Published online Apr 15, 2012. doi: 10.4291/wjgp.v3.i2.44
Revised: January 31, 2012
Accepted: April 10, 2012
Published online: April 15, 2012
Endoscopic resection is an effective treatment for non-invasive esophageal squamous cell neoplasms (ESCNs). Endoscopic mucosal resection (EMR) has been developed for small localized ESCNs as an alternative to surgical therapy because it shows similar effectiveness and is less invasive than esophagectomy. However, EMR is limited in resection size and therefore piecemeal resection is performed for large lesions, resulting in an imprecise histological evaluation and a high frequency of local recurrence. Endoscopic submucosal dissection (ESD) has been developed in Japan as one of the standard endoscopic resection techniques for ESCNs. ESD enables esophageal lesions, regardless of their size, to be removed en bloc and thus has a lower local recurrence rate than EMR. The development of new devices and the establishment of optimal strategies for esophageal ESD have resulted in fewer complications such as perforation than expected. However, esophageal stricture after ESD may occur when the resected area is larger than three-quarters of the esophageal lumen or particularly when it encompasses the entire circumference; such a stricture requires multiple sessions of endoscopic balloon dilatation. Recently, oral prednisolone has been reported to be useful in preventing post-ESD stricture. In addition, a combination of chemoradiotherapy (CRT) and ESD might be an alternative therapy for submucosal esophageal cancer that has a risk of lymph node metastasis because esophagectomy is extremely invasive; CRT has a higher local recurrence rate than esophagectomy but is less invasive. ESD is likely to play a central role in the treatment of superficial esophageal squamous cell neoplasms in the future.
- Citation: Honda K, Akiho H. Endoscopic submucosal dissection for superficial esophageal squamous cell neoplasms. World J Gastrointest Pathophysiol 2012; 3(2): 44-50
- URL: https://www.wjgnet.com/2150-5330/full/v3/i2/44.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v3.i2.44
Endoscopic resection (ER) is an effective treatment for esophageal squamous cell neoplasms (ESCNs) without nodal metastasis. A number of retrospective studies involving histopathological analyses of surgically resected specimens of esophageal squamous cell carcinomas (SCCs) have shown that cases of non-invasive epithelial carcinoma (EP, carcinoma in situ) and intra-mucosal invasive carcinoma limited to the lamina propria mucosae (LPM) had an extremely low risk of lymph node and distant metastasis[1-7]. Based on these findings, the Japanese guidelines state that the indication for ER of esophageal SCC is a lesion limited to EP or LPM. The lymph node metastasis rates of SCC invading to the muscularis mucosae (MM SCC) and SCC invading the submucosa less than 200 µm below the muscularis mucosae (SM1 SCC) are reported as 9.3% and 19.6%, respectively[8]. Thus, ER is a relative indication for MM-SM1 SCC according to the Japanese guidelines. On the other hand, SM2-grade cancer (that invading the submucosa more than 200 µm below the muscularis mucosae) has a high frequency of lymph node metastasis (around 40%) and therefore ER is not recommended[1,9].
Endoscopic mucosal resection (EMR) has been developed for small localized ESCNs as an alternative to surgical therapy because it shows similar effectiveness and is less invasive than esophagectomy[10-16]. However, EMR is limited in resection size and therefore piecemeal resection is performed for large lesions, resulting in an imprecise histological evaluation and a high frequency of local recurrence[17].
Endoscopic submucosal dissection (ESD) for ESCNs is an endoluminal therapeutic technique to dissect directly along the submucosal layer. ESD was developed in Japan for the surgical treatment of gastric cancer and gave high curative resection rates for early gastric cancer, regardless of tumor size[18-23]. Hence, the technique has also been introduced for the esophagus[24] and was approved for the treatment of esophageal cancer by the Japanese government in 2008. ESD allows en bloc resection regardless of the size and precise histological assessment of the specimens, which are excised in one piece with tumor-free lateral basal margins, therefore preventing residual disease and local recurrence[24,25]. Recent technical advances in ESD enable en bloc resection of lesions, even if they occupy the entire circumference of the esophageal lumen. However, according to the guidelines in Japan, ER is principally limited to lesions that do not exceed two-thirds of the luminal circumference because of postoperative esophageal stricture. Postoperative esophageal stricture after esophageal ESD might be observed frequently and this problem should be resolved before the widespread use of ESD for ESCNs.
In this review, we describe the present techniques, outcomes, complications and future perspectives of esophageal ESD for ESCNs.
EMR is a technique for resection of the mucosa containing the lesion via a snare wire. The lesion is strangulated by the snare wire and then resected after creating a submucosal cushion. To strangulate the lesion-containing mucosa firmly, several EMR methods, such as 2-channel EMR[26], EMR-C (EMR using a cap-fitted endoscope)[27] and esophageal endoscopic mucosal resection (EEMR)[28], have been developed. In 2-channel EMR, grasping forceps passed through another channel grasp the area near the lesion to help the snare wire strangulate the lesion. In EMR-C, the mucosa, including the lesion, is aspirated into a plastic cap attached to the tip of a forward-viewing endoscope; it is then strangulated by a small-diameter snare wire pre-looped within the cap and resected. These EMR methods are relatively easy and safe; thus, EMR has been used for the treatment of superficial ESCNs, especially small lesions that can be treated by EMR with en bloc resection. However, en bloc resection of lesions larger than 20 mm is difficult by EMR. Piecemeal resection of large lesions by EMR is insufficient for histological assessment and leads to local recurrence of ESCNs[17,29].
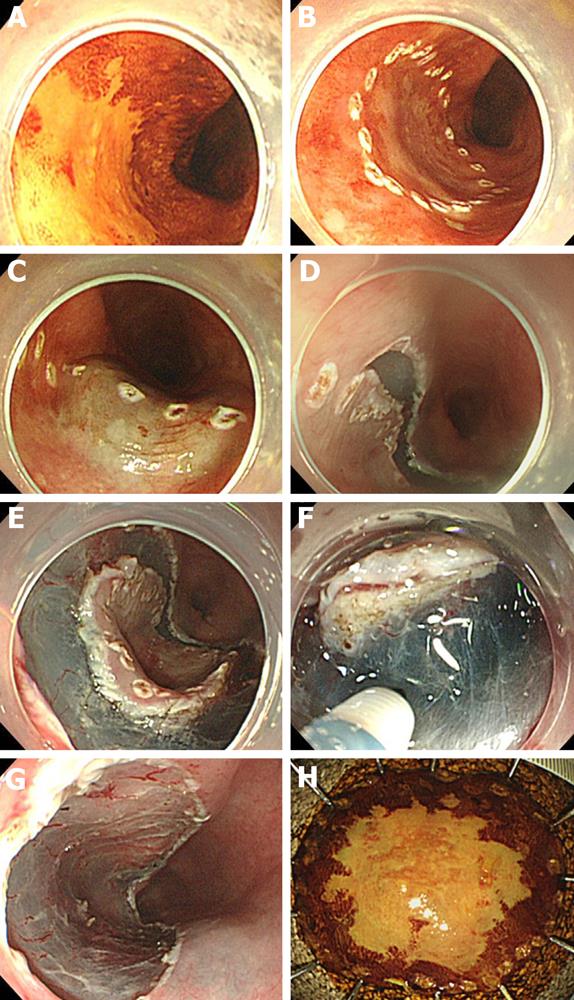
Detail of the ESD procedure has been described elsewhere[30,31]. Esophageal ESD comprises four steps (Figure 1): (1) circumferential marking: markings for the incision are made outside the margin with an electrosurgical knife; (2) submucosal injection: fluid such as sodium hyaluronate (0.5%) is injected into the submucosa to elevate the lesion from the muscle layer[32,33]. By mixing in a small amount of dye, the sodium hyaluronate can be easily distinguished from the non-injected area; (3) mucosal incision: a mucosal incision around the lesion is then made with an electrosurgical knife. Several knives have been developed for this purpose, such as the insulation-tip knife[34,35], hook knife[24,36], flex knife[37,38], flush knife[39], ball-tipped flush knife[40] and triangle-tipped knife[41]. Usually, the distal half of the mucosal incision is completed first, followed by the proximal half; and (4) submucosal dissection: dissection of the submucosa proceeds from the proximal to the distal end, using the same knife that was used for the mucosal incision.
Esophageal ESD is considered more difficult to perform than gastric ESD. The esophageal lumen is narrow and the esophageal wall moves continuously with respiratory movements and cardiac pulsation. Moreover, because the esophageal wall is thinner than that of the stomach, perforation during esophageal ESD occurs more frequently than during gastric ESD. This can result in mediastinal or subcutaneous emphysema and sometimes respiratory failure.
In Japan, the use of esophageal ESD has spread rapidly and has been attempted in several hospitals. As a result, the esophageal ESD procedure is now thought to be relatively straightforward. This is because: (1) it is easy to inject the fluid into the submucosal layer and to separate the mucosa from the muscle layer; (2) the submucosal layer is easily recognized because of the lesion located in a tangential direction; and (3) the submucosa of the esophagus contains few vessels that could lead to massive bleeding; thus, minimum hemostatic effort is required.
Esophageal ESD using conventional knives is a longer procedure and requires highly skilled endoscopists. This makes the acceptance of esophageal ESD in other countries more difficult. Grasping-type scissor forceps (GSF), which can grasp and incise the targeted tissue using an electrosurgical current, are a newly developed device for ESD[42-44]. Akahoshi et al[43] reported the usefulness of GSF for early gastrointestinal tract neoplasms because of their safety and simplicity. GSF can easily grasp the submucosal layer injected with fluid because the lesion is located in a tangential direction. Thus, GSF might be particularly useful for safely dissecting the submucosal layer in esophageal ESD.
The outcomes of esophageal ESD are shown in Tables 1 and 2. The en bloc resection rate is greater than 90% (90.6%-100%)[24,29,45-49]. En bloc resection, meaning resection in a single piece, facilitates an accurate histological assessment and reduces the risk of recurrence. In fact, the local recurrence rate after esophageal ESD is extremely low (0%-3.1%)[24,29,45-49]. In contrast, the local recurrence of SCCs after EMR was reported to be as high as 20% because en bloc resection by EMR is difficult and multiple resections are required for large lesions[17]. In a large scale study comparing 116 patients treated by ESD with 184 patients treated by EMR for superficial SCCs, Takahashi et al[48] reported that en bloc resection and the local resection rate were significantly better in the ESD group (100% and 0.9%, respectively) than in the EMR group (53.3% and 9.8%, respectively) (Table 2). In both groups, 19 of 300 patients experienced a local recurrence and 68.4% of all the local recurrences (13/19) were treated by piecemeal EMR. Thus, the EMR procedure is itself considered a risk for local recurrence. Ishihara et al[29,49] reported a comparison of ESD and EMR (EMR-C and 2-channel EMR) for esophageal cancers of both < 20 mm and ≥ 20 mm (Table 2). For the larger, latter group, they compared 32 lesions treated by ESD with 46 lesions treated by EMR. The en bloc resection rates of EMR and ESD were 10.9% (5/46 lesions) and 90.6% (29/32 lesions), respectively. Lesions treated by EMR also had significantly more recurrences (23.9%; 11/46) than those treated by ESD (3.1%; 1/32). There were no recurrences of lesions treated by en bloc resection. For the smaller lesions, Ishihara et al[29,49] compared 64 lesions treated by ESD with 36 lesions treated by EMR (21 EMR-C and 15 2-channel EMR). As shown in Table 2, the en bloc resection rate of ESD was superior to that of EMR-C or 2-channel EMR, even for a lesion < 20 mm in size.
| Authors | Year | Total | Mean size | Operation | En bloc | Local | Perforation | Stricture |
| lesions/cases | (mm) | time (min) | resection (%) | recurrence (%) | (%) | (%) | ||
| Oyama et al[24] | 2005 | 102/102 | 28 (4–64) | - | 95 (95/102) | 0 (0/102) | 0 (0/102) | 7.4 (7/95) |
| Ono et al[45] | 2009 | 107/84 | 22.9 (1–66) | - | 100 (107/107) | 1.2 (1/84) | 4 (4/107) | 18 (15/84) |
| Tamiya et al[47] | 2010 | 58/58 | 30.4 (4–67) | 180 | 100 (58/58) | 0 (0/58) | 0 (0/58) | 6.9 (4/58) |
| Nonaka et al[46] | 2010 | 27/25 | 21 (2–55) | 88 | 100 (27/27) | 0 (0/25) | 3.7 (1/27) | 12 (3/25) |
| Authors | Year | Method | Total | Mean size | Operation | En bloc | Local | Perforation |
| lesions | (mm) | time (min) | resection (%) | recurrence (%) | (%) | |||
| Takahashi et al[48] | 2010 | ESD | 116 | 30 (4-95) | 73.9 (21–307) | 100 (116/116) | 0.9 (1/116) | 2.6 (3/116) |
| EMR | 184 | 20 (4-60) | 44.4 (11–258) | 53.3 (98/184) | 9.8 (18/184) | 1.6 (3/184) | ||
| Ishihara et al[29] | 2008 | ESD | 32 | > 20 | 110 (30–245) | 90.6 (29/32) | 3.1 (1/32) | 0 (0/32) |
| EMR | 46 | > 20 | 35 (10–90) | 10.9 (5/46) | 23.9 (11/46) | 0 (0/46) | ||
| Ishihara et al[49] | 2008 | ESD | 31 | 16 | 64 | 100 (31/31) | 0 (0/31) | 3.2 (1/31) |
| EMR-C | 68 | 13 | 21 | 87 (59/68) | 0 (0/68) | 0 (0/68) | ||
| 2-channel | 72 | 12 | 15 | 71 (51/72) | 2.8 (2/72) | 0 (0/72) | ||
| EMR |
Although en bloc resection seems to be ideal for reducing the local recurrence rate, it is technically difficult to achieve by EMR. For en bloc resection of large lesions, ESD would be the best method. The procedure time for ESD is notably longer than that for EMR (as shown in Table 2). Further improvement to reduce the procedure time of this method is needed before ESD could become a standard treatment.
Minor bleeding during esophageal ESD is well controlled by hemostasis performed using the same knife as that used for submucosal dissection, hemostatic forceps (HDB2422/HDB2418; Pentax HOYA Co, Tokyo, Japan) or a coagrasper (FD-410LR; Olympus Medical Systems Co Tokyo, Japan). Massive bleeding complications are rarer in esophageal ESD than in gastric ESD.
As mentioned above, perforation during esophageal ESD has been considered to occur more frequently than during gastric ESD and can result in mediastinal or subcutaneous emphysema and sometimes respiratory failure. However, perforation is relatively rare (0%-4%), as shown in Tables 1 and 2. In these studies, all cases of perforation were cured conservatively without surgery. If perforation diagnosed by endoscopic findings of tearing of the muscle layer occurs, ESD can be completed after immediate closure of the perforation by endoscopic clipping.
On the other hand, pneumomediastinum (mediastinal emphysema) without perforation during esophageal ESD occurs frequently if the muscular layer is exposed. In a study of 58 patients treated for esophageal neoplasms by ESD, Tamiya et al[47] demonstrated that the incidence of pneumomediastinum detected by computed tomography (CT) was 56.3% (18/32) in the group with muscle exposure, although it was 0% (0/26) in the group without exposure of muscular layer. However, the presence of pneumomediastinum by CT did not imply overt esophageal perforation and did not influence the post-procedural clinical course. Thus, pneumomediastinum associated with muscle exposure is a minor complication.
Postoperative esophageal stricture might be observed more frequently after resections in the esophagus because ESD permits resection of large specimens, even completely circumferential resections. Stricture is reported to occur in 6.9%-18.0% of cases (Table 1). Stricture after esophageal ESD may occur when the resected area is larger than three-quarters of the circumference of the esophageal lumen. Mizuta et al[50] retrospectively evaluated 42 superficial esophageal cancer lesions in 33 patients who underwent ESD and showed that the predictive factors for post-ESD esophageal stricture were a circumferential mucosal defect size of more than 71% (sensitivity 100%, specificity 97.1%) and a circumferential tumor size of more than 59% (sensitivity 85.7%, specificity 97.1%).
Strictures have almost always been treated with repeated endoscopic balloon dilatation (EBD). Ono et al[45] reported that 15 of 84 (18%) patients treated by esophageal ESD experienced esophageal stricture and were successfully managed with EBD in a median of two sessions (range 1-20). Moreover, the authors recommended preventive EBD for cases of mucosal defect exceeding 75% of the esophageal circumference. However, the EBD procedure itself carries a risk of esophageal perforation. There are only a few studies concerned with dilatation of esophageal strictures after ESD. Takahashi et al[51] reported the risk of perforation and its specific risk factors during dilatation of post-EMR/ESD esophageal stricture. Seven perforations (1.1%, 7/648 procedures or 9.0%, 7/78 patients) were observed in this study. Two of these patients developed a thoracic abscess and needed drainage, although all the patients recovered without surgery.
Another treatment option for post-ESD esophageal stricture is endoscopic placement of a stent. Saito et al[52] reported two cases in whom biodegradable stents for post-ESD esophageal strictures were successfully placed. In both lesions, the mucosal defect had extended to seven-eighths of the circumference.
Recently, systemic steroid administration has been reported to be effective for post-EBD stricture and might resolve the problems of EBD, such as multiple EBD sessions reducing the patient’s quality of life and increasing the risk of esophageal perforation[53]. Isomoto et al[54] described seven patients with superficial SCC who underwent completely circumferential ESD. Of the seven patients, four were treated with an 8 wk course of oral prednisolone that was administered at a dose of 30 mg daily on the third post-ESD day and tapered off gradually (30 mg/d, 30 mg/d, 25 mg/d, 25 mg/d, 20 mg/d, 15 mg/d, 10 mg/d and 5 mg/d for 7 d each). Administration of oral prednisolone effectively either prevented esophageal stricture or reduced the number of EBD sessions. Two patients required no EBD and two patients required fewer EBD sessions (2 and 11 sessions, respectively) than the three patients (30, 20 and 48 sessions, respectively) who had not received oral prednisolone. In a retrospective study in 41 patients with esophageal stricture after complete circular or semicircular ESD for esophageal SCCs involving more than three-quarters of the lumen, Yamaguchi et al[55] compared an oral prednisolone group with a pre-emptive EBD group. Oral prednisolone was administered as in Isomoto et al[54]’s report. Pre-emptive EBD was started on the third day post-ESD and continued twice weekly for 8 wk. An additional EBD was performed on demand in both groups whenever dysphagia appeared. The average number of EBD sessions was significantly decreased in the oral prednisolone group compared with in the pre-emptive EBD group (1.7 vs 15.6, respectively). In this study, there were no complications related to the EBD itself in either group and no adverse events related to the oral prednisolone occurred. Oral prednisolone may offer a safe and effective option for the prevention of post-ESD stricture, potentially reducing or eliminating the need for EBD.
For esophageal ESD, in the 2007 annual meeting of the Japanese Gastroenterological Endoscopy Society, the 3 year survival rates for EP-LPM cancer and MM-SM1 cancer were 95.1% and 86.7%, respectively.
Ono et al[45] reported the long-term outcomes for 84 patients treated by ESD for ESCNs. Histopathologically, 58 patients were diagnosed with a high-grade intraepithelial neoplasm (HGIN), including EP or LPM cancer, and were followed-up without additional therapy. Only one patient, whose lesion was identified as HGIN with Rx (lateral) resection, had a local recurrence after 6 mo and was successfully treated with additional ESD. The cause-specific survival rate at 5 years was 100% for patients with HGIN or LPM cancer. On the other hand, 28 patients were histopathologically diagnosed with MM or SM cancer. Fifteen of the 28 patients underwent additional therapies [chemoradiotherapy (CRT)/radiotherapy, 6; surgery, 9]. Three patients with MM or SM cancer died of esophageal SCC after ESD. The cause-specific survival rate at 5 years was 85% for patients with MM or SM cancers.
Surgery is recommended for SM SCCs because of the high frequency of lymph node metastasis. However, esophagectomy is extremely invasive and is associated with significant morbidity and mortality, particularly in patients of advanced age or those with cardiac or pulmonary complications. Such patients may be treated with CRT. Although CRT has a favorable survival rate and mild toxicity in patients with a stage I lesion (UICC-TNM classification: T1N0M0), the local recurrence rate of CRT is higher than that of esophagectomy[56]. ESD with subsequent CRT for SM SCCs might be useful for preventing residual lesion or local recurrence. A randomized controlled study is now ongoing in Japan, in which patients with suspected SM1 or SM2 SCC are treated with ER, and whether subsequent CRT is performed is based on the histological findings. Patients with SCC occupying larger than three-quarters of the circumference of the esophageal lumen are excluded in this study.
In our experience, EBD for post-ESD stricture during subsequent CRT requires multiple sessions even if treated with steroids and therefore decreases the patient’s quality of life. Thus, CRT and subsequent ESD might be a useful therapeutic option for large esophageal lesions that have a risk of postoperative esophageal stricture and for residual or recurrent esophageal lesions.
Saito et al[57] reported three cases of superficial esophageal cancer treated with CRT followed by ESD. One patient refused surgery and the other two patients suffered from severe cardiopulmonary disease complications. In all three patients, CRT was effective in reducing tumor size and the residual tumors were completely resected by ESD. In this report, one patient had a superficial SCC occupying the entire circumference of the lumen at a site 15-25 cm from the upper incisor. The esophageal lesion was markedly reduced in size by CRT and the small residual lesion was resected en bloc by ESD. If ESD had been used initially in this case, esophageal stricture might have occurred and required several sessions of EBD. The combination of CRT plus subsequent ESD may also be useful for patients with superficial esophageal cancer who need completely circumferential ESD to avoid esophageal stricture.
In summary, ESD for the management of superficial ESCNs is an effective and safe therapeutic modality. ESD is well established in Japan, although esophageal ESD requires highly skilled surgeons. ESD is recommended for EP or LPM esophageal cancers, especially those larger than 2 cm. ESD is also indicated for lesions invading to the MM–SM or occupying the entire circumference of the lumen. ESD followed by CRT, or ESD after CRT, may be an alternative therapeutic option for patients unwilling to undergo esophagectomy or for high-risk patients with MM–SM cancer. The management of esophageal stricture after ESD is one of the major problems. The administration of prednisolone may be useful for esophageal stricture after ESD, reducing the requirement for EBD sessions. Although extensive controlled, randomized studies are necessary to evaluate the usefulness of these treatments, there is no doubt that ESD will play a central role in the treatment of superficial ESCNs in the future.
Peer reviewers: Dr. Joerg Zehetner, Department of Surgery, Keck School of Medicine, University of Southern California, 1510 San Pablo Street, Suite 514, Los Angeles, CA 90033, United States; Dr. James Patrick Dolan, Assistant Professor, Department of Surgery, Oregon Health and Science University, 3181 SW Sam Jackson Park Road, Portland, OR 97219, United States
S-Editor Wu X L- Editor Roemmele A E- Editor Wu X
| 1. | Japanese Society for Esophageal Diseases. Guidelines for diagnosis and treatment of esophageal carcinoma [in Japanese]. 2nd ed. Tokyo: Kanehara Shuppan 2007; . |
| 2. | Tajima Y, Nakanishi Y, Ochiai A, Tachimori Y, Kato H, Watanabe H, Yamaguchi H, Yoshimura K, Kusano M, Shimoda T. Histopathologic findings predicting lymph node metastasis and prognosis of patients with superficial esophageal carcinoma: analysis of 240 surgically resected tumors. Cancer. 2000;88:1285-1293. [PubMed] |
| 3. | Natsugoe S, Baba M, Yoshinaka H, Kijima F, Shimada M, Shirao K, Kusano C, Fukumoto T, Mueller J, Aikou T. Mucosal squamous cell carcinoma of the esophagus: a clinicopathologic study of 30 cases. Oncology. 1998;55:235-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Araki K, Ohno S, Egashira A, Saeki H, Kawaguchi H, Sugimachi K. Pathologic features of superficial esophageal squamous cell carcinoma with lymph node and distal metastasis. Cancer. 2002;94:570-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 121] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 5. | Eguchi T, Nakanishi Y, Shimoda T, Iwasaki M, Igaki H, Tachimori Y, Kato H, Yamaguchi H, Saito D, Umemura S. Histopathological criteria for additional treatment after endoscopic mucosal resection for esophageal cancer: analysis of 464 surgically resected cases. Mod Pathol. 2006;19:475-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 210] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 6. | Shimada H, Nabeya Y, Matsubara H, Okazumi S, Shiratori T, Shimizu T, Aoki T, Shuto K, Akutsu Y, Ochiai T. Prediction of lymph node status in patients with superficial esophageal carcinoma: analysis of 160 surgically resected cancers. Am J Surg. 2006;191:250-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 114] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 7. | Kim DU, Lee JH, Min BH, Shim SG, Chang DK, Kim YH, Rhee PL, Kim JJ, Rhee JC, Kim KM. Risk factors of lymph node metastasis in T1 esophageal squamous cell carcinoma. J Gastroenterol Hepatol. 2008;23:619-625. [PubMed] |
| 8. | Oyama T, Miyata Y, Shimatani S, Tomori A, Hotta K, Yoshida M. [Lymph nodal metastasis of m3, sm1 esophageal cancer]. Stomach Intest. 2002;37:71-74. |
| 9. | Kodama M, Kakegawa T. Treatment of superficial cancer of the esophagus: a summary of responses to a questionnaire on superficial cancer of the esophagus in Japan. Surgery. 1998;123:432-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 252] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 10. | Makuuchi H. Endoscopic mucosal resection for early esophageal cancer-indication and techniques. Dig Endosc. 1996;8:175-179. |
| 11. | Inoue H, Tani M, Nagai K, Kawano T, Takeshita K, Endo M, Iwai T. Treatment of esophageal and gastric tumors. Endoscopy. 1999;31:47-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 86] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Fujita H, Sueyoshi S, Yamana H, Shinozaki K, Toh U, Tanaka Y, Mine T, Kubota M, Shirouzu K, Toyonaga A. Optimum treatment strategy for superficial esophageal cancer: endoscopic mucosal resection versus radical esophagectomy. World J Surg. 2001;25:424-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 116] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Shimizu Y, Tsukagoshi H, Fujita M, Hosokawa M, Kato M, Asaka M. Long-term outcome after endoscopic mucosal resection in patients with esophageal squamous cell carcinoma invading the muscularis mucosae or deeper. Gastrointest Endosc. 2002;56:387-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 125] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 14. | Katada C, Muto M, Momma K, Arima M, Tajiri H, Kanamaru C, Ooyanagi H, Endo H, Michida T, Hasuike N. Clinical outcome after endoscopic mucosal resection for esophageal squamous cell carcinoma invading the muscularis mucosae--a multicenter retrospective cohort study. Endoscopy. 2007;39:779-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 129] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 15. | Pech O, May A, Gossner L, Rabenstein T, Manner H, Huijsmans J, Vieth M, Stolte M, Berres M, Ell C. Curative endoscopic therapy in patients with early esophageal squamous-cell carcinoma or high-grade intraepithelial neoplasia. Endoscopy. 2007;39:30-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 78] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 16. | Ciocirlan M, Lapalus MG, Hervieu V, Souquet JC, Napoléon B, Scoazec JY, Lefort C, Saurin JC, Ponchon T. Endoscopic mucosal resection for squamous premalignant and early malignant lesions of the esophagus. Endoscopy. 2007;39:24-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 84] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 17. | Katada C, Muto M, Manabe T, Ohtsu A, Yoshida S. Local recurrence of squamous-cell carcinoma of the esophagus after EMR. Gastrointest Endosc. 2005;61:219-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 98] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 18. | Hirao M, Masuda K, Asanuma T, Naka H, Noda K, Matsuura K, Yamaguchi O, Ueda N. Endoscopic resection of early gastric cancer and other tumors with local injection of hypertonic saline-epinephrine. Gastrointest Endosc. 1988;34:264-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 265] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 19. | Oda I, Gotoda T, Hamanaka H, Eguchi T, Saito Y, Matsuda T, Bhandari P, Emura F, Saito D, Ono H. Endoscopic submucosal dissection for early gastric cancer: technical feasibility, operation time and complications from a large consecutive series. Dig Endosc. 2005;17:54-58. |
| 20. | Kakushima N, Fujishiro M, Kodashima S, Muraki Y, Tateishi A, Omata M. A learning curve for endoscopic submucosal dissection of gastric epithelial neoplasms. Endoscopy. 2006;38:991-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 122] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 21. | Isomoto H, Shikuwa S, Yamaguchi N, Fukuda E, Ikeda K, Nishiyama H, Ohnita K, Mizuta Y, Shiozawa J, Kohno S. Endoscopic submucosal dissection for early gastric cancer: a large-scale feasibility study. Gut. 2009;58:331-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 470] [Cited by in RCA: 519] [Article Influence: 32.4] [Reference Citation Analysis (1)] |
