INTRODUCTION
The intrahepatic biliary tree is a complex three-dimensional network of interconnected ducts which starts at the level of canals of Hering, continues into intrahepatic ducts of increasing diameter and ends at the level of the extrahepatic bile ducts[1]. The intrahepatic biliary tree plays a critical role in many liver functions including bile formation, regeneration, injury repair, fibrosis, angiogenesis and regulation of blood flow. Most of these events are regulated by several neuropeptides, hormones, cytokines and growth factors which target cholangiocytes[2], the epithelial cells lining the biliary tree. Cholangiocytes are the preferential target of damage in a group of chronic cholestatic liver diseases called cholangiopathies, with a high social and economic impact due to their high prevalence and morbidity such as primary biliary cirrhosis (PBC), primary sclerosing cholangitis (PSC), polycystic liver disease (PCLD) and cholangiocarcinoma (CCA)[3-7]. In laboratory animals, ‘‘typical’’ cholangiocyte proliferation is achieved by a number of experimental models including bile duct ligation (BDL), partial hepatectomy, acute CCl4 feeding and chronic feeding of a-naphthylisothiocyanate (ANIT) or bile salts[8-11]. In these hyperplastic models, cholangiocyte proliferation is closely associated with increased secretin receptor gene expression and secretin-stimulated cAMP levels[12-18]. A variety of clinical and epidemiological observations have shown the involvement of sex hormones as inducers of growth and differentiation of target cells expressing their receptors[3]. The molecular mechanism of these complicated events, especially sex hormone-dependent growth enhancement, has been studied extensively. In MCF-7 cells, an estrogen-induced autocrine loop has been demonstrated to play an important role for estrogen-dependent growth. Androgen has been also found to promote the growth of SC-3 cell through the induction of several autocrine growth factors[4].
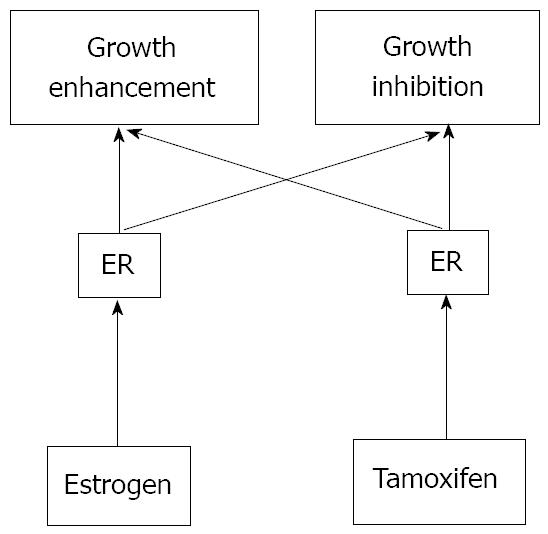
Sex hormones such as estrogens and androgens have been well known to regulate the growth of normal as well as transformed target cells[19-24]. Generally, they have been proposed to promote cell growth whereas so-called anti-hormones inhibit the hormone-dependent growth. For example, anti-androgens such as cyproterone acetate have also been known to accelerate the growth of prostate cancer in some circumstances[5]. Conversely, administration of a large amount of estrogens frequently causes the regression of estrogen-receptor-positive breast cancer[6]. Tamoxifen, the most widely used therapeutic agent of estrogen-dependent breast cancer, exhibits organ- and species-dependent differences in cell growth regulation[7]. Even in the same cells, growth response to tamoxifen has been observed to differ in a dose-dependent manner. One plausible explanation is that two pathways exist for estrogen-dependent growth in target cells, one for the stimulatory and another for the inhibitory signal[4] (Figure 1).
Figure 1 Two-pathway theory of estrogen-receptor-dependent growth.
Many estrogen target cells contain growth-stimulatory and -inhibitory pathways. Both pathways are mediated through ERs. Tamoxifen may possess relatively high affinity for the growth-inhibitory pathway whereas estrogen can mainly activate the growth-stimulatory pathway. However, any ligand for ER can stimulate both pathways.
In particular, estrogens exert a trophic action in several target organs such as liver[8] where they modulate growth and repair, intervening in neonatal liver growth and regeneration after injury in adults[3]. Moreover, chronic administration of estrogens for pharmacological purposes induces an enlargement of liver mass[25,26] and after partial hepatectomy, ERs expression in hepatocytes increases with subsequent transcription of genes involved in proliferation to restore a normal liver mass[25,27]. With this review, we aim to summarize the latest findings about the role of sex hormones on biliary epithelium function, their effects and alterations during cholestasis. The role and mechanism by which sex hormones modulate cholangiocyte functions have been explored only over the last few years at both experimental and clinical levels[28-50].
SEX HORMONES
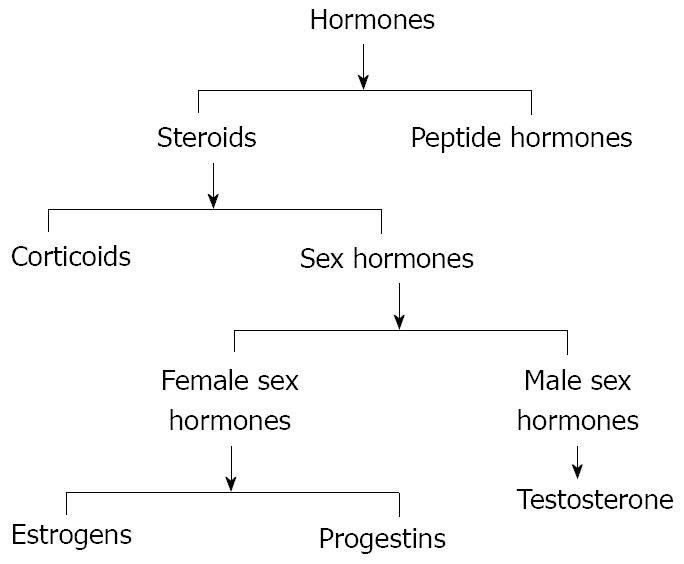
Hormones are the chemical messengers of the body since they are involved in transmission of information from one tissue to another and from cell to cell. Hormones circulate in the bloodstream and interact with target cells that possess receptors that can only be activated by a specific type of hormone. Several kinds of hormones exist for their structure or activity in the cell. Usually they are known as steroids and peptides (Figure 2). In general, steroids are sex hormones, chemical substances made from cholesterol and produced by a sex gland or other organ that has an effect on the sexual features of an organism[9]. Like many other kinds of hormones, they may also be artificially synthesized. On the other hand, peptides are made from long strings of amino acids to regulate other functions and are sometimes referred to as “protein” hormones.
Figure 2 Scheme of the two classes of hormones, steroids and peptide hormones that successively can be divided in corticoids and sex hormones.
Sex hormones are divided into 3 groups: (1) female sex hormones or estrogens; (2) male sex hormones or androgens; and (3) pregnancy hormones or progestins.
Estrogens
Estrogen is a generic term for estrus-producing compounds; the female sex hormones including estradiol, estriol and estrone (Figure 3). In humans, the estrogens are formed in the ovary, adrenal cortex, testis and fetoplacental unit and are responsible for female secondary sex characteristic development and, during the menstrual cycle, act on the female genitalia to produce an environment suitable for fertilization, implantation and nutrition of the early embryo[10]. Uses for estrogens include oral contraceptives, hormone replacement therapy, advanced prostate or postmenopausal breast carcinoma treatment and osteoporosis prophylaxis[51-55]. They also antagonize the effects of the parathyroid hormone minimizing the loss of calcium from bones and thus helping to keep bones strong[11]. Estradiol (E2) is the main female sex hormone. Its actions are mediated by two members of the nuclear receptor superfamily, estrogen receptor ERα and ERβ, and a recently discovered G protein-coupled membrane receptor, GPR30[56,57]. Mechanisms by which ERα and ERβ bind ligand, dimerize, associate with coactivators or corepressors and regulate gene transcription are typically referred to as “genomic” actions[12], which ultimately regulate both cell proliferation and survival[58-61]. Estrogens play biological activities in several organs[13] including the cardiovascular system, nervous system, digestive system and “male” organs such as the prostate. In target tissues, estrogens may exert opposite actions and heterogeneous effects[62-66]. In detail, overexpression of ERα has been associated with cancer development and progression in several organs[14]. The functions of ERβ are linked to a protective effect against uncontrolled or neoplastic cell proliferation[64,67]. In different types of cancer, estrogens synergize the effects of growth factors by acting at both receptor and post-receptor levels favouring the growth and spreading of tumour mass[68-73]. The liver is a hormone-sensitive organ. Both normal liver and hepatocellular carcinoma (HCC) tissues from male and female mammals have been shown to express specific ERs, stimulating both in vivo and in vitro hepatocyte proliferation[15]. Moreover, anti-estrogens like tamoxifen have been shown to reduce levels of ERs and to inhibit hepatocyte proliferation following partial hepatectomy[16]. Long-term use of oral contraceptives (OCs) and anabolic androgenic steroids (AASs) can induce both benign and hepatocellular tumors[17]. Other experimental findings suggest that estrogens have numerous neuroprotective actions. This responsiveness can diminish with age, reducing neuroprotective actions of estrogen[18]. Hormonal treatment plays an established role in several solid tumors, first of all in breast cancer where, for the last decades, the antiestrogen tamoxifen has been the most commonly used treatment for patients with estrogen receptor alpha (ER)-positive breast cancer[19]. Tamoxifen is characterized by a favourable toxicity profile which, together with the easy oral administration, makes this drug an interesting candidate for treatment of other solid tumors potentially responding to hormonal manipulation[74-77]. In addition, there is increasing evidence showing that adipose tissue is a site of steroid metabolism, including the interconversion of estrone (E1) and E2. The presence of both estrogen receptors (ERα and ERβ) in preadipocytes and mature adipocytes strongly suggest a role for active estrogen in these cells. For that reason, adipose tissue can be considered a significant source of estrogenic compounds.
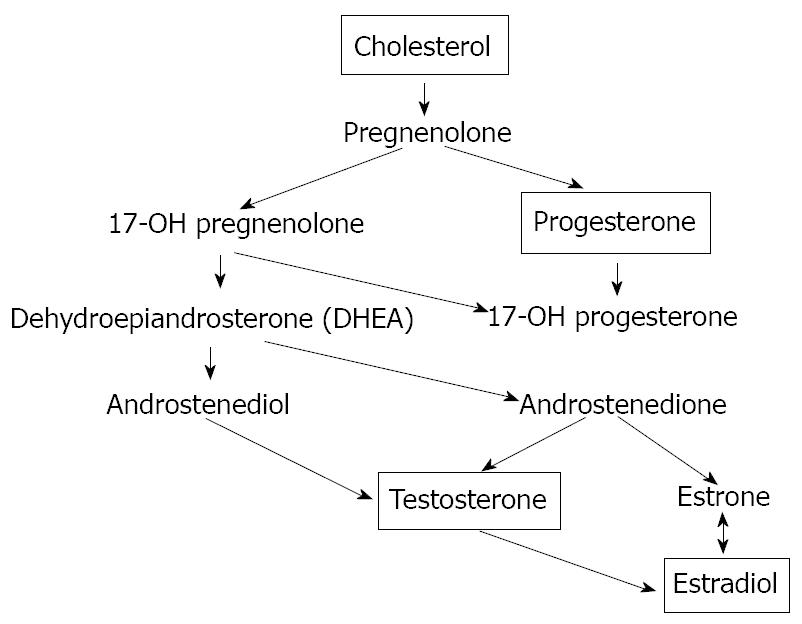
Figure 3 The biosynthesis of the sex hormones starts with the oxidation of the side chain of cholesterol, which is catalyzed by the enzyme cytochrome P450scc to form pregnenolone.
The next steps in the biosynthesis of testosterone can proceed via two different routes. Pregnenolone can be oxidised first by cytochrome P45017a to 17a-hydroxypregnenolone. The enzyme 3β-HSD also can convert pregnenolone first into progesterone. Both pregnenolone and progesterone are accepted as substrate by the enzyme cytochrome P45017a. In this way, after 3β-hydroxy-5-androstene-17-one (DHEA) synthesis, there is the testosterone and successively the estradiol formation.
Androgens
Androgens are a special kind of fat molecule with a four-ringed, carbon atom backbone or core[20]. A series of chemical reactions transform cholesterol first into the steroid pregnenolone and then into testosterone and other androgens (Figure 3). Like all steroid hormones, androgens produce effects by docking with receptors on the cell’s membrane surface or inside the cell in the liquid cytoplasm[20]. The steroid hormone/receptor unit moves into the nucleus activating specific genes. These genes drive the cell changes guiding androgen-controlled growth and development[21]. Scientists have studied androgens since the 18th century. John Hunter initially described androgenic actions in 1771. Almost two century later in 1935, Leopold Ruzicka worked out the chemical structure of the “androgenic principle” from the testes, calling it testosterone[22]. Testosterone and dihydrotestosterone (17-beta-hydroxy-5-alpha-androstan-3-one) are the most potent androgens in humans and four-legged vertebrates[20]. The weaker androgens androstenedione and dehydroepiandrosterone (DHEA) occur in small amounts in all vertebrates[23]. Testosterone is essential for the production of sperm and is manufactured by the interstitial Leydig cells of the testes. Its secretion increases sharply at puberty and is responsible for the development of the so-called secondary sexual characteristics of men during puberty[20]. Synthetic testosterone analogs are used in medicine to promote muscle and tissue growth in patients with muscular atrophy[24]. Testosterone therapy is indicated in adult men for the treatment of hypogonadism[25]. Over the last three decades it has become apparent that testosterone plays a significant role in the maintenance of bone and muscle mass, in erythropoiesis and in mental functions. Androgens are also key players in glucose homeostasis and lipid metabolism and exert an important role in liver. In fact, it has been observed that androgen receptors (ARs) are present in normal liver tissue from both males and females and that their expression is increased in tumor tissue[16]. Moreover, cross-sectional epidemiological studies have reported a direct correlation between plasma testosterone and insulin sensitivity and low testosterone levels are associated with an increased risk of type 2 diabetes mellitus, dramatically illustrated by androgen deprivation in men with prostate carcinoma[42,43]. Prostate cancer is one of the most common cancers among men and androgens are involved in controlling the growth of androgen-sensitive malignant prostatic cells[26]. The model of LNCaP prostate cancer cell line was used to study androgen and estrogen metabolism during the transformation process. It was discovered that substantial changes in androgen and estrogen metabolism occur in the cells during the process[45-47]. Recent evidence indicates androgen actions in protecting the brain against neurodegenerative diseases and their positive effects on age-related testosterone loss in men and increased risk for Alzheimer’s disease (AD)[27]. The successful use of hormone therapies in aging men and women to delay, prevent and/or treat Alzheimer’s disease will require additional research to optimize key parameters of hormone therapy[28].
Progestins
The term progestins is defined as the natural or synthetic progestational substances that mimics some or all of the actions of progesterone, a crude hormone of the corpus luteum from which progesterone can be isolated in pure form[29]. Progesterone is responsible for preparing the uterus for implementation of the fertilized egg. It also has an important role as a birth control agent[30]. It is a steroid hormone produced in the ovary under the control of the pituitary gonadotropins[78-80] (Figure 4). It has also been recently demonstrated that the synthetic progestogen, levonorgestrel, increases progesterone accumulation in cultured, stable porcine granulosa cells, the JC-410[81-93]. Results of those studies have been interpreted to suggest that progestins may affect progesterone synthesis by the regulation of steroidogenic enzymes, the cytochrome P450 side-chain cleavage (P450scc) and 3β-hydroxysteroid dehydrogenase (3β-HSD)[31]. The genomic action of progesterone is mediated by two progesterone receptor (PR) isoforms, A and B[84,85]. PR-B is a strong activator of gene transcription, whereas PR-A can act as a ligand-dependent trans-repressor of PR-B[32]. The large majority of PR target genes have been identified in breast cancer cells[87,88]. Different evidence indicates that this hormone also exerts neuroprotective effects on the central nervous system (CNS). Its neuroprotective actions make it a particularly promising therapeutic agent for neuroinjury and neurodegenerative diseases. Progesterone appears to exert its protective effects by protecting or rebuilding the blood-brain barrier, decreasing the development of cerebral edema, down-regulating the inflammatory cascade and limiting cellular necrosis and apoptosis[33]. The family of anti-progestins, i.e. mifepristone, includes pure agonists such as progesterone itself or progestins and, at the other end of the biological spectrum, pure progesterone receptor antagonists (PA). Selective progesterone receptor modulators (SPRM) have mixed agonist-antagonist properties and occupy an intermediate position of the spectrum. Mifepristone is used to terminate pregnancy[34]. Many PA and SPRM display direct antiproliferative effects in the endometrium although with variable actions which seem product- and dose-dependent. This property justifies their use in the treatment of myomas and endometriosis. Interestingly, clinical data show that treatment with these compounds is not associated with hypo-estrogenism and bone loss. The potential clinical applications of these compounds cover a broad field and are very promising in major public health areas. Further developments might also include hormone replacement therapy in post-menopausal women as well as the treatment of hormone-dependent tumors[35].
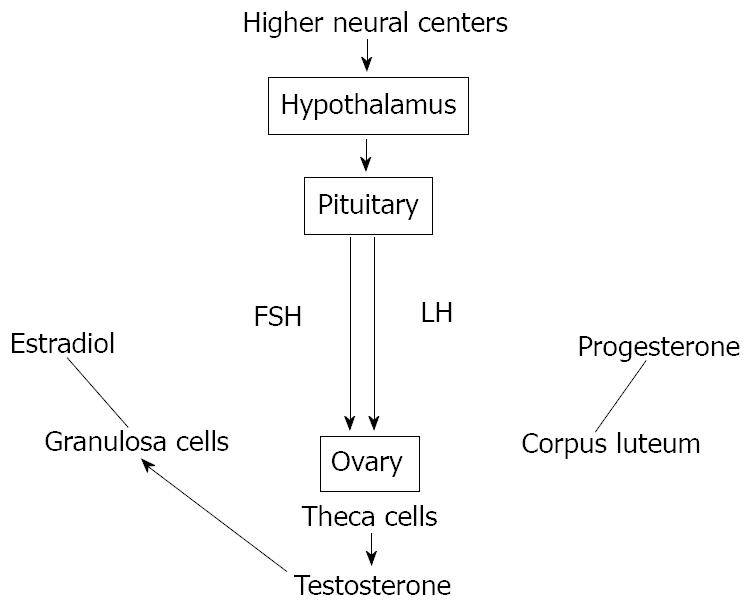
Figure 4 Scheme of hypothalamic-pituitary-gonadal (HPG) axis control exerted by both circulating and in situ locally produced estradiol in men.
This axis controls development, reproduction, and aging in animals. The hypothalamus produces gonadotropin-releasing hormone (GnRH). The pituitary gland produces luteinizing hormone (LH) and follicle-stimulating hormone (FSH), and the gonads produce estrogen, progesterone and testosterone from different kinds of cells.
Other sex hormones
Another group of substances secreted by the pituitary gland can be defined sex hormones. They include the follicle-stimulating hormone (FSH), the luteinizing hormone (LH) and the prolactin (Prl). The synthesis and secretion of estrogens is stimulated by FSH which is in turn controlled by the hypothalamic gonadotropin releasing hormone (GnRH) (Figure 4). High levels of estrogens suppress the release of GnRH providing a negative-feedback control of hormone levels[36]. Progesterone production is stimulated by LH which is also stimulated by GnRH[36]. Elevated levels of progesterone control themselves by the same negative feedback loop used by estrogen[37]. The two gonadotropins, FSH and LH, are key regulators of ovarian cell functions and the potential role of gonadotropins in the pathogenesis of ovarian cancer is suggested. The presence of gonadotropins in ovarian tumor fluid and their receptors expression suggests the importance of these factors in the transformation and progression of ovarian cancers as well as being prognostic indicators[94-97]. The recent cDNA microarray analyses and characterization in the molecular mechanisms of gonadotropin signaling have indicated the effects of gonadotropins on the regulation of some ovarian cancer cell growth, survival and metastasis that may involve other growth factors[38]. Prl is another hormone released by the pituitary gland that stimulates breast development and milk production in women[39]. It is secreted by so-called lactotrophs in the anterior pituitary as a prohormone. Although the pleiotropic actions of Prl are recognized, its role in regulating growth and differentiation of mammary tissues is better understood[98,99]. Several lines of evidence have also indicated that Prl acts as an autocrine, paracrine and endocrine progression factor for mammary carcinoma in vitro and in vivo in rodents and humans[100]. These data include recent epidemiologic studies indicating that postmenopausal women with ‘high-normal’ levels of Prl are at increased risk of breast cancer[101-105]. Elevated prolactin (hyperprolactinemia) may be due to a benign tumor in the pituitary gland called a prolactinoma. Abnormally low prolactin (hypoprolactinemia) can cause menstrual disorders and lead to inadequate lactation[31]. It is concluded that the rat is a predictive model for human mammary carcinogenesis and that rat mammary carcinogenesis induced by hyperprolactinaemic drugs may have greater importance in human toxicological risk than previously thought[40].
ESTROGENS AND BILIARY EPITHELIUM
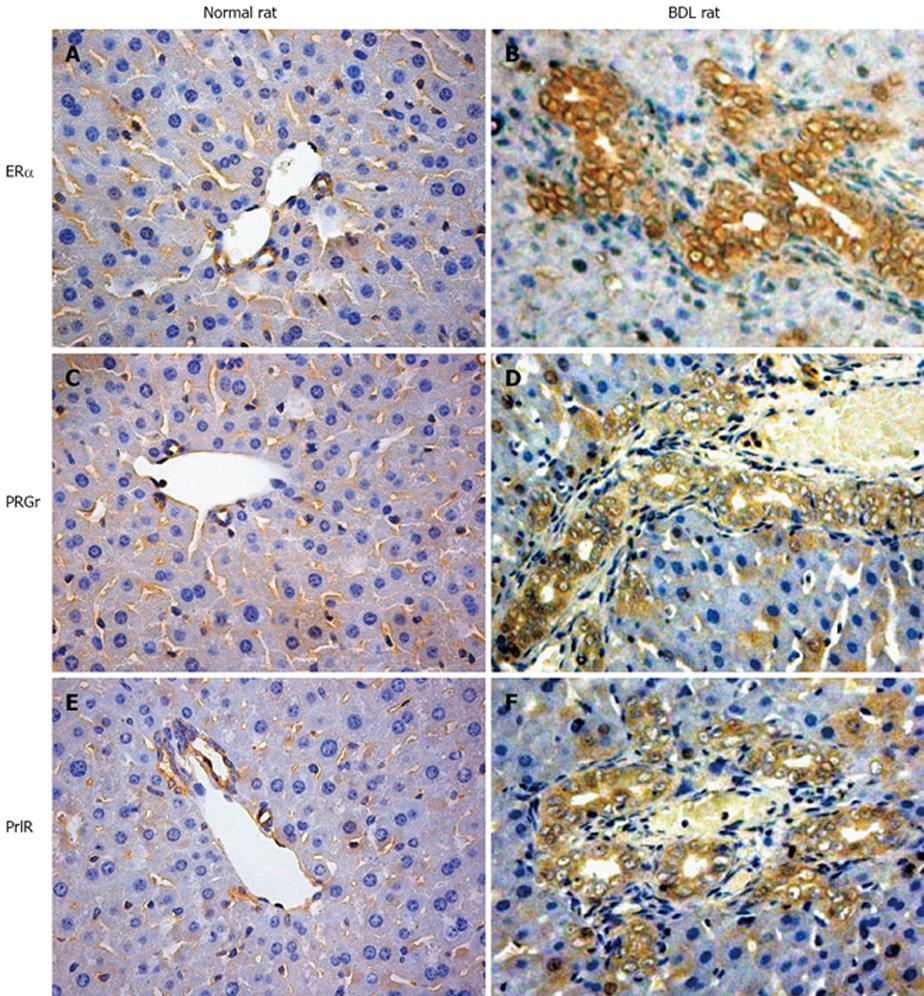
Estrogens and their metabolites have been hypothesized to have a pathogenic role in the diseases which preferentially affect the female sex[106-109]. Furthermore, marked alterations of estrogen hepatic metabolism occurs in cholestasis which is one of the hallmarks of cholangiopathies, including the decreased hepatic levels of P450-dependent microsomal enzymes with a consequent enhanced estradiol serum level[41]. Over the last years, we have discovered that rat cholangiocytes express both ERα and ERβ subtypes while hepatocytes only express ERα[28]. In addition to that, cholangiocyte proliferation after BDL is associated with a marked increase in the expression of ER and especially the ERβ while hepatocytes which do not proliferate after BDL display a decrease of ERα protein expression[42] (Figure 5). This important role of estrogens in modulating cholangiocyte proliferation during BDL is associated with enlarged bile duct mass and enhanced estradiol serum levels[29].
Figure 5 Some representative immunohistochemistry for Erα, progesterone and prolactin receptors in normal and bile duct ligation (BDL) rats.
A, B: Erα receptors; C, D: progesterone receptors; E, F: prolactin receptors. The expression of all these receptors is highly increased after BDL compared with that in the biliary epithelium of the normal animal. Original magnification × 40.
The role of estrogens in modulating cholangiocyte proliferation has been confirmed by experiments showing that when BDL rats were treated with tamoxifen or the pure ER antagonist, ICI 182,780, the intrahepatic bile duct mass was markedly decreased in comparison with the control rats by impaired proliferation and enhanced cholangiocyte apoptosis. In breast cancer and hepatocellular carcinoma, tamoxifen induces cell death by multiple mechanisms including the blocking of the mitogenic effect of estrogens and induction of apoptosis-related genes[43]. In fact, the Fas receptor/Fas ligand pathway plays a crucial role in tamoxifen-induced apoptosis in hepatocellular carcinoma and cholangiocarcinoma cell lines[110-112]. To support the positive modulatory effect of estrogens on cholangiocyte proliferation, in vitro experiments show that proliferation of isolated rat cholangiocytes were significantly increased by 17β-estradiol and that these effects were individually blocked by ER antagonists[28]. Regarding the role of endogenous estrogens on modulating cholangiocyte proliferation during experimental cholestasis, we also evaluated the effects of ovariectomy (OVX) and estrogen replacement treatment in BDL rats[44]. OVX rats were submitted to BDL and the bile duct mass was compared with control BDL rats submitted to sham-OVX and with BDL-OVX rats treated with exogenous administration of 17β estradiol. OVX induced a significant reduction of bile duct mass in BDL rats that was associated with a decreased expression of ERβ. Administration of 17β estradiol induced a normalization of bile duct mass, ER expression and cholangiocyte proliferation in comparison with untreated BDL rats. A probable cross-talk between estrogens and growth factors including IGF1 (insulin like growth factor) has been proposed and later demonstrated that result in a synergistic growth stimulation[4,112]. This signaling cascade, typically activated by growth factors acting through tyrosine kinase receptors, involves the recruitment of the steroid receptor-coactivator (Src) and adapter protein Shc (Src-homology/collagen protein) which act upstream to the mitogen-activated protein (MAP) kinase isoforms ERK1/2 (extracellular signal-regulated kinase)[113,114]. We found that cholangiocyte proliferation induced by BDL involves the activation of the Src/Shc/ERK signalling cascade blocked through administration of ER antagonists[45].
Normally, human cholangiocytes do not express ERs but they stain positive for ERα and β in different pathological conditions such as primary biliary cirrhosis (PBC)[31], polycystic liver disease[115] and cholangiocarcinoma[116-121]. All these conditions are characterized by reactive or neoplastic cholangiocyte proliferation, suggesting that estrogens and their receptors may play a role in modulating the proliferative activities of cholangiocytes and therefore the course of these diseases.
PBC is one of the chronic cholestatic liver diseases which represent the most frequent acquired cholangiopathy. This is an autoimmune liver disease in that the key pathology involves the attack upon the small, microscopic bile ducts by immune system inflammatory cells. The result is a chronic granulomatous inflammatory infiltrate invading and progressively destroying the small bile ducts within the portal tracts of the liver[46]. The disease predominantly affects females with a typical clinical presentation occurring during the peri- and post-menopausal period[47]. Recent findings suggest that estrogens may influence the course of PBC by directly modulating the pathophysiology of cholangiocytes[31]. In fact, in PBC, such as in other chronic cholestatic conditions, estrogen serum levels are increased as a consequence of impaired hepatic metabolism and biliary excretion of estrogens and their metabolites[48]. However, estrogen replacement therapy as osteoporosis treatment has been shown to be safe in PBC patients[49]. These clinical studies allowed summarizing the concept that administration of estrogens in PBC patients exerts deleterious effects on the liver but they can improve liver function. During PBC, the ER expression varies according to different stages and correlates with markers of proliferation and apoptosis. ERα expression increases from 1% of cholangiocytes in PBC stage I to 12% in stage III while ERβ is stably high in all histological stages. Interestingly, in stages I-III, ERα expression co-localizes with PCNA indicating that the expression of this receptor subtype is a typical feature of proliferating cholangiocytes. Furthermore, in stage IV of PBC where there is the maximal degree of ductopenia, cholangiocytes are negative for ERα and express the lowest proliferation/apoptosis ratio[31]. We can speculate that a relative proliferative deficiency of cholangiocytes in the terminal ductopenic stages of PBC is associated with the disappearance of ERα. These findings could have important therapeutic implication by the modulation of ERs. To this latter regard, preliminary clinical observations indicate that tamoxifen improves biochemical parameters of cholestatis in PBC patients. Interestingly, through the ERα, estrogens can positively modulate the GH/ IGF-1 axis[14].
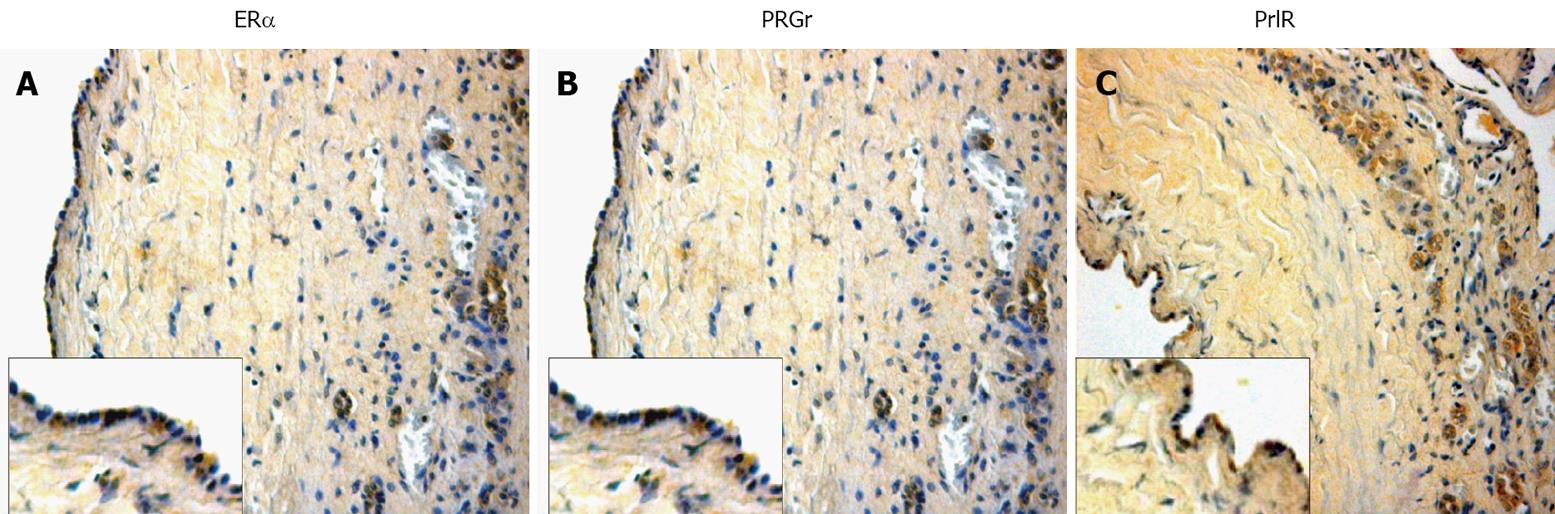
Autosomal dominant polycystic kidney disease (ADPKD) is one of the most prevalent human genetic diseases[50]. Hepatic cysts are the most common extra-renal clinical manifestation of ADPKD[51]. Estrogens have a role in the development and progression of hepatic cysts in ADPKD patients. The probability of developing hepatic cysts is higher in women than in men. Many studies and the clinical observations show a strict estrogen sensitivity of cyst formation and progression in ADPKD patients[122,123]. First of all, the epithelial layer of hepatic cysts presents the expression of ERβ and this occurs in all cysts examined, whereas the staining for ERα was less evident (Figure 6). Estrogens act not only directly but also by promoting the synthesis and release of growth factors from the cyst epithelium[115]. These findings show how the formation and progression of hepatic cysts is highly sensitive to changes in the estrogen status in the body[4,115].
Figure 6 Immunohistochemistry for ERα, progesterone and prolactin receptors in liver sections from patients affected with polycystic liver disease.
A: Erα receptors; B: progesterone recepors; C: prolactin receptors. Also in course of human cholangiopathies, these three considered receptors seem to play an important role in cholangiocyte physiology. Original magnification × 20.
Cholangiocarcinoma is a malignant tumor arising from cholangiocytes and characterized by a poor prognosis and scarce response to current therapies[124-132]. Human intrahepatic cholangiocarcinoma and the human intrahepatic cholangiocarcinoma cell line HuH-28 express ERs. The use of 17β-estradiol stimulates proliferation and inhibits apoptosis of HuH-28 cell lines, findings comparable with the proliferative response of MCF7, a breast cancer cell line. Proliferation of these cells induced by 17β-estradiol is associated with enhanced protein expression of ERα, p-ERK1/2 and pAKT but with decreased protein expression of ERβ[32,116]. This further supports the role of ERα in the estrogen-dependent modulation of neoplastic cell growth. Estrogens appear to act in several critical points of the IGF signal transduction pathway. ERα and IGF-1R have been shown to co-precipitate and their state of activation as well as the related signaling pathways have been shown to be potentiated by their coupling[52]. Finally, this mechanism may converge at different common transduction pathways modulating proliferation including ERK and phosphatidylinositol-3 kinase/Akt pathways[53]. Thus, the role played by estrogens and their receptors in the growth of ER-positive neoplasms represents the basis for the pharmacological treatment and/or prevention of different cancers with ER antagonists.
ANDROGENS AND BILIARY EPITHELIUM
The role of androgens on biliary epithelium has been poorly investigated. In fact, we have only preliminary data on the castration effects in normal and experimental rat model of BDL in which there is a decrease in androgen receptors expression and impairment in cholangiocyte growth especially after bile duct ligation to support the hypothesis that testosterone, as estrogens, may play a key role in biliary epithelium proliferation[54]. In human conditions, several studies exist on the use of anabolic androgenic steroid (AAS). They are frequently utilized at high doses by bodybuilders to achieve a rapid increase in muscle mass although they are associated with a number of side effects. Several liver disorders have been reported to be associated with AAS consumption such as cholestasis, peliosis hepatis and liver tumors[55]. In recent times, this use has also been proved to be involved in the development of hepatic adenomas (HA)[56]. Although more than 750 cases of oral contraceptive-induced HA have been reported, apparently androgen-induced HA are relatively rare. HA are not malignant tumors but surgical intervention may be required if sudden massive bleeding or liver failure occurs; rupture of HA with haemoperitoneum can be a life threatening complication[57]. A non-surgical approach should be considered for androgen-induced HA given that some tumors have regressed after AAS administration was stopped[58]. In any case, after a diagnosis of liver tumors the administration of AAS should cease[133-135].
PROGESTINS AND BILIARY EPITHELIUM
As previously summarized, a number of studies have shown that, not only estrogens and androgens but also progestins strongly regulate cholangiocyte functions. Glaser et al have been found that female and male rat cholangiocytes express nuclear and membrane receptors that bind progesterone (PR, PGRMC1, PGRMC2, and mPRα). Following chronic administration of progesterone to normal female and male rats, there is an increase in biliary growth which can be partly prevented by the simultaneous administration of the nuclear progesterone receptor antagonist RU-486[59] or with administration of a neutralizing anti-progesterone antibody[60]. Finally, this study also demonstrated for the first time that the biliary epithelium possesses the enzymatic pathway for the steroidogenesis of progesterone and secrete progesterone, indicating that, in addition to a paracrine pathway, cholangiocytes regulate their growth in an autocrine mechanism[136-142] (Figure 6). In humans, the concentrations in serum of sulfated metabolites of progesterone are known to be elevated in patients with intrahepatic cholestasis of pregnancy (ICP)[61]. Some studies propose that patients with ICP have a selective defect in this secretion into bile probably for a genetic polymorphism of canalicular transporters for steroid sulphates or their regulation. Interaction with estrogen metabolites may further enhance the process triggering ICP in genetically predisposed individuals[62]. Ursodeoxycholic acid, an important bile acid, stimulates the biliary excretion of these metabolites, particularly those with a 3alpha-hydroxy-5alpha (H) configuration and disulphates. The effect appears to be independent of the stimulation of bile acid secretion. An effect of ursodeoxycholic acid on the reductive metabolism of progesterone cannot be excluded[63].
OTHER SEX HORMONES AND BILIARY EPITHELIUM
Information on the role of FSH in liver pathophysiology is limited[64]. A study has demonstrated that liver cirrhosis is associated with endocrine dysfunction, notably in the gonadal axis[65]. In males it has been recognized that cirrhotic liver disease is associated with hypogonadism and feminization parallel with impairments in the serum level of sex hormones[66]. The derangement of hypothalamic-pituitary function may play a role in the sexual dysfunction and changes in sex hormones in male patients with cirrhosis[64]. For the first time, we have shown that the biliary epithelium expresses FSHR and that FSH is a trophic factor for the biliary epithelium since chronic administration of FSH to normal rats increased cholangiocyte proliferation and intrahepatic ductal mass by cAMP-dependent phosphorylation of ERK1/2 and Elk-1[12-13]. In support of the findings that FSH treatment increases cholangiocyte FSH receptor expression, it has been demonstrated that it induces follicular growth and ovulation together with an increase in FSH binding and mRNA levels in ovaries[143,144]. In addition, another study has demonstrated that treatment of these cells with FSH increases the levels of two FSH receptor mRNA transcripts[67]. Although FSH may modulate cholangiocyte growth by a paracrine mechanism, our studies support the novel concept that FSH is a key player in the autocrine loop regulating the balance between cholangiocyte proliferation and loss. These findings have important pathological implications since modulation of cholangiocyte expression and secretion of the trophic factor FSH may be important in the management of chronic cholestatic liver diseases[145].
Regarding the other gonadotropin, it has been observed that with a reduction in the plasma level of testosterone there is an elevation of the LH level in BDL rats demonstrating a primary defect in testosterone production by testes[146].
If the BDL rats were treated with L-NAME, a NO inhibitor, to reduce its over production during bile duct ligation, there is an interesting effect on the LH levels[68]. Prolonged L-NAME treatment could not decrease the elevated level of LH in BDL rats while it could increase the level of testosterone in those rats. These data suggest that the primary effect of bile duct ligation is at the level of Leydig cells and the increase of LH is secondary to the decrease in circulating testosterone. One interpretation is that L-NAME has only a partial effect on the NO inhibited Leydig cells which can produce normal levels of testosterone after being stimulated by an increased level of LH. The other interpretation is based on the complex effect of NO on gonadotropin secretion. In fact, it has previously been clearly demonstrated that NO stimulates LHRH secretion by activating guanylate cyclase and supports a potential role of NO as a neuroactive agent involved in the control of LHRH secretion and, thereby, reproductive functions[69]. It has also been suggested that the endogenous level of NO may determine the sensitivity of GnRH-stimulated gonadotropin released by the anterior pituitary[146-151].
In addition, Prl participates in the regulation of liver function. Their receptors (PrlR) are expressed by rat hepatocytes in the sinusoidal domain of cellular membranes and in perinuclear areas[70]. They are also expressed by human hepatocytes of patients with obstructive jaundice of different etiology but prolactin receptor expression is lower in hepatocytes compared to human cholangiocytes[71]. The expression pattern and regulation of PrlR isoforms is totally different in cholangiocytes compared to hepatocytes. In fact, mature rat cholangiocytes express low levels of PrlR while it is very high in hepatocytes; only the long isoform is detected in cholangiocytes while the short isoform predominates in hepatocytes; and PrlR levels in cholangiocytes are induced by obstructive cholestasis while it is the opposite in hepatocytes. From these data, the actions of prolactin on liver are anticipated to exhibit strong cell-type specificity in both normal and pathological conditions[72] (Figure 6).
Taffetani et al have demonstrated that Prl regulates the growth of female cholangiocytes, presumably by an autocrine mechanism. In fact, cholangiocytes from normal and BDL female and male rats express prolactin receptors. Furthermore, Prl has a trophic effect on the growth of normal female cholangiocytes by phosphorylation of PKCβ-I and dephosphorylation of PKCα. In addition, cholangiocytes express the protein for and secrete prolactin, suggesting that prolactin participates by an autocrine mechanism in the modulation of cholangiocyte proliferation and that it may be an important therapeutic approach for the management of cholangiopathies[152-159].
CONCLUSION
A large body of evidence supports the therapeutic potential of sex hormones in animal models and human clinical conditions in the modulation of cholangiocyte growth/loss. Mechanisms of action for most of them have been studied and others are in the course of study. Further investigations are needed to elucidate the precise mechanism of androgens, progestins and their receptors in regulating normal liver physiology and pathophysiology of cholestatic diseases. All of this interestingly suggests that sex hormones represent novel and important treatment options that could beneficially affect the pathophysiology of the biliary epithelium. Sex hormones clearly function as more than reproductive compounds by exhibiting a myriad of roles that are also essential to protect liver and biliary functions. In particular, the main concept is that estrogens and probably other hormones act by synergizing the effects of growth factors. This interaction may have more clinical implications for diseases involving the biliary epithelium in which cholangiocyte proliferation is a typical hallmark influencing disease progression and may also be relevant in the course of the neoplastic transformation. In conclusion, sex hormones are regulators of cholangiocyte proliferation in cholestasis and their modulation could represent a future therapeutic strategy for the management of cholangiopathies.
Supported partly by the University Federate Athenaeum Funds from University of Rome “La Sapienza” and PRIN 2007 to Gaudio E, University Funds to Onori P, the Dr. Nicholas C Hightower Centennial Chair of Gastroenterology from Scott and White, the VA Research Scholar Award, a VA Merit Award and the NIH Grants DK58411, and DK76898 to Dr. Alpini, a NIH RO1 Grant Award to Dr. Glaser (DK081442), a NIH K01 Grant Award (DK078532) to Dr. DeMorrow
Peer reviewer: Wei-Biao Cao, MD, Assistant Professor, Department of Medicine and Pathology, Rhode Island Hospital and the Warren Alpert Medical School of Brown University 55 Claverick St, Room 337, Providence, RI 02903, United States
S- Editor Zhang HN L- Editor Roemmele A E- Editor Liu N