Published online Mar 28, 2021. doi: 10.4329/wjr.v13.i3.64
Peer-review started: January 29, 2021
First decision: March 1, 2021
Revised: March 2, 2021
Accepted: March 22, 2021
Article in press: March 22, 2021
Published online: March 28, 2021
As we continue to fight against the current coronavirus disease-2019 (COVID-19) pandemic, healthcare professionals across the globe are trying to answer questions surrounding how to best help patients with the up-to-date available science while awaiting the development of new therapies and mass vaccination. Since early in the pandemic, studies indicated a heightened risk of venous thromboembolism (VTE) in COVID-19 infected patients. There have been differing expert opinions about how to assess pretest probability of VTE in this patient population. This has been partly due to the high prevalence of respiratory failure in this patient population and the use of D-dimer as a prognostic test which is also frequently elevated in patients with COVID-19 in absence of VTE. Some experts have argued for an approach similar to usual care with testing if clinical suspicion is high enough. Some have argued for more routine screening at different points of care. Others have even suggested empiric therapeutic anti-coagulation in moderate to severely ill COVID-19 patients. In the following article, we review and summarize the most current literature in hopes of assisting clinicians in decision making and guidance for when to be concerned for VTE in COVID-19 patients. We also discuss research gaps and share pathways currently being used within our institution.
Core Tip: As we continue to fight against the current coronavirus disease-2019 (COVID-19) pandemic, healthcare professionals across the globe are trying to answer questions surrounding how to best help patients with the up-to-date available science while awaiting the development of new therapies and mass vaccination. Since early in the pandemic, studies indicated a heightened risk of venous thromboembolism (VTE) in COVID-19 infected patients. There have been differing expert opinions about how to assess pretest probability of VTE in this patient population. This has been partly due to the high prevalence of respiratory failure in this patient population and the use of D-dimer as a prognostic test which is also frequently elevated in patients with COVID-19 in absence of VTE. Some experts have argued for an approach similar to usual care with testing if clinical suspicion is high enough. Some have argued for more routine screening at different points of care. Others have even suggested empiric therapeutic anticoagulation in moderate to severely ill COVID-19 patients. In the following paper we review and summarize the most current literature in hopes of assisting clinicians in decision making and guidance for when to be concerned for VTE in COVID-19 patients. We also discuss research gaps and share pathways currently being used within our institution.
- Citation: Patel L, Gandhi D, Westergard E, Ornes M, Lillyblad M, Skeik N. COVID-19 and venous thromboembolism: Known and unknown for imaging decisions. World J Radiol 2021; 13(3): 64-74
- URL: https://www.wjgnet.com/1949-8470/full/v13/i3/64.htm
- DOI: https://dx.doi.org/10.4329/wjr.v13.i3.64
At the time this paper was written, globally there are over 100 million patients who have tested positive for coronavirus disease-2019 (COVID-19) infection, with around 2.1 million patients having lost their lives due to this disease[1]. COVID-19 is caused by the novel coronavirus named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Though COVID-19 infections have a tendency to involve multiple organ systems, the respiratory system is primarily affected resulting in inflammatory infiltrates, and in severe cases leading to hypoxemia and respiratory failure. High risk of venous thromboembolism (VTE) in COVID-19 patients was recognized early on in the pandemic, with one study suggesting enoxaparin prophylaxis was associated with lower mortality[2]. However, despite thromboprophylaxis, the risk for VTE remains high[3]. Timely identification of deep vein thrombosis (DVT) and pulmonary embolism (PE) is critical in making clinical decisions regarding therapeutic anticoagulation. Computed tomography pulmonary angiography (CTPA) is considered the gold standard test for diagnosis of pulmonary artery clot. In patients presenting with COVID-19 infection, deciding when to screen or rule out pulmonary artery thromboembolism remains a challenge for physicians due to frequently fluctuating oxygenation requirements. Different approaches have been suggested and debated by experts including use of clinical decision-making tools, the use of D-dimer testing, universal CTPA or lower extremity ultrasound screening on admission to the hospital or at the time of admission to critical care units, and empiric higher than prophylactic anticoagulation. In the following review, we will explore current literature regarding clinical decision-making for imaging in the diagnosis of VTE in COVID-19 patients in the form of common clinical questions. We will also share our institution’s pathway for diagnosing VTE in this patient population.
Since the beginning of the pandemic, studies have indicated increased risk of both venous and arterial thromboembolism in COVID-19 patients, including DVT, PE, ischemic stroke, myocardial infarction and peripheral arterial thromboembolism[4]. One study, which compared national databases of viral pneumonias, showed COVID-19 was associated with higher incidences of thrombotic complications compared to other viral pneumonias[5]. The reported frequency of pulmonary embolism in critically ill COVID-19 patients is approximately 20%-30%[6,7]. Evidence suggests that small vessel pulmonary thrombi are more common than large pulmonary vessel involve-ment in COVID-19[8,9]. Pulmonary embolism is a serious thrombotic complication of COVID-19 pneumonia, with mortality rates for patients with COVID-19 and PE estimated at approximately 45%[10]. With available literature it is clear that risk of VTE is very high in patients with COVID-19, especially those requiring intensive care during hospitalization. Patients with COVID-19 and VTE have high risk of mortality.
The pathway for clot formation in acutely ill patients is Virchow’s Triad, which includes the predisposing factors of venous stasis, hypercoagulability and endothelial damage. All critically ill patients, despite underlying etiology, usually face a combination of the above factors and are therefore considered high risk for VTE. Post-mortem studies have raised significant concerns regarding microvascular thrombosis, as well as macrovascular involvement in COVID-19 patients[11,12]. Data suggests SARS-CoV-2 can infect pulmonary endothelial cells, triggering a cascade of local immune response involving leukocyte activation, complement deposition and platelet aggregation[13]. In a small study of 25 patients with COVID-19 who were admitted to the intensive care unit, screening bilateral lower extremity venous ultrasounds between days 5 and 10 of admission showed an overall incidence of proximal DVT of 24%, indicating lower extremity thrombosis is also a major contributor for pulmonary embolism in COVID-19 patients[14]. Apart from known factors that put critically ill patients at high risk of VTE, direct injury to the endothelium by the virus and strong local immune response seems to play a large role, especially in small pulmonary vessel in-situ thromboses’ in patients with COVID-19. Several studies also reported other hemostatic abnormalities in COVID-19 patients, including positive antiphospholipid antibodies, abnormal platelet function and abnormal coagulation parameters that likely add a complex interplay further increasing risk of thromboembolism[15].
Clinical probability scores have been shown to assist in determining pretest probability of pulmonary embolism with more accuracy than clinician gestalt[16,17]. The Well’s criteria are the most popular and commonly used tool internationally used to aid in clinical decision making for diagnosis of VTE. Studies on the use of clinical probability scoring in COVID-19 patients is thus far very limited. One study indicated a Wells score > 2 had a higher correlation for VTE on imaging in critically ill COVID-19 patients[18]. Despite the limited evidence, use of a clinical prediction scoring tool should be considered in conjunction with clinical judgement when defining pretest probability of VTE in COVID-19 patients.
D-dimer is a soluble fibrin degradation product resulting from fibrinolysis of thrombi. It is frequently elevated in acute VTE, but is non-specific, being frequently elevated in many other non-thrombotic conditions including pregnancy, cancer and inflam-mation[19,20]. Cochrane review suggests D-dimer sensitivity ranging from 80%-100% and specificity from 23%-63% in prediction for VTE[21]. Due to lack of specificity and high false positive results, D-dimer is a good test to rule out VTE in low pretest probability patients if D-dimer results are normal, but should not be used to establish diagnosis of VTTE when levels are elevated[22].
In multiple studies D-dimer has shown to be frequently elevated in COVID-19 positive patients in the absence of VTE. Studies indicate up to 40%-50% of patients with COVID-19 will have elevated D-dimer during hospitalization[23,24]. In one study, admission D-dimer was found to be the same in those patients who were found to have VTE during hospitalization vs those without evidence of VTE[25]. Many retrospective studies suggest significantly higher D-dimer levels in patients with confirmed pulmonary embolism on CTPA vs patients without pulmonary embolism on CTPA[26,27]. Though data clearly indicates higher D-dimer values are associated with higher probability for pulmonary embolism on CTPA, there is ongoing debate about serial D-dimer testing and the cut-off value for D-dimer at which imaging to evaluate for VTE should be performed. Based on available studies, we think absolute D-dimer levels and changes over time should be taken into account in decision making on when to obtain imaging to evaluate for VTE despite the absence of significant clinical suspicion, but exact cut off values or percentage of change from initial D-dimer at which imaging should be performed remains controversial.
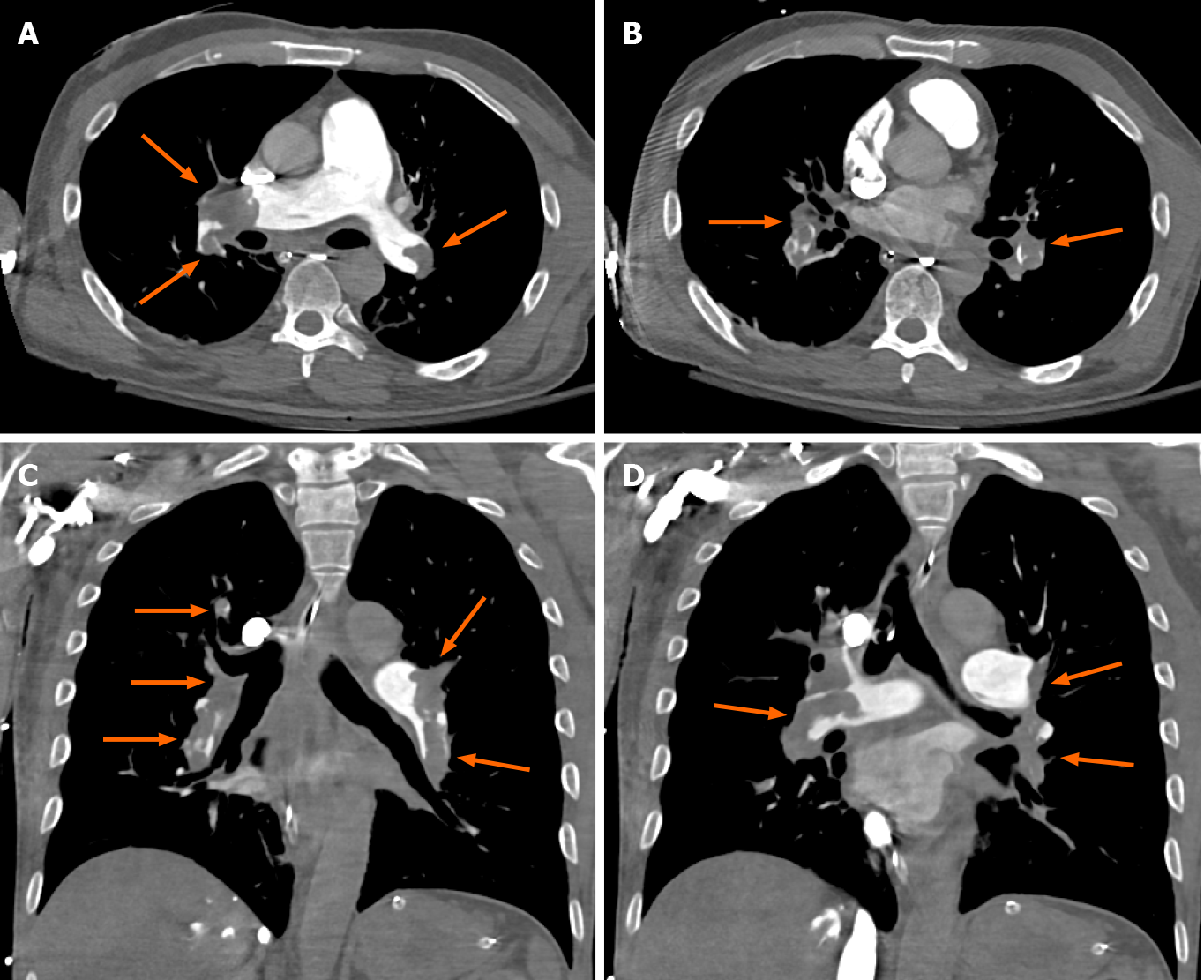
Currently, CTPA is considered the gold standard for diagnosis of pulmonary embolism and venous duplex ultrasound is considered standard for diagnosis of DVT. Pulmonary embolism is seen as a hypodense filling defect on CTPA (Figure 1). A normal CTPA effectively rules out pulmonary embolism with high negative predictive values of around 99%[28]. However, like any other testing modality, CTPA carries risks including exposure to ionizing radiation and use of intravenous iodinated contrast, placing the patient at risk for renal toxicity especially in patients with existing kidney disease and hypersensitivity reactions. Overtreatment of clinically insignificant pulmonary emboli also comes with significant risk given the need for therapeutic anticoagulation medications and resulting risk of bleeding[16].
High resolution CT of lungs can identify ground glass opacities with significant accuracy. In the early part of pandemic there was concern regarding accuracy of reverse transcriptase- polymerase chain reaction (RT-PCR) which led to studies suggesting lung CT scan as a more sensitive modality for diagnosing COVID-19 pneumonia[29]. Though RT-PCR remains the gold standard for confirmation of COVID-19 diagnosis, it has been advised to consider CT chest as a primary modality for diagnosis, especially if RT-PCR availability is limited or there is a delay in testing results[30]. Many institutions use CT modalities over chest x-ray due to the poor sensitivity of chest x-ray in diagnosing COVID-19 pneumonia. Questions naturally arise if one should consider CTPA as a triage test due to the potential added value of evaluating for pulmonary embolism in addition to imaging the lung parenchyma, especially in patients presenting to the emergency department.
Studies evaluating the role of CTPA as a triage and universal evaluation strategy in emergency departments are limited. In a single center retrospective study in the United Kingdom, 48 patients with COVID-19 like symptoms, but without clinical concern for PE, were screened with non-contrast CT. All patients who had findings concerning for COVID-19 or RT-PCR confirmed COVID-19 underwent CTPA. Overall, there was only one positive CTPA (2%) for pulmonary embolism[31]. On the other side in one retrospective study, emergency department clinicians referred COVID-19 patients for CTPA based on clinical suspicion for PE with results showing detection of PE on CTPA in 18% of patients[32]. Of note, data from early in the pandemic in France suggested that PE’s in COVID-19 positive patients typically occurred around day 6 of infection (median)[33]. Currently there are no studies to our knowledge evaluating venous ultrasound as mandatory screening in emergency room in patients with COVID-19.
Though there are many studies indicating high prevalence of VTE in COVID-19 patients, most studies were performed involving patients in the intensive care setting. Data suggests that critically ill patients are at high risk for VTE despite primary cause of that illness[34,35].
Due to the risk associated with intravenous contrast exposure, the role of CTPA as universal testing for all emergency room or hospitalized patients with COVID-19 is not advisable. Similarly routine lower extremity ultrasound of all COVID-19 patients in emergency department does not have cost benefits and is also associated with risks, especially in regards to detecting small distal DVTs with unknown clinical significance.
A high index of clinical suspicion especially in patients with significant hypoxemia along with the use of clinical probability scores and D-dimer should be the driving factors in determining when to obtain CTPA or lower extremity ultrasound in COVID-19 patients.
Hospitalization due to medical illness is associated with increased risk of VTE. Critically ill patients have an even higher risk of VTE despite underlying diagnosis, with many critically ill patients unable to express their symptoms. Physical diagnosis can be challenging and usually not very high yield in diagnosis of DVT[36].
In Prophylaxis for Thromboembolism in Critical Care Trial study pre-COVID 3764 intensive care unit (ICU) patients were randomized to receive either prophylactic low molecular weight heparin, dalteparin or unfractionated heparin. Patients underwent mandatory twice weekly lower extremity ultrasound. The overall VTE rate in the study was 9.1% and DVT rate was 5.5%[37]. In a study published recently involving medical-surgical critically ill patients, twice weekly surveillance with lower extremity ultrasonography lead to 9.6% rate of DVT and was associated with higher detection of DVT compared to non-surveillance standard care group and a lower 90 d mortality (adjusted HR: 0.75, 95%CI: 0.57 to 0.98)[38].
To date, there are not many studies involving systematic screening ultrasound for detection of DVT in COVID-19 patients. In a study involving 26 critically ill patients with COVID-19, when surveillance ultrasonography was mandated, DVT rate was close to 50%. In this study, all patients were mechanically ventilated and about 90% of patients were on vasopressor therapy[39]. Based on limited available data, some institutes and expert groups recommend screening lower extremity ultrasound for patients with COVID-19 who need ICU level care[40,41]. Factors such as size of the hospital, as well as location and local treatment cultures can play a role in which patients are cared for in ICU settings. Smaller hospitals may treat patients on high oxygen or noninvasive mechanical ventilation in intensive care units. Flow management can lead to patients spending some time in intensive care beds due to lack of availability of beds on medical wards. These factors should all be considered when making surveillance imaging decisions.
This is one area where there is agreement amongst most experts and professional societies. The European Society of Radiology, European Society of Thoracic Imaging and European Society of Cardiology suggested that CTPA should be performed to evaluate for pulmonary embolism in COVID-19 patients with limited extent of disease on non-contrast imaging and significant supplemental oxygen needs[42,43].
Given the prevalence of VTE in COVID-19 patients, many physicians and professional societies have contemplated the role of empiric therapeutic anticoagulation for all hospitalized COVID-19 patients. Currently, randomized data is lacking to support the use of empiric therapeutic anticoagulation even amongst critically ill patients. One recent randomized controlled trial comparing therapeutic and prophylactic enoxaparin showed therapeutic enoxaparin improved gas exchange and need for mechanical ventilation in severe COVID-19 patients[44], Many institutions have created alternate guidelines supporting the use of “ intermediate” or full therapeutic anticoagulation[6]. Of course, the use of higher intensity anticoagulation comes with its own set of risks, with several small retrospective studies showing major bleeding events and even fatalities associated with its use[45]. Current guidelines recommend prophylactic dose anticoagulation for hospitalized adults with COVID-19[46]. In addition, guidelines recommend empiric treatment of suspected PE if imaging is expected to take > 4 h or for DVT if imaging is expected to take > 24 h[47]. Currently optimal dosing of intermediate anticoagulation with goal of pharmacoprophylaxis in COVID-19 patients remains unknown[48]. Randomized controlled trials are underway to answer these questions. Results of these trials will help clarify more precise use of anticoagulation strategy in near future[49]. At this point, at our institution, we do not recommend universal intermediate or therapeutic anticoagulation for all patients with COVID-19. We suggest universal pharmacologic prophylactic anticoagulation (if bleeding risk is acceptable) and maintaining a high index of clinical suspicion to help in early diagnosis of VTE events and escalation to appropriate therapeutic dosing when indicated.
Point of care ultrasound (POCUS) in care of all patients is rapidly evolving. Currently, training in POCUS is variable across different medical institutions. Availability of good quality ultrasound machines for point of care use is an additional challenge. Evaluation of the lower extremity deep veins with POCUS for evaluation of deep venous thrombosis is reasonable if the provider has the skills for acquisition and interpretation of images. At our institute, bedside clinicians are not trained and do not use venous ultrasound for thrombosis evaluation. Although periodic screening for deep venous thromboses in medical patients was performed in previous VTE prophylaxis efficacy trials, it has not been studied as an intervention, and, therefore, cannot be recommended[49-55]. For providers with this set of skills, however, lower extremity venous POCUS can be considered in critically ill patients with COVID-19. For patients with moderate to severe COVID-19 with hemodynamic worsening or sudden instability, POCUS use is recommended for rapid evaluation of cor pulmonale[41].
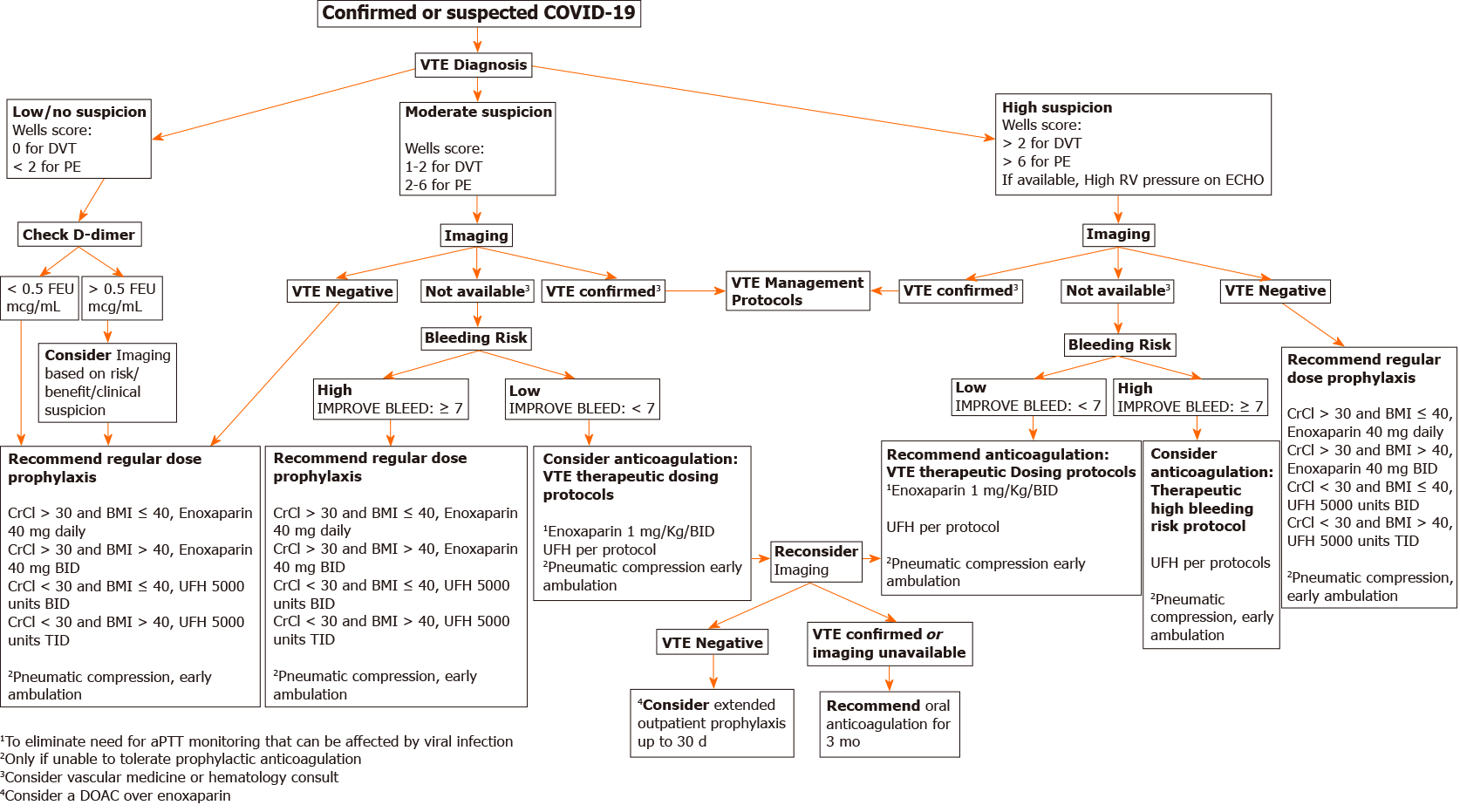
Due to the coronavirus pandemic, a disaster preparedness group of health system experts came together to form the COVID Clinical Content Group. A new webpage on COVID care was created on the health systems website to assist providers with current evidence and local expert guidance. Evidence on risk of thrombosis and management is routinely evaluated panel of experts which include hospitalists, intensivist, vascular medicine specialist, hematologist and anticoagulation pharmacist. Consensus recommendations are posted on this webpage, and periodic educational webinars are hosted. Our current algorithm is described below (Figure 2).
VTE remains a concerning complication in patients with COVID-19 infections. