Published online May 28, 2017. doi: 10.4329/wjr.v9.i5.245
Peer-review started: December 13, 2016
First decision: January 16, 2017
Revised: February 23, 2017
Accepted: March 12, 2017
Article in press: March 13, 2017
Published online: May 28, 2017
Processing time: 160 Days and 8.2 Hours
To assess the safety and efficacy of transarterial chemoembolization (TACE) of hepatocellular carcinoma (HCC) using a new generation of 40 μm drug eluting beads in patients not eligible for curative treatment.
Drug eluting bead TACE (DEB-TACE) using a new generation of microspheres (embozene tandem, 40 μm) preloaded with 100 mg of doxorubicin was performed on 48 early or intermediate HCC patients with compensated cirrhosis. Response to therapy was assessed with Response Evaluation Criteria in Solid Tumors (RECIST) and modified RECIST (mRECIST) guidelines applied to computed tomography or magnetic resonance imaging. Eleven out of the 48 treated patients treated progressed on to receive liver orthotopic transplantation (OLT). This allowed for histological analysis on the treated explanted nodules.
DEB-TACE with 40 μm showed a good safety profile without major complications or 30-d mortality. The objective response rate of treated tumors was 72.6% and 26.7% according to mRECIST and RECIST respectively. Histological examination in 11 patients assigned to OLT showed a necrosis degree > 90% in 78.6% of cases. The overall time to progression was 13 mo (11-21).
DEB-TACE with 40 μm particles is an effective treatment for the treatment of HCC in early-intermediate patients (Barcelona Clinic Liver Cancer stage A/B) with a good safety profile and good results in term of objective response rate and necrosis.
Core tip: This is the first study exploring the safety and efficacy of 40 μm drug eluting bead transarterial chemoembolization for the treatment of hepatocellular carcinoma (HCC) in a series of 48 patients not suitable for ablation or surgical therapies. The use of microspheres smaller than 100 μm is not common practice in the western countries due to skepticism and fear of non-target embolization. Our aim is to present our initial experiences when treating with smaller microspheres so we all can test the potential advantages inherent to them and evaluate the effectiveness in the treatment of HCC nodules.
- Citation: Greco G, Cascella T, Facciorusso A, Nani R, Lanocita R, Morosi C, Vaiani M, Calareso G, Greco FG, Ragnanese A, Bongini MA, Marchianò AV, Mazzaferro V, Spreafico C. Transarterial chemoembolization using 40 µm drug eluting beads for hepatocellular carcinoma. World J Radiol 2017; 9(5): 245-252
- URL: https://www.wjgnet.com/1949-8470/full/v9/i5/245.htm
- DOI: https://dx.doi.org/10.4329/wjr.v9.i5.245
Transarterial chemoembolization (TACE) is the current standard of care for hepatocellular carcinoma (HCC) in patients with multinodular disease, classified as intermediate stage (stage B) of the Barcelona Clinic Liver Cancer (BCLC) staging system[1]. Furthermore, in clinical practice, a number of patients with early stage (stage A) disease, not eligible for curative treatment (surgery, transplantation or ablation) are commonly treated with TACE[2,3].
Conventional TACE (c-TACE) has shown superiority over basic supportive care in unresectable HCC in two randomised studies published in the early 2000s[4,5] and in a meta-analysis published in 2003[6].
Recently developed drug eluting beads (DEB) have the ability to bind and carry up to double the doxorubicin dose[7,8] thus overcoming the common drawbacks of c-TACE such as the release of the chemotherapeutic agent into the systemic circulation.
DEB-TACE superiority over c-TACE or TACE superiority over transarterial embolization has not been proven in recent studies[9,10] both in terms of survival and as objective response to treatment. These new microspheres have ensured a reduction in the systemic concentration of the loaded chemotherapeutic agent, with a lower rate of post-procedural toxicity compared to c-TACE[11-13]. The first available microspheres had a diameter ranging between 500 and 900 μm that has gradually reduced over the years to let DEB penetrate deeper into tumor circulation arterioles. This theory is supported by recently published controlled studies on smaller microspheres that show encouraging preliminary data on the radiological response in terms of extensive intratumoral necrosis[14,15].
Embozene tandem 40 μm (Boston Scientific, Minneapolis, MA, United States) are a new size of tightly calibrated spherical drug-eluting beads able to load up 100 mg of doxuribicin in a 2 mL syringe, or 150 mg in a 3 mL syringe. These biocompatible, non-resorbable, hydrogel microspheres are coated with an inorganic perfluorinated polymer (Polyzene®-F). They show a small increase in size (< 5% of the original diameter) during drug loading and storage if compared with similar DEB on the market. Dc-Beads M1 (initial diameter 70-150 μm) show a dehydration and loss in size after loading drug; Hepashere (initial diameter 30-60 μm), instead, show an increase in size up to 4 times of the initial diameter, resulting in a final diameter between 120-240 μm after loading drug. Smaller microspheres theoretically allow for more distal vascular penetration and more homogeneous intratumoral drug distribution, with no meaningful evidence of better results in terms of objective response if compared to 100-300 μm particles[16].
The aim of this study was to assess the efficacy and safety of 40 μm DEB-TACE in a series of 48 early-intermediate HCC patients complying with eligibility criteria. Primary endpoint was the evaluation of adverse events and complications related to TACE as well as the tumor response rate, considered as best achieved response. Secondary outcomes were the time to progressions (TTP) and time to response (TTR).
Data from 48 early-intermediate HCC patients (BCLC stage A/B) referred to our two tertiary centers between May 2013 and May 2015 and treated with DEB-TACE using 40 μm microspheres were retrospectively analysed (Table 1). All patients signed a dedicated informed consent form. A multidisciplinary team made up of interventional radiologists, oncologists, hepatologists, pathologists and hepatic surgeons selected candidates for the treatment.
| Age (yr) | 67 (49-95) |
| Sex | |
| Male | 42 (87.5) |
| Female | 6 (13.5) |
| Aetiology | |
| HCV | 27 (56.2) |
| HBV | 9 (18.75) |
| Alcohol | 6 (12.5) |
| Cryptogenetic | 3 (6.25) |
| NASH | 3 (6.25) |
| Child-Pugh | |
| A | 45 (93.4) |
| B | 3 (6.6) |
| MELD | 8 (6-14) |
| BCLC | |
| A | 21 (43.8) |
| B | 27 (56.2) |
| ECOG 0 | 48 (100) |
| Portal hypertension | |
| Yes | 22 (45.9) |
| No | 26 (54.1) |
| Tumour extension | |
| Unilobar | 29 (60.4) |
| Bilobar | 19 (39.6) |
| No. of tumors (target) | |
| Total | 128 |
| Median | 2 (1-4) |
| Max. diameter (mm) | 30 (10-96) |
| Sum of diameters (mm) | 44 (13-130) |
| TACE | |
| Total cycles | 73 |
| Segmental | 47 (64.4) |
| Bisegmental | 22 (30.1) |
| Trisegmental | 4 (5.5) |
All patients were asymptomatic at enrolment (performance status 0) with cirrhotic disease related to hepatitis C in 56.2% of cases (27/48). Fifty-six point two percent of patients were in BCLC B stage, while 43.8% were in early stage A. All patients presented with a preserved liver function (93.4% Child-Pugh A and 6.6% Child-Pugh B7). Other comorbidities were reported in approximately half of the study population (notably Diabetes, Arterial Hypertension and Chronic Obstructive Pulmonary Disease). No tumors receiving DEB-TACE had been previously treated. The mean number of tumors was 2 (range 1-4) with 30 mm (range 10-96) maximum mean diameter, and the mean sum of all maximum diameters came up to 44 mm (range 13-130).
Patient eligibility was established with the following inclusion criteria: Age > 18 years; HCC diagnosis according to the current guidelines[1-3]: Early/intermediate patients not eligible for percutaneous or surgical ablative therapies; well compensated cirrhosis with Child-Pugh Score up to B7; performance status 0 according to the Eastern Cooperative Oncology Group.
Exclusion criteria included bilirubin > 2 mg/dL; principal (main trunk) or segmental portal thrombosis; previous treatments on target tumors (ablation, TACE, Sorafenib); intolerance to doxorubicin (leukocyte count < 3000/mm3; cardiac ejection fraction < 50%); aspartate amino transferase and alanine amino transferase levels > 270 IU/mL, and patients receiving angiogenesis agents or affected by uncorrectable coagulation disorders.
To assess the disease extent in the liver, its vascular pattern and possible intrahepatic vascular invasions, all patients received pre-treatment abdominal imaging with computed tomography (CT) 128 slices (Somaton Definition Flash, Siemens Healthcare, Erlangen, Germany) or with magnetic resonance imaging (MRI) 1.5 T (Achieva, Philips Healthcare, Best, the Netherlands). In addition to that, they received a CT scan of the chest for a complete staging of the extrahepatic disease.
CT image acquisition technique, before and after treatment, required both a baseline abdominal scan and the arterial, portal and late venous phase study after intravenous administration of a 120-140 mL bolus of iodinated contrast medium (Iopamiro 370 mg/dL, Bracco, Milan, Italy) at an injection flow of 4 mL/s with the Bolus Tracking technique.
The protocol for abdominal MRI required the acquisition of in-phase and out-of-phase T1 weighted sequences, T2 weighted Half-Fourier acquisition Single-shot Turbo-spin Echo (HASTE) and Fat-Saturated (FAT-SAT) sequences, diffusion study and Tissue High Resolution Isotropic Voxel Excitation sequences [T1 weighted FAT-SAT and 3D GRE (3D GradientEcho)] both before and after infusion of Gadolinium-EthOxyBenzyl-Diethylene Triamine Pentaacetic Acid (Gd-EOB-DTPA) 0.025 mmol/mL (Primovist, Bayer, Leverkusen, Germany) with acquisitions up to 20 min during the hepatospecific phase.
TACE was performed using transfemoral arterial access route with a micro-puncture system by placing a 5F vascular introducer (Boston Scientific, Natick, MA, United States). The angiographic study of the superior mesenteric artery and the celiac trunk for the characterisation of hepatic vascular anatomy was performed using an angiography unit (Axiom Angiographic Unit, Siemens Healthcare, Erlanger, Germany), and a 5F catheter (Cobra or Simmons, Boston Scientific, Natick, MA, United States). The angiographic study of extrahepatic pathological branches in some HCC tumors (usually peripheral tumors) was based on a careful study of pre-TACE imaging or on missing parts of the pathological tumor vascularization at the selective angiographic study.
Selective studies of segmental and pathological feeding vessels were also performed using a coaxial micro catheter (Progreat 2.7F, Terumo, Tokyo, Japan), with a highly selective administration of the treatment.
In cases presenting multifocal disease, the treatment never targeted more than three hepatic segments per session. DEB-TACE was performed using a 2 mL/100 mg of doxorubicin (Adriblastina, Pfizer, New York, NY, United States) loaded dose on embozene tandem 40 μm microspheres.
In all performed DEB-TACE treatments embozene tandem 40 μm microspheres were diluted in 20-30 mL of iodinated contrast medium (Iopamiro 370 mg/dL), slowly injected manually with a 3 mL syringe, applying gentle pressure, until blood flow stasis was induced[17].
Vasodilator drugs via transcatheter intra-arterial were not administered before starting chemoembolization with a view to expand to the maximum the neoplastic vascular network and theoretically increase the penetration of the particles and, accordingly, the potential effectiveness of the treatment as reported by some authors[18]. Permanent or temporary embolizing materials were not used to complete DEB-TACE in some tumors of greater dimensions where vascular stasis with only 40 μm drug eluting beads was not achieved. In such cases, a second treatment session has been scheduled after performing a CT/MRI investigation to assess that some conditions leading to the impossibility of repeating the treatment, such as the onset of ascites or portal vein thrombosis, had not occurred.
Access haemostasis was achieved by a mechanical system, as Exoseal (Cordis, Miami Lakes, FL, United States), and a subsequent manual compression for about 3-5 min until haemostasis was achieved.
Premedication included 100 mg of paracetamol (Paracetamolo 10 mg/mL S.A.L.F., Bergamo, Italy), 8 mg of ondansetron (Ondansetron 8 mg/4 mL Hikma, Fervença, Portugal) and 50 mg of ranitidine (Ranitidina 50 mg/5 mL, S.A.L.F., Bergamo, Italy). Intravenous antibiotic prophylaxis was administered with 2 g of cefazolin (Cefamizin, Pfizer, New York, NY, United States) consistent with the hospital internal guidelines.
Patients were discharged after a brief observation period (48-72 h). Clinical evaluation and assessment of treatment-related toxicity were performed on an outpatient basis with physician’s visits and laboratory tests 12, 24 and 48 h after TACE, 4 wk later and every 3 mo thereafter. AE were defined as treatment related if occurred during hospital stay or within 30 d from treatment. Safety parameters were classified according to the Common Terminology Criteria for Adverse Events 4.0[19] at each follow-up visit.
Two radiologists (Dr. Carlo Spreafico and Dr. Giorgio Greco) both experienced in interventional radiology and interventional hepatic imaging performed all radiological assessments independently.
Tumoral response to treatment was assessed according to RECIST and mRECIST with a CT scan or MRI investigation performed 4 wk after DEB-TACE and, then, every 3 mo during the follow-up period[20].
A second treatment session, according to the “on demand” policy, was scheduled in case of partial response (PR) or stable disease (SD) after performing blood chemistry tests documenting good preserved hepatic function and continuity in the eligibility criteria for treatment.
In case of repeated DEB-TACE sessions, only the best response was considered for analytical purposes since this has been recently proved a better predictor of survival than the initial response[21]. In patients submitted to OLT, the treated tumors were histologically analysed during the months after treatment with targeted definition of necrosis induced by TACE.
The descriptive statistical analysis was expressed as median and range in the case of continuous variables and absolute numbers and percentage in the case of categorical ones. Time to best response and TTP were calculated with the Kaplan-Meyer method, computed from the time of the first treatment and censored to the day of transplantation in transplant patients. All calculations were obtained with the SPSS software (IBM, Armonk, NY, United States). The statistical review of the study was performed by a biomedical statistician.
All procedures were performed without technical impediments that would prevent treatment of the target tumor. The two study sites performed an overall number of 73 TACE (47 segmental, 22 bisegmental and 4 trisegmental) on a total number of 128 tumors. 31 patients (64.7%) underwent one treatment cycle, 10 patients (20.8%) to 2 treatment cycles, 6 patients (12.5%) to 3 treatment cycles, and 1 patient to 4 treatment cycles (2%), with a mean number of treatments per patient of 1.45.
Response to treatment was assessed by classifying the tumors into three classes according to dimensional criteria, as specified in Table 2 (according to mRECIST) and Table 3 (according to RECIST). The objective response rate (CR + PR) was 26.8% and 69% for tumors smaller than 3 cm, 32.1% and 85.7% for tumors with diameters between 3 and 5 cm, 10% and 70% for tumors with diameter over 5 cm according to RECIST and mRECIST, respectively.
| Ø nodules | No. of nodules | CR | PR | SD | PD |
| Ø < 3 cm | 77 | 47.4% | 21.6% | 27.8% | 3.2% |
| 3 ≤ Ø ≤ 5 cm | 22 | 42.8% | 42.8% | 10.8% | 3.6% |
| Ø > 5 cm | 3 | 40% | 30% | 30% | 0% |
| Overall response | 102 | 46% | 26.6% | 24.4% | 3% |
| Ø nodules | No. of nodules | CR | PR | SD | PD |
| Ø < 3 cm | 77 | 6.2% | 20.6% | 66% | 7.2% |
| 3 ≤ Ø ≤ 5 cm | 22 | 3.5% | 28.6% | 64.4% | 3.5% |
| Ø > 5 cm | 3 | 0% | 10% | 90% | 0% |
| Overall response | 102 | 5.2% | 21.5% | 67.4% | 5.9% |
Considering all the treated tumors, the overall objective response rate (CR + PR) was 26.7% according to RECIST and 72.6% according to mRECIST. These data include all the 48 patients of our series. These results were calculated with RECIST and mRECIST criteria, based on the last available CT scan/MRI, with an overall mean follow-up period of 357 d (range 30-810).
Eleven patients qualified for OLT after 15 overall cycles of DEB-TACE, with a mean number of treatments per patient of 1.36. Seven out of 11 patients received 1 treatment, with remaining 4 receiving 2 treatments. Median time elapsed between TACE and OLT was 4.8 mo (95%CI: 2.3-6.5). The histological examination (Table 4) performed on 11 explanted livers reported a total number of 14 tumors of HCC, 10 of which were ≤ 3 cm and 4 were between 3 and 5 cm.
| Ø nodules | Degree of necrosis | ||
| 100% | > 90% | < 50% | |
| Ø < 3 cm | 7 | - | 3 |
| 3 ≤ Ø ≤ 5 cm | 2 | 2 | - |
| Overall necrosis | 9 (64.3%) | 2 (14.3%) | 3 (21.4%) |
Among the tumors smaller than 3 cm, 7 presented 100% necrosis and 3 presented a necrosis rate below 50%. Two out of 4 tumors > 3 cm presented a 100% necrosis rate (Figure 1), while the other 2 were above 90%.
Toxicity data are reported in Table 5. All the observed AE were mild and transient, with no grade 3/4 toxicity reported. There were no cases of post procedure mortality within 30 d.
| Toxicity | Grade 1/2 | Grade 3/4 |
| Clinical findings | ||
| Post embolization syndrome | 11/73 (15%) | - |
| Ascites | 3/73 (4.1%) | - |
| Abdominal pain | 18/73 (24.6%) | - |
| Nausea/vomiting | 9/73 (12.3%) | - |
| Laboratory tests | ||
| Bilirubin | 5/73 (6.8%) | - |
| Transaminase | 10/73 (13.7%) | - |
No major AE were recorded, neither systemically (pulmonary embolism, splenic infarction, gastrointestinal mucosal tumors, acute pancreatitis or cholecystitis, spinal cord injury) related to non-target embolization nor locally (hepatic infection or abscesses, ischemic hepatitis and bile duct injuries) due to local toxicity or ischemia[22]. Median hospital stay was 2 d (range 2-4). Post-embolization syndrome (PES) occurred in 15% of treatments (11/73). Other common AE were abdominal pain (24.6%) and nausea/vomiting (12.3%), which were treated with analgesic drugs and anti-emetics, and mild ascites (4.1%). Transient post procedure increase in transaminase levels occurred in 13.7% of cases (10/73). Other 1/2 grade laboratory tests alterations included a transient increase in bilirubin levels (6.8%).
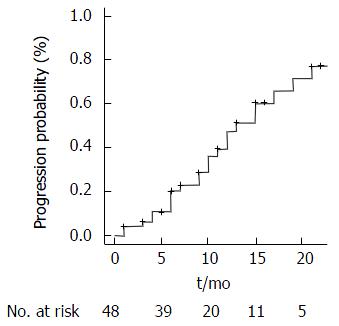
The TTR for all patients was of 4 mo (95%CI: Range 1-4). Overall 24 patients (50%) experienced tumor progression through the study period. One-year progression free survival (PFS) was 64.5% whereas 2-year PFS was 52%. Median TTP was 13 mo (95%CI: Range: 11-21), calculated on mRECIST, as described in Figure 2.
DEB-TACE is the standard of care for HCC intermediate stage patients and a valuable therapeutic option in BCLC A stage when curative approach is unfeasible[1,23]. Several consecutive sessions are usually needed for DEB-TACE to be effective, i.e., the complete tumor response, so that the optimal treatment should lead to higher tumor necrosis rate with the lowest incidence of adverse event. DEB-TACE showed a low incidence of PES[13] and systemic toxicity than in previous reports[8], but its superiority over c-TACE is still a matter of debate[10,24-26].
Since the diameter of chemo loaded microspheres seems to be related to their therapeutic action[17,18], studies on pharmacological kinetics have focused on producing smaller particles that could penetrate deeper into the tumor’s vascular network. The most distal penetration of these microspheres reduces the phenomenon of hypoxic-ischaemic neoangiogenesis[27,28]. However, for embolization not associated with any drug (bland embolization), the use of particles with a diameter < 100 μm presented a concerning rate of complications, especially in the treatment of large tumors[29,30]. Some complications in this type of procedures are related to the “non-target embolization” that is, the unwanted escape of microspheres outside the optimal area for treatment, which can affect other organs or unwanted areas of the same organ. Acute pancreatitis (0.88%-15.2%), acute cholecystitis (0.2%-5.4%), pulmonary embolism (0.17%-2.7%), splenic infarction (0.08%-1.4%), gastrointestinal mucosal tumors (0.22%-0.7%), spinal cord injury (0.3%-1.2%) are among the possible extrahepatic complications[31]. Unwanted hepatic complications such as ischemic hepatitis (0.26%-15.4%), liver infarction or abscess (0.5%-2.7%) or bile duct injuries[31] are connected to local ischemic damages. Some recent studies have proven a very high degree of safety in the use of loadable particles with diameters below 100 μm, with good preliminary efficacy results in terms of radiological and histological response to treatment[14,15,18].
A new generation of microspheres (embozene tandem 40 μm) has been recently marketed for selective intra-arterial treatment even though data on the efficacy and safety profile of the product is yet to be published. To our knowledge, this is the first report on the safety and efficacy of 40 μm particles preloaded with doxorubicin in the treatment of HCC with DEB-TACE. The overall objective response rate (CR + PR) obtained has been of 26.7% to RECIST and 72.6% to mRECIST. This is comparable to the rates according to mRECIST of two recent series carried out with 70-150 μm[14,15] and 30-60 μm[18] (initial diameters) particles loaded with doxorubicin by Spreafico et al[14] and Malagari et al[18] respectively.
Cases of failed response to locoregional therapy, defined as progressive disease, were around 5.9% and 3% with RECIST and mRECIST, respectively. Median TTP was 13 mo (11-21), an interesting and slightly better result if compared with previous published trials using other microspheres. Moreover, it is to be considered that we did not restrict progression analysis only to local progression of target tumors, but also distant intrahepatic progressions and/or metastases occurrence were investigated.
In 11 patients out of the recruited 48, DEB-TACE was used as bridging therapy for OLT with a complete pathologic response (meant as a 100% necrosis in the histological evaluation) in 64.3% cases (9/14 tumors). Tumors smaller than 3 cm shown a better response in term of histological necrosis (70% complete necrosis), compared to those larger than 3 cm (50% complete necrosis). These histological results are consistent with those reported elsewhere[32,33].
The best radiological response was obtained with a single cycle in 60% of patients, with two cycles in 30% of cases and with three cycles in 10% of patients, with a TTR of about 4 mo (95%CI: Range: 4-6). The effectiveness of HCC treatment using TACE on demand has been proved in our series in the event of detection of SD or PR during the follow-up by CT or MRI, in line with data in the literature. The very low toxicity rates observed in our series are probably a consequence of the high selectivity of the procedure ensured by the use of smaller particles.
The procedures were generally well tolerated. Recorded toxicity levels were lower than recent studies using larger diameter microspheres and consistent with two other studies concerning microspheres with a pre-loading diameter between 70-150 μm[14,15] and 30-60 μm[18].
The incidence of PES was to be lower than the percentages published in other series[11,12,14,18] with particles having similar or larger dimensions, most likely due to the selectivity of the procedure and possible sparing of a larger area of peritumoral hepatic parenchyma.
To our knowledge this is the first series regarding the use of 40 μm DEB in HCC treatment. This is interesting for world community and especially for western countries where there is skepticism about using particles smaller than 100 μm for DEB-TACE due to the non-target embolization danger. Our preliminary experience shows that 40 μm DEB-TACE is a highly effective and safe technique for HCC non suitable to ablation or surgery therapies with a low rate of PES and no major complications, either local or systemic. The results are complete for all the 48 patients and for 11 of them a histologically proven response to DEB-TACE on surgical specimen is available. Objective local response reached 72.6% and 26.7% according to mRECIST and RECIST without damage to adjacent healthy liver as evidenced by imaging, histology and liver biochemistry. The study has some limitations such as the retrospective nature, the single series and the small sample of patients. Further studies with a longer follow-up period and a bigger sample should be planned to confirm our results.
The results of this retrospective study indicate that DEB-TACE with 40 μm particles is an effective and safe treatment for early-intermediate HCC patients not eligible for curative treatment with good results in term of objective response rate and necrosis.
Transarterial chemoembolization (TACE) is the current standard of care for hepatocellular carcinoma (HCC) in patients with multinodular disease, classified as intermediate stage (stage B) to Barcelona Clinic Liver Cancer (BCLC) Staging System or in patients in early stage (stage A) not eligible for curative treatment (surgery, liver transplantation or percutaneous ablative treatments). Conventional TACE (c-TACE) has shown superiority over basic supportive care in unresectable HCC in literature since the early 2000s. Actually there is no evidence of superiority of drug eluting bead TACE (DEB-TACE) on c-TACE or transarterial embolization in literature. In the last fifteen years many particles with diameters gradually smaller have been developed for DEB-TACE. These new 40 μm diameter drug eluting beads theoretically penetrate deeper into tumor circulation arterioles reducing the phenomenon of hypoxic-ischaemic neoangiogenesis due to transarterial embolization. There is no evidence in literature of DEB-TACE superiority over c-TACE in terms of efficacy. The only demonstrated vantage is the reduced rate of post embolic syndrome.
The authors’ report on 40 μm DEB-TACE for HCC is the first experience in literarature. The weakness points are the small sample of patients and the retrospective design of the study but it can represent an interesting report for scientific community as first evaluation of safety and efficacy of this new generation of microparticles. Further studies with a larger number of patients will be needed to confirm data and confirm or deny the theoretical benefits of this new generation of micro-particles in the treatment of HCC.
The study confirms a degree of objective response to treatment, defined as complete or partial response according to Response Evaluation Criteria in Solid Tumors (RECIST) and modified RECIST applied to computed tomography and magnetic resonance imaging, consistent with that of previous studies in the literature with a slightly higher caliber particles (40-60 and 70-150 μm). The data is comforting when you consider the lack of intra or extrahepatic complications due to the phenomenon of non-target embolization, cause for concern in Western countries where the DEBTACE is widespread with larger gauge particles.
This study suggests that 40 μm DEBTACE is safe and effective in early-intermediate HCC patients with compensated cirrhosis.
DEB-TACE: Drug eluting bead TACE; Nontarget embolization: Unwanted escape of particles outside the territory seat of treatment.
This paper presented about the efficacy of TACE using drug eluting beads for HCC patients. This topic could be interesting for readers.
Manuscript source: Unsolicited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Edeline J, Jin B, Ohira M S- Editor: Ji FF L- Editor: A E- Editor: Li D
| 1. | EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-943. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4059] [Cited by in F6Publishing: 4399] [Article Influence: 366.6] [Reference Citation Analysis (2)] |
| 2. | Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429-442. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2207] [Cited by in F6Publishing: 2213] [Article Influence: 105.4] [Reference Citation Analysis (0)] |
| 3. | Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5972] [Cited by in F6Publishing: 6392] [Article Influence: 491.7] [Reference Citation Analysis (1)] |
| 4. | Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734-1739. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2502] [Cited by in F6Publishing: 2526] [Article Influence: 114.8] [Reference Citation Analysis (0)] |
| 5. | Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, Fan ST, Wong J. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164-1171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1904] [Cited by in F6Publishing: 1923] [Article Influence: 87.4] [Reference Citation Analysis (0)] |
| 6. | Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907-1917. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3241] [Cited by in F6Publishing: 3235] [Article Influence: 154.0] [Reference Citation Analysis (0)] |
| 7. | Poon RT, Tso WK, Pang RW, Ng KK, Woo R, Tai KS, Fan ST. A phase I/II trial of chemoembolization for hepatocellular carcinoma using a novel intra-arterial drug-eluting bead. Clin Gastroenterol Hepatol. 2007;5:1100-1108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 8. | Varela M, Real MI, Burrel M, Forner A, Sala M, Brunet M, Ayuso C, Castells L, Montañá X, Llovet JM. Chemoembolization of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol. 2007;46:474-481. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 694] [Cited by in F6Publishing: 687] [Article Influence: 40.4] [Reference Citation Analysis (1)] |
| 9. | Brown KT, Do RK, Gonen M, Covey AM, Getrajdman GI, Sofocleous CT, Jarnagin WR, D’Angelica MI, Allen PJ, Erinjeri JP. Randomized Trial of Hepatic Artery Embolization for Hepatocellular Carcinoma Using Doxorubicin-Eluting Microspheres Compared With Embolization With Microspheres Alone. J Clin Oncol. 2016;34:2046-2053. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 219] [Cited by in F6Publishing: 283] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 10. | Sacco R, Bargellini I, Bertini M, Bozzi E, Romano A, Petruzzi P, Tumino E, Ginanni B, Federici G, Cioni R. Conventional versus doxorubicin-eluting bead transarterial chemoembolization for hepatocellular carcinoma. J Vasc Interv Radiol. 2011;22:1545-1552. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 11. | Lammer J, Malagari K, Vogl T, Pilleul F, Denys A, Watkinson A, Pitton M, Sergent G, Pfammatter T, Terraz S. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol. 2010;33:41-52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 12. | Song MJ, Chun HJ, Song DS, Kim HY, Yoo SH, Park CH, Bae SH, Choi JY, Chang UI, Yang JM. Comparative study between doxorubicin-eluting beads and conventional transarterial chemoembolization for treatment of hepatocellular carcinoma. J Hepatol. 2012;57:1244-1250. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 181] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 13. | Facciorusso A, Di Maso M, Muscatiello N. Drug-eluting beads versus conventional chemoembolization for the treatment of unresectable hepatocellular carcinoma: A meta-analysis. Dig Liver Dis. 2016;48:571-577. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 153] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 14. | Spreafico C, Cascella T, Facciorusso A, Sposito C, Rodolfo L, Morosi C, Civelli EM, Vaiani M, Bhoori S, Pellegrinelli A. Transarterial chemoembolization for hepatocellular carcinoma with a new generation of beads: clinical-radiological outcomes and safety profile. Cardiovasc Intervent Radiol. 2015;38:129-134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 15. | Odisio BC, Ashton A, Yan Y, Wei W, Kaseb A, Wallace MJ, Vauthey JN, Gupta S, Tam AL. Transarterial hepatic chemoembolization with 70-150 µm drug-eluting beads: assessment of clinical safety and liver toxicity profile. J Vasc Interv Radiol. 2015;26:965-971. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Blümmel J, Reinhardt S, Schäfer M, Gilbert C, Sun L, Ren J. Drug-eluting Beads in the Treatment of Hepatocellular Carcinoma and Colorectal Cancer Metastases to the Liver. Eur Oncol Haematol. 2012;8:162-166. [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Lencioni R, de Baere T, Burrel M, Caridi JG, Lammer J, Malagari K, Martin RC, O’Grady E, Real MI, Vogl TJ. Transcatheter treatment of hepatocellular carcinoma with Doxorubicin-loaded DC Bead (DEBDOX): technical recommendations. Cardiovasc Intervent Radiol. 2012;35:980-985. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 199] [Cited by in F6Publishing: 215] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 18. | Malagari K, Pomoni M, Moschouris H, Kelekis A, Charokopakis A, Bouma E, Spyridopoulos T, Chatziioannou A, Sotirchos V, Karampelas T. Chemoembolization of hepatocellular carcinoma with HepaSphere 30-60 μm. Safety and efficacy study. Cardiovasc Intervent Radiol. 2014;37:165-175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 19. | National Cancer Institute. Common terminology criteria for adverse events v4.0. NCI, NIH, DHHS. May 29, 2009. NIH publication 09-7473. Available from: http://www.hrc.govt.nz/sites/default/files/CTCAE manual - DMCC.pdf. [Cited in This Article: ] |
| 20. | Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2583] [Cited by in F6Publishing: 3001] [Article Influence: 214.4] [Reference Citation Analysis (36)] |
| 21. | Kim BK, Kim SU, Kim KA, Chung YE, Kim MJ, Park MS, Park JY, Kim do Y, Ahn SH, Kim MD. Complete response at first chemoembolization is still the most robust predictor for favorable outcome in hepatocellular carcinoma. J Hepatol. 2015;62:1304-1310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 128] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 22. | Xia J, Ren Z, Ye S, Sharma D, Lin Z, Gan Y, Chen Y, Ge N, Ma Z, Wu Z. Study of severe and rare complications of transarterial chemoembolization (TACE) for liver cancer. Eur J Radiol. 2006;59:407-412. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 124] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 23. | Malagari K, Alexopoulou E, Chatzimichail K, Hall B, Koskinas J, Ryan S, Gallardo E, Kelekis A, Gouliamos A, Kelekis D. Transcatheter chemoembolization in the treatment of HCC in patients not eligible for curative treatments: midterm results of doxorubicin-loaded DC bead. Abdom Imaging. 2008;33:512-519. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 24. | Golfieri R, Giampalma E, Renzulli M, Cioni R, Bargellini I, Bartolozzi C, Breatta AD, Gandini G, Nani R, Gasparini D. Randomised controlled trial of doxorubicin-eluting beads vs conventional chemoembolisation for hepatocellular carcinoma. Br J Cancer. 2014;111:255-264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 410] [Cited by in F6Publishing: 435] [Article Influence: 43.5] [Reference Citation Analysis (1)] |
| 25. | Xie ZB, Wang XB, Peng YC, Zhu SL, Ma L, Xiang BD, Gong WF, Chen J, You XM, Jiang JH. Systematic review comparing the safety and efficacy of conventional and drug-eluting bead transarterial chemoembolization for inoperable hepatocellular carcinoma. Hepatol Res. 2015;45:190-200. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 26. | Facciorusso A, Mariani L, Sposito C, Spreafico C, Bongini M, Morosi C, Cascella T, Marchianò A, Camerini T, Bhoori S. Drug-eluting beads versus conventional chemoembolization for the treatment of unresectable hepatocellular carcinoma. J Gastroenterol Hepatol. 2016;31:645-653. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 27. | Wang B, Xu H, Gao ZQ, Ning HF, Sun YQ, Cao GW. Increased expression of vascular endothelial growth factor in hepatocellular carcinoma after transcatheter arterial chemoembolization. Acta Radiol. 2008;49:523-529. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 215] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 28. | Kobayashi N, Ishii M, Ueno Y, Kisara N, Chida N, Iwasaki T, Toyota T. Co-expression of Bcl-2 protein and vascular endothelial growth factor in hepatocellular carcinomas treated by chemoembolization. Liver. 1999;19:25-31. [PubMed] [Cited in This Article: ] |
| 29. | Bonomo G, Pedicini V, Monfardini L, Della Vigna P, Poretti D, Orgera G, Orsi F. Bland embolization in patients with unresectable hepatocellular carcinoma using precise, tightly size-calibrated, anti-inflammatory microparticles: first clinical experience and one-year follow-up. Cardiovasc Intervent Radiol. 2010;33:552-559. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 30. | Maluccio MA, Covey AM, Porat LB, Schubert J, Brody LA, Sofocleous CT, Getrajdman GI, Jarnagin W, Dematteo R, Blumgart LH. Transcatheter arterial embolization with only particles for the treatment of unresectable hepatocellular carcinoma. J Vasc Interv Radiol. 2008;19:862-869. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 131] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 31. | López-Benítez R, Richter GM, Kauczor HU, Stampfl S, Kladeck J, Radeleff BA, Neukamm M, Hallscheidt PJ. Analysis of nontarget embolization mechanisms during embolization and chemoembolization procedures. Cardiovasc Intervent Radiol. 2009;32:615-622. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 32. | Nicolini A, Martinetti L, Crespi S, Maggioni M, Sangiovanni A. Transarterial chemoembolization with epirubicin-eluting beads versus transarterial embolization before liver transplantation for hepatocellular carcinoma. J Vasc Interv Radiol. 2010;21:327-332. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 111] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 33. | Nicolini D, Svegliati-Baroni G, Candelari R, Mincarelli C, Mandolesi A, Bearzi I, Mocchegiani F, Vecchi A, Montalti R, Benedetti A. Doxorubicin-eluting bead vs conventional transcatheter arterial chemoembolization for hepatocellular carcinoma before liver transplantation. World J Gastroenterol. 2013;19:5622-5632. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 45] [Cited by in F6Publishing: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |