Copyright
©The Author(s) 2021.
World J Gastrointest Pathophysiol. Jul 22, 2021; 12(4): 59-83
Published online Jul 22, 2021. doi: 10.4291/wjgp.v12.i4.59
Published online Jul 22, 2021. doi: 10.4291/wjgp.v12.i4.59
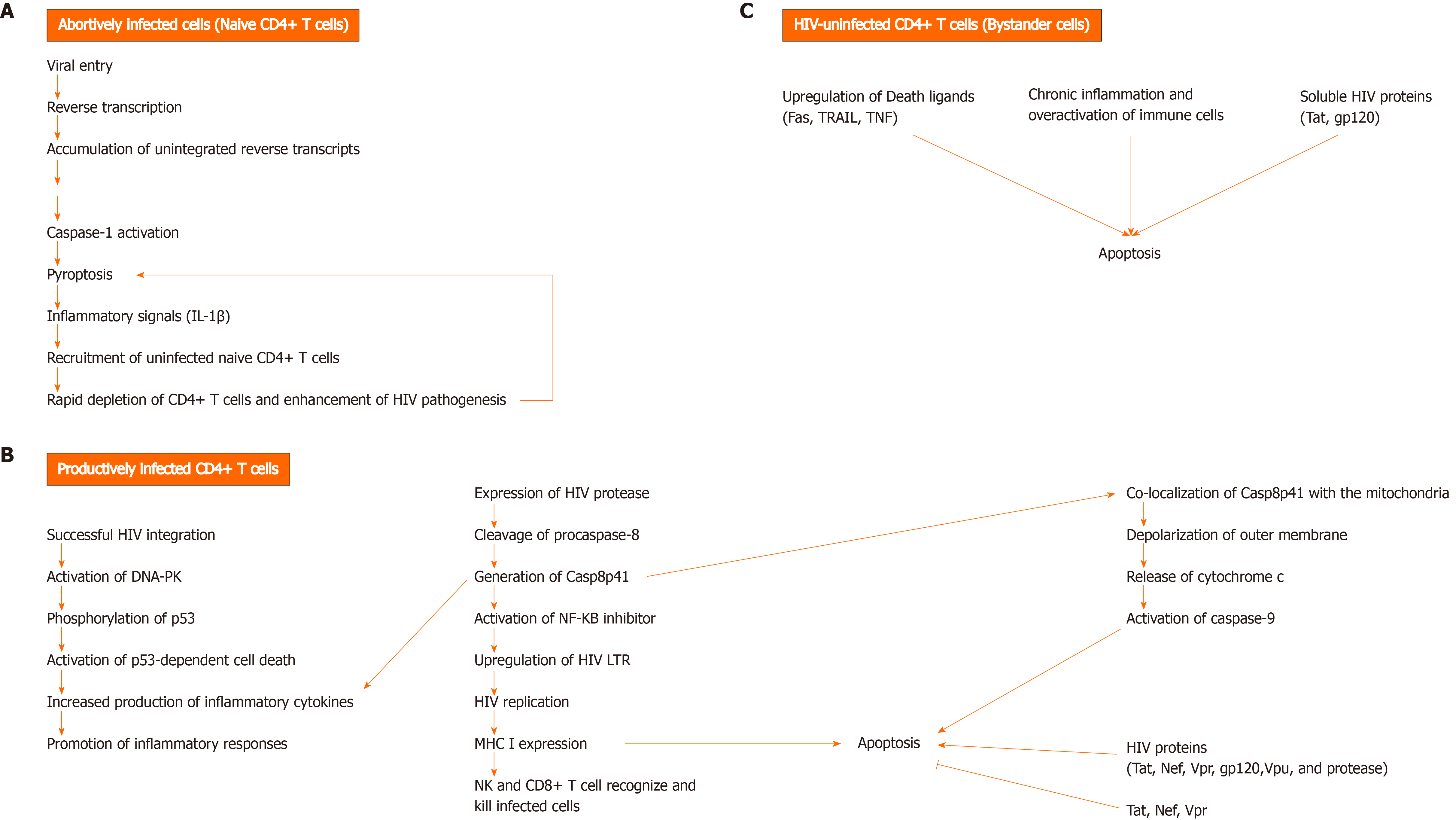
Figure 1 Mechanisms of CD4+ T cell death during human immunodeficiency virus infection.
A: Unsuccessful human immunodeficiency virus (HIV) infection can lead to HIV-infected CD4+ T cell death by pyroptosis, an inflammatory programmed cell death that occurs via caspase-1 activation. As a result, inflammatory signals, such as Interleukin-1β are released. The accumulation of unintegrated reverse transcripts, following viral entry and reverse transcription, can indirectly activate caspase-1 and induce pyroptosis in resting CD4+ T cells with abortive viral infection. Pyroptosis is thought to greatly contribute to the rapid depletion of CD4+ T cells and development of chronic inflammation, as a result of proinflammatory cytokine release from dying CD4+ T cells, which in turn causes the recruitment of uninfected and naïve CD4+ T cells into the lymphoid tissues. These cytokines trigger pyroptosis in the recruited cells, leading to a vicious cycle of inflammation, thereby enhancing HIV pathogenesis by creating an overactive immune environment and further cell death; B: Cell death can occur in productively infected CD4+ T cells, following successful HIV integration and expression of HIV protease (PR). HIV PR can cleave cellular procaspase-8 and generate Casp8p41. This fragment activates the transcription factor NF-kappaB inhibitor and thus, induces HIV replication by upregulating HIV long terminal repeats (LTRs). Casp8p41 expression promotes inflammatory responses by enhancing the production of pro-inflammatory cytokines. Besides, Casp8p41 induces apoptosis by directly co-localizing with the mitochondria and depolarizing its outer membrane. The subsequent release of cytochrome c from mitochondria leads to activation of caspase 9 and cell death. Another mechanism by which viral integration triggers cell death is through the activation of deoxyribonucleic acid-dependent protein kinase, resulting in phosphorylation of p53 and activation of p53-dependent cell death program. HIV Tat, Nef, and Vpr can have both pro- and anti- apoptotic effects. On the other hand, gp120, Vpu, and protease have pro-apoptotic effects. HIV-infected cells can be killed by cytotoxic lymphocytes, natural killer cells and CD8+ T cells, which become highly active during infection; C: HIV-uninfected cells, known as bystander cells, usually die by apoptosis during the course of infection, due to either: upregulation of death ligands (Fas, TRAIL, TNF), activation-induced cell death due to chronic inflammation and over activation of immune cells, or direct cytotoxic effects of soluble HIV proteins (Tat, gp120). DNA-PK: DNA-dependent protein kinase.
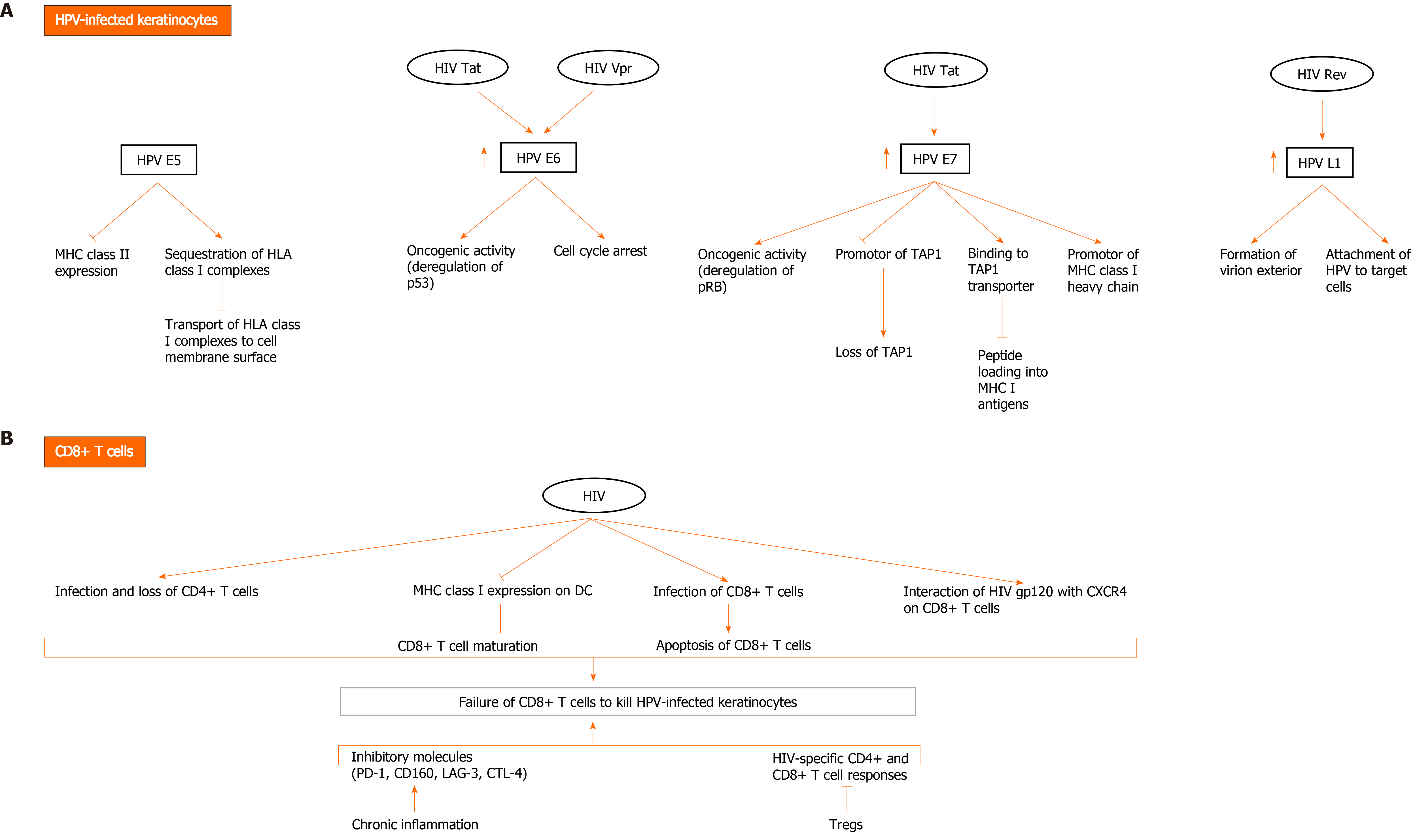
Figure 2 Possible mechanisms of direct and indirect interactions between human papillomavirus and human immunodeficiency virus to evade the immune system and mediate human papillomavirus carcinogenesis.
A: Human immunodeficiency virus (HIV) and human papillomavirus (HPV) contribute to HPV-related carcinogenesis and evasion of immune cells through several mechanisms involving direct interaction between HIV and HPV proteins in HPV-infected keratinocytes. HPV E5 oncoprotein downregulates major histocompatibility complex (MHC) II expression and sequesters human leukocyte antigen (HLA) class I complexes in keratinocytes, thereby blocking transport of HLA class I complexes to cell membrane surface. HPV E6 exerts oncogenic effects, mainly through deregulation of p53 and induction of cell cycle arrest. HPV E7 exerts oncogenic activity through deregulating pRB. It promotes downregulation of MHC class I expression through downregulating peptide transporter 1 associated with antigen processing (TAP1) and binding to TAP transporter, and thus inhibiting peptide loading into MHC I antigens. It also downregulates promotor of MHC I heavy chain. Importantly, HIV Tat upregulates the expression of HPV16 E6 and E7, enhancing their oncogenic effects. It also increases the expression of HPV L1. HIV Rev indirectly upregulates HPV L1 expression. HIV Vpr interacts with HPV E6 protein to induce cell cycle arrest and oncogenesis; B: HIV infection diminishes immune response to HPV infection, resulting in HPV persistence and pathogenesis. Failure of CD8+ T cells to kill HPV-infected keratinocytes is a major event in HIV and HPV co-infection and occurs through multiple mechanisms. In addition to loss of CD4+ T cells due to infection with HIV, downregulation of MHC I expression on dendritic cells (DC) by HIV inhibits CD8+ T cell maturation. A small fraction of CD8+ T cells become infected with HIV and are susceptible to the direct cytotoxic effects of the virus. Moreover, HIV gp120 interacts with CXCR4 on CD8+ T cells and affects their function. Other factors also contribute to inhibition of HIV-specific CD4+ and CD8+ T cell responses and include activation of Tregs and the expression of inhibitory molecules (programmed death-1 [PD-1], CD160, lymphocyte activation gene 3, cytotoxic T lymphocyte antigen-4 [CTLA-4]) by CD8+ T cells during chronic inflammation induced by HIV.
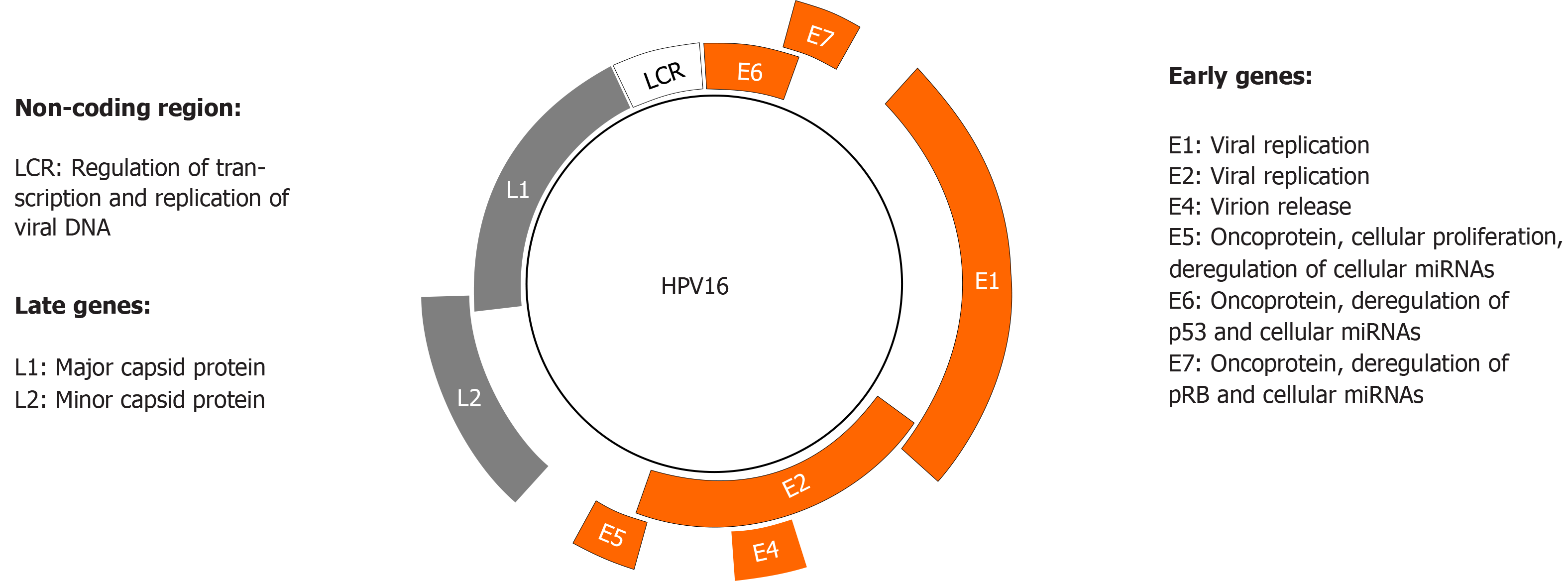
Figure 3 Human papillomavirus genome organization and function.
Human papillomavirus (HPV) has a circular double-stranded genome, which is divided into three regions: early, late, and non-coding long control region. The latter regulates transcription and replication of viral deoxyribonucleic acid. The early and late regions encode eight proteins whose major functions are shown.
- Citation: Al Bitar S, Ballouz T, Doughan S, Gali-Muhtasib H, Rizk N. Potential role of micro ribonucleic acids in screening for anal cancer in human papilloma virus and human immunodeficiency virus related malignancies. World J Gastrointest Pathophysiol 2021; 12(4): 59-83
- URL: https://www.wjgnet.com/2150-5330/full/v12/i4/59.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v12.i4.59