| 22. | Kato M, Nishida T, Tsutsui S, Komori M, Michida T, Yamamoto K, Kawai N, Kitamura S, Zushi S, Nishihara A. Endoscopic submucosal dissection as a treatment for gastric noninvasive neoplasia: a multicenter study by Osaka University ESD Study Group. J Gastroenterol. 2011;46:325-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 89] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 23. | Toyokawa T, Fujita I, Morikawa T, Okamoto A, Miyasaka R, Watanabe K, Horii J, Gobaru M, Terao M, Murakami T. Clinical outcomes of ESD for early gastric neoplasms in elderly patients. Eur J Clin Invest. 2011;41:474-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Oyama T, Tomori A, Hotta K, Morita S, Kominato K, Tanaka M, Miyata Y. Endoscopic submucosal dissection of early esophageal cancer. Clin Gastroenterol Hepatol. 2005;3:S67-S70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 459] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 25. | Fujishiro M, Yahagi N, Kakushima N, Kodashima S, Muraki Y, Ono S, Yamamichi N, Tateishi A, Shimizu Y, Oka M. Endoscopic submucosal dissection of esophageal squamous cell neoplasms. Clin Gastroenterol Hepatol. 2006;4:688-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 258] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 26. | Tada M, Murakami A, Karita M, Yanai H, Okita K. Endoscopic resection of early gastric cancer. Endoscopy. 1993;25:445-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 277] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 27. | Inoue H, Takeshita K, Hori H, Muraoka Y, Yoneshima H, Endo M. Endoscopic mucosal resection with a cap-fitted panendoscope for esophagus, stomach, and colon mucosal lesions. Gastrointest Endosc. 1993;39:58-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 368] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 28. | Makuuchi H. Esophageal endoscopic mucosal resection (EEMR) tube. Surg Laparosc Endosc. 1996;6:160-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Ishihara R, Iishi H, Takeuchi Y, Kato M, Yamamoto S, Yamamoto S, Masuda E, Tatsumi K, Higashino K, Uedo N. Local recurrence of large squamous-cell carcinoma of the esophagus after endoscopic resection. Gastrointest Endosc. 2008;67:799-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 89] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 30. | Yamamoto H. Technology insight: endoscopic submucosal dissection of gastrointestinal neoplasms. Nat Clin Pract Gastroenterol Hepatol. 2007;4:511-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 31. | Fujishiro M, Kodashima S, Goto O, Ono S, Niimi K, Yamamichi N, Oka M, Ichinose M, Omata M. Endoscopic submucosal dissection for esophageal squamous cell neoplasms. Dig Endosc. 2009;21:109-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 32. | Yamamoto H, Sekine Y, Higashizawa T, Kihira K, Kaneko Y, Hosoya Y, Ido K, Saito K, Sugano K. Successful en bloc resection of a large superficial gastric cancer by using sodium hyaluronate and electrocautery incision forceps. Gastrointest Endosc. 2001;54:629-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 89] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 33. | Kita H, Yamamoto H, Miyata T, Sunada K, Iwamoto M, Yano T, Yoshizawa M, Hanatsuka K, Arashiro M, Omata T. Endoscopic submucosal dissection using sodium hyaluronate, a new technique for en bloc resection of a large superficial tumor in the colon. Inflammopharmacology. 2007;15:129-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 34. | Gotoda T, Kondo H, Ono H, Saito Y, Yamaguchi H, Saito D, Yokota T. A new endoscopic mucosal resection procedure using an insulation-tipped electrosurgical knife for rectal flat lesions: report of two cases. Gastrointest Endosc. 1999;50:560-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 330] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 35. | Ono H, Kondo H, Gotoda T, Shirao K, Yamaguchi H, Saito D, Hosokawa K, Shimoda T, Yoshida S. Endoscopic mucosal resection for treatment of early gastric cancer. Gut. 2001;48:225-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1134] [Cited by in RCA: 1148] [Article Influence: 47.8] [Reference Citation Analysis (4)] |
| 36. | Oyama T, Kikuchi Y. Aggressive endoscopic mucosal resection in the upper GI tract-Hook knife EMR method. Minim Invasive Ther Allied Technol. 2002;11:291-295. |
| 37. | Yahagi N, Fujishiro M, Kakushima N, Kobayashi K, Hashimoto T, Oka M, Iguchi M, Enomoto S, Ichinose M, Niwa H. Endoscopic submucosal dissection for early gastric cancer using the tip of an electro-surgical snare (thin type). Dig Endosc. 2004;16:34-38. [RCA] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 175] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 38. | Kodashima S, Fujishiro M, Yahagi N, Kakushima N, Omata M. Endoscopic submucosal dissection using flexknife. J Clin Gastroenterol. 2006;40:378-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 57] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 39. | Takashi T, Eisei N, Takashi H, Toshio D, Takeshi S, Yoshinori I, Wataru O, Chie U, Masafumi T, Tomoomi H. Use of short needle knife for esophageal endoscopic submucosal dissection. Dig Endosc. 2005;17:246–252. [RCA] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 40. | Toyonaga T, Man-I M, Fujita T, Nishino E, Ono W, Morita Y, Sanuki T, Masuda A, Yoshida M, Kutsumi H. The performance of a novel ball-tipped Flush knife for endoscopic submucosal dissection: a case-control study. Aliment Pharmacol Ther. 2010;32:908-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 41. | Inoue H, Minami H, Kaga M, Sato Y, Kudo SE. Endoscopic mucosal resection and endoscopic submucosal dissection for esophageal dysplasia and carcinoma. Gastrointest Endosc Clin N Am. 2010;20:25-34, v-vi. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 42. | Akahoshi K, Honda K, Akahane H, Akiba H, Matsui N, Motomura Y, Kubokawa M, Endo S, Higuchi N, Oya M. Endoscopic submucosal dissection by using a grasping-type scissors forceps: a preliminary clinical study (with video). Gastrointest Endosc. 2008;67:1128-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 43. | Akahoshi K, Akahane H. A new breakthrough: ESD using a newly developed grasping type scissor forceps for early gastrointestinal tract neoplasms. World J Gastrointest Endosc. 2010;2:90-96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 44. | Akahoshi K, Honda K, Motomura Y, Kubokawa M, Okamoto R, Osoegawa T, Nakama N, Kashiwabara Y, Higuchi N, Tanaka Y. Endoscopic submucosal dissection using a grasping-type scissors forceps for early gastric cancers and adenomas. Dig Endosc. 2011;23:24-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 45. | Ono S, Fujishiro M, Niimi K, Goto O, Kodashima S, Yamamichi N, Omata M. Long-term outcomes of endoscopic submucosal dissection for superficial esophageal squamous cell neoplasms. Gastrointest Endosc. 2009;70:860-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 334] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 46. | Nonaka K, Arai S, Ishikawa K, Nakao M, Nakai Y, Togawa O, Nagata K, Shimizu M, Sasaki Y, Kita H. Short term results of endoscopic submucosal dissection in superficial esophageal squamous cell neoplasms. World J Gastrointest Endosc. 2010;2:69-74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 47. | Tamiya Y, Nakahara K, Kominato K, Serikawa O, Watanabe Y, Tateishi H, Takedatsu H, Toyonaga A, Sata M. Pneumomediastinum is a frequent but minor complication during esophageal endoscopic submucosal dissection. Endoscopy. 2010;42:8-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 48. | Takahashi H, Arimura Y, Masao H, Okahara S, Tanuma T, Kodaira J, Kagaya H, Shimizu Y, Hokari K, Tsukagoshi H. Endoscopic submucosal dissection is superior to conventional endoscopic resection as a curative treatment for early squamous cell carcinoma of the esophagus (with video). Gastrointest Endosc. 2010;72:255-64, 264.e1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 243] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 49. | Ishihara R, Iishi H, Uedo N, Takeuchi Y, Yamamoto S, Yamada T, Masuda E, Higashino K, Kato M, Narahara H. Comparison of EMR and endoscopic submucosal dissection for en bloc resection of early esophageal cancers in Japan. Gastrointest Endosc. 2008;68:1066-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 231] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 50. | Mizuta H, Nishimori I, Kuratani Y, Higashidani Y, Kohsaki T, Onishi S. Predictive factors for esophageal stenosis after endoscopic submucosal dissection for superficial esophageal cancer. Dis Esophagus. 2009;22:626-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 150] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 51. | Takahashi H, Arimura Y, Okahara S, Uchida S, Ishigaki S, Tsukagoshi H, Shinomura Y, Hosokawa M. Risk of perforation during dilation for esophageal strictures after endoscopic resection in patients with early squamous cell carcinoma. Endoscopy. 2011;43:184-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 52. | Saito Y, Tanaka T, Andoh A, Minematsu H, Hata K, Tsujikawa T, Nitta N, Murata K, Fujiyama Y. Novel biodegradable stents for benign esophageal strictures following endoscopic submucosal dissection. Dig Dis Sci. 2008;53:330-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 108] [Article Influence: 6.4] [Reference Citation Analysis (1)] |
| 53. | Yamaguchi N, Isomoto H, Shikuwa S, Nakayama T, Hayashi T, Ohnita K, Takeshima F, Kohno S, Nakao K. Effect of oral prednisolone on esophageal stricture after complete circular endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma: a case report. Digestion. 2011;83:291-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 54. | Isomoto H, Yamaguchi N, Nakayama T, Hayashi T, Nishiyama H, Ohnita K, Takeshima F, Shikuwa S, Kohno S, Nakao K. Management of esophageal stricture after complete circular endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma. BMC Gastroenterol. 2011;11:46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 109] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 55. | Yamaguchi N, Isomoto H, Nakayama T, Hayashi T, Nishiyama H, Ohnita K, Takeshima F, Shikuwa S, Kohno S, Nakao K. Usefulness of oral prednisolone in the treatment of esophageal stricture after endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma. Gastrointest Endosc. 2011;73:1115-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 248] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 56. | Yamamoto S, Ishihara R, Motoori M, Kawaguchi Y, Uedo N, Takeuchi Y, Higashino K, Yano M, Nakamura S, Iishi H. Comparison between definitive chemoradiotherapy and esophagectomy in patients with clinical stage I esophageal squamous cell carcinoma. Am J Gastroenterol. 2011;106:1048-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 57. | Saito Y, Takisawa H, Suzuki H, Takizawa K, Yokoi C, Nonaka S, Matsuda T, Nakanishi Y, Kato K. Endoscopic submucosal dissection of recurrent or residual superficial esophageal cancer after chemoradiotherapy. Gastrointest Endosc. 2008;67:355-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |