Published online Jan 22, 2021. doi: 10.4291/wjgp.v12.i1.1
Peer-review started: October 26, 2020
First decision: November 20, 2020
Revised: November 30, 2020
Accepted: December 8, 2020
Article in press: December 8, 2020
Published online: January 22, 2021
Processing time: 86 Days and 23.9 Hours
Anastomotic leakage is a serious complication following gastrointestinal surgery and is associated with increased morbidity and mortality. The incidence of anastomotic leakage is determined by anatomy and is reported to be between 4%-33% for colon anastomosis and 1%-3% for small intestine anastomosis. The etiology of anastomotic leakage of the intestine has been divided into three main factors: healing disturbances, communication between intra- and extra-luminal compartments, and infection. All three factors interact, and one factor will inevitably lead to the other two factors resulting in tissue ischemia, tissue necrosis, and anastomotic leakage.
To evaluate ischemic metabolites and cefuroxime concentrations in both anastomosis and non-anastomosis ileum and colon in a porcine model.
Eight healthy female pigs (Danish Landrace breed, weight 58-62 kg) were included in this study. Microdialysis catheters were placed for sampling of ischemic metabolites (glucose, lactate, glycerol, and pyruvate) and cefuroxime concentrations in both anastomosis and non-anastomosis ileum and colon. Cefuroxime 1.5 g was administered as an intravenous infusion over 15 min. Subsequently, dialysates and blood samples were collected over 8 h and the ischemic metabolites and cefuroxime concentrations were quantified in all samples. The concentrations of glucose, lactate, glycerol and pyruvate were determined using the CMA 600 Microdialysis Analyzer with Reagent Set A (M Dialysis AB, Sweden), and the concentrations of cefuroxime and meropenem were quantified using a validated ultra-high-performance liquid chromatography assay.
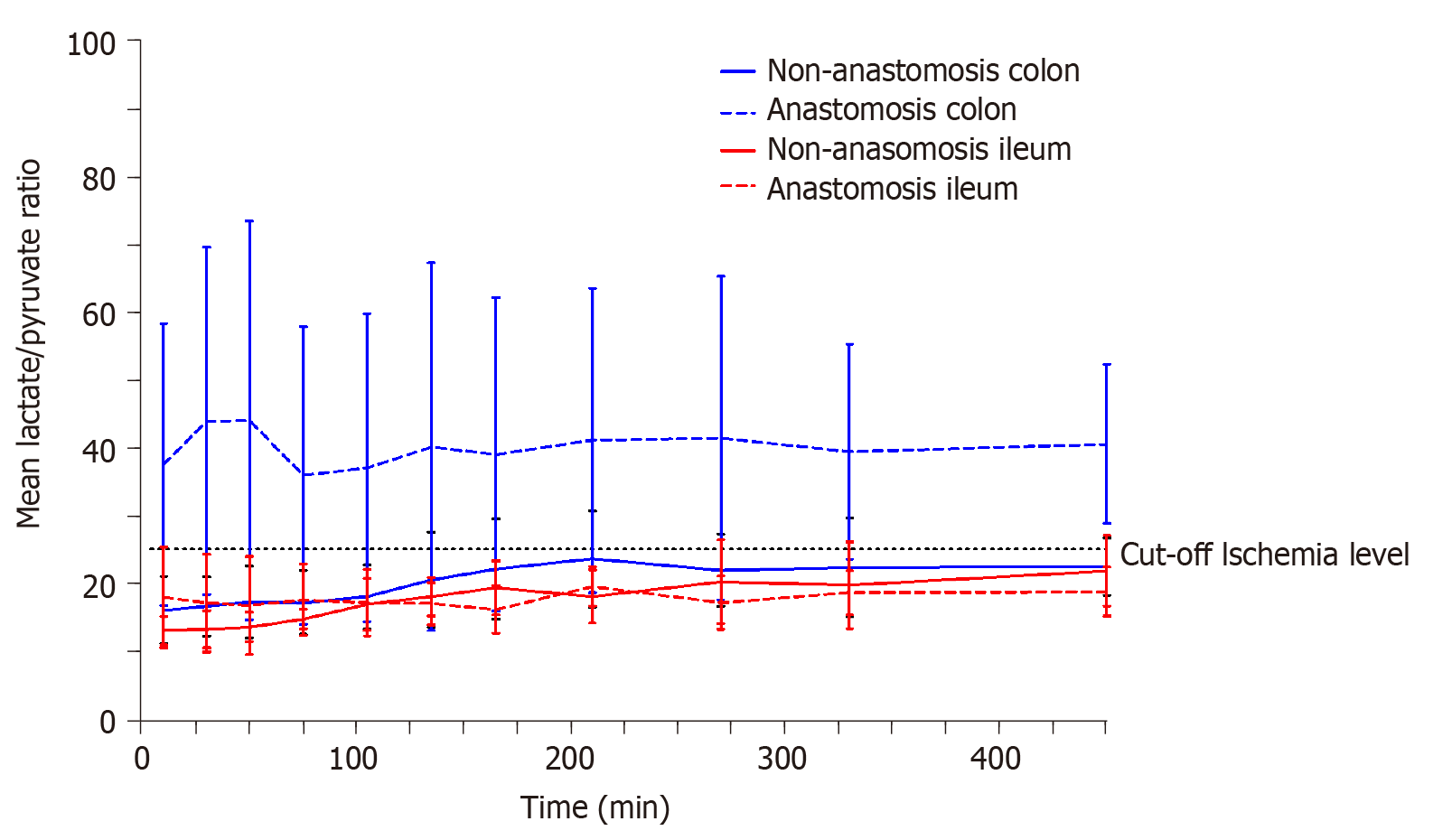
Only the colon anastomosis induced mean ischemic lactate/pyruvate ratios above 25 (ischemic cut-off) throughout the entire sampling interval, and simultaneously decreased glucose concentrations. The mean time for which cefuroxime concentrations were maintained above the clinical breakpoint minimal inhibitory concentration for Escherichia coli (8 µg/mL) ranged between 116-128 min across all the investigated compartments, and was similar between the anastomosis and non-anastomosis ileum and colon. For all pigs and in all the investigated compartments, a cefuroxime concentration of 8 µg/mL was reached within 10 min after administration. When comparing the pharmacokinetic parameters between the anastomosis and non-anastomosis sites for both ileum and colon, only colon Tmax and half-life differed between anastomosis and non-anastomosis (P < 0.03). Incomplete tissue penetrations were found in all tissues except for the non-anastomosis colon.
Administering 1.5 g cefuroxime 10 min prior to intestine surgery seems sufficient, and effective concentrations are sustained for approximately 2 h. Only colon anastomosis was locally vulnerable to ischemia.
Core Tip: We found that only colon anastomosis was locally vulnerable to ischemia but reached similar cefuroxime concentrations to those in the remaining investigated intestine compartments. Our study suggests that administering 1.5 g cefuroxime 10 min prior to intestine surgery is sufficient, and that effective concentrations are sustained for approximately 2 h. This is the first study to investigate the influence of anastomoses on ileum and colon ischemic metabolites and cefuroxime concentrations in a simultaneous paired design.
- Citation: Hanberg P, Bue M, Thomassen M, Løve US, Kipp JO, Harlev C, Petersen E, Søballe K, Stilling M. Influence of anastomoses on intestine ischemia and cefuroxime concentrations: Evaluated in the ileum and colon in a porcine model. World J Gastrointest Pathophysiol 2021; 12(1): 1-13
- URL: https://www.wjgnet.com/2150-5330/full/v12/i1/1.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v12.i1.1
Anastomotic leakage is a serious complication following gastrointestinal surgery and is associated with increased morbidity and mortality[1]. The incidence of anastomotic leakage is determined by anatomy and is reported to be between 4%-33% for colon anastomosis and 1%-3% for small intestine anastomosis[1-4]. The etiology of anastomotic leakage is multifactorial, and to some extent not fully understood[3]. Nonetheless, previous studies have suggested that the etiology is due to three main factors: healing disturbances, communication between intra- and extra-luminal compartments, and infection[3,5,6]. With this theory, one of these three factors can often be identified as the primary cause of the anastomotic leakage. However, it is believed that all three factors interact and one factor will lead to the two other factors resulting in tissue ischemia, tissue necrosis, and anastomotic leakage[3,7].
Gastrointestinal surgery is predisposed to infection given its vicinity to the bacterial load within the intestine. Sufficient antimicrobial prophylaxis is considered an essential preventive measure in protecting surgical anastomoses from bacterial overgrowth and relies on the achievement of therapeutic antimicrobial target site concentration[8]. While antimicrobial concentrations have been evaluated in various tissues and settings[9,10], intestine antimicrobial concentrations remain poorly investigated. Cephalosporins, e.g., cefuroxime, is frequently used both prophylactically and in the treatment of infections within gastrointestinal surgery, due to its broad-spectrum efficacy against gram-positive as well as gram-negative bacteria[11].
Microdialysis is a membrane-bearing method, which allows continuous sampling of ischemic metabolites and the free antimicrobial concentrations in the interstitial space of various tissues[12,13]. It has previously been employed in various abdominal relevant sites for the study of ischemic metabolites[14-18], and for sampling cefuroxime concentrations in various extra-abdominal tissues[19,20]. We hypothesized that anastomoses of the ileum and colon would present an immediate postoperative local increase in ischemic metabolites and lower cefuroxime concentrations in comparison to the non-anastomosis intestine. To test this, we conducted a porcine study applying microdialysis for the evaluation of ischemic metabolites and cefuroxime concentrations in both anastomosis and non-anastomosis ileum and colon.
This study was conducted at the Institute of Clinical Medicine, Aarhus University Hospital, Denmark. The study was carried out according to existing laws and approved by the Danish Animal Experiments Inspectorate (license No.: 2017/15-0201-01184). All appropriate measures were taken to minimize animal pain and discomfort. Chemical analyses were performed at the Department of Clinical Biochemistry, Aarhus University Hospital, Denmark.
Glucose, lactate, glycerol, and pyruvate can easily and promptly be analyzed when linked to an appropriate analytical assay[12,21,22]. Under anaerobic conditions, glucose levels decrease due to a combination of increased glucose consumption, which is required in order to maintain adenosine triphosphate (ATP) production, and a decreased organ or tissue supply due to reduced perfusion[21]. Lactate is produced from pyruvate under anaerobic conditions resulting in increased lactate concentrations, decreased pyruvate concentrations, and ultimately increased lactate/pyruvate ratios[21]. A lactate/pyruvate ratio above 25 is considered to signify ischemia[22]. Glycerol is a basic component of the cell membrane. When the cell membrane is damaged, glycerol is released, and is therefore used as marker of cell damage[21].
Microdialysis: Microdialysis is a catheter-based technique with a semipermeable membrane at the tip of the catheter, which allows for continuous and simultaneous sampling of interstitial fluid from multiple sites[23]. Due to continuous perfusion of the semipermeable membrane, equilibrium never occurs, and the dialysate concentration only represents a fraction of the actual concentration. This fraction is referred to as the relative recovery, which can be determined by various calibration methods[23]. In this study, meropenem was used as an internal calibrator for cefuroxime[13]. Relative recovery was not determined for the ischemic metabolites. Changes in the concentration ratios between interventions or compartments, for comparison between anastomosis and non-anastomosis tissue and for ratios between metabolites (lactate/pyruvate) are quantitative measures and independent of relative recovery[24].
Equipment from M Dialysis AB (Stockholm, Sweden) was used. The microdialysis catheters consisted of CMA 70 membranes (membrane length: 20 mm, 20 kDa molecule cut-off), and CMA 107 precision pumps produced a flow rate of 2 µL/min.
Animals, anesthetic, and surgical procedure: Eight healthy female pigs (Danish Landrace breed, weighing 58-62 kg) were included in the study. The pigs received general anesthesia during the study with the combination of propofol (500-600 mg/h, continuous infusion) and fentanyl (0.60-0.75 mg/h, continuous infusion). Temperature and pH where monitored for each pig and were kept within the range of 36.4-38.5°C and 7.40-7.50, respectively.
After induction of anesthesia, surgery was initiated. The intestines were presented via a midline abdominal incision. A 5 cm ileum resection, approximately 50 cm orally from the ileocaecal valve, was performed. The ileum was anastomosed end-to-end with a continuous (Monocryltm 4-0) suture using the extramucosal technique ad modum Davos (hand-sewn end-to-end extramucosal running suture). Good blood supply to the intestine ends was visualized by brisk bleeding from the arcade artery prior to suturing. One microdialysis catheter was placed in the ileum wall parallel to and approximately 0.5 cm from the anastomosis. An adjacent microdialysis catheter was placed approximately 50 cm orally from the ileum anastomosis. Subsequently, a 5 cm colon resection was performed approximately 10 cm anally from the ileocecal valve. Good blood supply to the colon ends was visualized by brisk bleeding from the arcade artery. The colon was similarly anastomosed end-to-end with a continuous (Monocryltm 4-0) suture. One microdialysis catheter was placed in the colon wall parallel to and approximately 0.5 cm from the anastomosis. An adjacent microdialysis catheter was placed approximately 30 cm anally from the colon anastomosis. All catheters were placed using splitable introducers. After placement of all catheters, the abdominal wall was carefully closed.
Following placement of the microdialysis catheters, all catheters were perfused with 0.9% NaCl containing 5 µg/mL meropenem, allowing for continuous calibration, and 30 min tissue equilibration was allowed for.
Sampling procedures: Cefuroxime 1.5 g was administered intravenously over 15 min, marking time zero. Dialysates were collected at 20 min intervals from time 0-60 min, at 30 min intervals from time 60-180 min, and at 60 min intervals from time 180-360 min and from time 420-480 min, giving a total of 11 samples during 8 h. Blood samples were collected from a central venous catheter at the midpoint of the sampling intervals.
Dialysate samples were instantly stored at -80°C until analysis. The venous blood samples were stored at 5°C for a maximum of 6 h before being centrifuged at 3000 rpm for 10 min. Plasma aliquots were then stored at -80°C until analysis.
For the ischemic metabolites, the primary endpoint was evaluation of lactate/pyruvate ratios. For cefuroxime concentrations, the primary endpoint was assessment of the time for which the free cefuroxime was maintained above the clinical breakpoint minimal inhibitory concentration (T>MIC) for Escherichia coli (8 µg/mL)[25].
Cefuroxime and meropenem concentrations: The concentrations of cefuroxime and meropenem were quantified using a validated ultra-high-performance liquid chromatography assay[26]. Inter-run imprecisions (percent coefficients of variation) were 4.7% at 2.5 µg/mL for quantification of cefuroxime and 3.0% at 2.0 µg/mL for quantification of meropenem. The lower limits of quantification were 0.06 µg/mL for cefuroxime and 0.5 µg/mL for meropenem.
Assessment of ischemic metabolites: The concentrations of glucose, lactate, glycerol and pyruvate were determined using the CMA 600 Microdialysis Analyzer with Reagent Set A (M Dialysis AB, Sweden).
Pharmacokinetic parameters were determined for each compartment in all animals using noncompartmental analysis in Stata (v. 15.1, StataCorp LLC, College Station, TX, United States). The area under the concentration-time curves (AUC) were calculated using the trapezoidal rule. The maximum of all the recorded concentrations was defined as peak drug concentration (Cmax), enabling calculation of the time to Cmax (Tmax). Half-life (T1/2) was calculated as ln(2)/λeq, where λeq is the terminal elimination rate constant estimated by linear regression of the log concentration on time. The AUCtissue/AUCplasma ratio was calculated as a measure of tissue penetration. Microsoft Excel was used to estimate the T>MIC using linear interpolation. A general comparison of the pharmacokinetic parameters and T>MIC was conducted using a repeated measurements analysis of variance followed by pairwise comparisons made by linear regression. The Kenward-Roger approximation method was used for correction of degrees of freedom due to the small sample size. The model assumptions were tested using visual diagnosis of residuals, fitted values, and estimates of random effects. A significance level of 5% was used. Microsoft Excel was used to calculate the mean concentration difference in percentage for the ischemic markers between the anastomosis and non-anastomosis (anastomosis/non-anastomosis) ileum and colon. The measured cefuroxime and ischemic marker concentrations in the dialysate were attributed to the midpoint of the sampling intervals.
All pigs completed the study. The relative recovery (SD) was 24% (5) for non-anastomosis ileum, 18% (3) for ileum anastomosis, 28% (9) for non-anastomosis colon, and 27% (7) for colon anastomosis.
The lactate/pyruvate ratio for each compartment is depicted in Figure 1. Only the mean lactate/pyruvate ratio for the colon anastomosis was above the ischemic cut-off level of 25, and remained above 25 throughout the entire sampling interval.
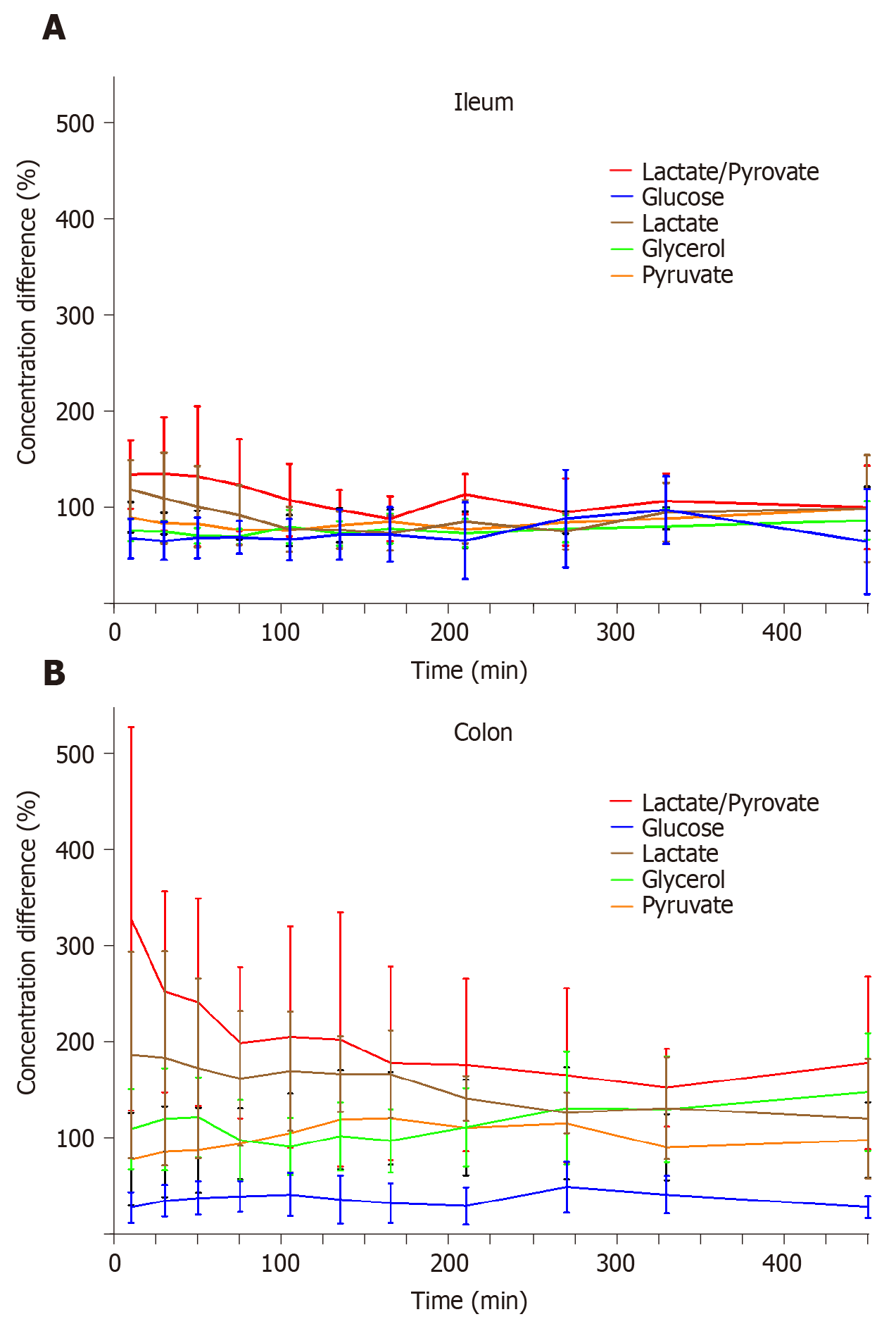
The mean concentration differences (%) for glucose, lactate, glycerol, pyruvate, and lactate/pyruvate ratios between both anastomosis and non-anastomosis (anastomosis/non-anastomosis) ileum and colon are depicted in Figure 2 and Table 1. For the colon, the lactate/pyruvate ratio between anastomosis and non-anastomosis was increased in the first 75 min after placement of the microdialysis catheters, which was primarily driven by increased lactate concentrations. The lactate/pyruvate ratio then normalized. No differences were observed for the lactate/pyruvate ratio between anastomosis and non-anastomosis ileum. The glucose ratio between anastomosis and non-anastomosis colon was decreased throughout the 8 h sampling interval with a mean ratio range of 28%-49%. For the ileum, the glucose ratio (anastomosis/non-anastomosis) was only decreased in the first 135 min after placement of the microdialysis catheters and then normalized. While glycerol concentrations were similar in anastomosis and non-anastomosis colon, decreased glycerol concentrations were found in anastomosis compared to non-anastomosis ileum. The mean concentration of the ischemic metabolites for both non-anastomosis and anastomosis ileum and colon are shown in Table 2 and 3.
| Glucose | Lactate | Glycerol | Pyruvate | Lactate/pyruvate | ||||||
| Time | Ileum (%) | Colon (%) | Ileum (%) | Colon (%) | Ileum (%) | Colon (%) | Ileum (%) | Colon (%) | Ileum (%) | Colon (%) |
| 10 | 68 (47; 89) | 28 (13; 44) | 119 (88; 149) | 186 (79; 293) | 77 (65; 88) | 110 (68; 151) | 90 (74; 106) | 78 (30; 126) | 134 (99; 170) | 327 (129; 526) |
| 30 | 66 (46; 85) | 35 (19; 51) | 110 (63;157) | 183 (72; 294) | 75 (64; 87) | 120 (67; 173) | 84 (73; 95) | 86 (39; 133) | 135 (76; 194) | 252 (148; 356) |
| 50 | 69 (48; 90) | 38 (21; 55) | 101 (59; 143) | 173 (79; 266) | 71 (63; 79) | 122 (80; 163) | 83 (69; 97) | 88 (44; 132) | 133 (60; 205) | 241 (133; 348) |
| 75 | 69 (52; 86) | 40 (24; 56) | 92 (61; 123) | 162 (92; 232) | 70 (62; 78) | 98 (56; 140) | 77 (67; 87) | 94 (58; 131) | 123 (76; 171) | 199 (120; 277) |
| 105 | 67 (46; 89) | 42 (19; 64) | 78 (54; 101) | 170 (108; 232) | 80 (63; 98) | 91 (62; 121) | 77 (61; 93) | 105 (65; 146) | 108 (71; 145) | 205 (90; 320) |
| 135 | 72 (46; 98) | 37 (12; 61) | 77 (58; 96) | 167 (127; 206) | 73 (61; 86) | 102 (67; 137) | 82 (64; 100) | 119 (68; 170) | 98 (77; 118) | 202 (71; 334) |
| 165 | 72 (44; 101) | 33 (12; 53) | 74 (56; 92) | 166 (121; 212) | 78 (63; 94) | 98 (65; 130) | 85 (73; 98) | 121 (73; 168) | 89 (66; 112) | 178 (78; 278) |
| 210 | 66 (26; 106) | 30 (11; 49) | 86 (63; 108) | 141 (118; 165) | 74 (59; 88) | 111 (71; 152) | 78 (59; 96) | 111 (61; 160) | 114 (93; 135) | 176 (86; 266) |
| 270 | 89 (39; 139) | 49 (23; 76) | 75 (57; 94) | 126 (105; 147) | 78 (64; 92) | 131 (73; 189) | 85 (73; 96) | 115 (57; 173) | 96 (61; 130) | 165 (75; 255) |
| 330 | 98 (63; 133) | 42 (22; 61) | 95 (65; 126) | 131 (79; 184) | 81 (63; 99) | 130 (75; 185) | 89 (78; 100) | 90 (56; 125) | 107 (79; 135) | 152 (112; 193) |
| 450 | 65 (10; 119) | 29 (17; 40) | 99 (44; 155) | 120 (58; 182) | 87 (67; 107) | 148 (87; 209) | 99 (76; 122) | 98 (59; 137) | 100 (57; 144) | 178 (89; 268) |
| Glucose | Lactate | Glycerol | Pyruvate | Lactate/pyruvate | ||||||
| Time | Ileum | Ileum anastomosis | Ileum | Ileum anastomosis | Ileum | Ileum anastomosis | Ileum | Ileum anastomosis | Ileum | Ileum anastomosis |
| 10 | 1.63 (1.11; 2.15) | 1.03 (0.70; 1.35) | 0.86 (0.65; 1.06) | 0.99 (0.68; 1.31) | 62.29 (52.23; 72.34) | 47.00 (38.06; 55.94) | 65.00 (57.58; 72.42) | 57.86 (47.66; 68.05) | 13.00 (10.90; 15.11) | 17.94 (10.50; 25.37) |
| 30 | 1.45 (0.99; 1.90) | 0.85 (0.65; 1.05) | 0.82 (0.61; 1.03) | 0.87 (0.52; 1.22) | 63.29 (56.37; 70.20) | 47.00 (40.42; 53.58) | 62.00 (54.03; 69.97) | 51.14 (44.50; 57.79) | 13.20 (10.43; 15.96) | 17.05 (9.83; 24.28) |
| 50 | 1.41 (0.85; 1.96) | 0.83 (0.64; 1.03) | 0.81 (0.61; 1.01) | 0.79 (0.50; 1.08) | 63.57 (55.13; 72.02) | 44.86 (37.85; 51.86) | 60.29 (50.19; 70.38) | 48.43 (42.28; 54.58) | 13.52 (11.36; 15.69) | 16.68 (9.45; 23.93) |
| 75 | 1.28 (0.78; 1.79) | 0.80 (0.64; 0.96) | 0.84 (0.70; 0.99) | 0.77 (0.51; 1.03) | 59.29 (52.26; 66.31) | 41.14 (35.48; 46.81) | 57.14 (49.82; 64.45) | 43.43 (37.29; 49.57) | 14.71 (13.25; 16.18) | 17.55 (12.25; 22.85) |
| 105 | 1.01 (0.82; 1.21) | 0.64 (0.47; 0.81) | 0.97 (0.71; 1.23) | 0.71 (0.51; 0.91) | 57.71 (49.13; 66.30) | 45.57 (34.14; 57.00) | 57.43 (47.06; 67.80) | 43.14 (34.92; 51.36) | 16.87 (13.03; 20.71) | 17.12 (12.20; 22.04) |
| 135 | 0.82 (0.69; 0.96) | 0.58 (0.37; 0.79) | 1.06 (0.74; 1.37) | 0.77 (0.56; 0.98) | 63.00 (53.30; 72.70) | 46.14 (34.93; 57.36) | 58.29 (44.04; 72.53) | 45.86 (35.97; 55.74) | 17.99 (15.12; 20.86) | 16.98 (13.88; 20.08) |
| 165 | 0.96 (0.62; 1.30) | 0.69 (0.38; 1.00) | 1.09 (0.82; 1.36) | 0.78 (0.57; 0.99) | 64.42 (51.05; 77.81) | 48.29 (38.94; 57.63) | 57.43 (45.68; 69.18) | 47.86 (39.49; 56.22) | 19.31 (15.34; 23.28) | 16.14 (12.64; 19.64) |
| 210 | 0.98 (0.55; 1.41) | 0.55 (0.23; 0.87) | 1.13 (0.83; 1.42) | 0.93 (0.67; 1.18) | 71.00 (48.40; 93.60) | 51.86 (33.18; 70.54) | 62.57 (51.12; 74.02) | 47.71 (36.57; 58.86) | 18.04 (14.11; 21.97) | 19.54 (16.66; 22.42) |
| 270 | 0.88 (0.34; 1.43) | 0.59 (0.25; 0.92) | 1.41 (0.95; 1.88) | 0.99 (0.71; 1.23) | 79.29 (51.38; 107.19) | 57.86 (42.24; 73.47) | 69.86 (57.67; 82.04) | 58.14 (48.69; 67.60) | 20.24 (14.07; 26.40) | 17.12 (13.21; 21.03) |
| 330 | 1.43 (0.34; 2.52) | 0.96 (0.39; 1.53) | 1.44 (0.95; 1.93) | 1.27 (0.82; 1.72) | 77.57 (54.66; 100.48) | 58.14 (46.42; 69.87) | 74.71 (57.03; 92.13) | 65.86 (49.75; 81.96) | 19.68 (13.31; 26.05) | 18.58 (15.32; 21.83) |
| 450 | 0.67 (0.41; 0.93) | 0.52 (0.22; 0.82) | 1.36 (1.02; 1.69) | 1.14 (0.73; 1.55) | 67.14 (48.62; 85.67) | 55.14 (43.26; 67.03) | 63.67 (50.17; 77.17) | 58.86 (45.97; 71.74) | 21.83 (16.66; 27.01) | 18.74 (15.13; 22.35) |
| Glucose | Lactate | Glycerol | Pyruvate | Lactate/pyruvate | ||||||
| Time | Colon | Colon anastomosis | Colon | Colon anastomosis | Colon | Colon anastomosis | Colon | Colon anastomosis | Colon | Colon anastomosis |
| 10 | 2.63 (1.06; 4.21) | 0.58 (0.23; 0.93) | 1.65 (1.36; 1.94) | 3.22 (1.21; 5.23) | 99.86 (72.69; 127.02) | 118.00 (55.70; 180.30) | 115.71(79.45; 151.98) | 85.83 (31.36; 140.31) | 16.02 (11.05; 21.00) | 61.18 (11.78; 110.57) |
| 30 | 2.21 (1.15; 3.27) | 0.62 (0.19; 1.05) | 1.60 (1.38; 1.82) | 2.85 (0.98; 4.72) | 96.29 (70.78; 121.79) | 114.50 (56.00; 173.00) | 104.29 (81.34; 126.83) | 86.83 (40.02; 133.64) | 16.56 (12.16; 20.95) | 43.94 (18.28; 69.59) |
| 50 | 2.13 (1.12; 3.14) | 0.75 (0.07; 1.42) | 1.48 (1.21; 1.74) | 2.43 (0.95; 3.91) | 91.29 (65.75; 116.82) | 110.50 (55.85; 165.15) | 91.57 (75.05; 108.10) | 78.00 (37.62; 118.38) | 17.21 (11.91; 22.52) | 44.01 (14.62; 73.41) |
| 75 | 2.09 (1.02; 3.16) | 0.92 (-0.01; 1.86) | 1.36 (1.07; 1.66) | 2.07 (1.01; 3.13) | 89.00 (69.11; 108.89) | 92.33 (42.55; 142.11) | 81.71 (70.21; 93.22) | 76.33 (41.95; 110.72) | 17.14 (12.41; 21.88) | 35.87 (13.91; 57.84) |
| 105 | 1.73 (1.14; 2.31) | 0.77 (0.08; 1.45) | 1.29 (1.06; 1.51) | 2.06 (1.23; 2.88) | 93.71 (69.64; 117.79) | 91.17 (47.73; 134.60) | 75.00 (62.82; 87.18) | 76.17 (43.34; 109.00) | 17.96 (13.26; 22.67) | 37.02 (14.28; 59.75) |
| 135 | 1.55 (1.10; 1.99) | 0.65 (0.12; 1.18) | 1.35 (1.08; 1.62) | 2.14 (1.46; 2.81) | 99.00 (68.25; 129.75) | 99.17 (54.54; 143.80) | 71.86 (57.66; 86.06) | 81.17 (41.82; 120.50) | 20.51 (13.46; 27.55) | 40.10 (12.97; 67.24) |
| 165 | 1.54 (1.04; 2.05) | 0.53 (0.22; 0.84) | 1.52 (1.21; 1.84) | 2.40 (1.47; 3.32) | 95.00 (65.60; 124.40) | 94.67 (51.95; 137.39) | 75.14 (57.85; 92.44) | 84.33 (47.06; 121.61) | 22.10 (14.74; 29.46) | 39.03 (15.89; 62.17) |
| 210 | 1.53 (0.65; 2.41) | 0.43 (0.15; 0.70) | 1.89 (1.50; 2.29) | 2.46 (1.91; 3.00) | 103.43 (64.19;142.67) | 103.83 (62.12; 145.55) | 88.00 (63.70; 112.30) | 87.50 (43.41; 131.59) | 23.57 (16.49; 30.65) | 41.06 (18.57; 63.54) |
| 270 | 1.00 (0.56; 1.43) | 0.48 (0.09; 0.88) | 2.07 (1.75; 2.39) | 2.51 (1.97; 3.06) | 100.00 (66.94; 133.06) | 114.50 (69.08; 159.92) | 94.86 (60.15; 129.57) | 88.67 (45.00; 132.34) | 21.90 (16.58; 27.21) | 41.39 (17.54; 65.25) |
| 330 | 1.51 (0.35; 2.68) | 0.70 (-0.36; 1.75) | 2.59 (1.92; 3.27) | 2.90 (1.85; 3.96) | 94.57 (74.34; 114.80) | 109.83 (69.68; 149.98) | 104.43 (70.89; 137.97) | 82.5 (49.76; 115.24) | 22.34 (15.05; 29.63) | 39.43 (23.56; 55.30) |
| 450 | 0.62 (0.28; 0.96) | 0.15 (0.11; 0.20) | 2.49 (2.19; 2.78) | 2.89 (1.54; 4.23) | 80.43 (59.32; 101.53) | 110.67 (63.49; 157.84) | 94.00 (52.20; 135.80) | 75.17 (43.75; 106.59) | 22.44 (18.23; 26.65) | 40.55 (28.83; 52.27) |
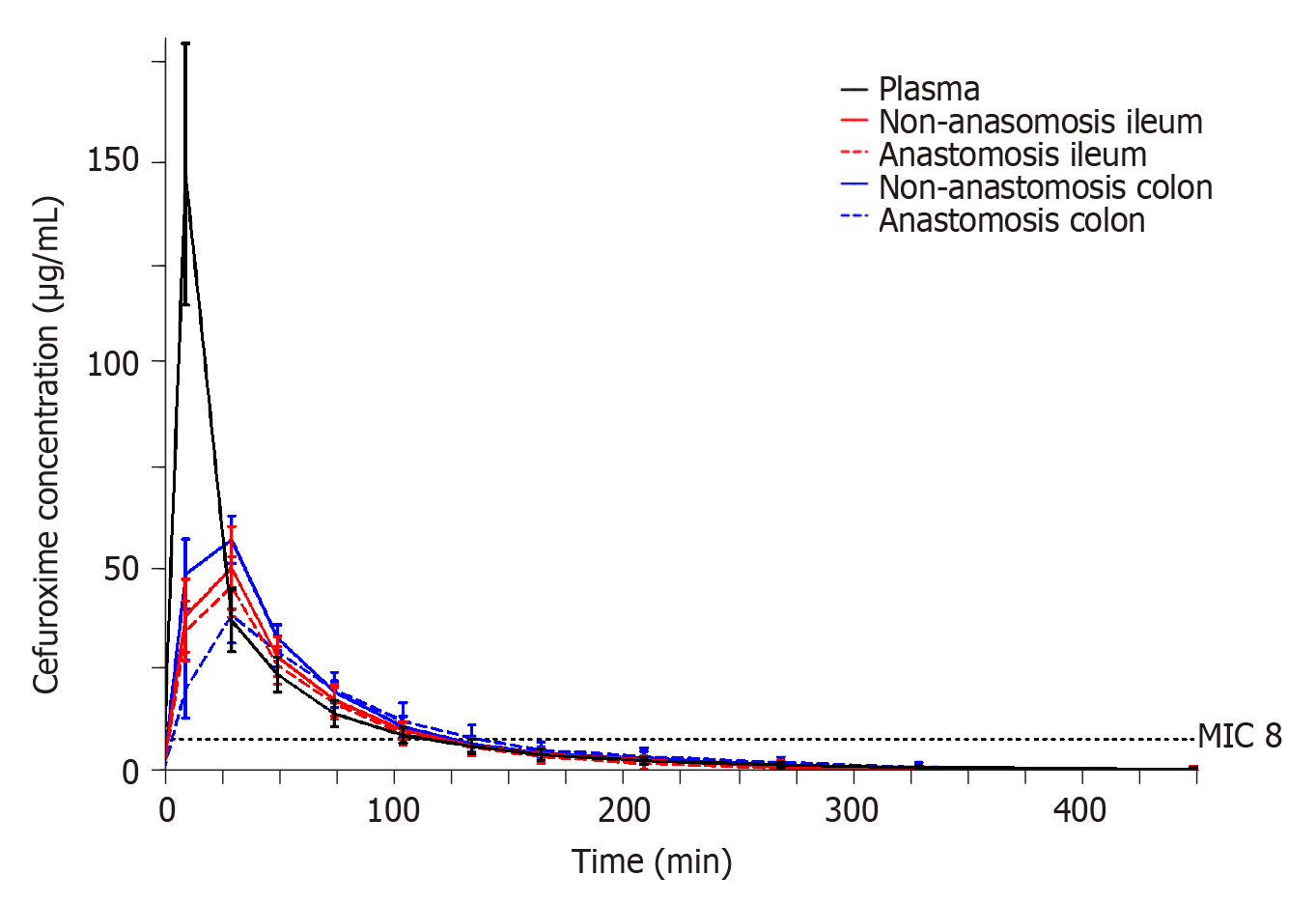
The T>MIC (8 µg/mL) results for each compartment are shown in Table 4. The mean T>MIC (8 µg/mL) ranged between 116-128 min across all investigated compartments. A similar T>MIC (8 µg/mL) was found between both anastomosis and non-anastomosis ileum and colon (P > 0.6). For all pigs and in all intestine compartments, a cefuroxime concentration of 8 µg/mL was reached within 10 min after administration.
| Compartment | Non-anastomosis | Anastomosis | P values |
| Plasma | 116 (96; 135) | - | - |
| Ileum | 120 (101; 140) | 116 (97; 136) | 0.61 |
| Colon | 126 (106; 145) | 128 (108; 148) | 0.77 |
The resulting pharmacokinetic parameters are shown in Table 5 and individual concentration time profiles are depicted in Figure 3. When comparing the pharmacokinetic parameters between the anastomosis and non-anastomosis sites for both ileum and colon, only colon Tmax and half-life differed between anastomosis and non-anastomosis (P < 0.03). Incomplete tissue penetrations were found in all tissues except for the non-anastomosis colon with a mean penetration of 0.90 (95% confidence interval 0.73; 1.06). When comparing plasma to the intestine compartments, plasma AUC and Cmax were higher and Tmax was shorter (P < 0.02). Only non-anastomosis colon AUC was similar to plasma AUC (P = 0.10).
| Compartment | Non-anastomosis | Anastomosis | P value |
| Plasma AUC (min μg/mL) | 4849 (4003; 5786)a | - | - |
| Ileum AUC (min μg/mL) | 3678 (2786; 4570) | 3327 (2436; 4219) | 0.28 |
| Colon AUC (min μg/mL) | 4219 (3327; 5110) | 3542 (2622; 4462) | 0.61 |
| Plasma Cmax (μg/mL) | 147 (131; 163)b | - | - |
| Ileum Cmax (μg/mL) | 51 (35; 66) | 46 (30; 62) | 0.65 |
| Colon Cmax (μg/mL) | 58 (42; 74) | 39 (22; 56) | 0.08 |
| Plasma Tmax (min) | 10 (5; 15)b | - | - |
| Ileum Tmax (min) | 28 (23; 32) | 28 (23; 32) | 1.00 |
| Colon Tmax (min) | 25 (20; 30) | 33 (27; 38) | 0.03 |
| Plasma T1/2 (min) | 58 (44; 73) | - | - |
| Ileum T1/2 (min) | 54 (39; 68) | 52 (37; 66) | 0.70 |
| Colon T1/2 (min) | 53 (38; 67) | 66 (52; 81) | 0.02 |
| Ileum AUCtissue/AUCplasma | 0.74 (0.57; 0.91) | 0.68 (0.50; 0.85) | 0.56 |
| Colon AUCtissue/AUCplasma | 0.90 (73; 1.07) | 0.72 (0.53; 0.90) | 0.12 |
This is the first study to investigate the influence of anastomoses on ileum and colon ischemic metabolites and cefuroxime concentrations in a simultaneous paired design. The main findings were increased lactate/pyruvate ratios in the colon anastomosis and similar T>MIC (8 µg/mL) for cefuroxime in all the investigated intestine compartments.
Microdialysis is a well-known sampling tool for the study of ischemic metabolites and have been applied in various abdominal relevant sites, e.g., intraperitoneal, mediastinal, intrahepatic, and in intestine walls[14-18]. A systemic review investigated whether intraperitoneal placed microdialysis could be used for early detection of colon and rectal anastomotic leakage[27]. The study concluded that increasing intraperitoneal lactate concentrations could be associated with anastomotic leakage, but with low predictive values[27]. No studies have previously investigated ischemic metabolites in anastomotic intestine tissue. The present study does not investigate the ischemic changes related to an anastomotic leakage, but only the ischemic conditions related to anastomoses of ileum and colon. Interestingly, our data suggest that colon anastomosis is more vulnerable to ischemia, depicted by an increased lactate/pyruvate ratio and decreased glucose concentrations. This may indirectly correlate with the inherent higher risk of colon anastomosis leakage than that of the small intestine[1-4]. Although these findings may not be surprising, it may lead to a better future understanding of anastomotic leakage.
Despite a predisposed risk of infections in gastrointestinal surgery, antimicrobial tissue concentrations in the intestines remain poorly investigated. For cefuroxime, it is generally recommended that the antimicrobial tissue concentrations exceed MIC values of relevant bacteria throughout surgery in order to be efficient in a prophylactic setting[8,11]. In gastrointestinal surgery, the most commonly encountered bacterium is Escherichia coli, which exhibits a clinical breakpoint MIC for cefuroxime of 8 µg/mL[25]. In the present study, cefuroxime concentrations of 8 µg/mL were reached within 10 min in all the investigated compartments and were maintained above 8 µg/mL for approximately 2 h. Thus, cefuroxime displayed prompt penetration into the intestines and similar elimination rates compared to that of plasma. This indicates that administering 1.5 g cefuroxime 10 min prior to surgery is sufficient, and that effective concentrations are sustained for approximately 2 h. For gastrointestinal procedures lasting longer than 2 h, and in cases with a need for postoperative concentrations above relevant MIC or to accommodate higher MIC targets, increasing or alternative dosing regimens, e.g., continuous infusion, should be considered.
There is an interesting discrepancy between the ischemic metabolite findings and cefuroxime concentrations. We found an increased vulnerability to ischemia in the colon anastomosis but almost identical pharmacokinetic cefuroxime endpoints in all intestine compartments. This may imply that cefuroxime penetration, to some extent, is independent of the local ischemic conditions. However, it is unknown whether a threshold exists, in which cefuroxime penetration decreases with increasing intestine ischemia. This calls for further investigation.
Surgery and sampling were performed on healthy juvenile pigs (aged 5 mo). Although pigs have been shown to parallel human physiology and anatomy to a large extent[28], more data are needed to firmly evaluate the translational potential of these findings. Infection and inflammation have previously been correlated with decreased antimicrobial tissue concentrations in other settings[9,10]. However, all pigs in the present study had a presumed good intestinal blood supply without any influence of fibrotic or inflamed intestine tissue. Therefore, future studies assessing the effect of influenced blood flow, inflammation, fibrosis, atherosclerosis etc. on the ischemic metabolites and antimicrobial concentrations in larger animal studies are warranted. Finally, we investigated the ischemic and cefuroxime properties in relation to a sutured anastomosis. The use of stapled anastomoses has increased over the past years and results from the present study cannot directly be extrapolated to stapled anastomoses.
In conclusion, we found that only colon anastomosis induced increased lactate/pyruvate ratios and decreased glucose concentrations, suggesting that colon anastomoses are more vulnerable to ischemia. Moreover, we found a similar T>MIC (8 µg/mL) in all the investigated compartments. Sufficient cefuroxime intestine concentrations were reached within 10 min after administration and were maintained for approximately 2 h.
Anastomotic leakage is a serious complication following gastrointestinal surgery and is associated with increased morbidity and mortality. The etiology of anastomotic leakage is multifactorial, and to some extent, is not fully understood.
Previous studies have suggested that the etiology is due to three main factors: healing disturbances, communication between intra- and extra-luminal compartments, and infection. However, no studies have previously investigated ischemic metabolites in anastomotic intestine tissue and the intestine antimicrobial concentrations.
To evaluate ischemic metabolites and cefuroxime concentrations in both anastomosis and non-anastomosis ileum and colon in a porcine model.
Eight healthy female pigs were included. Microdialysis catheters were placed for sampling ischemic metabolites and cefuroxime concentrations in both anastomosis and non-anastomosis ileum and colon. Cefuroxime 1.5 g was administered as an intravenous infusion over 15 min.
Only the colon anastomosis induced mean ischemic lactate/pyruvate ratios above 25 (ischemic cut-off) throughout the entire sampling interval, and simultaneously decreased glucose concentrations. The mean time for which cefuroxime concentrations were maintained above the clinical breakpoint minimal inhibitory concentration for Escherichia coli (8 µg/mL) ranged between 116-128 min across all the investigated compartments, and was similar between the anastomosis and non-anastomosis ileum and colon. For all pigs and in all the investigated compartments, a cefuroxime concentration of 8 µg/mL was reached within 10 min after administration.
Administering 1.5 g cefuroxime 10 min prior to intestine surgery seems sufficient, and effective concentrations are sustained for approximately 2 h. Only colon anastomosis was locally vulnerable to ischemia.
The present study demonstrates that microdialysis can be used to investigate ischemic metabolites and cefuroxime concentrations in both anastomosis and non-anastomosis intestines. This method may therefore have the potential to result in a better future understanding of anastomotic leakage.
We would like to thank the Department of Orthopaedic Surgery, Horsens Regional Hospital, and the Orthopaedic Research Unit, Aarhus University Hospital for supporting this study. Finally, we would like to thank Baatrup A for helping with the chemical analyses.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Denmark
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cimen SG S-Editor: Gao CC L-Editor: Webster JR P-Editor: Wang LYT
| 1. | Snijders HS, Wouters MW, van Leersum NJ, Kolfschoten NE, Henneman D, de Vries AC, Tollenaar RA, Bonsing BA. Meta-analysis of the risk for anastomotic leakage, the postoperative mortality caused by leakage in relation to the overall postoperative mortality. Eur J Surg Oncol. 2012;38:1013-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 2. | Hyman N, Manchester TL, Osler T, Burns B, Cataldo PA. Anastomotic leaks after intestinal anastomosis: it's later than you think. Ann Surg. 2007;245:254-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 454] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 3. | Sparreboom CL, Wu ZQ, Ji JF, Lange JF. Integrated approach to colorectal anastomotic leakage: Communication, infection and healing disturbances. World J Gastroenterol. 2016;22:7226-7235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 4. | Zafar SN, Khan MR, Raza R, Khan MN, Kasi M, Rafiq A, Jamy OH. Early complications after biliary enteric anastomosis for benign diseases: a retrospective analysis. BMC Surg. 2011;11:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Meyer J, Naiken S, Christou N, Liot E, Toso C, Buchs NC, Ris F. Reducing anastomotic leak in colorectal surgery: The old dogmas and the new challenges. World J Gastroenterol. 2019;25:5017-5025. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 78] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (3)] |
| 6. | Sciuto A, Merola G, De Palma GD, Sodo M, Pirozzi F, Bracale UM, Bracale U. Predictive factors for anastomotic leakage after laparoscopic colorectal surgery. World J Gastroenterol. 2018;24:2247-2260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 263] [Cited by in RCA: 224] [Article Influence: 32.0] [Reference Citation Analysis (2)] |
| 7. | Oikonomakis I, Jansson D, Hörer TM, Skoog P, Nilsson KF, Jansson K. Results of postoperative microdialysis intraperitoneal and at the anastomosis in patients developing anastomotic leakage after rectal cancer surgery. Scand J Gastroenterol. 2019;54:1261-1268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Uçkay I, Harbarth S, Peter R, Lew D, Hoffmeyer P, Pittet D. Preventing surgical site infections. Expert Rev Anti Infect Ther. 2010;8:657-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 75] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 9. | Bue M, Hanberg P, Koch J, Jensen LK, Lundorff M, Aalbaek B, Jensen HE, Søballe K, Tøttrup M. Single-dose bone pharmacokinetics of vancomycin in a porcine implant-associated osteomyelitis model. J Orthop Res. 2018;36:1093-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Tøttrup M, Bue M, Koch J, Jensen LK, Hanberg P, Aalbæk B, Fuursted K, Jensen HE, Søballe K. Effects of Implant-Associated Osteomyelitis on Cefuroxime Bone Pharmacokinetics: Assessment in a Porcine Model. J Bone Joint Surg Am. 2016;98:363-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for Prevention of Surgical Site Infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control. 1999;27:97-132; quiz 133. [PubMed] |
| 12. | Bue M, Hanberg P, Thomassen MB, Tøttrup M, Thillemann TM, Søballe K, Birke-Sørensen H. Microdialysis for the Assessment of Intervertebral Disc and Vertebral Cancellous Bone Metabolism in a Large Porcine Model. In Vivo. 2020;34:527-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Hanberg P, Bue M, Öbrink-Hansen K, Kabel J, Thomassen M, Tøttrup M, Søballe K, Stilling M. Simultaneous Retrodialysis by Drug for Cefuroxime Using Meropenem as an Internal Standard-A Microdialysis Validation Study. J Pharm Sci. 2020;109:1373-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Emmertsen KJ, Wara P, Soerensen FB, Stolle LB. Intestinal microdialysis--applicability, reproducibility and local tissue response in a pig model. Scand J Surg. 2005;94:246-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Sommer T, Larsen JF. Intraperitoneal and intraluminal microdialysis in the detection of experimental regional intestinal ischaemia. Br J Surg. 2004;91:855-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Ansorge C, Regner S, Segersvärd R, Strömmer L. Early intraperitoneal metabolic changes and protease activation as indicators of pancreatic fistula after pancreaticoduodenectomy. Br J Surg. 2012;99:104-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 17. | Ellebæk M, Qvist N, Fristrup C, Mortensen MB. Mediastinal microdialysis in the diagnosis of early anastomotic leakage after resection for cancer of the esophagus and gastroesophageal junction. Am J Surg. 2014;208:397-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Nowak G, Ungerstedt J, Wernerman J, Ungerstedt U, Ericzon BG. Clinical experience in continuous graft monitoring with microdialysis early after liver transplantation. Br J Surg. 2002;89:1169-1175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 57] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Tøttrup M, Søballe K, Bibby BM, Hardlei TF, Hansen P, Fuursted K, Birke-Sørensen H, Bue M. Bone, subcutaneous tissue and plasma pharmacokinetics of cefuroxime in total knee replacement patients - a randomized controlled trial comparing continuous and short-term infusion. APMIS. 2019;127:779-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Hanberg P, Bue M, Öbrink-Hansen K, Thomassen M, Søballe K, Stilling M. Timing of Antimicrobial Prophylaxis and Tourniquet Inflation: A Randomized Controlled Microdialysis Study. J Bone Joint Surg Am. 2020;102:1857-1864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Korth U, Merkel G, Fernandez FF, Jandewerth O, Dogan G, Koch T, van Ackern K, Weichel O, Klein J. Tourniquet-induced changes of energy metabolism in human skeletal muscle monitored by microdialysis. Anesthesiology. 2000;93:1407-1412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Ungerstedt U, Rostami E. Microdialysis in neurointensive care. Curr Pharm Des. 2004;10:2145-2152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 107] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 23. | Kho CM, Enche Ab Rahim SK, Ahmad ZA, Abdullah NS. A Review on Microdialysis Calibration Methods: the Theory and Current Related Efforts. Mol Neurobiol. 2017;54:3506-3527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 24. | Chaurasia CS, Müller M, Bashaw ED, Benfeldt E, Bolinder J, Bullock R, Bungay PM, DeLange EC, Derendorf H, Elmquist WF, Hammarlund-Udenaes M, Joukhadar C, Kellogg DL Jr, Lunte CE, Nordstrom CH, Rollema H, Sawchuk RJ, Cheung BW, Shah VP, Stahle L, Ungerstedt U, Welty DF, Yeo H. AAPS-FDA workshop white paper: microdialysis principles, application and regulatory perspectives. Pharm Res. 2007;24:1014-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 180] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 25. | European Society of Clinical Microbiology and Infectious Diseases. Available from: https://mic.eucast.org/Eucast2/SearchController/search.jsp?action=performSearch&BeginIndex=0&Micdif=mic&NumberIndex=50&Antib=46&Specium=-1,2020. |
| 26. | Hanberg P, Öbrink-Hansen K, Thorsted A, Bue M, Tøttrup M, Friberg LE, Hardlei TF, Søballe K, Gjedsted J. Population Pharmacokinetics of Meropenem in Plasma and Subcutis from Patients on Extracorporeal Membrane Oxygenation Treatment. Antimicrob Agents Chemother. 2018;62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 27. | Ellebæk MB, Daams F, Jansson K, Matthiessen P, Cosse C, Fristrup C, Ellebæk SB, Sabroe JE, Qvist N. Peritoneal microdialysis as a tool for detecting anastomotic leakage in patients after left-side colon and rectal resection. A systematic review. Scand J Gastroenterol. 2018;53:1625-1632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Swindle MM, Makin A, Herron AJ, Clubb FJ Jr, Frazier KS. Swine as models in biomedical research and toxicology testing. Vet Pathol. 2012;49:344-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 816] [Cited by in RCA: 954] [Article Influence: 68.1] [Reference Citation Analysis (0)] |