Published online May 12, 2020. doi: 10.4291/wjgp.v11.i3.43
Peer-review started: December 31, 2019
First decision: February 19, 2020
Revised: March 5, 2020
Accepted: April 8, 2020
Article in press: April 8, 2020
Published online: May 12, 2020
Processing time: 132 Days and 19.8 Hours
The etiology and pathogenesis of inflammatory bowel disease (IBD), including ulcerative colitis and Crohn’s disease, are not fully understood so far. Therefore, IBD still remains incurable despite the fact that significant progress has been achieved in recent years in its treatment with innovative medicine. About 20 years ago, selective granulocyte and monocyte apheresis (GMA) was invented in Japan and later approved by the Japanese health authority for IBD treatment. From then on this technique was extensively used for IBD patients in Japan and later in Europe. Clinical trials from Japan and European countries have verified the effectiveness and safety of GMA therapy in patients with IBD. In 2013, GMA therapy was approved by China State Food and Drug Administration for therapeutic use for the Chinese IBD patients. However, GMA therapy has not been extensively used in China, although a few clinical studies also showed that it was effective in clinical and endoscopic induction of remission in Chinese IBD patients with a high safety profile. This article reviews past history, present clinical application as well as the future prospective of GMA therapy for patients with IBD.
Core tip: Conventional therapies for inflammatory bowel disease (IBD) patients including mesalazine, corticosteroids, and immunosuppressants have been used for decades with unsatisfactory outcomes due to ineffectiveness or side effects. Although the emerging biologic agents have revolutionized IBD treatment, severe opportunistic infections, primary or secondary loss of response, etc. are the major clinical concerns of clinicians and patients. In recent years, selective granulocyte and monocyte apheresis has been used in Japan, Europe, China and elsewhere for its advantages of satisfactory efficacy and high safety profile. Granulocyte and monocyte apheresis therapy is an important and promising therapeutic option for IBD patients.
- Citation: Chen XL, Mao JW, Wang YD. Selective granulocyte and monocyte apheresis in inflammatory bowel disease: Its past, present and future. World J Gastrointest Pathophysiol 2020; 11(3): 43-56
- URL: https://www.wjgnet.com/2150-5330/full/v11/i3/43.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v11.i3.43
Inflammatory bowel disease (IBD) includes two chronic relapsing and remitting diseases, i.e. ulcerative colitis (UC) and Crohn’s disease (CD). Millions of individuals worldwide are currently affected by this disease in terms of function and quality of life[1]. Although it is thought to be an immune disorder of the gastrointestinal tract in genetically susceptible individuals exposed to environmental risk factors[1-4], IBD is sometimes difficult to define due to either its history of unusual complexity or poor understanding of its etiology and pathogenesis. Therefore, IBD still remains incurable despite significant progress in recent years due to its treatment with innovative medicine. Clinical and experimental studies have indicated that the distressful flare-ups of IBD might be triggered by the impaired function of the intestinal barrier and a dysregulated immune response to the gut microbiota[5,6].
Treatment of IBD with aminosalicylates and corticosteroids has been used for decades with unsatisfactory outcomes due to either their poor effectiveness or side effects. In recent years, an array of emerging medical therapies including biologic agents, immune cell modulators, mucosal barrier enhancers and stem cells have revolutionized IBD treatment[7]. Nevertheless, therapies for IBD have been mostly empirical rather than based on understanding of the disease etiology. Furthermore, side effects of long-term medications are inevitable, even though medications are often initially effective in the majority of patients[8].
It is currently believed that an imbalance between pro-inflammatory and anti-inflammatory cytokines exist in patients with IBD, which is closely related to the onset and development of the disease[9,10]. Additionally, myeloid leukocytes, especially granulocytes and monocytes, have been shown to play an important role in the occurrence and development of IBD[11,12]. Based on this theory, selective granulocytes and monocytes apheresis (GMA) therapy targeting these cells and subsequently on inflammatory cytokines was invented and applied to IBD patients in Japan about 20 years ago. It has been generally accepted that GMA therapy is a non-pharmacological therapeutic option for IBD patients due to its good therapeutic effect and incomparable safety, especially when conventional therapies are ineffective[13,14]. This article reviews the past history, present application, and future prospect of GMA therapy for patients with IBD.
The term “apheresis” is from the Greek “apairesos” or Roman “aphairesis” meaning to remove or take away something by force or a withdrawal. Before modern medicine, it was a common way to treat diseases, e.g., by bloodletting. At that time, it was thought that the goal of treatment could be achieved by eliminating pathogenic factors from the blood[15]. The removal of plasma and return of red blood cells was first demonstrated in research animals at Johns Hopkins University in 1914. Since then, apheresis technology was gradually used in clinics, including centrifugation, plasmapheresis, plateletpheresis, photopheresis, etc. The technique of selective GMA originated from the separation of blood cells.
As early as the beginning of the 20th century, centrifugation was used to selectively remove blood components that were thought to activate or promote the occurrence of diseases to achieve a therapeutic goal through non-drug therapy. Separation of blood cells was first used for leukemia, tumors, rheumatoid arthritis and other diseases and later gradually applied to treat IBD[16]. However, centrifugation has certain disadvantages of being expensive to perform and difficult to handle.
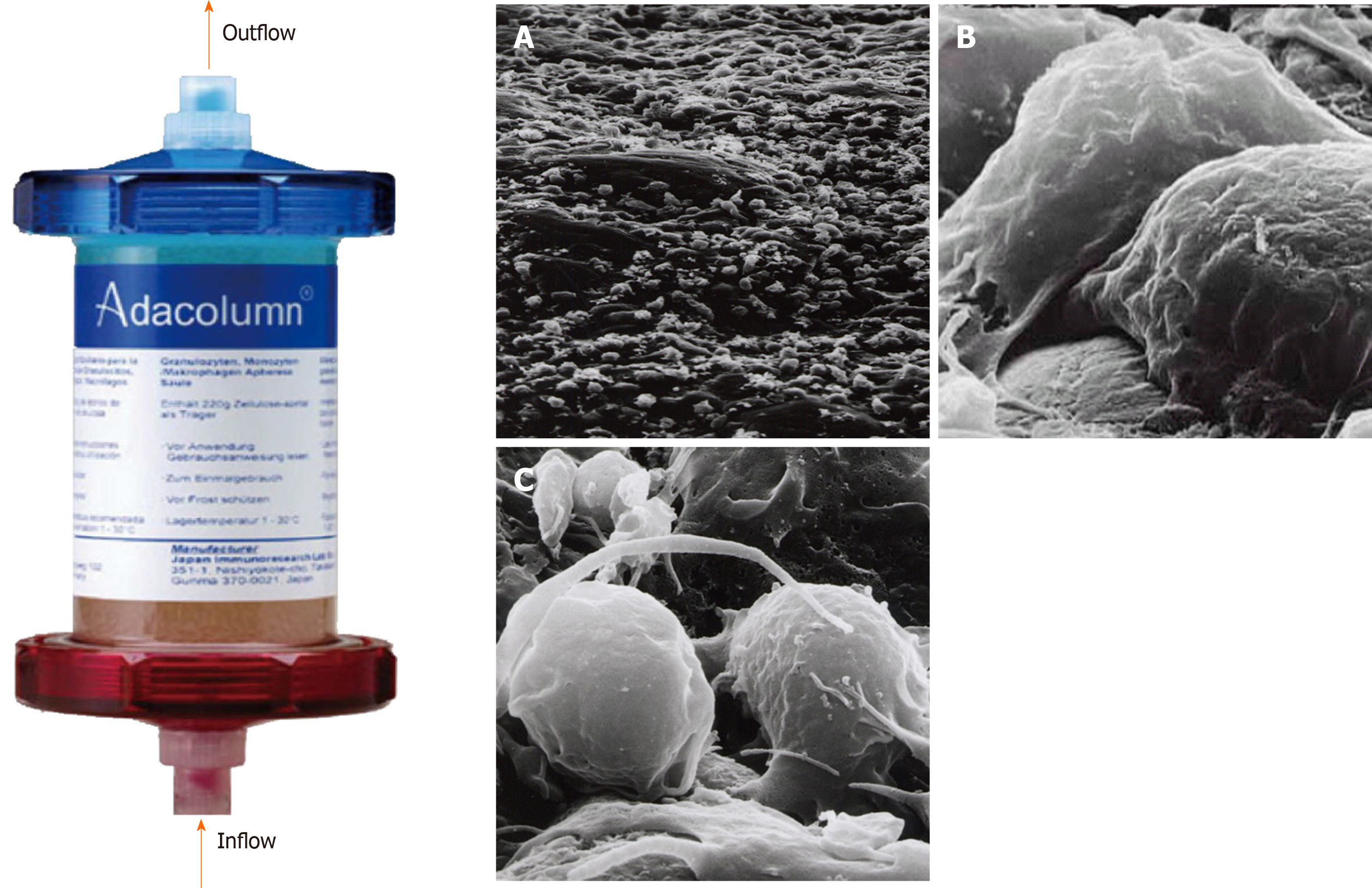
In the 1980s, a novel extracorporeal leukocyte removal filter with a commercial name of Cellsorba was developed by Asahi Medical Co. Japan. The filter consists of non-woven polyester cylindrical fabric. Using this filter, one could partially remove leukocytes from the whole blood during extracorporeal circulation, and it was approved for therapeutic use by the Japanese government in 1989. Cellsorba is simpler to operate and more efficient in the removal of granulocytes and monocytes and hence has greater clinical efficacy than centrifugation[16]. It has been found that the Cellsorba column is capable of removing about 1.6 × 1010 white blood cells per session including almost 100% of neutrophils and monocytes and 30%-60% of lymphocytes.
Studies have shown that the leukocyte removal filter could reduce the number of activated leukocytes as well as serum levels of pro-inflammatory cytokines[17-19]. Selective GMA, also known as granulocytapheresis, could selectively remove granulocytes and monocytes using a device commercially named Adacolumn, which was developed by the Japan Immunoresearch Laboratories Co., Ltd. of Takasaki, Japan. The column (G-1 granulocyte removal column) is packed with cellulose acetate beads. About 65% of granulocytes, 55% of monocytes and a significant fraction of lymphocytes are removed from peripheral blood passing through the column[20]. In the early 2000s, Japan national health reimbursement scheme introduced GMA as an induction of remission therapy for UC patients[21]. In the same year, Adacolumn was approved by the Ministry of Health of Japan for treating UC patients. Afterwards, Adacolumn became available for clinical application for IBD patients in the European Union countries after it was CE marked[22]. From then on, there have been accumulating reports on clinical efficacy and safety of Adacolumn in patients with IBD from Japan as well as from European countries[23-28]. In 2013, GMA therapy was approved for Chinese IBD patients by the Government Health Authority, and since then it has been applied in clinics to treat IBD patients in China mainland[29].
The Adacolumn (G-1 column), 206 mm in length, 60 mm in diameter and 335 mL in capacity, is made of polycarbonate and filled with 220 g of cellulose acetate beads of 2 mm in diameter (adsorptive carriers) bathed in 130 mL sterile saline[30] (Figure 1)[31]. The Adacolumn apheresis system consists of four components: The column, the blood circuit lines, Adamonitor and the pump. The column and its blood circuit lines are allowed for single use. The Adamonitor is the center of the system and is formed by a blood pump and four other functional units[20,22]. The pump of the Adacolumn system has special functional units, including a flow rate and time setting panel, a pressure monitor as well as a fault detecting alarm system. With the help of these functional units, if the actual pressure of the apheresis blood does not match the preset values, then the system will alarm and automatically stop working. Likewise, in the event of other abnormal conditions, the system will recognize it and alarm. Sometimes it will automatically switch off to ensure the safety of apheresis procedures[22].
GMA reduces inflammatory leukocytes and inhibits their infiltration: Elevation in number and activity of neutrophils and monocytes in peripheral blood contributes to the basic pathophysiology of IBD. In patients with IBD, peripheral circulating activated granulocytes, monocytes and macrophages are increased in number and subsequently lead to an increased level of circulating pro-inflammatory cytokines and infiltration of intestinal mucosa by these inflammatory cells, which are significantly correlated with intestinal inflammation level[11]. Therefore, removal of these inflammatory cells should be theoretically beneficial to patients with IBD[9].
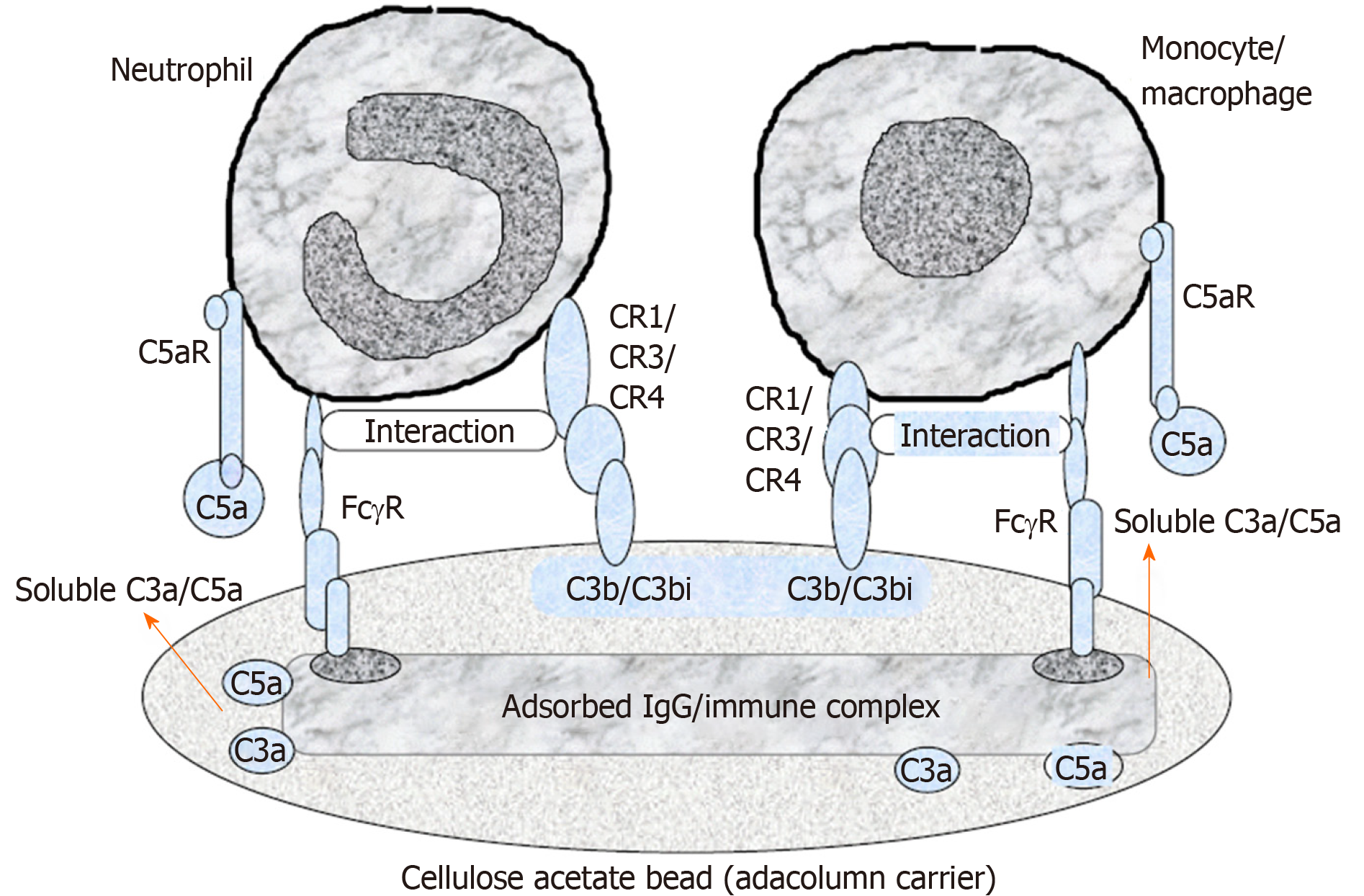
Cellulose acetate beads inside the Adacolumn are capable of selectively adsorbing circulating neutrophils and monocytes by binding to IgG fragments (Fcγ) and immune complement complexes[32], which serve as a “connecting bridge” between leukocytes and the beads (Figure 2). A significant reduction of CD14(+) CD16(+) monocytes in peripheral blood of GMA-treated IBD patients can be observed[33]. One study showed that soluble cell adhesion molecule-1 and soluble vascular cell adhesion molecule-1 were significantly increased in the peripheral blood of patients with IBD and closely related to the degree of tissue inflammation[34]. An in vitro study observed that the concentration of soluble cell adhesion molecule-1 and soluble vascular cell adhesion molecule-1 in blood samples was significantly decreased after incubation with acetate beads as adsorption carrier at different temperatures when compared with that in the control group without acetate beads incubation[35]. Therefore, GMA is also capable of inhibiting leukocyte migration by downregulating expression of leukocyte related adhesion molecules and thereby affecting the adhesion between cells and vascular endothelium at the initial stage. One study indicated that CX3CR1 played an important role in regulating the dynamic balance of intestinal macrophages, bacterial translocation and inflammatory effector Th17 response in patients with IBD[36]. Pro-inflammatory monocytes are able to highly express α4 integrin and CX3CR1 in patients with active UC, while GMA is able to selectively remove CD14+CD16+CX3CR1+ monocytes and increase CD14hiCD16-CCR2low “immature” monocytes and consequently inhibiting the adhesion and chemotaxis of pro-inflammatory monocytes to a certain extent[37].
GMA affects functions of other immune cells: One study observed that granulocytes removable by the Adacolumn from peripheral blood were mostly CD10-positive granulocytes, but the number of myeloid CD10-negative premature granulocytes with low pro-inflammatory function increased significantly after GMA therapy. This indicates that GMA therapy may play its therapeutic role indirectly by promoting the migration of “less-inflamed” premature granulocytes from bone marrow to peripheral blood making the granulocyte level in the peripheral blood unchanged[38]. Another study showed that the Adacolumn adsorption column was involved in the increased induction of myeloid-derived suppressor cells, which are strong anti-inflammatory cells, thus regulating the inflammatory response through immune cells to relieve the disease[39]. CD4+CD25+Foxp3+Treg cells are necessary for the maintenance of autoimmune tolerance. Kamikozuru et al[40] showed that after five sessions of GMA in active UC patients who achieved remission, the number of CD4+CD25+Foxp3+Treg in the peripheral blood increased to the level of the normal control group at the 10th wk. A clinical study by Muratov et al[41] showed that CD4+ T cells producing IFN-γ in peripheral blood of active IBD patients were significantly reduced after GMA therapy, and Waitz et al[42] found that the number of myeloid dendritic cells in the peripheral blood of patients with active UC was significantly higher than that of the normal control group. However, the number of myeloid dendritic cells in peripheral blood after GMA therapy was significantly lower. The mechanism may be that dendritic cells can express a variety of receptors, including Fc gamma, which can be absorbed by the cellulose acetate beads of the Adacolumn, resulting in a transient decline of dendritic cells in the peripheral blood and increase intestinal tolerance to different antigens.
GMA regulates pro-inflammatory and anti-inflammatory cytokines: As a pro-inflammatory cytokine, TNF-α plays a very important role in IBD. Its level in active IBD patients is significantly higher than that in normal subjects. One study showed that GMA therapy could significantly decrease peripheral blood levels of TNF-α, IL-1β, IL-6 and IL-8 in active IBD patients to alleviate the disease[38], and the intestinal tissue levels of these cytokines were significantly lowered in IBD patients who had achieved clinical or endoscopic remission but remained unchanged in patients without remission after GMA therapy[43]. It is known that TGF-β1 is a kind of pleiotropic cytokine as well as the most powerful intestinal immunosuppressant. Cellulose acetate in the Adacolumn can absorb soluble human leukocyte antigen I in peripheral blood, and soluble human leukocyte antigen I induces the production and release of soluble Fas ligand, which further leads to the production of TGF-β1[44,45], hence inducing the immune suppression effect. On one hand, IL-1, including IL-1α and IL-1β, is the major pro-inflammatory cytokine in the early inflammatory response and is significantly enhanced in its expression in the inflamed intestinal tissues, which is correlated well with the disease activity. On the other hand, IL-1Ra, a strong anti-inflammatory inhibitor of IL-1, is increased in active UC patients who responded well to GMA therapy, but no significant change is seen in IL-1Ra levels in patients who did not respond to GMA therapy[46]. This means that balance of IL-1Ra/IL-1 is very important in regulating inflammatory response in IBD patients.
GMA for UC: The first clinical trial of GMA for UC was reported in 2001. It was a multicenter controlled study with a total of 53 patients with active UC receiving five sessions in consecutive weeks of GMA therapy in combination with prednisolone at 14 hospitals in Japan[21]. By the 7th wk, 58.5% of the patients had achieved remission or improved, and prednisolone dosage was gradually reduced. Only eight non-severe adverse events in 5 patients were reported. Therefore, the first clinical trial indicated that GMA was a potentially effective and safe way to induce remission as well as tapering steroid dosage[21]. Since then, a large number of clinical applications and studies have been conducted in Japan, most of which showed satisfactory clinical and endoscopic outcomes and proved that GMA is an effective and safe way for UC patients who experienced tapering of corticosteroids as well as lowering the colon resection rate[24,47-54]. A significant study was reported in 2009 by Hibi et al[55] in which 656 severe or refractory UC patients in 53 medical institutions were observed over 7 years. The results showed the clinical response rates of severe, moderate and mild patients were 63.2%, 65.7% and 80.4%, respectively. Patients treated with GMA had a lower clinical recurrence rate and longer sustained remission.
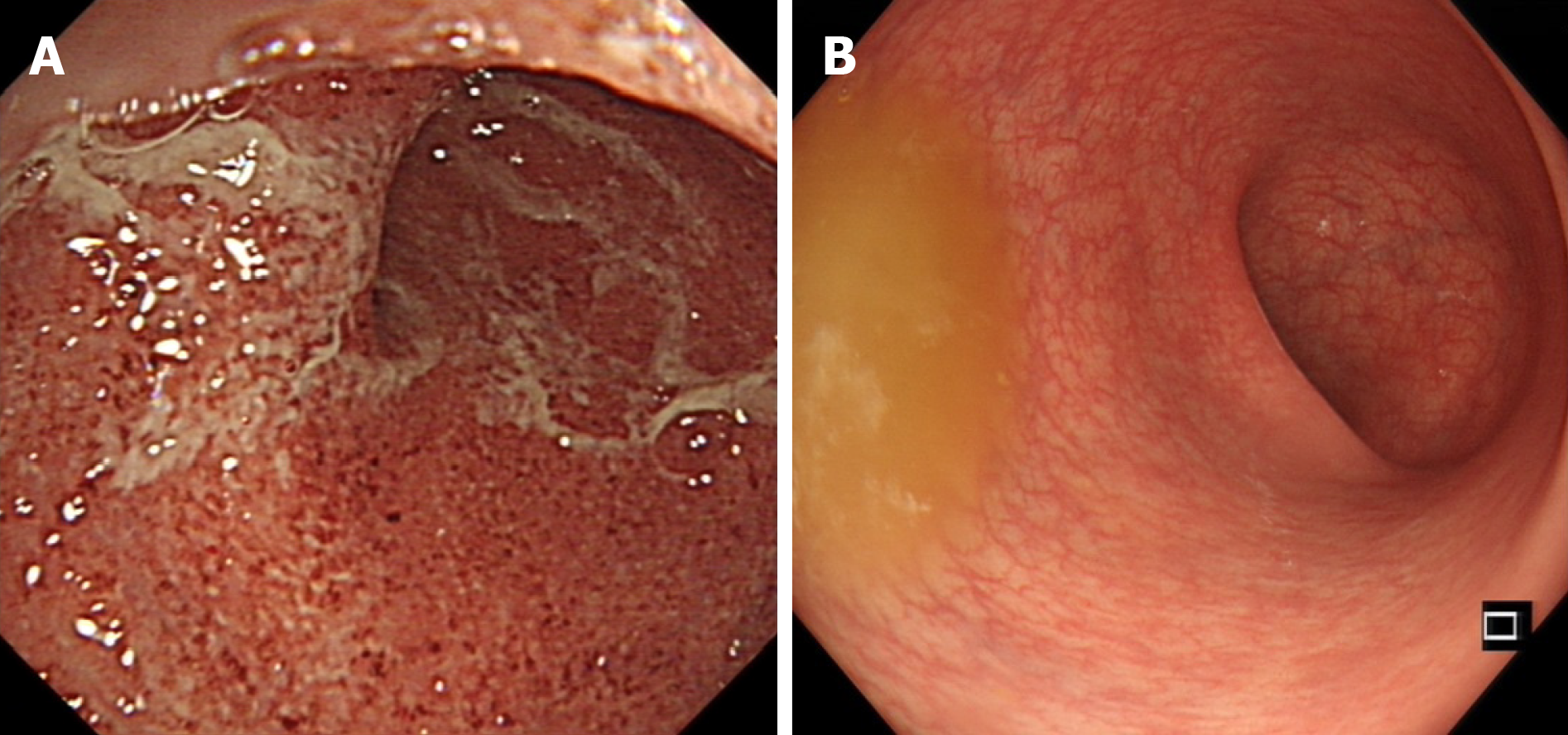
In recent years, biologic agents and immunosuppressants have been increasingly used in IBD. However, some IBD patients responded poorly to these agents. Many clinical trials were conducted to observe the effect of GMA for IBD patients who failed or were refractory to pharmacological therapy. D'Ovidio et al[24] treated 12 mild to moderate steroid-dependent/refractory UC patients by GMA. After 6 wk, mucosal healing was accomplished in 3 patients, partial mucosal healing in 8 patients, and 1 patient had no response. In 2016, in a single-arm, open-label, multicenter trial[56] conducted in 18 centers in the United Kingdom, France and Germany, 84 moderate-to-severe active UC patients having poor response or intolerance to immuno-suppressant and/or biologics were enrolled. Each patient received GMA therapy of one session per week with the Adacolumn. After continuous apheresis for 12 wk, 33 of the 84 patients achieved clinical remission, and 47 achieved a clinical response. For patients with previous immunosuppressant and/or biologic failure, the clinical remission rate was 30.0%. Similar results were also found from other reports[50,57]. These studies indicated that the Adacolumn apheresis is effective in induction of remission in patients with steroid-dependent UC who failed immunosuppressant and/or biological agents.
Apart from these clinical trials aiming at induction of remission, the effectiveness of maintenance in UC patients treated with GMA was also observed. In a multicenter study at 24 medical institutions in Italy[58], a total of 230 patients (including 194 UC patients) received at least one session of GMA therapy and were then followed up for 1 year. The results showed that 77.7% of UC patients obtained positive outcomes at 3 mo and 87.1% at 12 mo. Similar results were also observed in other studies[27,41]. Furthermore, it seems to be equally effective in relapsed patients who have achieved remission by previous GMA therapy[59].
In 2011, a retrospective, observational, multicenter study was conducted for cytomegalovirus (CMV) positive UC patients[60]. In this study, CMV-positive UC patients were treated with either additional GMA (11 patients) or immuno-suppressant (9 patients) after ineffective antiviral treatment. In the GMA group, 9 patients achieved remission and 2 underwent colectomy. In the immunosuppressant group, 4 achieved remission but 5 underwent colectomy. Therefore, it was concluded from this study that GMA was more effective in UC patients with opportunistic CMV infection as compared with conventional drugs like immunosuppressants. Nevertheless, additional studies for GMA therapy for CMV-positive IBD patients need be performed in the future because a conclusion should be reached with care from this retrospective study with a small sample size. However, this study demonstrated that GMA was safer than immunosuppressants in patients with opportunistic infections.
Relapse is an unlucky clinical feature of patients with UC. An open-label, prospective, randomized, controlled study[61] from the United Kingdom was conducted aiming at prevention of relapse in UC patients by GMA therapy. Sixty UC patients in remission but with fecal calprotectin over 250 mg/g (at high risk of clinical relapse) were enrolled. Twenty-nine patients received five sessions of weekly GMA, and thirty-one patients were kept on maintenance therapy. After 6 mo follow-up, 72.4% of the GMA-treated patients were still in remission, while only 32.3% in the control group were still in remission. Therefore, it was concluded from the studies above that selective leukocytapheresis significantly reduces recurrence rates and delays the time to relapse. Furthermore, GMA in combination with biologics also yielded satisfactory clinical outcomes. In a retrospective study reported by Tanida et al[62] in 2018, nine refractory UC patients received combination therapy with adalimumab plus intensive GMA. Over half of the nine patients displayed clinical remission at 10 wk, and 33.3% displayed remission at 52 wk under subsequent maintenance monotherapy of adalimumab.
Although GMA therapy has been proven effective and safe in patients with UC, there was a report with contradictory results. In a randomized, prospective, double-blind, sham-controlled trial[63] conducted at 36 centers in the United States and Canada, 168 patients with moderate to severe active UC were enrolled and assigned randomly to either GMA group (84 patients) or sham-treatment group (84 patients). The results showed that the clinical remission and response rate in the GMA group were 17% and 44%, respectively, while the clinical remission and response rate in the sham-treatment group were 11% and 39%, respectively. No differences between the two groups were found indicating that GMA was unsatisfactory in treating patients with moderate to severe UC. The conclusion from this study contradicts that from the majority of other studies. It is known that the best responders to GMA therapy are UC patients of short disease duration with no previous medications and steroid naïve UC patients[53,64]. So the possible explanation for this contradiction may be that patients enrolled in this trial did not fall into the category of best responders.
In summary, GMA is an effective and safe therapeutic option for moderate to severe UC patients, particularly for those who are refractory to or dependent on corticosteroids, which can be tapered or avoided. At present, no one can conclude that GMA can be used as a first-line therapy, but at least as an alternative choice for patients with UC (Figures 3 and 4).
GMA for CD: The efficacy of GMA in patients with CD was first reported by Matsui et al[65], who treated 7 CD patients refractory to conventional therapy. The patients received five or six sessions of weekly GMA in combination with the previous conventional therapy. The results showed that 5 patients achieved clinical remission while the other 2 patients did not respond. In another clinical study that enrolled 21 active CD patients, all of the patients achieved significant improvement 7 wk after weekly GMA for 5 consecutive weeks as an adjunct to conventional therapy evaluated by CDAI, IOIBD and IBDQ scores[66]. Apart from the studies showing the usefulness of GMA, other clinical studies including case reports also verified the effectiveness of GMA in patients with CD[67-70]. In 2018, Tanida et al[71] reported three patients with refractory CD treated with intensive GMA plus ustekinumab. At wk 10, clinical remission was achieved for all the patients. Therefore, at present it is believed that GMA or in combination with biologics can be used to induce remission in CD patients.
As for maintenance of remission, a clinical case report from Spain showed that one steroid-dependent CD patient experienced no clinical and endoscopic relapse for 12 mo with the combination of infliximab and GMA therapy[25]. Another CD patient with severe fistula refractory to conventional therapy also achieved sustained remission by GMA therapy[28]. However, there were a number of CD patients who obtained disappointing outcomes with GMA therapy. In 2013, a randomized, double-blind, sham-controlled study was reported by Sands et al[72]. They enrolled 235 patients with moderate to severe active CD from the United States and Canada, and all the patients finished ten sessions of GMA therapy. The results showed that clinical remission and response rate in the GMA group was 17.8% and 28.0%, respectively, while the clinical remission and response rate in the sham-treatment group was 19.2% and 26.9%, respectively. In another clinical study involving 12 patients with steroid dependent CD who received weekly GMA therapy, only 1 patient experienced no relapse within 6 mo of follow-up in spite of the initial clinical remission in 70% of the patients[57]. From the above data, it seems that the effectiveness of GMA in CD patients is not as good as in UC as illustrated by a meta-analysis, which concluded that GMA therapy in UC demonstrated a significant higher clinical efficacy than CD[13]. The possible reasons for the difference of effectiveness of GMA therapy between UC and CD await explanation. One possible reason may lie perhaps in the different intestinal neutrophil infiltration between the small intestine and the colon[14].
Optimizing GMA for IBD: Based on clinical trials, the efficacy rate was as high as 100% for initial UC patients and over 80% for steroid naïve patients[52,53]. The best responders to GMA therapy are UC patients with short disease duration and no previous medications and steroid naïve UC patients[32,52,53,73]. In non-responders, deep colonic lesions or loss of extensive mucosal tissues are usually observed by endoscopic examinations[52,64]. GMA is a time dependent therapy for IBD patients; several weeks may be needed before achieving favorable clinical outcomes. In addition, five sessions of GMA therapy are generally good for patients with a short course of the disease, while patients with recurrent episodes, especially steroid dependent or refractory, usually require ten sessions to achieve remission[32,49,56].
Based on its frequency of sessions, GMA therapy is classified into two therapeutic protocols: Regular and intensive GMA. In regular GMA, one session per week is carried out for five to ten sessions, whereas in intensive GMA, two sessions per week are required for a total of five to ten sessions[52,55]. Sakuraba et al[74] reported a prospective multicenter study involving 112 UC patients who were divided into two groups: Regular GMA group and intensive GMA group. The results showed that intensive GMA therapy has a significantly higher remission rate than regular GMA therapy. Treatment duration and volume of blood infusion in the Adacolumn of a single GMA session may also influence the effectiveness of GMA as seen in the study by Kanke et al[51]. They showed that 90 min for each GMA session has a significant better outcome as compared to routine 60 min of treatment. Yoshimura et al[75] attempted to increase the blood volume perfusion from 1800 mL to over 3000 mL per GMA session, which seemed to have yielded a significantly better clinical outcome with no safety concerns.
GMA for children and adolescent IBD patients: IBD is featured by its high morbidity in children and adolescents in whom growth and development may be affected by the disease and by the supposed life-long pharmacological treatment as well. Therefore, non-pharmacological treatment appears to bear significant importance for children and adolescent IBD patients[76].
In 2003, the first clinical report of GMA therapy for pediatric IBD patients was published by Tomomasa et al[77]. They treated 12 steroid-refractory IBD children with an average age of 12 years using GMA therapy (five to ten sessions). Nine of the twelve patients achieved clinical remission, and two patients had no response. The dosage of steroid was tapered in all patients. Four of the nine patients relapsed in an average of 3.5 mo after the last GMA session. The other patients remained in remission until an average of 22.8 mo. Similar results had also been reported by Ruuska et al[78]. In a single center trial[79], a total of 24 children and adolescents with IBD who failed mesalazine or sulphasalazine were enrolled. After GMA therapy in combination with prednisolone, all the patients obtained remission. Furthermore, in a clinical trial[80] involving 53 pediatric/adolescent patients, the incidence of adverse events was 18.9%, a figure, however, higher than that in all 437 patients (11.4%).
From most of the above studies, one can see that GMA is effective and well tolerated in children and adolescent IBD patients who have failed conventional drug therapy, and GMA in combination corticosteroids yielded better clinical outcomes. However, there have been no sufficient clinical data to verify the effectiveness of GMA therapy in children and adolescents IBD patients, so more clinical studies are needed to address this question.
GMA for pregnant IBD patients: Clinical and epidemiological studies have shown that the fertility period was usually at the peak incidence of IBD patients, and the disease itself is an important risk factor for pregnancy. The fertility rate of IBD patients is significantly lower than that of healthy people due to disease activity, nutritional status, surgery and drug treatment. Therefore, it is challenging to manage pregnant IBD patients[81,82]. Theoretically speaking, GMA therapy could be safe and effective for pregnant IBD patients. However, at present most of the published studies were case reports. In 2006, Okada et al[83] reported a 30-year-old pregnant woman of 13 wk gestation with severe steroid-dependent UC who was treated by Cellsorba leukocytapheresis. The patient successfully achieved a rapid improvement after the first GMA session, and clinical remission was obtained 2 wk later. The patient delivered a full-term healthy baby during the remission stage. Another three case reports involving 5 pregnant UC patients showed satisfactory responses to GMA therapy with smooth delivery and an absence of adverse events[84-86].
Although evidence of effectiveness of GMA in pregnant IBD patients are based upon case reports, it might become the first-line therapy for pregnant IBD patients due to its safety. Of course, more research is needed before GMA therapy is generally accepted as the first-line therapy for pregnant IBD patients.
As a non-pharmacological therapy, GMA is incomparable to other therapies regarding its safety[87]. The largest clinical study to date was performed by Hibi et al[55] who followed 656 UC patients treated by GMA from 53 centers in Japan starting in 2009 for 7 years. The results showed that GMA therapy had a very high safety profile with only mild or moderate adverse events related to GMA. In a multicenter study[21], a total of 53 patients were treated with GMA therapy in combination with prednisone for 5 wk. Only eight mild adverse events were observed in 5 patients, and no patients ceased the treatment due to adverse events. In another study, no significant differences regarding safety were found between patients receiving five and ten GMA sessions as reported by Dignass et al[88] who divided 196 patients with moderate to severe steroid-dependent or steroid-refractory UC into two groups of five and ten GMA sessions.
In a meta-analysis report involving nine randomized trials of GMA therapy[13,89], the most common adverse events were headache and flushing, and patients treated with GMA had a significant lower incidence of side effects than conventional therapies, i.e. corticosteroids. Besides, no serious adverse reactions had been reported in the children and pregnant women IBD patients who received GMA therapy[78,84,90].
The use of anticoagulant is indispensable for GMA therapy. Sawada et al[91] analyzed 832 patients from 116 medical facilities in Japan for safety of anticoagulant use of nafamostat mesylate or heparin. The main side effects in patients using nafamostat mesylate were mild headache (2.2%), nausea (1.3%) and fever (0.9%), while in patient using heparin, the main side effects were decreased platelet count (2.7%), nasal congestion (1.8%) and pain in the vascular access sites (1.8%). Apart from these mild side effects, no serious adverse events were observed in patients using either nafamostat mesylate or heparin. In summary, adverse events of GMA therapy are rare, mild and well tolerated.
As a non-pharmacological therapy, GMA has been demonstrated to be effective and safe for patients with IBD. Nevertheless, most of the clinical trials and literature of GMA therapy came from Japan. It has not been used extensively on a global scale, particularly in China, although it was approved by China State Food and Drug Administration for IBD patients in 2013. Furthermore, its effectiveness in IBD patients, particularly in CD patients, was doubted by some authors especially in a biological era when many biologic agents and immunosuppressants have been extensively used for IBD. Besides, price is also one of the main factors limiting the clinical usage of GMA therapy when cost-effectiveness perplexes both doctors and patients. Therefore, much needs to be done before it can be accepted as a therapeutic option for IBD patients worldwide.
GMA targets inflammatory immune cells such as granulocytes and monocytes /macrophages to alleviate intestinal inflammation in IBD patients[14]. In addition, other immune cells such as T cells, B cells and dendritic cells are also involved in the pathogenesis of IBD[40,42]. At present, although there have been a few studies on the influence of GMA on these immune cells[40-42], it is unknow how they contribute to the clinical effectiveness of GMA in patients with IBD. It is known that gut microbiota play an important role in the pathogenesis of IBD[6,92], but research must be done to determine whether GMA therapy influences the gut microbiota. One study (our unpublished data) has shown that the unfavorable gut microbiota could be improved by GMA therapy in patients with UC. It is possible that GMA therapy could exert its therapeutic effect by improving the steady state of gut microbiota in IBD patients. However, more studies are needed before we are able to answer these questions.
Re-evaluation of GMA: Many reports have verified the effectiveness of GMA for induction of remission in IBD especially in UC patients. However, most of them were case reports or clinical trials of small size from Japan and Europe. Therefore, more large-scale, multicenter prospective studies are needed to further verify the effectiveness of GMA therapy for IBD. Furthermore, the effectiveness of GMA therapy in CD patients is controversial in limited clinical trials to date. Although GMA therapy may be effective for induction of remission in patients with relapsed UC, future studies should be focused on its effectiveness as a maintenance therapy[76,93]. Besides, it is also worth looking for predictive factors for responders to GMA therapy.
The effectiveness of GMA has been verified in children and adolescent IBD patients[94]. In theory, GMA can be used in IBD patients of any age, but there are limited clinical trials of GMA in children and adolescent IBD patients. Due to the differences in disease characteristics, body weight and circulatory status between children and adults, future studies are needed to determine the appropriate candidates as well as safety of GMA therapy for children and adolescent IBD patients. Fetal safety in pregnant patients with IBD who receive pharmacological treatment during pregnancy has always been a common concern of both doctors and patients. Although most of the drugs for IBD patients are at relatively low risk to fetal safety, there are still insufficient data to prove that various drugs are safe for patients with IBD in pregnancy in terms of miscarriage or malformation. Therefore, the choice of drugs for patients with IBD during pregnancy is difficult for both doctors and patients[83,84]. Because it is a non-pharmacological technique in which no any medications are involved during the procedures, GMA is much safer for IBD patients in pregnancy as evidenced by several clinical reports[83-85]. However, future clinical trials and observation of large-scale trials are needed before GMA becomes accepted as a safe and effective therapy for IBD patients during pregnancy.
GMA therapy was invented by Japanese scholars for patients with IBD. Due to its effectiveness and safety, especially its effect on anti-inflammatory cytokines, GMA therapy has also been used to treat other autoimmune diseases. In Italy, Morabito et al[95] treated nine patients with alcoholic hepatitis with GMA. The results showed that GMA therapy could reduce circulating inflammatory markers and improve the patient’s clinical status. In addition, GMA also has therapeutic effects on patients with Bechet’s disease[96] and rheumatoid arthritis[97]. With more clinical applications, it is hopeful that GMA therapy could be used clinically for other autoimmune diseases apart from IBD.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Tanida S, Zaltman C S-Editor: Ma YJ L-Editor: Filipodia E-Editor: Qi LL
| 1. | Mulder DJ, Noble AJ, Justinich CJ, Duffin JM. A tale of two diseases: the history of inflammatory bowel disease. J Crohns Colitis. 2014;8:341-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 130] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 2. | Lerebours E, Bussel A, Modigliani R, Bastit D, Florent C, Rabian C, René E, Soulé JC. Treatment of Crohn's disease by lymphocyte apheresis: a randomized controlled trial. Groupe d'Etudes Thérapeutiques des Affections Inflammatoires Digestives. Gastroenterology. 1994;107:357-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 43] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Bicks RO, Groshart KD. The current status of T-lymphocyte apheresis (TLA) treatment of Crohn's disease. J Clin Gastroenterol. 1989;11:136-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 33] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Klein HG. Adoptive immunotherapy: novel applications of blood cell separators. J Clin Apher. 1988;4:198-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 5. | Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2894] [Cited by in RCA: 3351] [Article Influence: 186.2] [Reference Citation Analysis (11)] |
| 6. | Morgan XC, Tickle TL, Sokol H, Gevers D, Devaney KL, Ward DV, Reyes JA, Shah SA, LeLeiko N, Snapper SB, Bousvaros A, Korzenik J, Sands BE, Xavier RJ, Huttenhower C. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 2012;13:R79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1756] [Cited by in RCA: 2063] [Article Influence: 158.7] [Reference Citation Analysis (0)] |
| 7. | Cohen BL, Sachar DB. Update on anti-tumor necrosis factor agents and other new drugs for inflammatory bowel disease. BMJ. 2017;357:j2505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 110] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 8. | Present DH. How to do without steroids in inflammatory bowel disease. Inflamm Bowel Dis. 2000;6:48-57; discussion 58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Rogler G, Andus T. Cytokines in inflammatory bowel disease. World J Surg. 1998;22:382-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 385] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 10. | Powell N, Lo JW, Biancheri P, Vossenkämper A, Pantazi E, Walker AW, Stolarczyk E, Ammoscato F, Goldberg R, Scott P, Canavan JB, Perucha E, Garrido-Mesa N, Irving PM, Sanderson JD, Hayee B, Howard JK, Parkhill J, MacDonald TT, Lord GM. Interleukin 6 Increases Production of Cytokines by Colonic Innate Lymphoid Cells in Mice and Patients With Chronic Intestinal Inflammation. Gastroenterology. 2015;149:456-467.e15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 11. | Levine AP, Segal AW. What is wrong with granulocytes in inflammatory bowel diseases? Dig Dis. 2013;31:321-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Segal AW. The role of neutrophils in the pathogenesis of Crohn's disease. Eur J Clin Invest. 2018;48 Suppl 2:e12983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | Liu Z, Jiang X, Sun C. The efficacy and safety of selective granulocyte and monocyte apheresis for inflammatory bowel disease: A meta-analysis. Eur J Intern Med. 2016;36:e26-e27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Saniabadi AR, Tanaka T, Yamamoto T, Kruis W, Sacco R. Granulomonocytapheresis as a cell-dependent treatment option for patients with inflammatory bowel disease: Concepts and clinical features for better therapeutic outcomes. J Clin Apher. 2019;34:51-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Seigworth GR. Bloodletting over the centuries. N Y State J Med. 1980;80:2022-2028. [PubMed] |
| 16. | Takenaka Y. Lymphocytapheresis. Artif Organs. 1996;20:914-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Sawada K, Ohnishi K, Fukui S, Yamada K, Yamamura M, Amano K, Amano K, Wada M, Tanida N, Satomi M. Leukocytapheresis therapy, performed with leukocyte removal filter, for inflammatory bowel disease. J Gastroenterol. 1995;30:322-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 115] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 18. | Sawada K, Ohnishi K, Kosaka T, Chikano S, Yokota Y, Egashira A, Izawa H, Yamamura M, Amano K, Satomi M, Shimoyama T. Leukocytapheresis with leukocyte removal filter as new therapy for ulcerative colitis. Ther Apher. 1997;1:207-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 60] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Kosaka T, Sawada K, Ohnishi K, Egashira A, Yamamura M, Tanida N, Satomi M, Shimoyama T. Effect of leukocytapheresis therapy using a leukocyte removal filter in Crohn's disease. Intern Med. 1999;38:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Sawada K, Shimoyama T. Therapeutic cytapheresis for inflammatory bowel disease. Ther Apher. 1998;2:90-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Shimoyama T, Sawada K, Hiwatashi N, Sawada T, Matsueda K, Munakata A, Asakura H, Tanaka T, Kasukawa R, Kimura K, Suzuki Y, Nagamachi Y, Muto T, Nagawa H, Iizuka B, Baba S, Nasu M, Kataoka T, Kashiwagi N, Saniabadi AR. Safety and efficacy of granulocyte and monocyte adsorption apheresis in patients with active ulcerative colitis: a multicenter study. J Clin Apher. 2001;16:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 202] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 22. | Saniabadi AR, Hanai H, Takeuchi K, Umemura K, Nakashima M, Adachi T, Shima C, Bjarnason I, Lofberg R. Adacolumn, an adsorptive carrier based granulocyte and monocyte apheresis device for the treatment of inflammatory and refractory diseases associated with leukocytes. Ther Apher Dial. 2003;7:48-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 206] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 23. | Hanai H. Positions of selective leukocytapheresis in the medical therapy of ulcerative colitis. World J Gastroenterol. 2006;12:7568-7577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | D'Ovidio V, Aratari A, Viscido A, Marcheggiano A, Papi C, Capurso L, Caprilli R. Mucosal features and granulocyte-monocyte-apheresis in steroid-dependent/refractory ulcerative colitis. Dig Liver Dis. 2006;38:389-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | González Carro P, Pérez Roldán F, Roncero García Escribano O, Lafuente R, Legaz Huidobro ML, Amigo Echenagusía A. Case report: combination therapy with granulocyte apheresis and infliximab for refractory Crohn's disease. J Clin Apher. 2006;21:249-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Wittig BM, Zeitz M. Critical comment: analyzing the effect of novel therapies on cytokine expression in inflammatory bowel disease: do cytokine levels reflect clinical response? Int J Colorectal Dis. 2006;21:505-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 27. | Bresci G, Parisi G, Mazzoni A, Scatena F, Capria A. Granulocytapheresis versus methylprednisolone in patients with acute ulcerative colitis: 12-month follow up. J Gastroenterol Hepatol. 2008;23:1678-1682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Tate D, Cairnes V, Valori R, Makins R. First successful use of leukocyte apheresis as maintenance therapy for Crohn's disease in the United Kingdom. J Clin Apher. 2014;29:181-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 29. | Lai YM, Yao WY, He Y, Jiang X, Gu YB, Chen MH, Liu YL, Yuan YZ, Qian JM. Adsorptive Granulocyte and Monocyte Apheresis in the Treatment of Ulcerative Colitis: The First Multicenter Study in China. Gut Liver. 2017;11:216-225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | Pineda AA. Developments in the apheresis procedure for the treatment of inflammatory bowel disease. Inflamm Bowel Dis. 2006;12 Suppl 1:S10-S14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 31. | Saniabadi AR, Hanai H, Suzuki Y, Ohmori T, Sawada K, Yoshimura N, Saito Y, Takeda Y, Umemura K, Kondo K, Ikeda Y, Fukunaga K, Nakashima M, Beretta A, Bjarnason I, Lofberg R. Adacolumn for selective leukocytapheresis as a non-pharmacological treatment for patients with disorders of the immune system: an adjunct or an alternative to drug therapy? J Clin Apher. 2005;20:171-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 81] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 32. | Saniabadi AR, Hanai H, Fukunaga K, Sawada K, Shima C, Bjarnason I, Lofberg R. Therapeutic leukocytapheresis for inflammatory bowel disease. Transfus Apher Sci. 2007;37:191-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 70] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 33. | Hanai H, Iida T, Takeuchi K, Watanabe F, Yamada M, Kikuyama M, Maruyama Y, Iwaoka Y, Hirayama K, Nagata S, Takai K. Adsorptive depletion of elevated proinflammatory CD14+CD16+DR++ monocytes in patients with inflammatory bowel disease. Am J Gastroenterol. 2008;103:1210-1216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 88] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 34. | Nanau RM, Neuman MG. Metabolome and inflammasome in inflammatory bowel disease. Transl Res. 2012;160:1-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 35. | Nishise S, Takeda Y, Nara H, Abe Y, Sasaki Y, Asao H, Ueno Y. Adsorption of Soluble Immunoglobulin-Type Adhesion Molecules to Cellulose Acetate Beads. Ther Apher Dial. 2018;22:261-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 36. | Nishimura M, Kuboi Y, Muramoto K, Kawano T, Imai T. Chemokines as novel therapeutic targets for inflammatory bowel disease. Ann N Y Acad Sci. 2009;1173:350-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 82] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 37. | Takeda S, Sato T, Katsuno T, Nakagawa T, Noguchi Y, Yokosuka O, Saito Y. Adsorptive depletion of alpha4 integrin(hi)- and CX3CR1hi-expressing proinflammatory monocytes in patients with ulcerative colitis. Dig Dis Sci. 2010;55:1886-1895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 38. | Kashiwagi N, Sugimura K, Koiwai H, Yamamoto H, Yoshikawa T, Saniabadi AR, Adachi M, Shimoyama T. Immunomodulatory effects of granulocyte and monocyte adsorption apheresis as a treatment for patients with ulcerative colitis. Dig Dis Sci. 2002;47:1334-1341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 129] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 39. | Sakanoue M, Higashi Y, Kanekura T. Inhibition of Inflammatory Cytokines and Induction of Myeloid-Derived Suppressor Cells by the Effects of Granulocyte and Monocyte Adsorption Apheresis. Ther Apher Dial. 2017;21:628-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 40. | Kamikozuru K, Fukunaga K, Hirota S, Hida N, Ohda Y, Yoshida K, Yokoyama Y, Tozawa K, Kawa K, Iimuro M, Nagase K, Saniabadi AR, Nakamura S, Miwa H, Matsumoto T. The expression profile of functional regulatory T cells, CD4+CD25high+/forkhead box protein P3+, in patients with ulcerative colitis during active and quiescent disease. Clin Exp Immunol. 2009;156:320-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 41. | Muratov V, Lundahl J, Ulfgren AK, Elvin K, Fehrman I, Ahlborg N, Ost A, Hittel N, Saniabadi A, Löfberg R. Down-regulation of interferon-gamma parallels clinical response to selective leukocyte apheresis in patients with inflammatory bowel disease: a 12-month follow-up study. Int J Colorectal Dis. 2006;21:493-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 42. | Waitz G, Petermann S, Liebe S, Emmrich J, Ramlow W. Reduction of dendritic cells by granulocyte and monocyte adsorption apheresis in patients with ulcerative colitis. Dig Dis Sci. 2008;53:2507-2515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 43. | Yamamoto T, Saniabadi AR, Umegae S, Matsumoto K. Impact of selective leukocytapheresis on mucosal inflammation and ulcerative colitis: cytokine profiles and endoscopic findings. Inflamm Bowel Dis. 2006;12:719-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 44. | Kamato D, Burch ML, Piva TJ, Rezaei HB, Rostam MA, Xu S, Zheng W, Little PJ, Osman N. Transforming growth factor-β signalling: role and consequences of Smad linker region phosphorylation. Cell Signal. 2013;25:2017-2024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 213] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 45. | Contini P, Negrini S, Bodini G, Trucchi C, Ubezio G, Strada P, Savarino V, Ghio M. Granulocytes and monocytes apheresis induces upregulation of TGFβ1 in patients with active ulcerative colitis: A possible involvement of soluble HLA-I. J Clin Apher. 2017;32:49-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 46. | Sakimura K, Omori T, Iwashita E, Yoshida T, Tsuzuki Y, Fujimori K, Konishi F, Yoshida Y, Anzai H, Suzuki H, Sugawara S, Takeda Y, Hiraishi K, Saniabadi AR, Ide T, Miura S, Ota S. Clinical response is associated with elevated plasma interleukin-1 receptor antagonist during selective granulocyte and monocyte apheresis in patients with ulcerative colitis. Dig Dis Sci. 2006;51:1525-1531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 47. | Hanai H, Watanabe F, Saniabadi AR, Matsushitai I, Takeuchi K, Iida T. Therapeutic efficacy of granulocyte and monocyte adsorption apheresis in severe active ulcerative colitis. Dig Dis Sci. 2002;47:2349-2353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 64] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 48. | Fukunaga K, Fukuda Y, Sawada K, Hori K, Matoba Y, Sagayama K, Ohnishi K, Fukui S, Shimoyama T. Poorly controlled ulcerative colitis treated by colectomy during remission induced by extracorporeal leukocyte removal therapy. J Gastroenterol. 2003;38:684-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 49. | Hanai H, Watanabe F, Takeuchi K, Iida T, Yamada M, Iwaoka Y, Saniabadi A, Matsushita I, Sato Y, Tozawa K, Arai H, Furuta T, Sugimoto K, Bjarnason I. Leukocyte adsorptive apheresis for the treatment of active ulcerative colitis: a prospective, uncontrolled, pilot study. Clin Gastroenterol Hepatol. 2003;1:28-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 159] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 50. | Hanai H, Watanabe F, Yamada M, Sato Y, Takeuchi K, Iida T, Tozawa K, Tanaka T, Maruyama Y, Matsushita I, Iwaoka Y, Kikuch K, Saniabadi AR. Adsorptive granulocyte and monocyte apheresis versus prednisolone in patients with corticosteroid-dependent moderately severe ulcerative colitis. Digestion. 2004;70:36-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 108] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 51. | Kanke K, Nakano M, Hiraishi H, Terano A. Clinical evaluation of granulocyte/monocyte apheresis therapy for active ulcerative colitis. Dig Liver Dis. 2004;36:811-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 77] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 52. | Suzuki Y, Yoshimura N, Saniabadi AR, Saito Y. Selective granulocyte and monocyte adsorptive apheresis as a first-line treatment for steroid naïve patients with active ulcerative colitis: a prospective uncontrolled study. Dig Dis Sci. 2004;49:565-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 117] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 53. | Suzuki Y, Yoshimura N, Fukuda K, Shirai K, Saito Y, Saniabadi AR. A retrospective search for predictors of clinical response to selective granulocyte and monocyte apheresis in patients with ulcerative colitis. Dig Dis Sci. 2006;51:2031-2038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 54. | Watanabe K, Oshitani N, Kamata N, Inagawa M, Yamagami H, Higuchi K, Arakawa T. Efficacy and endoscopic prediction of cytapheresis therapy in patients with refractory and steroid-dependent ulcerative colitis. Aliment Pharmacol Ther. 2007;24:147-152. [DOI] [Full Text] |
| 55. | Hibi T, Sameshima Y, Sekiguchi Y, Hisatome Y, Maruyama F, Moriwaki K, Shima C, Saniabadi AR, Matsumoto T. Treating ulcerative colitis by Adacolumn therapeutic leucocytapheresis: clinical efficacy and safety based on surveillance of 656 patients in 53 centres in Japan. Dig Liver Dis. 2009;41:570-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 56. | Dignass A, Akbar A, Hart A, Subramanian S, Bommelaer G, Baumgart DC, Grimaud JC, Cadiot G, Makins R, Hoque S, Bouguen G, Bonaz B. Safety and Efficacy of Granulocyte/Monocyte Apheresis in Steroid-Dependent Active Ulcerative Colitis with Insufficient Response or Intolerance to Immunosuppressants and/or Biologics [the ART Trial]: 12-week Interim Results. J Crohns Colitis. 2016;10:812-820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 57. | Domènech E, Hinojosa J, Esteve-Comas M, Gomollón F, Herrera JM, Bastida G, Obrador A, Ruiz R, Saro C, Gassull MA; Spanish Group for the Study of Crohn's Disease and Ulcerative Colitis (GETECCU). Granulocyteaphaeresis in steroid-dependent inflammatory bowel disease: a prospective, open, pilot study. Aliment Pharmacol Ther. 2004;20:1347-1352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 89] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 58. | Passalacqua S, Ferraro PM, Bresci G, D'Ovidio V, Astegiano M, Principi M, Testa R, D'Incà R, Valpiani D, Armuzzi A, Sablich R, Cavallaro F, Costa F, Di Leo V, Colombo E, Santini A, Aratari A, Lecis P, Saladino V, Riegler G, Marco M, Calella F, Ricci C, Guidi ML, Repaci G, Silla M. The Italian Registry of Therapeutic Apheresis: granulocyte-monocyte apheresis in the treatment of inflammatory bowel disease. A multicentric study. J Clin Apher. 2011;26:332-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 59. | Lindberg A, Eberhardson M, Karlsson M, Karlén P. Long-term follow-up with Granulocyte and Monocyte Apheresis re-treatment in patients with chronically active inflammatory bowel disease. BMC Gastroenterol. 2010;10:73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 60. | Yoshino T, Nakase H, Matsuura M, Matsumura K, Honzawa Y, Fukuchi T, Watanabe K, Murano M, Tsujikawa T, Fukunaga K, Matsumoto T, Chiba T. Effect and safety of granulocyte-monocyte adsorption apheresis for patients with ulcerative colitis positive for cytomegalovirus in comparison with immunosuppressants. Digestion. 2011;84:3-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 61. | Maiden L, Takeuchi K, Baur R, Bjarnason I, O'Donohue J, Forgacs I, Chung-Faye G, Sanderson J, Bjarnason I. Selective white cell apheresis reduces relapse rates in patients with IBD at significant risk of clinical relapse. Inflamm Bowel Dis. 2008;14:1413-1418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 62. | Tanida S, Mizoshita T, Nishie H, Ozeki K, Katano T, Kubota E, Kataoka H, Kamiya T, Joh T. Combination Therapy With Adalimumab Plus Intensive Granulocyte and Monocyte Adsorptive Apheresis in Patients With Refractory Ulcerative Colitis. J Clin Med Res. 2015;7:884-889. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 63. | Sands BE, Sandborn WJ, Feagan B, Löfberg R, Hibi T, Wang T, Gustofson LM, Wong CJ, Vandervoort MK, Hanauer S; Adacolumn Study Group. A randomized, double-blind, sham-controlled study of granulocyte/monocyte apheresis for active ulcerative colitis. Gastroenterology. 2008;135:400-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 162] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 64. | Tanaka T, Okanobu H, Kuga Y, Yoshifuku Y, Fujino H, Miwata T, Moriya T, Nishida T, Oya T. Clinical and endoscopic features of responders and non-responders to adsorptive leucocytapheresis: a report based on 120 patients with active ulcerative colitis. Gastroenterol Clin Biol. 2010;34:687-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 65. | Matsui T, Nishimura T, Matake H, Ohta T, Sakurai T, Yao T. Granulocytapheresis for Crohn's disease: a report on seven refractory patients. Am J Gastroenterol. 2003;98:511-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 66. | Fukuda Y, Matsui T, Suzuki Y, Kanke K, Matsumoto T, Takazoe M, Matsumoto T, Motoya S, Honma T, Sawada K, Yao T, Shimoyama T, Hibi T. Adsorptive granulocyte and monocyte apheresis for refractory Crohn's disease: an open multicenter prospective study. J Gastroenterol. 2004;39:1158-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 76] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 67. | Kato S, Kani K, Takabayashi H, Yamamoto R, Ogawa T, Matsuda A, Yakabi K. Treatment of refractory Crohn's disease by intensive granulocyte and monocyte adsorption apheresis: a report on two drug refractory cases. Intern Med. 2011;50:1591-1593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 68. | Ozeki K, Tanida S, Mizushima T, Mizoshita T, Tsukamoto H, Hirata Y, Murakami K, Shimura T, Kataoka H, Kamiya T, Joh T. Combination therapy with adalimumab plus intensive granulocyte and monocyte adsorptive apheresis induced clinical remission in a Crohn's disease patient with the loss of response to scheduled adalimumab maintenance therapy: a case report. Intern Med. 2012;51:595-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 69. | Kato S, Hosomi E, Amano F, Kobayashi T, Kani K, Yamamoto R, Ogawa T, Matsuda A, Sato Y, Izaki S, Mitarai T, Yakabi K. The efficacy of intensive granulocyte and monocyte adsorption apheresis in a patient with Crohn's disease complicated by extensive subcutaneous aseptic neutrophilic abscesses. J Crohns Colitis. 2012;6:787-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 70. | Fukunaga K, Yokoyama Y, Kamikozuru K, Yoshida K, Kikuyama R, Nagase K, Nakamura S, Takei Y, Miwa H, Matsumoto T. Selective depletion of peripheral granulocyte/monocyte enhances the efficacy of scheduled maintenance infliximab in Crohn's disease. J Clin Apher. 2010;25:226-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 71. | Tanida S, Mizoshita T, Ozeki K, Katano T, Tanaka M, Nishie H, Shimura T, Okamoto Y, Kubota E, Kataoka H, Joh T. Combination Therapy With Intensive Granulocyte and Monocyte Adsorptive Apheresis Plus Ustekinumab in Patients With Refractory Crohn's Disease. Ther Apher Dial. 2018;22:295-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 72. | Sands BE, Katz S, Wolf DC, Feagan BG, Wang T, Gustofson LM, Wong C, Vandervoort MK, Hanauer S. A randomised, double-blind, sham-controlled study of granulocyte/monocyte apheresis for moderate to severe Crohn's disease. Gut. 2013;62:1288-1294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 73. | Yokoyama Y, Kawai M, Fukunaga K, Kamikozuru K, Nagase K, Nogami K, Kono T, Ohda Y, Iimuro M, Hida N, Nakamura S, Miwa H, Matsumoto T. Looking for predictive factors of clinical response to adsorptive granulocyte and monocyte apheresis in patients with ulcerative colitis: markers of response to GMA. BMC Gastroenterol. 2013;13:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 74. | Sakuraba A, Motoya S, Watanabe K, Nishishita M, Kanke K, Matsui T, Suzuki Y, Oshima T, Kunisaki R, Matsumoto T, Hanai H, Fukunaga K, Yoshimura N, Chiba T, Funakoshi S, Aoyama N, Andoh A, Nakase H, Mizuta Y, Suzuki R, Akamatsu T, Iizuka M, Ashida T, Hibi T. An open-label prospective randomized multicenter study shows very rapid remission of ulcerative colitis by intensive granulocyte and monocyte adsorptive apheresis as compared with routine weekly treatment. Am J Gastroenterol. 2009;104:2990-2995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 106] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 75. | Yoshimura N, Tadami T, Kawaguchi T, Sako M, Yoshimoto H, Yamaka T, Takazoe M. Processed blood volume impacts clinical efficacy in patients with ulcerative colitis undergoing adsorptive depletion of myeloid lineage leucocytes. J Gastroenterol. 2012;47:49-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 76. | Tanaka T, Yamamoto T, Sawada K, Sacco R. Treatment options for children and adolescents with inflammatory bowel disease: is granulomonocytapheresis an effective alternative to drug therapy? Expert Rev Gastroenterol Hepatol. 2017;11:749-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 77. | Tomomasa T, Kobayashi A, Kaneko H, Mika S, Maisawa S, Chino Y, Syou H, Yoden A, Fujino J, Tanikawa M, Yamashita T, Kimura S, Kanoh M, Sawada K, Morikawa A. Granulocyte adsorptive apheresis for pediatric patients with ulcerative colitis. Dig Dis Sci. 2003;48:750-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 78. | Ruuska T, Wewer V, Lindgren F, Malmborg P, Lindquist M, Marthinsen L, Browaldh L, Casswall T, Kalliomäki M, Grönlund J. Granulocyte-monocyte adsorptive apheresis in pediatric inflammatory bowel disease: results, practical issues, safety, and future perspectives. Inflamm Bowel Dis. 2009;15:1049-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 79. | Tanaka T, Sugiyama S, Goishi H, Kajihara T, Akagi M, Miura T. Treatment of children and adolescents with ulcerative colitis by adsorptive depletion of myeloid lineage leucocytes as monotherapy or in combination with low dose prednisolone after failure of first-line medications. BMC Gastroenterol. 2013;13:130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 80. | Motoya S, Tanaka H, Shibuya T, Osada T, Yamamoto T, Hongo H, Mizuno C, Saito D, Aoyama N, Kobayashi T, Ito H, Tanida S, Nojima M, Kokuma S, Hosoi E. Safety and effectiveness of granulocyte and monocyte adsorptive apheresis in patients with inflammatory bowel disease in special situations: a multicentre cohort study. BMC Gastroenterol. 2019;19:196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 81. | Dubinsky M, Abraham B, Mahadevan U. Management of the pregnant IBD patient. Inflamm Bowel Dis. 2008;14:1736-1750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 82. | Bortoli A, Pedersen N, Duricova D, D'Inca R, Gionchetti P, Panelli MR, Ardizzone S, Sanroman AL, Gisbert JP, Arena I, Riegler G, Marrollo M, Valpiani D, Corbellini A, Segato S, Castiglione F, Munkholm P; European Crohn-Colitis Organisation (ECCO) Study Group of Epidemiologic Committee (EpiCom). Pregnancy outcome in inflammatory bowel disease: prospective European case-control ECCO-EpiCom study, 2003-2006. Aliment Pharmacol Ther. 2011;34:724-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 128] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 83. | Okada H, Makidono C, Takenaka R, Hiraoka S, Fujiwara A, Kato J, Kawahara Y, Kawamoto H, Mizuno M, Shiratori Y. Therapeutic efficacy of leukocytapheresis in a pregnant woman with severe active ulcerative colitis. Digestion. 2006;74:15-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 84. | D'Ovidio V, Meo D, Gozer M, Bazuro ME, Vernia P. Ulcerative colitis and granulocyte-monocyte-apheresis: safety and efficacy of maintenance therapy during pregnancy. J Clin Apher. 2015;30:55-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 85. | Takahashi H, Sugawara K, Sugimura M, Iwabuchi M, Mano Y, Ukai K, Tadokoro K. Flare up of ulcerative colitis during pregnancy treated by adsorptive granulocyte and monocyte apheresis: therapeutic outcomes in three pregnant patients. Arch Gynecol Obstet. 2013;288:341-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 86. | Shibuya T, Haga K, Kamei M, Okahara K, Ito S, Takahashi M, Nomura O, Murakami T, Makino M, Kodani T, Ishikawa D, Sakamoto N, Osada T, Ogihara T, Watanabe S, Nagahara A. Successful remission of ulcerative colitis flare-up during pregnancy with adsorptive granulomonocytapheresis plus tacrolimus. Intest Res. 2018;16:484-488. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 87. | Saniabadi AR, Tanaka T, Ohmori T, Sawada K, Yamamoto T, Hanai H. Treating inflammatory bowel disease by adsorptive leucocytapheresis: a desire to treat without drugs. World J Gastroenterol. 2014;20:9699-9715. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 55] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 88. | Dignass AU, Eriksson A, Kilander A, Pukitis A, Rhodes JM, Vavricka S. Clinical trial: five or ten cycles of granulocyte-monocyte apheresis show equivalent efficacy and safety in ulcerative colitis. Aliment Pharmacol Ther. 2010;31:1286-1295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 89. | Yoshino T, Nakase H, Minami N, Yamada S, Matsuura M, Yazumi S, Chiba T. Efficacy and safety of granulocyte and monocyte adsorption apheresis for ulcerative colitis: a meta-analysis. Dig Liver Dis. 2014;46:219-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 90. | Martín de Carpi J, Vilar P, Prieto G, García Novo MD, Ribes C, Varea V. Safety and efficacy of granulocyte and monocyte adsorption apheresis in paediatric inflammatory bowel disease: a prospective pilot study. J Pediatr Gastroenterol Nutr. 2008;46:386-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 91. | Sawada K, Ohdo M, Ino T, Nakamura T, Numata T, Shibata H, Sakou J, Kusada M, Hibi T. Safety and Tolerability of Nafamostat Mesilate and Heparin as Anticoagulants in Leukocytapheresis for Ulcerative Colitis: Post Hoc Analysis of a Large-Scale, Prospective, Observational Study. Ther Apher Dial. 2016;20:197-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 92. | Vindigni SM, Zisman TL, Suskind DL, Damman CJ. The intestinal microbiome, barrier function, and immune system in inflammatory bowel disease: a tripartite pathophysiological circuit with implications for new therapeutic directions. Therap Adv Gastroenterol. 2016;9:606-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 145] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 93. | Sakuraba A, Sato T, Morohoshi Y, Matsuoka K, Okamoto S, Inoue N, Takaishi H, Ogata H, Iwao Y, Hibi T. Intermittent granulocyte and monocyte apheresis versus mercaptopurine for maintaining remission of ulcerative colitis: a pilot study. Ther Apher Dial. 2012;16:213-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 94. | Tomomasa T, Tajiri H, Kagimoto S, Shimizu T, Yoden A, Ushijima K, Uchida K, Kaneko H, Abukawa D, Konno M, Maisawa S, Kohsaka T, Kobayashi A; Japanese Study Group for Pediatric Ulcerative Colitis. Leukocytapheresis in pediatric patients with ulcerative colitis. J Pediatr Gastroenterol Nutr. 2011;53:34-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 95. | Morabito V, Novelli S, Poli L, Ferretti G, Ruberto F, Pugliese F, Pretagostini R, Berloco PB, Rossi M. Adacolumn Granulocyte-Apheresis for Alcoholic Hepatitis: Preliminary Study. Transplant Proc. 2016;48:352-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 96. | Higashi Y, Shimokawa M, Kawai K, Kanekura T. Granulocyte and monocyte adsorption apheresis for Behçet's disease in a pregnant woman. J Dermatol. 2013;40:1042-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 97. | Kitagaichi M, Kusaoi M, Tsukahara T, Murayama G, Nemoto T, Sekiya F, Kon T, Ogasawara M, Kempe K, Yamaji K, Tamura N, Tsuda H, Takasaki Y. Safety and efficacy of the leukocytapheresis procedure in eighty-five patients with rheumatoid arthritis. Transfus Apher Sci. 2016;55:225-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |