Published online Jun 20, 2020. doi: 10.4291/wjgp.v11.i4.78
Peer-review started: January 10, 2020
First decision: January 28, 2020
Revised: March 27, 2020
Accepted: May 27, 2020
Article in press: May 27, 2020
Published online: June 20, 2020
Processing time: 158 Days and 8.2 Hours
Traditional serrated adenoma was first reported by Longacre and Fenoglio-Presier in 1990. Their initial study described main features of this lesion, but the consensus diagnostic criteria were not widely adopted until recently. Traditional serrated adenoma presents with grossly protuberant configuration and pinecone-like appearance upon endoscopy. Histologically, it is characterized by ectopic crypt formation, slit-like serration, eosinophilic cytoplasm and pencillate nuclei. Although much is now known about the morphology and molecular changes, the mechanisms underlying the morphological alterations are still not fully understood. Furthermore, the origin of traditional serrated adenoma is not completely known. We review recent studies of the traditional serrated adenoma and provide an overview on current understanding of this rare entity.
Core tip: This mini-review summarizes recent findings of traditional serrated adenoma. The origin of traditional serrated adenoma and its molecular pathogenesis are discussed in details.
- Citation: Gui H, Husson MA, Mannan R. Correlations of morphology and molecular alterations in traditional serrated adenoma. World J Gastrointest Pathophysiol 2020; 11(4): 78-83
- URL: https://www.wjgnet.com/2150-5330/full/v11/i4/78.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v11.i4.78
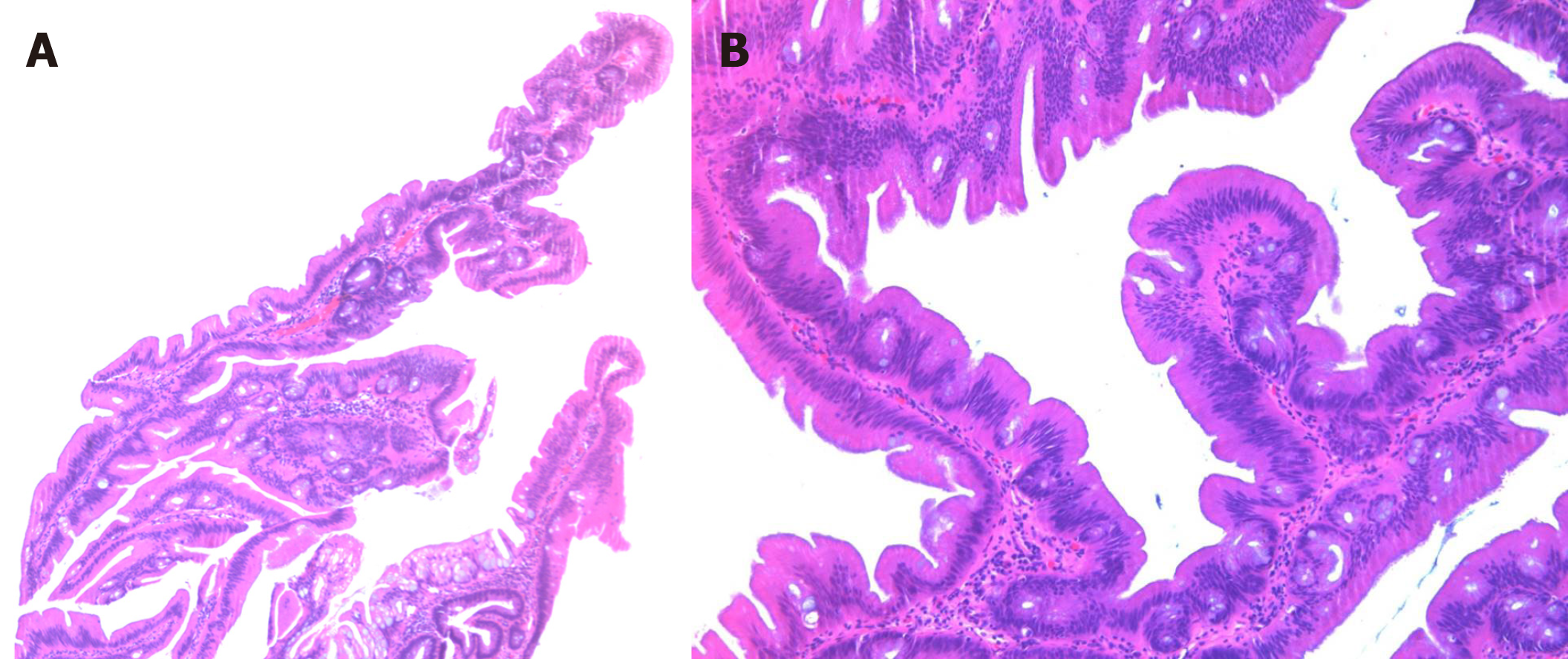
Colorectal carcinoma (CRC) is a heterogeneous disease in terms of its molecular pathways of carcinogenesis. Most, if not all CRCs, arise from conventional adenomas or serrated lesions, the latter accounting for 5%-35% of CRC[1,2]. Serrated polyps include hyperplastic polyps (HPs), sessile serrated lesions (SSLs), traditional serrated adenomas (TSAs) and unclassified serrated adenomas according to 2019 World Health Organization classification of colonic epithelial neoplasms[3]. Sessile serrated lesions and TSAs are regarded as precursors to CRC, while small HPs are considered to have little risk for neoplastic progression. TSAs comprise 0.56%-1.9% of colorectal polyps and are the least characterized serrated lesions in the colorectal carcinogenesis[4-6]. Endoscopically, TSAs show exophytic protuberant configuration and pinecone-like appearance[7,8]. Histologically, TSAs feature architectures of complex filiform or villiform growth pattern, slit-like or flat-top serration and ectopic crypt formation (ECF) which is defined as small rudimental crypts located on the side of villous structure and displaced from bottom muscularis mucosa. Cytologically, TSA is characterized by lining of epithelium with abundant eosinophilic cytoplasm, pseudostratified pencillate nuclei and dispersed chromatin (Figure 1)[9]. Although it is debatable which feature is the most sensitive and which one is the most specific, it is agreed that none of them alone is sufficient or required for diagnosing TSA. Fulfilling 2 out of 3 core features (ECF, eosinophilic cytoplasm and slit-like serration) may be more reproducible in making the diagnosis of TSA[10].
The molecular pathogenesis of TSA is poorly understood due to its rarity. Less is known about the mechanism that drives precursor lesions and their subsequent risk of progression. In this review, we will present the currently available literature, focusing on the origin of TSA. We will also attempt to correlate the molecular changes with morphologic features, which might help us understand how TSAs develop from precursor lesions or de novo.
TSAs are probably underdiagnosed by pathologists for several reasons. TSAs are the rarest among the three serrated colonic polyps, comprising of about 5% serrated polyps and 0.56%-1.9% of all colorectal polyps[4-6], and widely accepted consensus criteria for diagnosing TSA were not available until recently. Chetty[11] listed a constellation of architectural and cytological features of TSA in a succinct review of the entity. However, none of these features are unique or specific for TSA. The minimal criteria for diagnosing TSA are also not specified in many studies. Additionally, TSAs are often admixed with HP or SSL[8,12,13], causing difficulty in recognition. Three variants of TSA were described, the prototypical filiform TSA, the less common flat TSA[12] and the rare mucin-rich TSA[9].
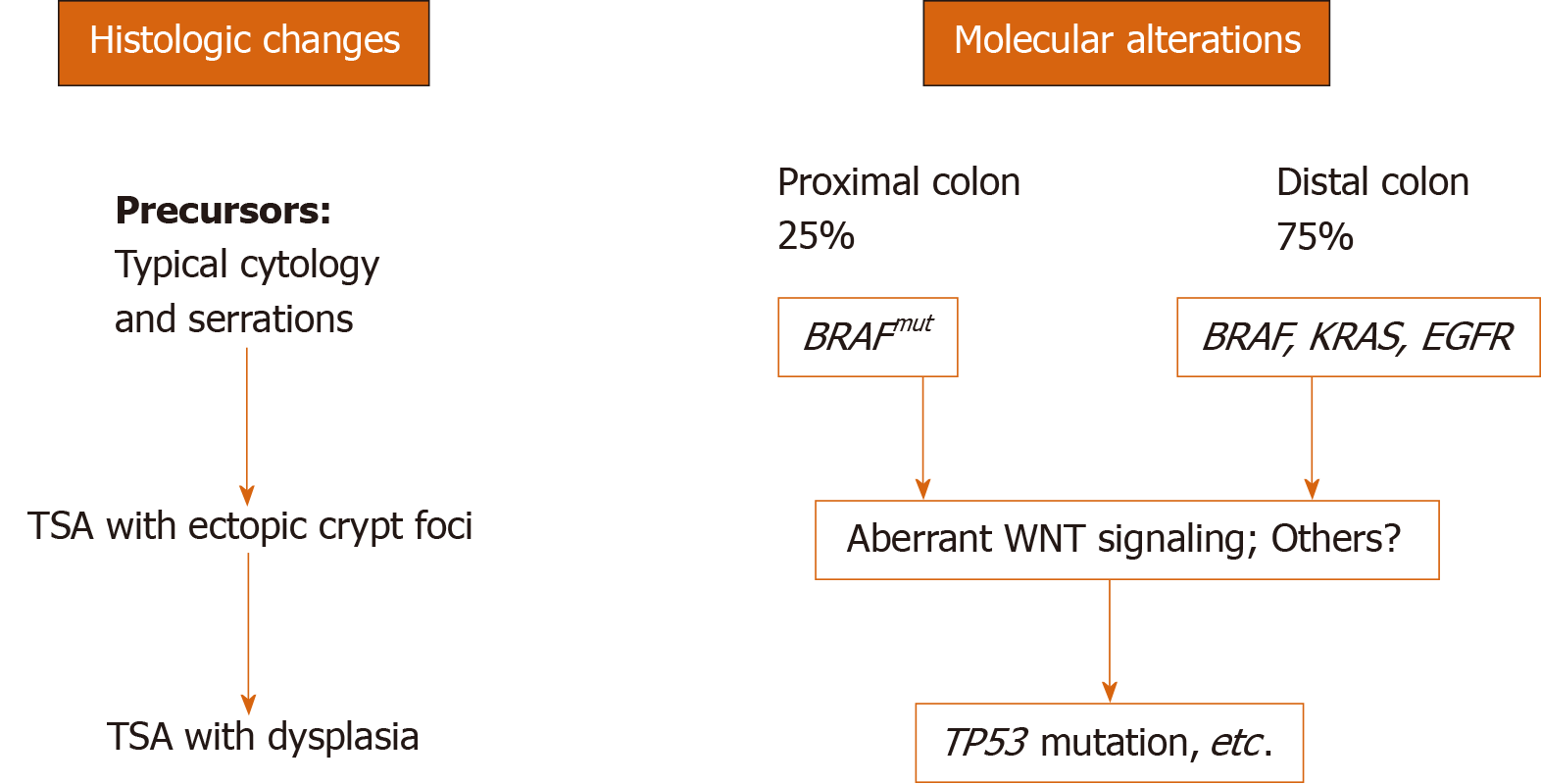
Genetic heterogeneity of TSAs contributes to the variation in cytomorphology. Almost 90% of TSAs develop through two mutually exclusive pathways: BRAF mutation (56.4%) and KRAS mutation (31.9%)[8,12-15] (Table 1). The remaining 10% may have other pathways involved such as EGFR (Figure 2)[16] that appears to segregate with KRAS-mutated polyps[12]. BRAF gene encodes an anti-apoptotic serine-threonine kinase. BRAF V600E activating mutation is an early event that drives serrated lesion into CRC[17,18]. TSAs with BRAF mutation often show a flat growth pattern with serrated dysplasia, high CpG island methylator phenotype (CIMP) and are more likely located in the proximal colon than KRAS-mutated TSA[8,12]. KRAS-mutated TSA are usually distally located and exophytic with adenomatous dysplasia. In addition to KRAS mutation, TSAs from distal colon show selective methylation of SMOC1 gene and loss of its expression, which are also frequently associated with high-grade adenoma and CIMP-low/microsatellite stable CRC[19].
| Country/Territory | Polyp | Distal | BRAF | KRAS | Wild type | ECF | Slit-like serration | Typical cytology | Ref. |
| United States | 24 | 96% (23) | 29% (7) | 46% (11) | 25% (6) | NA | NA | 79% (19) | [14] |
| South Korea | 107 | 74.8% (80) | 55.1% (59) | 33.6% (36) | 11.2% (12) | 79.4% (85) | 100% (107) | 100% (107) | [8] |
| Taiwan | 60 | 61.7% (37) | 35% (21) | 52% ( 31) | 13.3% (8) | NA | NA | NA | [13] |
| Australia | 200 | 71% (142) | 67% (134) | 22% (43) | 11% (23) | 89% (178) | 98% (196) | 100% (200) | [12] |
| Japan | 129 | 82.2% (106) | 61.2% (79) | 34.8% (45) | 3.9% (5) | NA | NA | NA | [15] |
| Australia | 70 | 71% (50) | 47% (33) | 31% (22) | 21% (15) | 67% (47) | 81% (57) | NA | [20] |
| Total | 590 | 74.2% (438) | 56.4% (333) | 31.9%(188) | 11.7% (69) | 82% (310/377) | 95% (360/377) | 98.5% (326/331) |
TSAs may arise from precursor lesions of microvesicular HP or SSL or may occur de novo. BRAF mutated TSAs are also more likely admixed or associated with HP or SSL-like lesions, which are identified in TSA in 38%-52.3% of cases[8,12,20]. One early study suggested that serrated precursor lesions adjacent to distal TSA are distinguished from SSL by lack of Annexin A10 despite shared morphologic and molecular features[21]. Annexin A10 is normally expressed in upper gastrointestinal tract[22]. It is identified as a marker of SSL[23] and is expressed in colorectal cancer of serrated pathway undergoing gastric programming[24]. Thus, it is not surprising that the serrated precursor lesions of TSA in this study, arising predominantly from distal colon, are distinctive from proximal colonic SSL[21]. It is more likely that small flat TSAs identified in proximal colon would be expressing Annexin A10. More recently, Bettington and colleagues compared small polyps (< 1 cm) (71% from the distal colon) and shoulder lesion in large TSAs, demonstrating similar immunophenotypic and molecular profiles[20]. These findings support that small TSAs do exist and may arise at least partially from some HP/SSL-like precursors.
WNT signaling is the main driver of colon cancer and physiological proliferation of colonic crypts[25]. Alterations in components of WNT pathway including mutations of RNF43, APC and CTNNB1, and overexpression of RSPO (due to fusion gene or amplification), can all lead to stabilization and nuclear localization of β-catenin and activation of WNT signaling[26]. Nuclear β-catenin staining, as well as p53 positivity, loss of p16 and MLH1 promoter methylation is seen in the late development of polyps with dysplastic features[12,13]. However some molecular studies showed that components of WNT signaling are frequently altered in TSAs (30%-70%) regardless of the degree of dysplasia[15,27]. A recent study using microdissection to interrogate genetic changes revealed a stepwise molecular change in TSAs and associated precursors[28]. Clonally, the HP/SSL-like precursors share the identical mitogen-activated protein kinase (MAPK) pathway gene mutations (BRAF or KRAS) with TSAs. However, these precursors exhibit fewer mutated WNT pathway genes or heterozygotic mutations (i.e., RNF43, APC, and CTNNB1) than TSA with biallelic inactivation. This study supports the sequence of MAPK to WNT alterations in TSA developing from HP and SSL-like lesions (Figure 2). One drawback of this study is that only one out of 15 polyps had KRAS mutation. Hence TSAs with KRAS mutation, which are predominantly found in the distal colon and typically are large in size, remain of uncertain in terms of origin and critical molecular alterations during development.
The presence of histologic features in TSAs is highly variable depending on their size and location. ECF is considered relatively more specific whereas slit-like serration and typical cytology are more sensitive features (Table 1). Serration is the common feature of HP, SSL and TSA. However, the cytomorphology of these three entities differs, reflecting distinct mechanisms underlying their development. BRAF or KRAS are two initiating mutations commonly seen in serrated polyps, activating MAPK pathway. HPs are characterized by saw-toothed serration in the upper half to third of the crypts and absence of basal crypt dilation[29,30]. Epithelial proliferation with defective apoptosis[5], delayed crypt cellular migration and maturation toward the surface leads to infolding of epithelial lining and formation of HPs. The majority of HPs are innocuous, largely because KRAS mutation in HP does not expand the stem cell pool but instead increases transit-amplifying cells in the mid and upper regions of crypts[31].
By contrast, SSLs have irregular proliferative zones and bidirectional maturation toward both surface and base of the crypt, causing pathognomonic basal crypt dilation and lateral spread of crypt base[32]. This architectural change was suggested to be similar to gastric foveolar growth pattern characterized by a mid-level proliferative compartment and bidirectional differentiation[11]. Another salient feature of SSLs is prominent inhibition of apoptosis in contrast to HPs and TSAs[33].
Compared to HP and SSL, TSAs have slit-like, flat-top serration rather than saw-toothed serration. Eosinophilic cells in TSA with luminal brush border and a prominent villiform growth pattern are the features reminiscent of small intestine morphology[9]. It was believed that eosinophilic cytoplasm seen in TSA is due to cellular senescence[32]. Senescence and apoptosis are two protecting approaches of cells and tissue in response to oncogenic stresses[34]. They are the barriers that must be overcome in precursor lesions to promote and progress into fully developed TSAs. In TSAs, depending on locations, BRAF or KRAS are the initiating mutations activating MAPK pathway. Both, however, may cause cellular senescence and cell cycle arrest through p53/p21 axis or p16INK4 activation[29,34]. SSL is also well known to have high rate of BRAF mutation[18]. Therefore, it is not uncommon to observe occasional eosinophilic atypical cells in SSLs[32]. Animal models supported that BRAF V600E mutation causes cellular senescence after first wave of proliferation[35] and a shift of balance from proliferation to differentiation, which can be rescued by a loss of additional differentiation-promoting factors (CDX2, SMAD4 and p16) or activation of WNT signaling[36]. In SSLs and TSAs located in proximal colon, hypermethylation of P16INK4 promoter and loss of p16 expression are the late events[12] that may cause evasion of senescence program implemented by BRAF mutation, whereas activation of WNT is likely the pathway employed for the progression of distally located TSAs.
ECF is a key feature of TSAs, especially in large protuberant ones in the distal colon. The presence of ECF in TSAs ranges from 67% to 89%, depending on the location and size of the polyps[8,12,20]. ECF is defined as small crypts displaced from muscularis mucosa, likely representing a progression step by disrupting the signalings of colonic crypt homeostasis. Bone morphogenetic protein (BMP4) signaling is probably a good candidate. BMP signaling plays an important role in villus morphogenesis and is known to promote cell differentiation and repress crypt formation[37,38]. Studies using human pluripotent stem cells demonstrated that BMP signaling is only transiently required for colonic differentiation, while small intestinal differentiation is the default program in the absence of BMP signaling[10]. Loss of BMP signaling in animal model[37] leads to ectopic crypt foci that resembles the phenotype of juvenile polyposis syndrome, which is known to harbor BMPR1A and SMAD4 mutations in human[39,40]. Therefore, it is possible that ECF and villiform growth of TSA represent dysplastic transformation of colonic crypts into small intestinal villous morphology. This morphology may arise owing to aberrant molecular pathways such as BMP signaling, which controls villus-crypt homeostasis of gastrointestinal tract. Along with early events of BRAF or KRAS mutation, additional molecular changes drive proliferation of intestinal epithelium and shape them into TSA with distinct cytomorphology. Further accumulations of aberration in p53 and WNT signaling lead to progression of TSA into prominent dysplasia and CRC.
TSAs are rare serrated polyps located predominantly in the distal colon. At least two pathways have been identified, converging on activation of MAPK by BRAF or KRAS mutations. Small HP/SSL-like lesions with BRAF mutations might initiate as TSA precursors. Whether it is the same case occurring in the small serrated lesion with KRAS mutation awaits further investigation. Because TSA-derived colorectal cancer is considered very aggressive[30], study of the TSA-carcinoma sequence, its progression from lack of dysplasia to high-grade atypia and malignancy, is also warranted in the future.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aoki H, Li WB S-Editor: Wang JL L-Editor:A E-Editor: Wu YXJ
| 1. | Bettington M, Walker N, Clouston A, Brown I, Leggett B, Whitehall V. The serrated pathway to colorectal carcinoma: current concepts and challenges. Histopathology. 2013;62:367-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 351] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 2. | Snover DC. Update on the serrated pathway to colorectal carcinoma. Hum Pathol. 2011;42:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 439] [Cited by in RCA: 478] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 3. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2441] [Article Influence: 488.2] [Reference Citation Analysis (3)] |
| 4. | Bettington M, Walker N, Rosty C, Brown I, Clouston A, Wockner L, Whitehall V, Leggett B. Critical appraisal of the diagnosis of the sessile serrated adenoma. Am J Surg Pathol. 2014;38:158-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 5. | Higuchi T, Sugihara K, Jass JR. Demographic and pathological characteristics of serrated polyps of colorectum. Histopathology. 2005;47:32-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 160] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 6. | Spring KJ, Zhao ZZ, Karamatic R, Walsh MD, Whitehall VL, Pike T, Simms LA, Young J, James M, Montgomery GW, Appleyard M, Hewett D, Togashi K, Jass JR, Leggett BA. High prevalence of sessile serrated adenomas with BRAF mutations: a prospective study of patients undergoing colonoscopy. Gastroenterology. 2006;131:1400-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 410] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 7. | Sweetser S, Smyrk TC, Sinicrope FA. Serrated colon polyps as precursors to colorectal cancer. Clin Gastroenterol Hepatol. 2013;11:760-767; quiz e54-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 85] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 8. | Kim MJ, Lee EJ, Suh JP, Chun SM, Jang SJ, Kim DS, Lee DH, Lee SH, Youk EG. Traditional serrated adenoma of the colorectum: clinicopathologic implications and endoscopic findings of the precursor lesions. Am J Clin Pathol. 2013;140:898-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 9. | Childs BG, Baker DJ, Kirkland JL, Campisi J, van Deursen JM. Senescence and apoptosis: dueling or complementary cell fates? EMBO Rep. 2014;15:1139-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 464] [Cited by in RCA: 652] [Article Influence: 59.3] [Reference Citation Analysis (0)] |
| 10. | Múnera JO, Sundaram N, Rankin SA, Hill D, Watson C, Mahe M, Vallance JE, Shroyer NF, Sinagoga KL, Zarzoso-Lacoste A, Hudson JR, Howell JC, Chatuvedi P, Spence JR, Shannon JM, Zorn AM, Helmrath MA, Wells JM. Differentiation of Human Pluripotent Stem Cells into Colonic Organoids via Transient Activation of BMP Signaling. Cell Stem Cell. 2017;21:51-64.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 193] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 11. | Chetty R. Traditional serrated adenoma (TSA): morphological questions, queries and quandaries. J Clin Pathol. 2016;69:6-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Bettington ML, Walker NI, Rosty C, Brown IS, Clouston AD, McKeone DM, Pearson SA, Klein K, Leggett BA, Whitehall VL. A clinicopathological and molecular analysis of 200 traditional serrated adenomas. Mod Pathol. 2015;28:414-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 135] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 13. | Tsai JH, Liau JY, Lin YL, Lin LI, Cheng YC, Cheng ML, Jeng YM. Traditional serrated adenoma has two pathways of neoplastic progression that are distinct from the sessile serrated pathway of colorectal carcinogenesis. Mod Pathol. 2014;27:1375-1385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 14. | Fu B, Yachida S, Morgan R, Zhong Y, Montgomery EA, Iacobuzio-Donahue CA. Clinicopathologic and genetic characterization of traditional serrated adenomas of the colon. Am J Clin Pathol. 2012;138:356-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 15. | Sekine S, Ogawa R, Hashimoto T, Motohiro K, Yoshida H, Taniguchi H, Saito Y, Yasuhiro O, Ochiai A, Hiraoka N. Comprehensive characterization of RSPO fusions in colorectal traditional serrated adenomas. Histopathology. 2017;71:601-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 16. | Bongers G, Muniz LR, Pacer ME, Iuga AC, Thirunarayanan N, Slinger E, Smit MJ, Reddy EP, Mayer L, Furtado GC, Harpaz N, Lira SA. A role for the epidermal growth factor receptor signaling in development of intestinal serrated polyps in mice and humans. Gastroenterology. 2012;143:730-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Barras D. BRAF Mutation in Colorectal Cancer: An Update. Biomark Cancer. 2015;7:9-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 18. | Yang HM, Mitchell JM, Sepulveda JL, Sepulveda AR. Molecular and histologic considerations in the assessment of serrated polyps. Arch Pathol Lab Med. 2015;139:730-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 19. | Aoki H, Yamamoto E, Takasawa A, Niinuma T, Yamano HO, Harada T, Matsushita HO, Yoshikawa K, Takagi R, Harada E, Tanaka Y, Yoshida Y, Aoyama T, Eizuka M, Yorozu A, Kitajima H, Kai M, Sawada N, Sugai T, Nakase H, Suzuki H. Epigenetic silencing of SMOC1 in traditional serrated adenoma and colorectal cancer. Oncotarget. 2018;9:4707-4721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Bettington M, Rosty C, Whitehall V, Leggett B, McKeone D, Pearson SA, Walker N. A morphological and molecular study of proposed early forms of traditional serrated adenoma. Histopathology. 2018;73:1023-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Wiland HO 4th, Shadrach B, Allende D, Carver P, Goldblum JR, Liu X, Patil DT, Rybicki LA, Pai RK. Morphologic and molecular characterization of traditional serrated adenomas of the distal colon and rectum. Am J Surg Pathol. 2014;38:1290-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 22. | Lu SH, Yuan RH, Chen YL, Hsu HC, Jeng YM. Annexin A10 is an immunohistochemical marker for adenocarcinoma of the upper gastrointestinal tract and pancreatobiliary system. Histopathology. 2013;63:640-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 23. | Gonzalo DH, Lai KK, Shadrach B, Goldblum JR, Bennett AE, Downs-Kelly E, Liu X, Henricks W, Patil DT, Carver P, Na J, Gopalan B, Rybicki L, Pai RK. Gene expression profiling of serrated polyps identifies annexin A10 as a marker of a sessile serrated adenoma/polyp. J Pathol. 2013;230:420-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 24. | Tsai JH, Lin YL, Cheng YC, Chen CC, Lin LI, Tseng LH, Cheng ML, Liau JY, Jeng YM. Aberrant expression of annexin A10 is closely related to gastric phenotype in serrated pathway to colorectal carcinoma. Mod Pathol. 2015;28:268-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 25. | Clevers H. The intestinal crypt, a prototype stem cell compartment. Cell. 2013;154:274-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 755] [Cited by in RCA: 893] [Article Influence: 74.4] [Reference Citation Analysis (0)] |
| 26. | Gehart H, Clevers H. Tales from the crypt: new insights into intestinal stem cells. Nat Rev Gastroenterol Hepatol. 2019;16:19-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 655] [Article Influence: 109.2] [Reference Citation Analysis (0)] |
| 27. | Sekine S, Yamashita S, Tanabe T, Hashimoto T, Yoshida H, Taniguchi H, Kojima M, Shinmura K, Saito Y, Hiraoka N, Ushijima T, Ochiai A. Frequent PTPRK-RSPO3 fusions and RNF43 mutations in colorectal traditional serrated adenoma. J Pathol. 2016;239:133-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 102] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 28. | Hashimoto T, Ogawa R, Yoshida H, Taniguchi H, Kojima M, Saito Y, Sekine S. Acquisition of WNT Pathway Gene Alterations Coincides With the Transition From Precursor Polyps to Traditional Serrated Adenomas. Am J Surg Pathol. 2019;43:132-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 29. | Bennecke M, Kriegl L, Bajbouj M, Retzlaff K, Robine S, Jung A, Arkan MC, Kirchner T, Greten FR. Ink4a/Arf and oncogene-induced senescence prevent tumor progression during alternative colorectal tumorigenesis. Cancer Cell. 2010;18:135-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 152] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 30. | Bettington ML, Chetty R. Traditional serrated adenoma: an update. Hum Pathol. 2015;46:933-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 31. | Feng Y, Bommer GT, Zhao J, Green M, Sands E, Zhai Y, Brown K, Burberry A, Cho KR, Fearon ER. Mutant KRAS promotes hyperplasia and alters differentiation in the colon epithelium but does not expand the presumptive stem cell pool. Gastroenterology. 2011;141:1003-1013.e1-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 103] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 32. | Torlakovic EE, Gomez JD, Driman DK, Parfitt JR, Wang C, Benerjee T, Snover DC. Sessile serrated adenoma (SSA) vs. traditional serrated adenoma (TSA). Am J Surg Pathol. 2008;32:21-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 232] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 33. | Cassese G, Amendola A, Maione F, Giglio MC, Pagano G, Milone M, Aprea G, Luglio G, De Palma GD. Serrated Lesions of the Colon-Rectum: A Focus on New Diagnostic Tools and Current Management. Gastroenterol Res Pract. 2019;2019:9179718. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 34. | Endo A, Koizumi H, Takahashi M, Tamura T, Tatsunami S, Watanabe Y, Takagi M. A significant imbalance in mitosis versus apoptosis accelerates the growth rate of sessile serrated adenoma/polyps. Virchows Arch. 2013;462:131-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 35. | Carragher LA, Snell KR, Giblett SM, Aldridge VS, Patel B, Cook SJ, Winton DJ, Marais R, Pritchard CA. V600EBraf induces gastrointestinal crypt senescence and promotes tumour progression through enhanced CpG methylation of p16INK4a. EMBO Mol Med. 2010;2:458-471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 117] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 36. | Tong K, Pellón-Cárdenas O, Sirihorachai VR, Warder BN, Kothari OA, Perekatt AO, Fokas EE, Fullem RL, Zhou A, Thackray JK, Tran H, Zhang L, Xing J, Verzi MP. Degree of Tissue Differentiation Dictates Susceptibility to BRAF-Driven Colorectal Cancer. Cell Rep. 2017;21:3833-3845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 37. | Haramis AP, Begthel H, van den Born M, van Es J, Jonkheer S, Offerhaus GJ, Clevers H. De novo crypt formation and juvenile polyposis on BMP inhibition in mouse intestine. Science. 2004;303:1684-1686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 563] [Cited by in RCA: 576] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 38. | He XC, Zhang J, Tong WG, Tawfik O, Ross J, Scoville DH, Tian Q, Zeng X, He X, Wiedemann LM, Mishina Y, Li L. BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt-beta-catenin signaling. Nat Genet. 2004;36:1117-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 805] [Cited by in RCA: 814] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 39. | Howe JR, Sayed MG, Ahmed AF, Ringold J, Larsen-Haidle J, Merg A, Mitros FA, Vaccaro CA, Petersen GM, Giardiello FM, Tinley ST, Aaltonen LA, Lynch HT. The prevalence of MADH4 and BMPR1A mutations in juvenile polyposis and absence of BMPR2, BMPR1B, and ACVR1 mutations. J Med Genet. 2004;41:484-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 183] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 40. | Woodford-Richens K, Bevan S, Churchman M, Dowling B, Jones D, Norbury CG, Hodgson SV, Desai D, Neale K, Phillips RK, Young J, Leggett B, Dunlop M, Rozen P, Eng C, Markie D, Rodriguez-Bigas MA, Sheridan E, Iwama T, Eccles D, Smith GT, Kim JC, Kim KM, Sampson JR, Evans G, Tejpar S, Bodmer WF, Tomlinson IP, Houlston RS. Analysis of genetic and phenotypic heterogeneity in juvenile polyposis. Gut. 2000;46:656-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 76] [Article Influence: 3.0] [Reference Citation Analysis (0)] |