Published online May 22, 2022. doi: 10.4291/wjgp.v13.i3.73
Peer-review started: August 9, 2021
First decision: October 16, 2021
Revised: October 26, 2021
Accepted: April 21, 2022
Article in press: April 21, 2022
Published online: May 22, 2022
Processing time: 281 Days and 20.1 Hours
Cardiac and hepatic functionality are intertwined in a multifaceted relationship. Pathologic processes involving one may affect the other through a variety of mechanisms, including hemodynamic and membrane transport effects.
To better understand the effect of extrahepatic cholestasis on regulations of membrane transporters involving digoxin and its implication for digoxin clearance.
Twelve adult rats were included in this study; baseline hepatic and renal laboratory values and digoxin pharmacokinetic (PK) studies were established before evenly dividing them into two groups to undergo bile duct ligation (BDL) or a sham procedure. After 7 d repeat digoxin PK studies were completed and tissue samples were taken to determine the expressions of cell membrane transport proteins by quantitative western blot and real-time polymerase chain reaction. Data were analyzed using SigmaStat 3.5. Means between pre-surgery and post-surgery in the same experimental group were compared by paired t-test, while independent t-test was employed to compare the means between sham and BDL groups.
Digoxin clearance was decreased and liver function, but not renal function, was impaired in BDL rats. BDL resulted in significant up-regulation of multidrug resistance 1 expression in the liver and kidney and its down-regulation in the small intestine. Organic anion transporting polypeptides (OATP)1A4 was up-regulated in the liver but down-regulated in intestine after BDL. OATP4C1 expression was markedly increased in the kidney following BDL.
The results suggest that cell membrane transporters of digoxin are regulated during extrahepatic cholestasis. These regulations are favorable for increasing digoxin excretion in the kidney and decreasing its absorption from the intestine to compensate for reduced digoxin clearance due to cholestasis.
Core Tip: The heart, kidney and liver are inextricably linked by virtue of blood flow and metabolism of medications. Cholestasis induced by bile duct ligation resulted in liver functional injury and a decrease in digoxin clearance. Quantitative western blot and real-time polymerase chain reaction demonstrated the up or down regulation of membrane transporters multidrug resistance 1, organic anion transporting polypeptides (OATP)1A4, and OATP4C1 in the liver, kidney, and intestine. Cell digoxin transporters are regulated during cholestasis which is favorable for increasing digoxin excretion.
- Citation: Giroux P, Kyle PB, Tan C, Edwards JD, Nowicki MJ, Liu H. Evaluating the regulation of transporter proteins and P-glycoprotein in rats with cholestasis and its implication for digoxin clearance. World J Gastrointest Pathophysiol 2022; 13(3): 73-84
- URL: https://www.wjgnet.com/2150-5330/full/v13/i3/73.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v13.i3.73
The heart and liver are inextricably linked by virtue of blood flow and metabolism of medications, respectively. Chronic cardiac failure is characterized by cholestatic liver disease, manifested as elevation of gamma-glutamyl transferase and bilirubin[1]. Conversely, cholestatic liver disease can lead to cardiac dysfunction. Drugs with biliary elimination may have a decreased clearance in patients with cholestasis[2]. In an experimental model of cholestasis, bile duct ligation (BDL) in rats results in cardiomyopathy characterized by impaired basal cardiac contractility and reduced left ventricular pressure[3]. Furthermore, obstructive cholestasis results in impaired excretion of digoxin[4,5].
The identification of a number of organic anion transporting polypeptides (OATP) and P-glycoproteins also known as multidrug resistance 1 (MDR1) has revolutionized our understanding of the transport of biologic compounds and medications. To date, three transporters have been identified which are integral in digoxin clearance - MDR1, OATP1A4, and OATP4C1.
The main route of elimination of digoxin is renal excretion, which is closely correlated with the glomerular filtration rate and combined with tubular secretion and reabsorption. Smaller portion of digoxin is eliminated by bile duct with certain degree of enterohepatic recycling[6]. The movement of digoxin in to and out of cells is mediated by different cell membrane transporters. In the rat, OATP1A4 (also known as OATP2) is found on the basolateral membrane of hepatocytes and the membrane of enterocytes serving as an influx transporter[7-9]. Administration of the OATP1A4 inhibitor, amiodarone, resulted in increased plasma levels of intravenously administered digoxin secondary to decreased biliary excretion, liver distribution, and intestinal distribution of digoxin[10]. Administration of phenobarbital increased expression of OATP1A4 mRNA and protein, resulting in a 4-fold increase in digoxin uptake[11].
The MDR1 transporter is found in the canaliculus of the liver, the apical membrane of mucosal cells in the intestine, and the apical membrane of proximal tubule epithelial cells in the kidney, and it has been shown as an efflux pump for digoxin[12,13]. In rodents MDR1 is coded for by 2 genes, MDR1A and MDR1B. MDR1A is highly expressed in the intestine, intermediately expressed in the brain, low expression in the kidney, and minimally expressed in the liver[14]. MDR1B is intermediately expressed in the kidney and has low expression in the brain and liver[14]. The ontogeny of MDR1A and MDR1B expression in the kidney correlates with digoxin clearance[15]. MDR1 is important in the elimination of digoxin. It is located on the canalicular membrane of hepatocytes, where it transports digoxin into the canaliculus. In the intestine, MDR1 is found on the apical membrane of enterocytes, where it serves an effluxer role to inhibit absorption of digoxin. In the kidney, MDR1 is found on the apical membrane of the proximal tubule, where it transports digoxin into the urine[16]. OATP4C1 is found in the kidney, located on the basolateral membrane of proximal tubule epithelia cells[17]. The physiological role of OATP4C1 in the kidney has been shown to be coupled with MDR1 to promote the renal clearance of digoxin[17].
The distributions of cell membrane transporters vary in different tissues, and a transporter may function differently among the tissues[18]. This makes it difficult to explain the body’s response to increased blood digoxin during cholestasis. Cholestasis results in increased expression of OATP1A4 and MDR1 in the liver which favors improved hepatobiliary excretion of digoxin[19-21]. The effect of cholestasis on OATP4C1 has not been studied to date.
We performed this study to determine the effect of cholestasis on the expression of transporters responsible for the uptake and excretion of digoxin in the liver, kidney, and intestine. The implications of the changes in the transporters for digoxin pharmacokinetics (PKs) are discussed.
Unless otherwise stated, all chemicals used in this study were purchased from Sigma Chemical Co. (St. Louis, MO, United States). Digoxin injection solution was purchased from Baxter Healthcare Corporation (Deerfield, IL, United States). Antibodies for western blot were purchased as follows: Anti-MDR1 (Cat: ab170904; Lot: GR21757-38) and anti-OATP1A4 antibody (Cat: ab224610; Lot: GR319515-7) were purchased from abcam (Cambridge, MA, Unite States). Anti-OATP4C1 (Cat: 24584-1-AP) was purchased from Proteintech (Rosemont, IL, United States).
Adult male Sprague Dawley rats (225-250 g, Harlan Sprague Dawley, Inc. Indianapolis, IN, United States) were used for the study. They were kept in plastic cages with free access to food and water with alternating 12-h periods of light and darkness. Rats were randomly divided into a sham group (n = 6) and a BDL group (n = 6).
BDL was performed as described in previous publications[22,23]. In brief, rats were anaesthetized with isoflurane, and a midline ventral incision was made through the linea alba and the bile duct was isolated. A ligature was placed to the proximal portion and another ligature to the distal portion of the bile duct and then the ligatures were tightened. The bile duct was divided between the ligatures. The abdomen was closed by double-layer running suture, and the animal was allowed to wake up on a heating pad. Sham-operated control rats underwent similar surgical procedures except the ligatures were withdrawn, leaving the bile duct intact. The animals were sacrificed post-surgery day 7 after a post-surgery PK study. Tissue samples (liver, small intestine, and kidney) were collected and saved at -80 °C and RNAlater solution (Ambion, Foster City, CA, United States). The study was approved by the Institutional Animal Care and Use Committee at the University of Mississippi Medical Center.
Digoxin clearance was examined by PK studies two days prior to BDL/sham surgery and seven days following the surgeries. In brief, digoxin 0.02 mg/kg was injected through penile vein. Blood samples were obtained via tail vein at 0, 2, 5, 10, 30, 60, 120, 240, and 360 min following administration of digoxin for the measurement of digoxin. A separate blood sample (250 μL) was collected from tail vein for the measurement of liver function and bilirubin. Biochemical measurements were performed using a Roche-cobas® c501 analyzer (Roche Diagnostics, Indianapolis, IN, United States) for serum digoxin, total protein, albumin, alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP), bilirubin, blood urea nitrogen (BUN), and creatinine.
RNA was isolated from the tissues (liver, small intestine, and kidney) using a PureLink RNA Mini Kit (Invitrogen, Waltham, MA, United States) following the manufacturer’s protocol. First-strand cDNA was synthesized through reverse transcription of 0.5 μg of total RNA using iScript cDNA Synthesis system (Bio-Rad Hercules, CA, United States). Controls without reverse transcriptase were performed for each sample to ensure absence of genomic DNA. Real time polymerase chain reaction (RT-PCR) was carried out in a real time thermal cycler (iCycler, Bio-Rad) using iQ SYBR Green Supermix (Bio-Rad). Cycling conditions were 3 min at 95 °C, followed by 40 cycles of 15 s at 95 °C, 20 s at 60 °C, then 30 s at 72 °C. PCR specificity was tested via analysis of the melting curve and agarose gel electrophoresis. To semi-quantify input amounts of templates, standard curves were constructed with serial dilutions of cDNA sample from a positive control (kidney cDNA for MDR1 and OATP4C1, liver cDNA for OATP1A4). To standardize results, interpolated values for each sample were divided by the value of the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase. Primers were designed with Primer 3 software[24] and checked for absence of cross-reactivity by BLAST search. The primer pairs used, product size, and positive controls are shown in Table 1.
| Target gene | Primer sequences (5’-3’) | Size (bp) | Positive control |
| MDR1 | ATCAACTCGCAAAAGCATCC (F) | 116 | Kidney |
| AATTCAACTTCAGGATCCGC (R) | |||
| OATP1A4 | TGTGATGACCTGTGATAATTTTCCA (F) | 81 | Liver |
| TTCTCCACATATAGTTGGTGCTGAA (R) | |||
| OATP4C1 | TCAAGCTGGCAAAACTTCCC (F) | 239 | Kidney |
| CCGCAAAGCTCGATGTCAAT (R) | |||
| GAPDH | AAGATGGTGAAGGTCGGTGT (F) | 98 | Liver |
| GTTGATGGCAACAATGTCCACT (R) |
Cell membrane proteins were extracted from liver, intestine, and kidney tissues by using a Mem-PER Plus kit (Thermo Scientific, Rockford, IL, United States) following the manufacturer’s protocol. Halt Protease & Phosphatase inhibitor cocktail (Thermo Scientific, Rockford, IL, United States) was added to the extracting buffer to avoid protein degradation during procedures. Sample protein concentration was determined by using a BCA Protein Assay kit (Thermo Scientific). The protein sample was prepared for western blot by a Pierce SDS-PAGE Sample Prep Kit (Thermo Scientific) for concentrating samples while removing interfering substances. After sample buffer treatment proteins were loaded and separated on a pre-casted 4%-20% gradient SDS-PAGE gel (Bio-Red, Hercules, CA, United States) and transferred to an Immobilon-FL PVDF membrane (Merck KGaA, Darmstadt, Germany). After transfer, membrane was stained with REVERT™ Total Protein Stain (LI-COR Biosciences, Lincoln, NE, United States) for 5 min at room temperature, and then the blot image was analyzed with the Odyssey CLx® infrared imaging system (LI-COR Biosciences, Lincoln, NE, United States). Following total protein stain, the membranes were incubated with Odyssey Blocking Buffer (Li-cor, Lincoln, NE, United States) for 1 h at room temperature for blocking nonspecific binding sites. Then membranes were incubated overnight at 4 °C with primary antibodies against MDR1 (1:1600, Cat: ab170904; Lot: GR21757-38, abcam Cambridge, MA, United States), anti-OATP1A4 antibody (1:1000, Cat: ab224610; Lot: GR319515-7, abcam)[25], and anti-OATP4C1 (1:600, Cat: 24584-1-AP, Proteintech, Rosemont, IL, United States). Following the primary antibody treatments, the membranes were incubated with secondary IR dye-800 conjugated anti-rabbit antibody (1:10000, IRDy 800CW, Li-cor, Lincoln, NE, United States) for 1 h at room temperature. Western blot images were captured with the Odyssey CLx® infrared imaging system (LI-COR Biosciences, Lincoln, NE, United States) and analyzed for fluorescence density using Odyssey 2.0 software. Validation tests for sample loading sizes of each tissue, primary antibodies and secondary antibody were performed before the measurements. MDR1, OATP1A4 and OATP4C1 signals were normalized to total protein of each sample.
Data were analyzed by SigmaStat 3.5. The paired t-test was used to compare the means between pre-surgery and post-surgery in the same experimental group sham or BDL. The independent t-test was employed to compare the means between sham and BDL groups. The values from 6 rats in each group showed normal distributions. All tests were two-sided. The PKs of digoxin was analyzed by non-compartmental techniques. The area under the plasma area under the curve (AUC) was calculated. Values are expressed as mean ± SD. Statistical significance was considered at P < 0.05. The statistical methods of this study were reviewed by Dr. Lei Zhang, a biostatistician, at University of Mississippi Medical Center, Jackson, MS, United States.
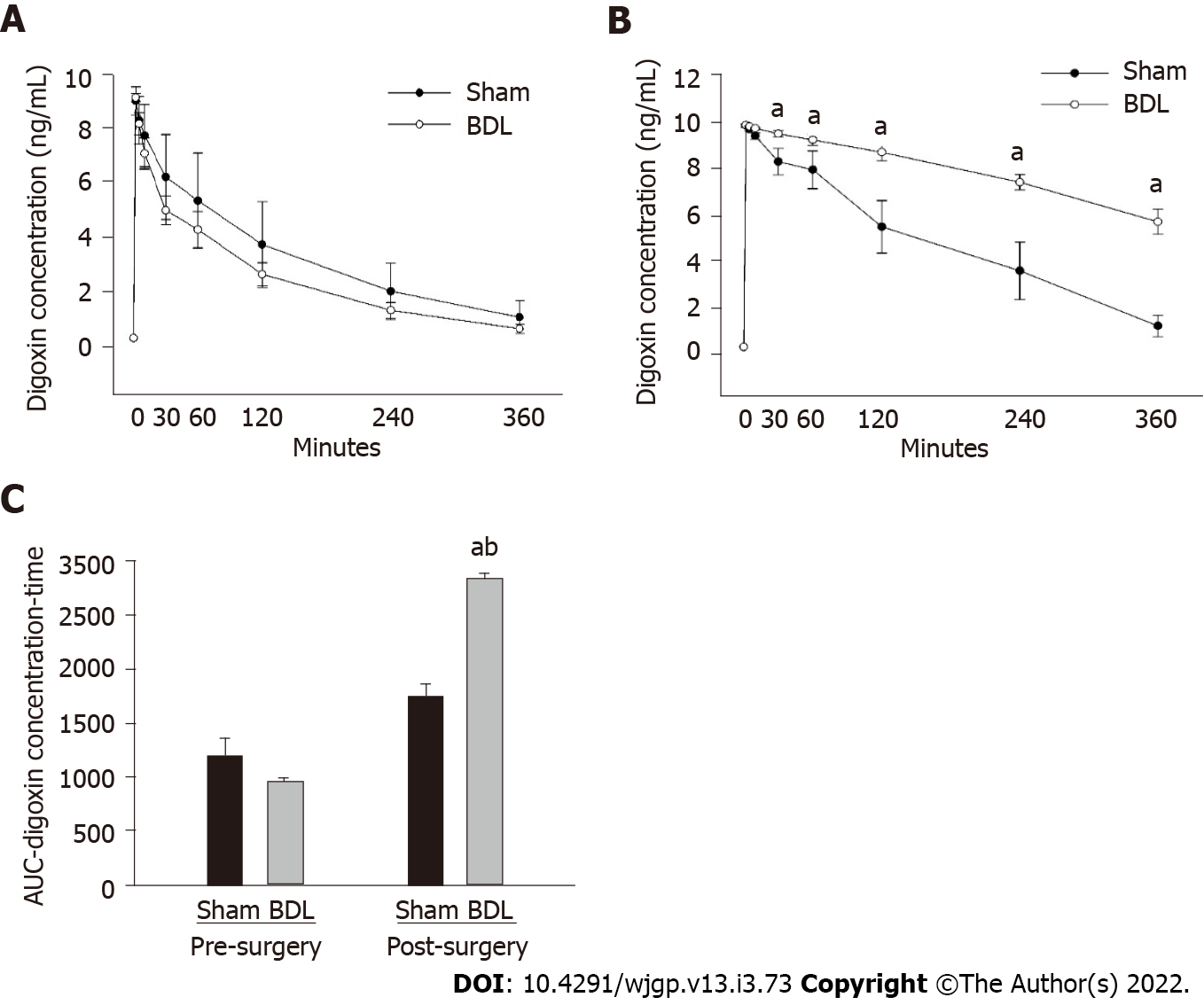
Digoxin PK studies were performed 2 d prior to BDL or sham surgery; the results were compared with digoxin PK studies performed 7 d following surgery. As shown in Figure 1, there was no difference in digoxin PKs between BDL and sham group prior surgery (Figure 1A). Following surgery, digoxin clearance was reduced in the BDL group as compared to the sham group (Figure 1B).
AUC of the post-BDL rats was significantly increased compared to the AUC of the pre-BDL and the post-surgery sham group (Figure 1C). AUC of the post-surgery sham group was slightly higher than that of the pre-surgery sham group but did not reach statistical significance. The change of AUC in the sham group following surgery may result from stress, change of gastrointestinal motility, or other factors induced by the sham surgery.
Biochemical parameters including serum total protein, albumin, ALT, AST, ALP, total bilirubin, direct bilirubin, BUN, and creatinine are represented in Table 2. There was significant liver functional injury in BDL rats as indicated by decreased serum albumin and increased ALT, AST and ALP. Obstructive jaundice developed in the post-BDL group as shown by increased total and direct bilirubin. Sham surgery did not affect liver function or bilirubin levels as compared to pre-surgery sham rats. Kidney function as measured by BUN and creatinine was not altered by BDL or sham surgery.
| Sham | BDL | |||
| Pre-surgery | Post-surgery | Pre-surgery | Post-surgery | |
| Tot protein | 6.63 ± 0.27 | 6.53 ± 0.35 | 6.53 ± 0.42 | 6.75 ± 0.23 |
| Albumin | 4.08 ± 0.17 | 3.85 ± 0.34 | 4.05 ± 0.14 | 3.40 ± 0.13a |
| ALP | 137.8 ± 19.78 | 122.3 ± 14.45 | 141.5 ± 12.74 | 467.2 ± 59.79a |
| Bilirubin, D | 0.04 ± 0.01 | 0.03 ± 0.01 | 0.03 ± 0.01 | 6.62 ± 1.72a |
| Bilirubin, T | 0.04 ± 0.02 | 0.06 ± 0.01 | 0.05 ± 0.02 | 11.67 ± 1.82a |
| ALT | 36.00 ± 12.02 | 57.00 ± 10.47 | 24.83 ± 8.28 | 191.8 ± 42.29a |
| AST | 71.83 ± 11.53 | 82.17 ± 4.92 | 64.17 ± 7.57 | 525.8 ± 107.11a |
| BUN | 17.54 ± 2.71 | 16.17 ± 3.13 | 18.23 ± 4.21 | 19.00 ± 5.57 |
| Creatinine | 0.27 ± 0.03 | 0.25 ± 0.02 | 0.29 ± 0.03 | 0.28 ± 0.04 |
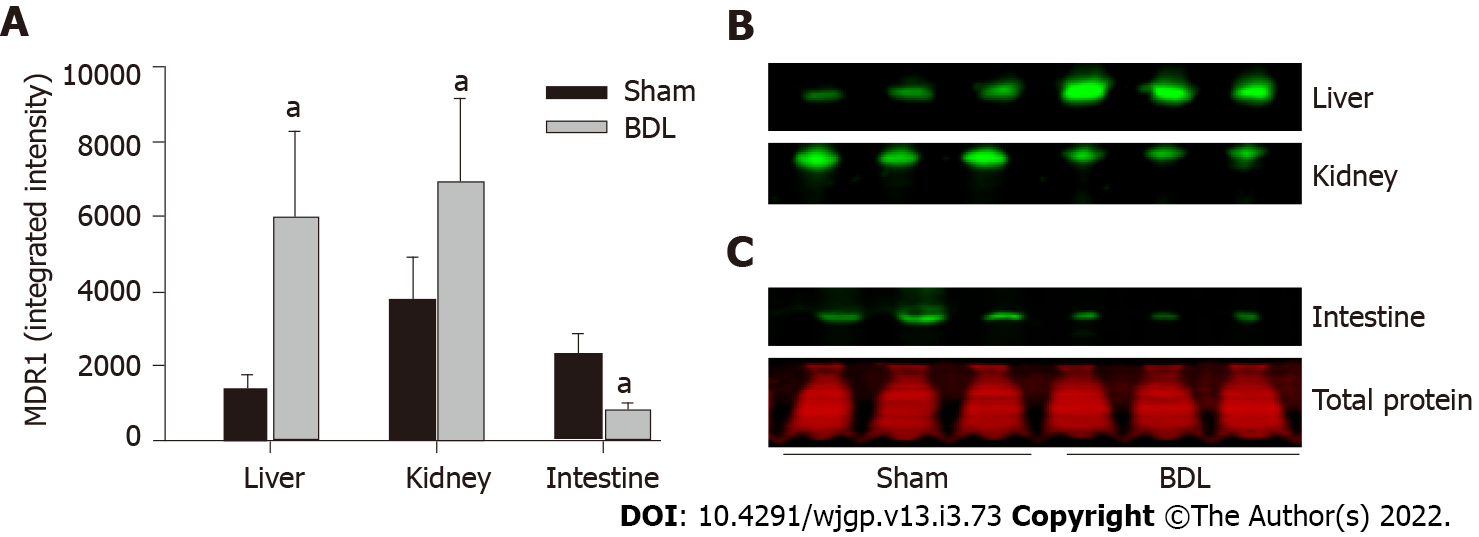
The expression of the organic anion transporters was analyzed by quantitative western blot as described in the methods. MDR1 was expressed in all the tissues examined: Liver, kidney, and small intestine (Figures 2A and 2B). BDL resulted in significant up-regulation of MDR1 expression in the liver and kidney and its down-regulation in the small intestine.
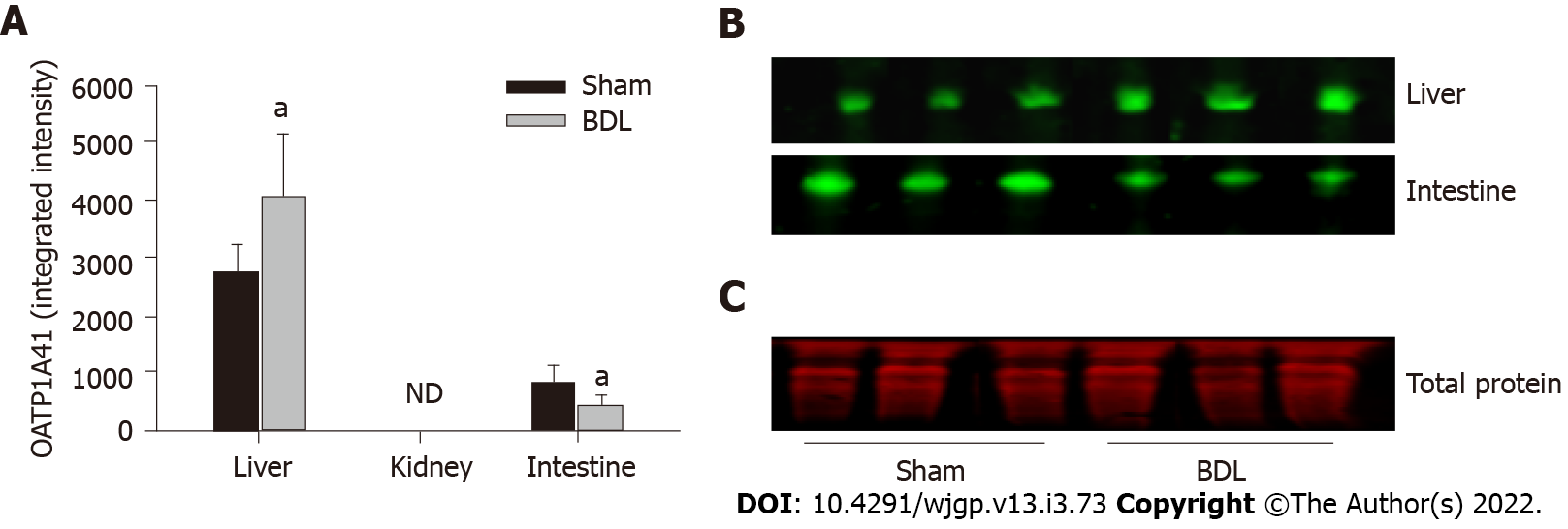
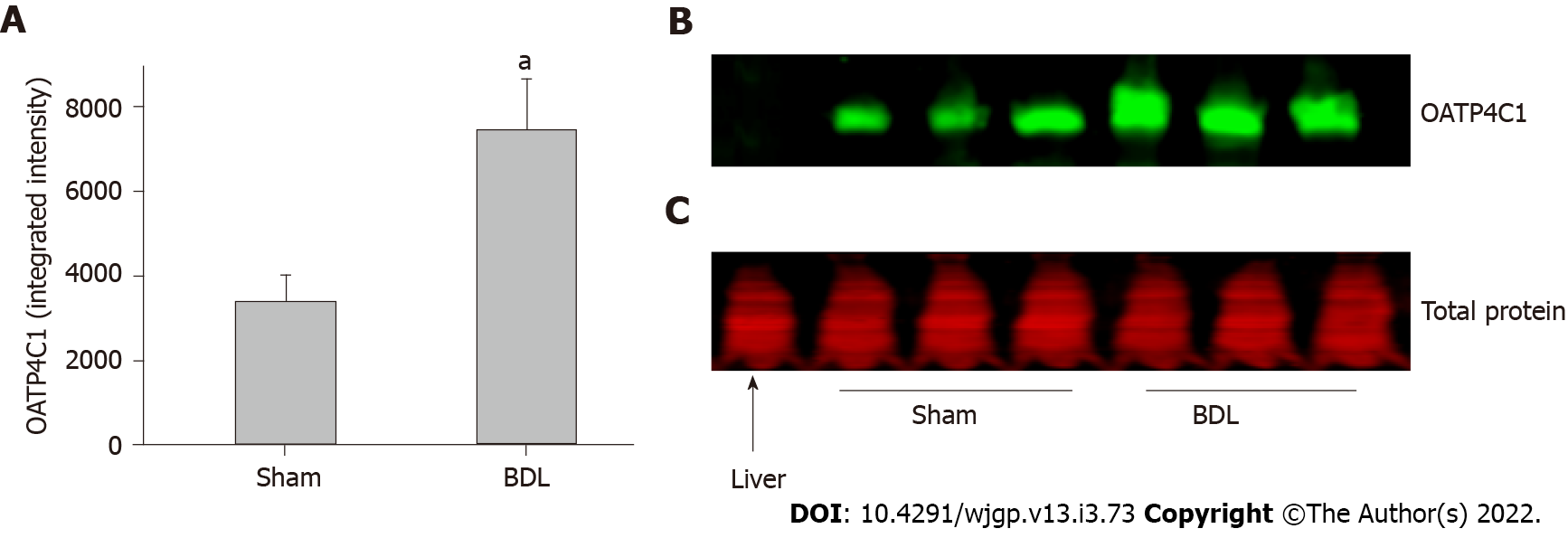
OATP1A4 protein was expressed in the liver and small intestine but it was not detectable in the kidney. OATP1A4 was significantly up-regulated by BDL in the liver and down-regulated in the small intestine (Figures 3A and 3B). The expression of the organic anion transporter OATP4C1 was tested in the kidney. BDL led to a significantly increased expression of OATP4C1 as compared with sham surgery rats (Figures 4A and 4B).
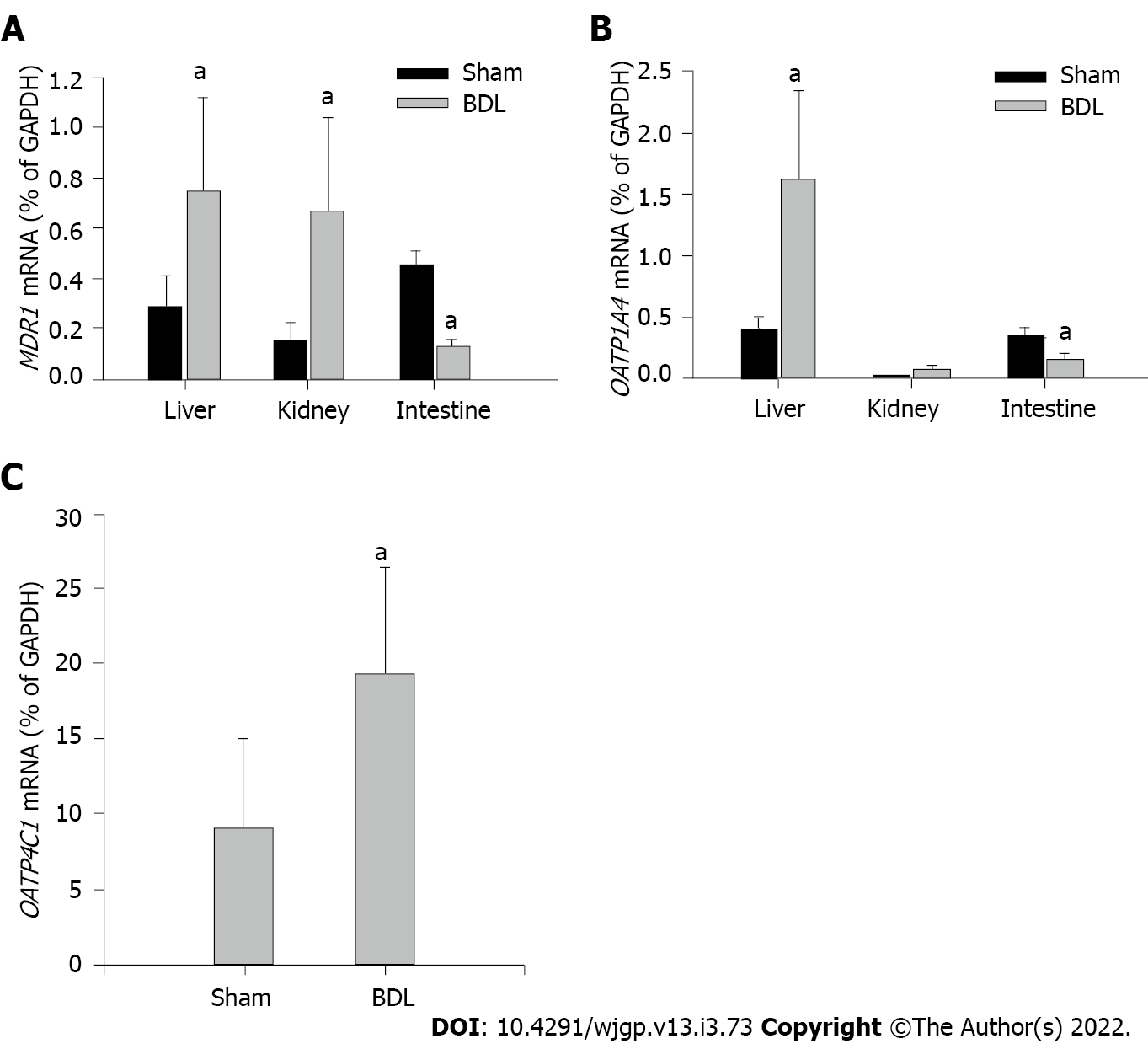
Transcription levels of MDR1, OATP1A4 and OATP4C1 were examined by mRNA expressions via RT-PCR. MDR1 mRNA was presented in all the tissues examined (Figure 5A). BDL markedly up-regulated MDR1 expression in the liver and kidney, down-regulated it in the small intestine as compared with sham surgery rats. OATP1A4 mRNA was expressed in the liver and small intestine (Figure 5B). A trace amount of OATP1A4 mRNA was tested in the kidney tissue. OATP1A4 mRNA was significantly up-regulated by BDL in the liver and down-regulated in the small intestine as compared with sham surgery rats. BDL did not alter OATP1A4 mRNA expression in the kidney (Figure 5B). OATP4C1 mRNA was expressed in the kidney and was significantly elevated after BDL surgery as compared with sham surgery rats (Figure 5C). A summary of the regulations of cell membrane transporters in kidney, intestine and liver, and potential effects on digoxin clearance are shown in Table 3.
| Efflux | Influx | Effects | |
| Kidney | MDR1: Up-regulated | OATP4C1: Up-regulated | Increase tubule exclusion |
| Intestine | MDR1: Down-regulated | OATP1A4: Down-regulated | Decrease intestinal absorption |
| Liver | MDR1: Up-regulated | OATP4C1: Up-regulated | Increase exclusion into bile duct |
Digoxin remains an important medication for treatment of cardiac dysfunction, a condition known to predispose to hepatic injury resulting in cholestasis. Cholestasis predisposes to elevated serum levels of digoxin with increased risk of toxicity. Clearance of digoxin is a complex process with differences between humans and rodents. In the rat about 60%-70% of digoxin is metabolized and the remainder excreted by the kidney (about 20%-30%) and liver (about 10%)[26,27]. In normal conditions, renal excretion of digoxin is closely correlated with the glomerular filtration rate with certain degree of tubular secretion and reabsorption. A small portion of digoxin eliminated by the bile duct goes through enterohepatic cycling[6]. The trafficking of digoxin in and out of cells is mediated by different cell membrane transporters. Previous studies have demonstrated that uptake and efflux of digoxin are mediated by OATP1A4 and MDR1, respectively, in the liver and intestine[7-9], and by OATP4C1 and MDR1 in the kidney[17]. Cholestasis alters expression of MDR1 and OATP1A4 in a manner favorable for an increase in excretion of digoxin[19-21], while the effect of cholestasis on OATP4C1 in the kidney has not been studied to date. We undertook this study to determine changes in these digoxin transporters in a model of cholestasis and their implications for digoxin clearance.
Cholestasis was induced by BDL as evidenced by elevated serum transaminase and bilirubin levels. Digoxin clearance was decreased in the BDL group in keeping with prior studies in a rabbit model[4,5]. In the earliest study, BDL also resulted in elevation of serum creatinine prompting the authors to propose decreased renal excretion of orally administered digoxin as the major mechanism for decreased clearance with disruption of the enterohepatic circulation as a potential complicating factor[4]. In a follow-up study, BDL led to decreased clearance of intravenously administered digoxin, but with absence of elevated serum creatinine. The authors concluded that impaired hepatic function and interruption of the enterohepatic circulation impaired digoxin elimination[5]. Discovery of MDR1, OATP1A4, and OATP4C1 has allowed more in-depth investigation into the mechanisms of digoxin absorption and clearance.
MDR1 is found on the apical membranes of proximal tubule cells, enterocytes, and hepatocytes where it is responsible for efflux of digoxin. In rodents MDR1 is the product of the MDR1 gene, which is made up of two forms, MDR1A and MDR1B[28]. Initial studies assessing the role of MDR1 in digoxin clearance focused on inhibiting the protein with quinidine, which inhibits intestinal excretion of digoxin[29]. To further study the role of MDR1 in digoxin clearance a knock-out model for MDR1A was created. In this model, fecal excretion of digoxin decreased and renal excretion increased compared to wild type animals, while there was no significant change in biliary excretion[30]. The authors concluded that the lower fecal excretion of digoxin was secondary to a decrease in drug excretion by the intestinal epithelium, rather than a decrease in biliary excretion. Increased renal excretion was surprising in the absence of MDR1A expression in the kidneys. The authors surmised that the increased renal clearance may be explained by other transporters (MDR1B) or increased glomerular filtration. They concluded that MDR1 contributes substantially to digoxin excretion via the intestinal epithelium and decreased re-uptake after biliary excretion[30].
Transport of digoxin in the liver is mediated by OATP1A4, responsible for uptake at the hepatocyte basolateral membrane, and MDR1, responsible for excretion into the bile at the apical membrane[7,14]. In the present study cholestasis/BDL led to increased expression of OATP1A4, increasing hepatic uptake of digoxin from the blood, and increased expression of MDR1, increasing biliary excretion of digoxin. Although these changes would predict increased clearance of digoxin through bile, ligation of the bile duct precludes this mode of clearance.
A carrier-mediated uptake of digoxin is responsible for its reabsorption of digoxin in intestine[31]. The carrier-mediated uptake was found to be sensitive to the OATP inhibitors BSP and apple juice, suggesting an OATP transporter as a likely candidate. Further support for an OATP transporter came from experiments using rat intestinal brush-border membrane vesicles which showed that an increased digoxin uptake in the presence of proton and bicarbonate gradients and outwardly directed glutathione gradient[31]. Recent studies demonstrated that intestinal OATP1A4 is a carrier protein that transports drugs from gut into the portal circulation[8], and digoxin has been shown as a substrate of OATP1A4[10]. Our result showed that BDL led to decreased expression of OATP1A4 in the intestine. Decreased expression of OATP1A4 in the intestine favors decreased absorption predicting improved drug clearance in the feces.
Although cholestasis results in changes in MDR1 and OATP1A4 favoring increased digoxin clearance, in the BDL model of cholestasis clearance of intravenously administered digoxin is limited to renal excretion. Although BDL led to changes that would predict increased clearance of digoxin through bile, ligation of the bile duct precludes this mode of clearance. Similarly, changes in the intestine following BDL favoring digoxin clearance in the feces are minimized by the study design. Digoxin administered intravenously would limit to amount of drug in the intestinal lumen. Further, BDL inhibits hepatic excretion of digoxin into the intestine.
In the kidney MDR1 is responsible for excretion of digoxin across the apical membrane of renal cells into urine[16]. Our result showed that OATP1A4 is not expressed in the kidney suggesting another transporter is responsible for transport of digoxin across the basolateral membrane into renal cells[17]. Mikkaichi et al[17] isolated an organic acid transporting peptide denoted OATP4C1 both in humans and rats. It is localized on the basolateral membrane of the proximal tubules of the kidney where it has been shown to be the primary transporter of digoxin into renal cells. MDR1 is co-localized with OATP4C1 in the proximal tubule. Renal failure leads to decreased expression in OATP4C1 but has no effect on expression of MDR1 suggesting that decreased digoxin clearance in renal failure is due to loss of OATP4C1 activity[17,32]. We have shown that cholestasis due to BDL results in increased expression in both MDR1 and OATP4C1 in the kidney favoring enhanced vectorial transport of digoxin from blood to urine by proximal tubule cells. To the best of our knowledge, the current report is the first study to investigate the regulation of OATP4C1 in kidney in a pathological model in vivo.
It is interesting that MDR1 and OATP1A4 participate in transport of both bile acids and digoxin[33]. Also, there is marked similarity in the method of excretion for bile acids and digoxin in obstructive cholestasis. OATP4C1 may also participate in the excretion of bile acids by the kidney through increased uptake at the basolateral membrane, although the data is conflicting. To date, two studies assessed the transport of bile acids in Madin-Darby canine kidney cells transfected with a plasmid containing OATP4C1, one showed no transport of taurocholate[17], while the other showed transport of both chenodeoxycholate and glycocholate[34]. Our study showed upregulation of OATP4C1 in cholestasis which would increase uptake of bile acids by proximal tubule cells with subsequent excretion at the apical membrane by MDR1.
Bile acids activate the nuclear hormone receptors farnesoid-X-receptor and pregnane-X-receptor (PXR) and in cholestasis there were increased activations of these receptors[35,36]. MDR1 and OATP1A4 are both PXR-responsive and their expression increased in cholestasis. OATP4C1 expression is induced through transitional factor Aryl hydrocarbon receptor (AhR) through binding of the xenobiotic responsive element[37]. Previous studies have shown that AhR is activated in cholestasis[38] through the action of PXR[39]. We propose that the increased expression of OATP4C1 in cholestasis is best explained by this mechanism.
This is an exploratory research to study how the body responds to increased digoxin during cholestasis. Further studies are needed to confirm the implications by measuring digoxin tissue distributions and digoxin concentrations in urine and along the intestinal tract from the duodenum to the ileum. We believe that the findings from the current study will serve as a base for future study of digoxin clearance mediated by renal-expressed OATP4C1 during cholestasis.
In conclusion, under physiological conditions, the main route of elimination of digoxin is renal excretion which is closely correlated with glomerular filtration rate. Biliary excretion is the major non-renal route. Enterohepatic cycle has minor importance[6]. Our finding demonstrated that under pathological condition, cholestasis in the current study, cell membrane digoxin transporters are regulated which is in favor of an increase in digoxin excretion in renal tubules and a decrease in its absorption from the tubules of intestine. These changes compensate the reduced digoxin clearance due to cholestasis. This finding could have clinical application by modifying transporters’ activities through pharmaceutical approaches for improving digoxin clearance during cholestasis.
The heart and liver are inextricably linked by virtue of blood flow and metabolism of medications. Drugs with biliary elimination, such as digoxin, decrease clearance with cholestasis.
We performed this study to better understand the effect of extrahepatic cholestasis on regulations of membrane transporters involving digoxin and its implication for digoxin clearance.
The efflux transporter, multidrug resistance 1 (MDR1), and influx transporters, organic anion transporting polypeptides (OATP)1A4 and OATP4C1 in kidney, intestine and liver were examined.
Twelve adult Sprague Dawley rats were included in this study; baseline hepatic and renal laboratory values and digoxin pharmacokinetic (PK) studies were established before evenly dividing them into two groups to undergo bile duct ligation (BDL) or a sham procedure. After 7 d repeat digoxin PK studies were completed and tissue samples were taken to determine the expressions of MDR1, OATP1A4 and OATP4C1 by quantitative western blot and real-time polymerase chain reaction.
Digoxin clearance was decreased and liver function, but not renal function, was impaired in BDL rats. BDL resulted in significant up-regulation of MDR1 expression in the liver and kidney and its down-regulation in the small intestine. OATP1A4 was up-regulated in the liver but down-regulated in intestine after BDL. OATP4C1 expression was markedly increased in the kidney following BDL.
The results suggest that cell membrane transporters of digoxin are regulated during cholestasis. These regulations are favorable for increasing digoxin excretion in kidney and decreasing its absorption from intestine in order to compensate the reduced digoxin clearance due to cholestasis.
The current study was designed as an exploratory research for providing clues for future study in this field. Previous studies on the transporters in kidney and intestine were done only by in vitro experiments. To the best of our knowledge, the current report is the first study to investigate the regulation of the digoxin transporters in kidney and intestine in animal model of cholestasis. Our results does demonstrate that the cell membrane transporters were regulated which is in favor of digoxin excretion during cholestasis. To confirm our finding, more detailed PK studies need to be done, for example, tissue distributions of digoxin and digoxin concentrations in urine and in intestine. Knock-out (KO) animal lacking the transporters, especially tissue-specific KO, will be a powerful tool in further study.
We thank Dr. Lei Zhang, a bio-statistician at University of Mississippi Medical Center, for review of the statistical methods.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: American Heart Association; American Society of Nephrology.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Chen L, China; Kreisel W, Germany; Tajiri K, Japan S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Naschitz JE, Slobodin G, Lewis RJ, Zuckerman E, Yeshurun D. Heart diseases affecting the liver and liver diseases affecting the heart. Am Heart J. 2000;140:111-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 182] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 2. | Delcò F, Tchambaz L, Schlienger R, Drewe J, Krähenbühl S. Dose adjustment in patients with liver disease. Drug Saf. 2005;28:529-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 145] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 3. | Yu W, Huang C, Wang Q, Zhao E, Ding Y, Huang T, Ma C, Meng B. Plasma BNP level combined with surgical Apgar score to predict operative major cardiac adverse events in malignant obstructive jaundice patients. Pak J Med Sci. 2016;32:1188-1193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 4. | Wójcicki M, Drozdzik M, Sulikowski T, Gawronska-Szklarz B, Wójcicki J, Rózewicka L, Skowron J, Zielinski S, Musial HD, Zakrzewski J. Pharmacokinetics of intragastrically administered digoxin in rabbits with experimental bile duct obstruction. J Pharm Pharmacol. 1997;49:1082-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 5. | Wójcicki M, Drozdzik M, Sulikowski T, Wójcicki J, Gawrońska-Szklarz B, Zieliński S, Rózewicka L. Pharmacokinetics of intravenously administered digoxin and histopathological picture in rabbits with experimental bile duct obstruction. Eur J Pharm Sci. 2000;11:215-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Iisalo E. Clinical pharmacokinetics of digoxin. Clin Pharmacokinet. 1977;2:1-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 143] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Reichel C, Gao B, Van Montfoort J, Cattori V, Rahner C, Hagenbuch B, Stieger B, Kamisako T, Meier PJ. Localization and function of the organic anion-transporting polypeptide Oatp2 in rat liver. Gastroenterology. 1999;117:688-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 149] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 8. | Koitabashi Y, Kumai T, Matsumoto N, Watanabe M, Sekine S, Yanagida Y, Kobayashi S. Orange juice increased the bioavailability of pravastatin, 3-hydroxy-3-methylglutaryl CoA reductase inhibitor, in rats and healthy human subjects. Life Sci. 2006;78:2852-2859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Naud J, Michaud J, Boisvert C, Desbiens K, Leblond FA, Mitchell A, Jones C, Bonnardeaux A, Pichette V. Down-regulation of intestinal drug transporters in chronic renal failure in rats. J Pharmacol Exp Ther. 2007;320:978-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 87] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 10. | Funakoshi S, Murakami T, Yumoto R, Kiribayashi Y, Takano M. Role of organic anion transporting polypeptide 2 in pharmacokinetics of digoxin and beta-methyldigoxin in rats. J Pharm Sci. 2005;94:1196-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Hagenbuch N, Reichel C, Stieger B, Cattori V, Fattinger KE, Landmann L, Meier PJ, Kullak-Ublick GA. Effect of phenobarbital on the expression of bile salt and organic anion transporters of rat liver. J Hepatol. 2001;34:881-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 41] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Bendayan R. Renal drug transport: a review. Pharmacotherapy. 1996;16:971-985. [PubMed] |
| 13. | Schinkel AH, Wagenaar E, van Deemter L, Mol CA, Borst P. Absence of the mdr1a P-Glycoprotein in mice affects tissue distribution and pharmacokinetics of dexamethasone, digoxin, and cyclosporin A. J Clin Invest. 1995;96:1698-1705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 841] [Cited by in RCA: 833] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 14. | Croop JM, Raymond M, Haber D, Devault A, Arceci RJ, Gros P, Housman DE. The three mouse multidrug resistance (mdr) genes are expressed in a tissue-specific manner in normal mouse tissues. Mol Cell Biol. 1989;9:1346-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 95] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Pinto N, Halachmi N, Verjee Z, Woodland C, Klein J, Koren G. Ontogeny of renal P-glycoprotein expression in mice: correlation with digoxin renal clearance. Pediatr Res. 2005;58:1284-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Thiebaut F, Tsuruo T, Hamada H, Gottesman MM, Pastan I, Willingham MC. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci U S A. 1987;84:7735-7738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1902] [Cited by in RCA: 1924] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 17. | Mikkaichi T, Suzuki T, Onogawa T, Tanemoto M, Mizutamari H, Okada M, Chaki T, Masuda S, Tokui T, Eto N, Abe M, Satoh F, Unno M, Hishinuma T, Inui K, Ito S, Goto J, Abe T. Isolation and characterization of a digoxin transporter and its rat homologue expressed in the kidney. Proc Natl Acad Sci U S A. 2004;101:3569-3574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 212] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 18. | Klaassen CD, Aleksunes LM. Xenobiotic, bile acid, and cholesterol transporters: function and regulation. Pharmacol Rev. 2010;62:1-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 660] [Cited by in RCA: 586] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 19. | Schrenk D, Gant TW, Preisegger KH, Silverman JA, Marino PA, Thorgeirsson SS. Induction of multidrug resistance gene expression during cholestasis in rats and nonhuman primates. Hepatology. 1993;17:854-860. [PubMed] |
| 20. | Slitt AL, Allen K, Morrone J, Aleksunes LM, Chen C, Maher JM, Manautou JE, Cherrington NJ, Klaassen CD. Regulation of transporter expression in mouse liver, kidney, and intestine during extrahepatic cholestasis. Biochim Biophys Acta. 2007;1768:637-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Donner MG, Schumacher S, Warskulat U, Heinemann J, Häussinger D. Obstructive cholestasis induces TNF-alpha- and IL-1 -mediated periportal downregulation of Bsep and zonal regulation of Ntcp, Oatp1a4, and Oatp1b2. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1134-G1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | Kountouras J, Billing BH, Scheuer PJ. Prolonged bile duct obstruction: a new experimental model for cirrhosis in the rat. Br J Exp Pathol. 1984;65:305-311. [PubMed] |
| 23. | Holmberg JT, Hederström E, Ihse I. A method to prevent recanalization of the transected bile duct in the rat. Scand J Gastroenterol. 1985;20:428-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1367] [Cited by in RCA: 5192] [Article Influence: 199.7] [Reference Citation Analysis (0)] |
| 25. | Dolezelova E, Sa ICI, Prasnicka A, Hroch M, Hyspler R, Ticha A, Lastuvkova H, Cermanova J, Pericacho M, Visek J, Lasticova M, Micuda S, Nachtigal P. High soluble endoglin levels regulate cholesterol homeostasis and bile acids turnover in the liver of transgenic mice. Life Sci. 2019;232:116643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Harrison LI, Gibaldi M. Pharmacokinetics of digoxin in the rat. Drug Metab Dispos. 1976;4:88-93. [PubMed] |
| 27. | Wirth KE, Frölich JC. Effect of spironolactone on excretion of 3H-digoxin and its metabolites in rats. Eur J Pharmacol. 1974;29:43-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Sukhai M, Yong A, Kalitsky J, Piquette-Miller M. Inflammation and interleukin-6 mediate reductions in the hepatic expression and transcription of the mdr1a and mdr1b Genes. Mol Cell Biol Res Commun. 2000;4:248-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 48] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 29. | Su SF, Huang JD. Inhibition of the intestinal digoxin absorption and exsorption by quinidine. Drug Metab Dispos. 1996;24:142-147. [PubMed] |
| 30. | Mayer U, Wagenaar E, Beijnen JH, Smit JW, Meijer DK, van Asperen J, Borst P, Schinkel AH. Substantial excretion of digoxin via the intestinal mucosa and prevention of long-term digoxin accumulation in the brain by the mdr 1a P-glycoprotein. Br J Pharmacol. 1996;119:1038-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 205] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 31. | Yao HM, Chiou WL. The complexity of intestinal absorption and exsorption of digoxin in rats. Int J Pharm. 2006;322:79-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 32. | Laouari D, Yang R, Veau C, Blanke I, Friedlander G. Two apical multidrug transporters, P-gp and MRP2, are differently altered in chronic renal failure. Am J Physiol Renal Physiol. 2001;280:F636-F645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 72] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 33. | Kullak-Ublick GA, Becker MB. Regulation of drug and bile salt transporters in liver and intestine. Drug Metab Rev. 2003;35:305-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 73] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 34. | Yamaguchi H, Sugie M, Okada M, Mikkaichi T, Toyohara T, Abe T, Goto J, Hishinuma T, Shimada M, Mano N. Transport of estrone 3-sulfate mediated by organic anion transporter OATP4C1: estrone 3-sulfate binds to the different recognition site for digoxin in OATP4C1. Drug Metab Pharmacokinet. 2010;25:314-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 35. | Sinal CJ, Tohkin M, Miyata M, Ward JM, Lambert G, Gonzalez FJ. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell. 2000;102:731-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1341] [Cited by in RCA: 1400] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 36. | Staudinger JL, Goodwin B, Jones SA, Hawkins-Brown D, MacKenzie KI, LaTour A, Liu Y, Klaassen CD, Brown KK, Reinhard J, Willson TM, Koller BH, Kliewer SA. The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc Natl Acad Sci U S A. 2001;98:3369-3374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1020] [Cited by in RCA: 1043] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 37. | Suzuki T, Toyohara T, Akiyama Y, Takeuchi Y, Mishima E, Suzuki C, Ito S, Soga T, Abe T. Transcriptional regulation of organic anion transporting polypeptide SLCO4C1 as a new therapeutic modality to prevent chronic kidney disease. J Pharm Sci. 2011;100:3696-3707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 38. | Volz DC, Kullman SW, Howarth DL, Hardman RC, Hinton DE. Protective response of the Ah receptor to ANIT-induced biliary epithelial cell toxicity in see-through medaka. Toxicol Sci. 2008;102:262-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 39. | Xie W, Evans RM. Orphan nuclear receptors: the exotics of xenobiotics. J Biol Chem. 2001;276:37739-37742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 158] [Article Influence: 6.6] [Reference Citation Analysis (0)] |