IL-6 were statistically significantly higher in Child class C patients with SBP, as compared to patients without SBP (P < 0.001, Figure 3). Furthermore, patients with SBP-RI showed the highest cytokine levels in serum and ascitic fluid (P < 0.001). Similarly serum and ascitic fluid TNF-α and IL-6 were significantly higher in Child class B patients with SBP as compared to patients without SBP (P < 0.001, Figure 4).
SBP is thought to develop as a consequence of impaired defense mechanisms against infections seen in cirrhotic patients such as: depressed reticulo-endothelial system, reduced phagocytic activity and serum complement levels with consequent low antibacterial activity in the ascitic fluid[4]. The presence of bacteria in serum and ascitic fluid is also thought to stimulate the immune system with the release of cytokines to augment immune defenses. However, as a consequence, this could in turn impact on hemodynamic and renal function, as well as survival. These cytokines behave like a double-edged sword; they can protect from bacterial infection but they can also initiate a sequence of events associated with adverse hemodynamic and metabolic consequences that can ultimately lead to the patient’s demise[1]. In this regard, cytokines, particularly TNF-α, and IL-6 are probably the most important mediators in sepsis[7,8].
Serum and ascitic fluid levels of TNF-α and IL-6 were higher in ascitic fluid complicated by SBP in comparison to patients with sterile ascites. Furthermore, when comparing ascitic fluid in patients with SBP-RI versus patients with SBP without renal impairment, it was found that serum and ascitic fluid levels of both TNF-α and IL-6 were higher in the former group than the latter group. Thus, renal impairment occurs in patients with the highest concentration of cytokines in plasma and ascitic fluid and is associated with marked activation of the rennin-angiotensin system. In fact, impairment of renal function was the most important predictor of hospital mortality in cirrhotic patients with SBP[9].
The pathogenesis of renal impairment in association with SBP is probably multifactorial[10]. Patients with liver cirrhosis and ascites have a circulatory dysfunction characterized by arteriolar vasodilatation, hypotension and decreased effective blood volume[9,10]. NO is a powerful vasodilatory agent that is likely to play a key role in the pathogenesis of the peripheral and splanchnic vasodilatation observed in cirrhosis[11]. Induction of NO production occurs by different cytokines synergistically. Therefore, it is likely that NO synthesis could be increased in patients with SBP[9,11]. Recent studies suggest that increased serum and ascitic fluid NO levels were observed in patients with infected ascites, and might have led to a deterioration of the increased peripheral vasodilatation, finally leading to the development of renal impairment with serious consequences in patients with SBP[12,13,14]. In this study, the renal impairment correlated well with the increased levels of TNF-α and IL-6 at the time of diagnosis of SBP, and this was reflected in the degree of inflammatory response. Furthermore, patients developing SBP-RI showed significantly higher ascitic fluid PMN concentration than patients without renal impairment.
It was also observed that patients with SBP-RI had a significantly higher Child-Pugh score (all patients were Child class C). Serum TNF-α and IL-6 levels among patients with Child class C showed that patients with SBP had higher serum and ascitic fluid levels of both TNF-α and IL-6 than patients without SBP. Moreover, patients with SBP-RI also had the highest TNF-α and IL-6 serum and ascitic fluid levels although the severity of liver disease was similar in both groups as estimated by the Child-Pugh score. Similarly, when comparing serum and ascitic fluid levels of both TNF-α and IL-6 in Child class B patients it was found that, patients with SBP had a significantly higher serum and ascitic fluid levels of both TNF-α and IL-6 than patients without SBP. This means that, serum and ascitic fluid levels of both TNF-α and IL-6 were significantly positively correlated with liver disease progression.
Our results are comparable to the results reported by Navasa et al[15], who also reported elevated plasma levels of TNF-α and IL-6 in patients with SBP despite the fact that the severity of liver disease was similar as estimated by the Child-Pugh score. In our study, as regards liver function tests, there was a significant positive correlation between serum and ascitic fluid levels of both TNF-α and IL-6 and serum ALT and AST. Moreover, there was also a significant positive correlation between serum and ascitic fluid levels of TNF-α, IL-6 and prolongation of prothrombin time. However, there was a significant negative correlation between serum and ascitic fluid levels of TNF-α, IL-6 and serum albumin. Only serum bilirubin did not show any significant correlation with both TNF-α and IL-6 serum and ascitic fluid levels. These results support the notion of a significant increase of both TNF-α and IL-6 in serum and ascitic fluid, according to the severity of liver disease represented by Child-Pugh score classes, which by definition again depends on these biochemical parameters. It thus seems logical that there is a proportional increase in both TNF-α and IL-6 level, with the severity of liver disease, and the positive and negative correlations with different laboratory parameters is related to their pivotal role in initiating and or perpetuating liver damage. TNF-α exerts a variety of effects that are mediated mainly by TNF-α receptor 1 (TNF-R1) in cell death. The activation of TNF-R1 leads to the activation of multiple apoptotic pathways involving the activation of reactive oxygen species[16]. These pathways are closely interlinked and mainly act on mitochondria, which release the apoptotic factors and other events resulting in apoptosis[16].
The healthy liver has well developed defense mechanisms that permit hepatocytes to adapt to cytokine-initiated stress, protecting them from cytokine-mediated lethality. TNF-α may cause liver injury when hepatocytes have been pre-exposed to toxins such as alcohol, that interfere with their usual protective responses. Thus antagonism of TNF-α and other injury-related cytokines in liver diseases merits evaluation as a possible treatment modality of these diseases. However, because the same cytokines are also necessary for the regeneration of tissue after liver injury, inhibition of these mediators might impair hepatic recovery[16].
We conclude that in cirrhotic patients, since the serum levels of both TNF-α and IL-6 are higher in SBP than in sterile ascites, they are likely to play an important role in the pathogenesis of SBP. It has been suggested that TNF-α production may enhance liver cell injury and that this will lead to renal impairment. This may account for the poor prognosis associated with SBP. We therefore recommend the measurement of serum TNF-α and IL-6 in cirrhotic patients with SBP, as they have both diagnostic and prognostic significance.
We sincerely thank the Hospital Administration for allowing the use of clinical and laboratory material for the purpose of this study.
Infections of the sterile ascitic fluid in patients with cirrhosis of the liver have a prognostic significance. Sepsis leads to the release of several inflammatory cytokines that have a deleterious effect, especially tumor necrosis factor-α (TNF-α). Furthermore, cytokines have been shown to impact on various metabolic functions, including causing liver and renal impairment and thereby affecting mortality.
Spontaneous bacterial peritonitis is one of the complications seen in patients with cirrhosis of the liver with ascites. Several mechanisms are suggested that cause bacterial translocation from the gut to infect the sterile ascitic fluid especially reduced immunity with subsequent impairment of the reticuloendothelial system. In this setting, overproduction of inflammatory cytokines can lead to multi-organ failure with a significant impact on morbidity and mortality, especially since a cirrhotic liver is unable to withstand the toxicity of pro-inflammatory cytokines such as TNF-α and Interleukin-6.
Several studies have examined the various etiologies affecting the pathogenesis of spontaneous bacterial peritonitis. Other investigators have studied the effects and outcomes of spontaneous bacterial peritonitis in patients with cirrhosis of the liver. This study was designed to examine the role of cytokines, namely TNF-α and Interleukin-6, in patients with cirrhosis of the liver having spontaneous bacterial peritonitis. Specifically the investigators wanted to correlate the presence of metabolic derangement with the occurrence, severity and consequences of these two inflammatory cytokines.
The study results indicate that the presence of metabolic derangement of liver and renal function is strongly correlated with the severity of the cytokine response, as well as mortality, in cirrhotic patients with spontaneous bacterial peritonitis.
Spontaneous bacterial peritonitis: It is defined as infection of a previously sterile ascitic fluid without any apparent intra-abdominal source of infection in cirrhotic patients with ascites.
This is a good study in cirrhotic patients having ascites with and without infection, but without any apparent source of infection i.e., spontaneous bacterial peritonitis. The study results show that mortality and poor outcome are associated with liver and renal impairment in those patients that overproduce pro-inflammatory cytokines like TNF-α and interleukin-6 and their liver which has been already exposed to toxins like alcohol is unable to cope with the lethality of these cytokines.
Peer reviewer: Chandana Herath, PhD, Senior Researcher, Department of Medicine, University of Melbourne, Austin Repatriation Hospital, Bldg 24, 300 Waterdale Road, Heidelberg Heights, VIC 3081, Australia
S- Editor Zhai HH L- Editor Hughes D E- Editor Zhang DN
| 1. | Runyon BA. Early events in spontaneous bacterial peritonitis. Gut. 2004;53:782-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Castellote J, López C, Gornals J, Tremosa G, Fariña ER, Baliellas C, Domingo A, Xiol X. Rapid diagnosis of spontaneous bacterial peritonitis by use of reagent strips. Hepatology. 2003;37:893-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 107] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 3. | Mowat C, Stanley AJ. Review article: spontaneous bacterial peritonitis--diagnosis, treatment and prevention. Aliment Pharmacol Ther. 2001;15:1851-1859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 4. | Ramachandran A, Balasubramanian KA. Intestinal dysfunction in liver cirrhosis: Its role in spontaneous bacterial peritonitis. J Gastroenterol Hepatol. 2001;16:607-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 5. | Rodríguez-Ramos C, Galan F, Díaz F, Elvira J, Martín-Herrera L, Girón-González JA. Expression of proinflammatory cytokines and their inhibitors during the course of spontaneous bacterial peritonitis. Dig Dis Sci. 2001;46:1668-1676. [PubMed] |
| 6. | Reuken PA, Pletz MW, Baier M, Pfister W, Stallmach A, Bruns T. Emergence of spontaneous bacterial peritonitis due to enterococci - risk factors and outcome in a 12-year retrospective study. Aliment Pharmacol Ther. 2012;35:1199-1208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 39] [Reference Citation Analysis (0)] |
| 7. | Rimola A, García-Tsao G, Navasa M, Piddock LJ, Planas R, Bernard B, Inadomi JM. Diagnosis, treatment and prophylaxis of spontaneous bacterial peritonitis: a consensus document. International Ascites Club. J Hepatol. 2000;32:142-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 8. | Wong F, Bernardi M, Balk R, Christman B, Moreau R, Garcia-Tsao G, Patch D, Soriano G, Hoefs J, Navasa M. Sepsis in cirrhosis: report on the 7th meeting of the International Ascites Club. Gut. 2005;54:718-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 279] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 9. | Grangé JD, Amiot X. Nitric oxide and renal function in cirrhotic patients with ascites: from physiopathology to practice. Eur J Gastroenterol Hepatol. 2004;16:567-570. [PubMed] [DOI] [Full Text] |
| 10. | Solà E, Ginès P. Renal and circulatory dysfunction in cirrhosis: current management and future perspectives. J Hepatol. 2010;53:1135-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 11. | Hung TH, Tsai CC, Hsieh YH, Tsai CC, Tseng CW, Tsai JJ. Effect of renal impairment on mortality of patients with cirrhosis and spontaneous bacterial peritonitis. Clin Gastroenterol Hepatol. 2012;10:677-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Bernardi M. Spontaneous bacterial peritonitis: from pathophysiology to prevention. Intern Emerg Med. 2010;5 Suppl 1:S37-S44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Such J, Hillebrand DJ, Guarner C, Berk L, Zapater P, Westengard J, Peralta C, Soriano G, Pappas J, Runyon BA. Tumor necrosis factor-alpha, interleukin-6, and nitric oxide in sterile ascitic fluid and serum from patients with cirrhosis who subsequently develop ascitic fluid infection. Dig Dis Sci. 2001;46:2360-2366. [PubMed] |
| 14. | Such J, Hillebrand DJ, Guarner C, Berk L, Zapater P, Westengard J, Peralta C, Soriano G, Pappas J, Francés R. Nitric oxide in ascitic fluid is an independent predictor of the development of renal impairment in patients with cirrhosis and spontaneous bacterial peritonitis. Eur J Gastroenterol Hepatol. 2004;16:571-577. [PubMed] |
| 15. | Navasa M, Follo A, Filella X, Jiménez W, Francitorra A, Planas R, Rimola A, Arroyo V, Rodés J. Tumor necrosis factor and interleukin-6 in spontaneous bacterial peritonitis in cirrhosis: relationship with the development of renal impairment and mortality. Hepatology. 1998;27:1227-1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 295] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 16. | Van Herreweghe F, Festjens N, Declercq W, Vandenabeele P. Tumor necrosis factor-mediated cell death: to break or to burst, that's the question. Cell Mol Life Sci. 2010;67:1567-1579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 165] [Article Influence: 11.0] [Reference Citation Analysis (0)] |