INTRODUCTION
The high accuracy of imaging madalities as computed tomography (CT) and magnetic resonance (MR) in the evaluation of the abdomen is usually able to detect incidental adrenal abnormalities; in this clinical scenario, the diagnostic goal is the differential diagnosis between benign and malignant lesions for the appropriate therapeutic management[1]. As initial diagnostic evaluation, clinical and laboratory assessment of adrenal function allows to detect hypersecreting adrenal tumors and, thus, to characterize such patients[2]. Conversely, an adrenal mass may not be associated to abnormal hormone hypersecretion or it may produces non-functional agents[1-3]. In these patients, tumor characterization is required; for this purpose, CT and MR well describe anatomic features of adrenal masses and may suggest presumptive imaging criteria for tissue characterization[4-6].
Nuclear adrenal scans with different radiolabeled compounds, which reflect specific metabolic pathway, are helpful to characterize adrenal tumors providing diagnostic information complementary to morphological imaging modalities[7,8]. For this purpose, different radiocompounds that reflects specific biological functions may be considered in nuclear medicine for adrenal radionuclide studies; among these agents, nor-cholesterol, metaiodobenzylguanidine (MIBG) and fluorine-deoxy-glucose (FDG) are the more commonly used in the clinical practice[7,9-15]. Furthermore, radiolabeled peptides such as somatostatin analogs have been reported to be able to characterize malignant adrenal lesions, as these express somatostatin receptors[16]. Limited comparative studies between radionuclide and MR imaging are available. A complementary role of MIBG and MR techniques in the diagnostic evaluation of patients with chromaffin-tissue tumors (CTT), such as pheochromocytoma or paraganglioma, has been suggested, although no data regarding the imaging characterization of such tumors are reported[17-19].
In this paper, we show the accuracy of nuclear scans with different radiocompounds in the assessment of adrenal tumors to perform lesion identification in comparison with MR images. The comparative results of nuclear and MR imaging studies in three different groups of patients with adrenal tumors, that is (1) patients with incidentalomas; (2) patients with adenomas; and (3) patients with CTT, are reported.
MATERIALS AND METHODS
Patient population
Patients were classified in three groups; in group 1, 30 patients (20 F and 10 M, 51 ± 13 years) with incidental non-hyperfunctioning adrenal tumors, detected on ultrasound and/or CT, were studied; in group 2, 34 patients (20 F and 14 M, 47 ± 15 years) with non-hypersecreting (n = 15) or hypersecreting (n = 19) adrenal adenomas; group 3 consisted of 18 patients (9 M and 9 M, mean age 37 ± 8 years) with CTT of which 14 were pheochromocytomas and 4 were paragangliomas. Patients with non-hypersecreting adrenal adenomas were separated into groups 1 and 2 for specific comparative diagnostic purposes. All patients underwent MR studies. In group 1, a total of 46 nuclear studies were analyzed using FDG (n = 11), MIBG (n = 15) and nor-cholesterol (n = 20) radiocompounds; in group 2, all patients underwent nor-cholesterol adrenal scintigraphy; in group 3 all patients underwent MIBG imaging. Pathology samples (n = 63) or follow-up data in adenomas (n = 19) were used as standard of reference for imaging studies interpretation. Informed patient consent was obtained in all cases.
MR imaging
MR was performed with a 3.0 Tesla magnet scan (Trio, Siemens Medical Systems, Hoffman Estates, IL); conventional TSE sequences were used to realize 5 mm contiguous sections of the abdomen in axial, coronal and sagittal views. T1 (TR/TE = 600/15 ms) and T2 (TR/TE = 2000/15-90 ms) images were obtained. T1 images were also acquired after intravenous injection of gadolinium-DTPA (0.2 mL/kg, Magnevist, Schering) using dynamic multi-phase acquisition. The tumor size was measured as maximal diameter (cm). For qualitative evaluation, signal intensity of tumor lesions was analyzed on T2 images as hypo-, iso- or hyper-intensity compared to liver signal intensity. Tumor lesions enhancement of gadolinium (yes or no) as well as signal intensity changes on chemical shift (CS) images were also analyzed. For quantitative analysis, signal intensity ratios (ratios of lesions signal intensity vs that of liver, fat, muscle and image background) was measured on T1 and T2-weighted images using region of interest analysis. The regions of interest considered for the analysis were placed on adrenal or lymph node neoplastic lesions as well as on right liver lobe, adrenal bed fat tissue, lumbar para-vertebral muscle tissue, and image background to obtain tumor lesion signal intensity ratios (SIRs).
Nor-cholesterol scintigraphy
Thyroid iodine uptake was blocked before intravenous tracer (37 MBq) injection with a saturated solution of potassium iodide (200 mg per os and per day, starting the day before tracer injection and continuing for 8 d); adrenal scanning was performed five and seven days after tracer administration using a large field of view gamma camera with a high-energy collimator (Orbiter, Siemens, Erlangen, Germany) and a 20% window centered at 364 KeV; posterior abdominal acquisitions were performed in preset time for 600 s for each scan. A mild laxative (bisacodyl) was given (10 mg) twice daily beginning 2 d before the first day of imaging to reduce interfering colonic iodine-131 activity. The grading of nor-cholesterol uptake by tumor lesions was visually and semi-quantitatively assessed by two individual and experienced evaluators; in case of disagreement, final interpretation was reached by consensus reading; in particular, nuclear images were assessed using the criteria by Gross et al[20]; for the semi-quantitative analysis, a 4-point scoring system was used to quantify nor-cholesterol concentration by adenomas as well as to perform a direct comparison between hypersecreting and non-hypersecreting lesions; the 4-point scoring system was: 0 = background tracer uptake, 1 = just visible tumor activity, 2 = increased tumor activity with faint uptake in the opposite gland and 3 = exclusive tumor activity with no uptake in the opposite gland.
MIBG scintigraphy
Thyroid iodine uptake was blocked before intravenous tracer (37 MBq) injection with a saturated solution of potassium iodide (200 mg per os and per day, starting the day before tracer injection and continuing for 8 d). Anterior and posterior all-body scans as well as abdominal spot views were acquired 24, 48, and 72 h after tracer administration using a dual head rotating gamma camera (ECAM, Siemens Medical Systems, Hoffman Estates, IL) with a high-energy collimator and a 20% window centered at 364 Kev. MIBG uptake was visually and quantitatively assessed in anatomic sites where tumor lesions were detected on MR images. For qualitative analysis, MIBG uptake was graded as mild, moderate, or intense; while for quantitative analysis, MIBG uptake was evaluated on 48 h images using a photographic densitometer (X-Rite Company, MI) for measuring optical density (OD), as previously described[21].
Fluorine-18 FDG PET
PET scan was performed using a EXACT 47 scanner (Siemens, Erlangen, Germany); patients were evaluated fasted since 6 h before tracer administration; patients were placed in the gantry using a computerized program localized on the superior abdomen; before tracer administration, transmission scan for the attenuation correction of emission scans was acquired for 20 min.; patients were intravenously injected with 370 MBq of F-18 FDG; emission scans were performed 30-45 min. after tracer injection; images were reconstructed using filtered backprojection smoothed with a Hann filter with a cutoff frequency of 0.4 cycles/pixels by SUN Workstation System generating PET scans as axial, coronal and sagittal views. The presence of abnormal FDG uptake was visually evaluated in the superior abdomen where tumor masses were localized by MR, while the grade of FDG uptake by the mass was assessed by two experienced evaluators; in case of disagreement, final interpretation was reached by consensus reading.
RESULTS
Group 1
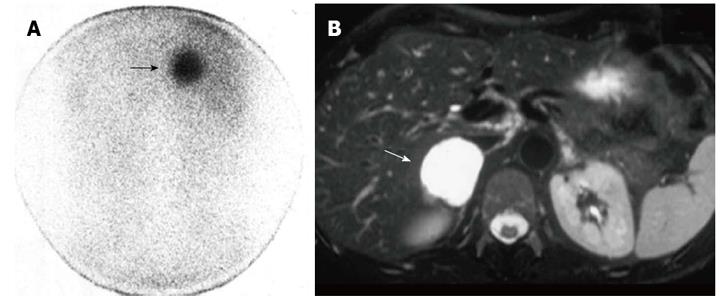
In this group, pathology examinations (n = 19), of which 16 post-surgical histology and 3 fine needle cytology samples, or follow-up controls (n = 11) demonstrated 22 benign adrenal tumors, of which 13 adenomas, 3 cysts, 2 myelolipomas, 4 pheochromocytomas (pheos), and 8 malignant adrenal lesions, of which 4 carcinomas, 1 sarcoma and 3 metastases. Qualitative MR analysis demonstrated: high signal intensity on T2 images in adenomas (46%), in pheos (100%) and adrenal malignancies (100%); no contrast enhancement (92%) and clear reduction of signal intensity on CSI (100%) in adenomas; significant contrast enhancement in pheos (100%) and malignant tumors (63%); no changes of signal intensity on CSI in pheos (100%) and malignancies (100%). Quantitative MR evaluation showed: higher signal intensity on T2 and post-contrast images in pheos vs adenomas and malignant tumors (P < 0.03). Radionuclide qualitative analysis demonstrated abnormal nor-cholesterol uptake only in adenomas (100%) (Figure 1) and, similarly, abnormal MIBG uptake only in pheos (100%) (Figure 2) as well as abnormal FDG uptake only in 100% of malignancies (100%) (Figure 3); no false positive or negative nuclear imaging results occurred.
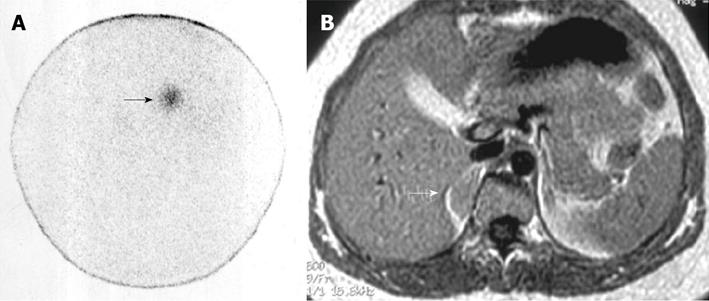
Figure 1 Right non-hypersecreting adrenal adenoma.
A: Abdominal posterior scan of I-131 nor-cholesterol scintigraphy demonstrates abnormal (faint) focal uptake in the right adrenal (black arrow) where a mass was detected by magnetic resonance (MR); no detectable tracer uptake in the left adrenal bed; B: T1-weighted axial MR detects a small lesion in the right adrenal bed (white arrow) isointense compared to liver signal intensity.
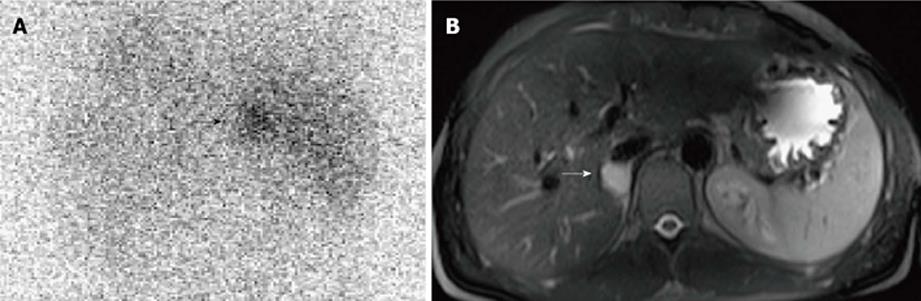
Figure 2 Right benign non-hypersecreting pheochromocytoma.
A: Abdominal posterior scan of I-131 metaiodobenzylguanidine scintigraphy demonstrates abnormal (faint) focal uptake in the right adrenal (black arrow) where a small mass was detected by magnetic resonance (MR); no detectable tracer uptake in the left adrenal bed; B: T2-weighted axial MR with fat-suppression detects a small right adrenal lesion hyperintense compared to liver signal intensity (white arrow).
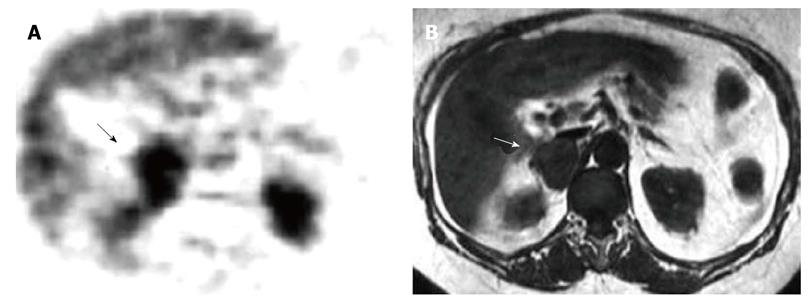
Figure 3 Right adrenal metastasis by melanoma.
A: Axial fluorine-18 fludeoxyglucose-positron emission tomography scan detects abnormal focal uptake in the right adrenal region (black arrow) where a large adrenal metastasis was detected by magnetic resonance (MR); diffuse normal liver tracer activity is detectable; physiologic tracer activity is also detectable in kidneys; B: T1-weighted axial MR detects a right adrenal tumor hypointense compared to liver signal intensity (white arrow).
Group 2
Of 34 patients included in this group, 19 presented hypersecreting and 15 had non-hypersecreting adenomas. In hypersecreting adenomas abnormal plasma amount of cortisol (n = 13) or aldosterone (n = 6) occurred. Pathology (n = 26) or follow-up data (n = 8) were obtained. MR detected 16 and 18 regular tumors of left and right adrenals, respectively. There was no significant difference in the quantitative evaluation of SIRs between hypersecreting (Figure 4A) and non-hypersecreting (Figure 1B) adenomas; however, no differences in lesion size (cm) were found between hypersecreting (2.7 ± 0.5) and non-hypersecreting (3.1 ± 0.9) tumors. For radionuclide studies, abnormal nor-cholesterol focal uptake occurred in all patients with adenomas, both hypersecreting and non-hypersecreting, showing exclusive or prevalent uptake. The accuracy of nor-cholesterol scanning was 100%. The semi-quantitative comparison of nor-cholesterol uptake between hypersecreting and non-hypersecreting lesions demonstrated a significant (P = 0.01) difference in tracer concentration between hypersecreting (2.8 ± 0.5) (Figure 4B) and non-hypersecreting (2.2 ± 0.6) lesions (Figure 1A).
Figure 4 Right hypersecreting adenoma of the adrenal gland.
A: Abdominal posterior scan of I-131 nor-cholesterol scintigraphy demonstrates abnormal (intense) focal uptake in the right adrenal (black arrow) where a mass was detected by magnetic resonance (MR); no uptake is detected on the contralateral side; normal bowel uptake is detectable on the left; B: T1-weighted axial MR detects a right adrenal lesion isointense compared to liver signal intensity (white arrow).
Group 3
Among the 10 patients with benign CTT, 8 had pheochromocytoma and 2 paraganglioma; while among the 8 patients with malignant CTT, 6 had pheochromocytoma and 2 paraganglioma. In patients with benign CTT, a total of 12 lesions were found since a para-aortic paraganglioma was bilateral and a pheochromocytoma was bilobated. In patients with malignant CTT, a total of 16 lesions were detected and the majority (75%) of these patients showed lymph nodes or bone involvement. At visual analysis, MR imaging and MIBG scintigraphy detected all tumor lesions. At quantitative analysis, MIBG uptake intensity ratio was significantly higher in malignant (Figure 5A) compared to benign (Figure 2A) lesions (5.2 ± 2.4 vs 2.9 ± 1.4, P < 0.01); on the contrary, at MR imaging no significant difference in tumor signal intensity ratios between malignant (Figure 5B) and benign (Figure 2B) lesions was observed; no significant difference in lesion size (cm) was found between benign (3.2 ± 0.5) and malignant (2.8 ± 0.8) tumors.
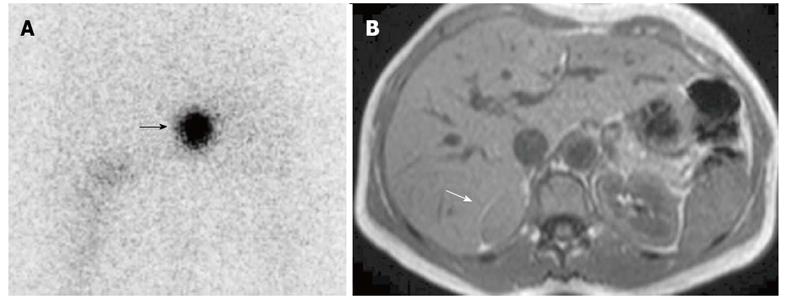
Figure 5 Right malignant hypersecreting pheochromocytoma.
A: Abdominal posterior scan of I-131 metaiodobenzylguanidine scintigraphy demonstrates abnormal (intense) focal uptake in the right adrenal (black arrow) where a large mass was detected by magnetic resonance (MR); no detectable tracer uptake in the left adrenal bed; B: T2-weighted axial MR with fat-suppression detects a large right adrenal lesion hyperintense compared to liver signal intensity (white arrow).
DISCUSSION
Tumor imaging characterization in patients with adrenal masses is clinically important since diagnostic evaluation currently consists not only of lesion detection, but also of tumor characterization in order to recognize lesion-type, to assess lesion endocrine activity as well as to differentiate between benign and malignant tumors. Advances in imaging techniques allow to reach these purposes. In this paper, we report the results obtained in three different groups of patients with adrenal tumors: (1) patients with incidentalomas; (2) patients with adenomas; and (3) patients with CTT. On the basis of our findings, nuclear imaging modalities using specific target agents are able to better characterize, compared with MR, adrenal tumors. Radionuclide techniques are able to identify the nature of adrenal incidentalomas and to differentiate between hypersecreting and non-hypersecreting adenomas as well as between benign and malignant CTT.
Group 1
Asymptomatic non-hypersecreting adrenal masses have recently increased as a result of the availability of high resolution abdominal imaging modalities as CT and MR[1]. However, CT- and MR- based imaging is useful for tumor detection and the differential diagnosis between benign and malignant lesions, as it provides accurate anatomic details useful for lesion characterization[4-6,22,23]. Despite CT and MR presumptive criteria for tissue characterization, complementary or alternative imaging modalities are still needed. In this setting, radionuclide techniques with different agents are able to in vivo characterize adrenal tumors and differentiate between benign and malignant abnormalities[4,7,9,12-14,24-26]; in particular, these agents are not related to each other and are concentrated in adrenal tumors depending by distinct metabolic pathways.
In adrenal adenomas, MR qualitative patterns were represented by signal intensity reduction on CS sequence and transient contrast enhancement with significant wash-out; in particular, CS signal intensity changes was the more appropriate criterion to characterize adenomas[4]. On the other hand, the analysis of signal intensity on T1 and T2 images showed inhomogeneous findings, which did not allow definite adenoma characterization. For pheos, T2 signal hyper-intensity and significant persistent gadolinium enhancement were both specific, as they were detected in all lesions. In malignant tumors T2 hyperintensity was observed in all lesions, while contrast enhancement was found only in 63% of these cases. These MR features suggest that high signal intensity, visually assessed on T2 scan, is not accurate in differentiating adrenal masses. Furthermore, since CS sequence was not useful to characterize pheos or adrenal malignancies, other technical methods are necessary in this setting. For this purpose, we used MR quantitative analysis to assess the degree of T2 high signal intensity as well as of contrast enhancement; this evaluation demonstrated higher values of these two parameters in pheos compared with adenomas and malignant tumours; however, these latter tumors were not differentiated using these parameters. These findings are concordant with those of other studies[4,6] suggesting that MR imaging offers only some indicative criteria for lesion characterization in patients with non-functioning adrenal tumors. In particular, the reduction of signal intensity on CS sequence seems to be the best marker for adenomas, while the quantitative evaluation of T2 or T1-contrast signal intensity allows to better identify pheos, while no specific MR signs for malignant tumors were selected.
Conversely, nuclear studies demonstrated more specific imaging results in comparison with MR for tumor characterization; in fact, the presence of nor-cholesterol concentration was observed only in adenomas, as well as that of MIBG in pheo and that of FDG in malignant tumors (Figures 1-3). Thus, nuclear imaging using different radiotracers is able to accurately and non-invasively characterize non-functioning adrenal tumors[9,12-14,25,26]; for this purpose, the choice of the radiocompound to perform adrenal scintigraphy is dependent by the clinical scenario. In patients with incidental occurrence of an adrenal mass, nor-cholesterol should be initially used because adenoma is the more frequent lesion; in case of absence of nor-cholesterol by the lesion, MIBG should be used as second choice, while FDG should be selected in case of clinical suspicion of malignant tumors. On the other hand, during the follow-up of oncologic patients with evidence of an adrenal mass, FDG should be initially selected; in case of absence of FDG by the lesion, nor-cholesterol and MIBG should be used as second and third choice, respectively. Currently, radionuclide scans are not frequently considered in the diagnostic management of patients with non-functioning adrenal tumors[27,28]; in particular, the results of nuclear imaging reflect important clinical implications; for example, in case of non-hypersecreting adenoma diagnosed on nor-cholesterol nuclear imaging, surgery is required only for large masses, while lesion size monitoring in follow-up is required in smaller tumors; otherwise, in case of pheo the pre-surgical diagnosis using MIBG scan allows to correctly prepare the patient or in case of malignant tumors the use of FDG PET may allow early diagnosis with subsequent immediate lesion resection with good prognosis. Therefore, nuclear scans with different radiocompounds represent a powerful diagnostic tool to specifically characterize non-functioning adrenal tumors.
Group 2
The characterization of adrenal adenomas comparing imaging techniques has been investigated in a previous study[29] using quantitative evaluation of nor-cholesterol uptake, CT attenuation value and CS MR signal intensity loss, but no comparative data between hypersecreting and non-hypersecreting lesions were reported. Our results showed that while the analysis of nor-cholesterol activity is able to distinguish between hyperfunctioning and non-hyperfunctioning adenomas, MR signal intensity ratios are not able in this setting. As cholesterol is the precursor molecule of all adrenocortical steroid hormones[30], nor-cholesterol scintigraphy has been clinically used in both hyperfunctioning and non-hyperfunctioning adenomas[31]. The diagnostic accuracy of nor-cholesterol imaging recorded in our study was high (100%) and concordant with previous findings. Interestingly, the analysis of radionuclide images showed a significant difference in nor-cholesterol uptake between hyperfunctioning and non-hyperfuntioning lesions (Figures 1 and 4), which reflects their different biosynthetic activity. Of note, the significantly higher nor-cholesterol concentration observed in hypersecreting adenomas was independent on tumor size. As non-hypersecreting adenomas showed increased nor-cholesterol uptake vs normal adrenal glands, but lower nor-cholesterol uptake compared to hypersecreting lesions, nor-cholesterol uptake may be more reliable than peripheral hormone levels to assess the functional index of non-hypersecreting adenomas. In this field, a nuclear diagnostic criterion, using the intensity of nor-cholesterol, of silent pre-clinical adrenal dysfunction has been previously suggested in adenomas when adrenal functional abnormality may be not yet detected by laboratory markers[32]. Semi-quantitative indexes of nor-cholesterol activity in adrenal adenomas have been indicated to be able to evaluate adrenal functional degree, especially when serum hormonal values are not inconclusive. Furthermore, assessment of nor-cholesterol uptake could identify those lesions at risk of causing an overt clinical syndrome as a result of hormonal hyperproduction[33,34], and it may be a powerful instrument to assess the biosynthetic activity of some adenomas, which have been shown to produce relevant amounts of adrenocortical hormones, yet insufficient to cause symptoms.
The comparative results of nor-cholesterol scintigraphy and MR imaging demonstrated a similar diagnostic sensitivity for the identification of cortical adenomas confirming previous studies[7,35]; in particular, MR imaging should be selected since this tecnique is radiation-free as well as it may be performed also without contrast administration using CS sequence. However, when imaging differentiation between hypersecreting and non-hypersecreting adenomas was investigated, no specific MR criteria for this topic were found; in fact, no differences in MR parameters were observed between these lesions; these findings suggest that MR parameters reflect tumor tissue anatomic features which are not related to endocrine function.
Group 3
Pheochromocytomas are CTT localized in the adrenal medulla with the specific characteristics to secrete cathecolamines. The most frequent form is the sporadic with unilateral benign involvement of a single gland, but the possibility of bilateral, extra-adrenal, or multiple localization as well as familiar or malignant forms occur in 10% of cases[36]. The distinction between benign and malignant pheochromocytoma based on histological analysis is challenging, and metastatic disease is the only established criterion for malignancy, according to the World Health Organization classification of solid tumors[36].
Tumor imaging characterization in terms of differentiation between benign and malignant lesions is currently attractive in oncology. Although benign lesions represent the majority of pheochromocytomas, malignancy may occur in 10% of patients as well as benign tumors may also rarely show malignant evolution[36]. Although no reliable CT and/or MR imaging features are available to detect malignant pheocromocytoma, these techniques can show invasive regional tumor growth and/or the presence of distant metastases[4]. Several diagnostic methods, such as biological fluid tests, molecular markers, imaging techniques, and genome studies, have been proposed to differentiate between benign and malignant pheochromocytoma[37,38]. Previous experiences suggested that radiolabeled somatostatin analogs might be used to characterize malignant pheochromocytomas[16,39]. Conversely, MIBG uptake has been demonstrated to reflect the concentration of neurosecretory storage granules in CTT[40]. Lesions that concentrate MIBG can be benign or malignant and the ability of MIBG scan for tumor detection depends on both tumor size and differentiation[7,41]. Our results demonstrated that quantitative analysis of MIBG uptake may differentiate between benign and malignant CTT such as pheochromocytoma and paraganglioma, while MR imaging using signal intensity ratio measurement is not useful for this purpose. In fact, MIBG uptake, measured as tumor lesion OD IR, was significantly higher in malignant lesions compared to benign disease (Figures 2 and 5); this finding could depend by larger size of malignant compared to benign CTT, however no difference in lesion size was found.
MIBG, a physiological analog of nor-epinephrine and guanetidine, shows a specific uptake by chromaffin-tissue cells in adrenal as well as extra-adrenal locations[41-43]; of note, storage catecholamine granules have been demonstrated within adrenal medullary tissue and pheochromocytoma tumor lesions[40]. Since MIBG uptake reflects the concentration of neurosecretory storage granules in chromaffin normal tissue and in the corresponding tumors, the higher MIBG uptake of lesions in patients with malignant compared to those of patients with benign tumors suggests a higher concentration of catecholamine in malignant pheochromocytoma. Thus, this difference could reflect different functional conditions with malignant lesions being more prone to catecholamine secretion crises compared to benign tumors. This observation might explain the severe hypertensive attacks that frequently occur in patients with malignant pheochromocytoma and might also justify the use of radiolabeled MIBG for therapeutic purposes in such patients when conventional treatments are not effective[44,45].
Previous comparative studies between MIBG and MR imaging demonstrated the complementary role of these techniques in the diagnostic evaluation of patients with pheochromocytoma or paraganglioma[17-19]. In these studies no data regarding the characterization and differentiation between benign and malignant tumors have been reported. Our results showed that there are no specific MR criteria, either on T1- or on T2-scans, to differentiate between benign and malignant CTT; no significant differences in MR signal intensity ratios were observed between tumor lesions of benign and malignant neoplasms; these findings suggest that signal intensity ratios reflects tumor tissue structural characteristics which are not related to metabolic and functional conditions.
In conclusion, nuclear imaging modalities using specific target agents are able to better characterize, compared with MR, adrenal tumors. Radionuclide techniques are able to identify the nature of adrenal incidentalomas and to differentiate between hypersecreting and non-hypersecreting adenomas as well as between benign and malignant CTT.
COMMENTS
Background
Imaging modalities such as computed tomography (CT), magnetic resonance imaging (MRI) and nuclear modalities are actually advanced as well as technically improved suggesting that qualitative and/or quantitative methods might be helpful for this purpose.
Research frontiers
Radionuclide techniques using specific tracers such as labeled nor-cholesterol, metaiodobenzylguanidine (MIBG) and fluorine-deoxy-glucose (FDG) may provide in vivo tissue characterization of adrenal tumours being able to differentiate between benign and malignant abnormalities; these agents have no relation to each other and are taken up by individual parts of adrenals on the basis of entirely separate mechanism and are able to differentiate different types of tumours.
Innovations and breakthroughs
Nuclear techniques using specific tracers such as nor-cholesterol, MIBG and FDG are able to provide in vivo tissue characterization of adrenal tumors being able to differentiate between benign and malignant lesions. Quantitative analysis of MIBG uptake may differentiate between benign and malignant chromaffin-tissue tumors such as pheochromocytoma and paraganglioma, while MR imaging using signal intensity ratio measurement is not useful in this field.
Terminology
Nuclear imaging: Nuclear imaging is part of nuclear medicine and consists of functional techniques used for diagnostic purposes. Imaging characterization: Imaging characterization consist of techniques able to provide diagnostic criteria and parameters to perform tissue diagnosis in terms of benign or malignant lesions. Adrenal tumors: Adrenal tumors are lesions of adrenal glands located into the retroperitoneum; these lesions may be benign or malignant and, thus, tumor characterization is clinically required for patient appropriate management.
Peer review
This study concerns a comparison of different nuclear imaging methods (nor-cholesterol, MIBG and FDG) to MR imaging for the diagnostic evaluation of patients with adrenal masses. The results show that nuclear imaging methods in general show higher sensitivity compared to MR in detecting adrenal tumors and should be useful in the clinic. This is well written.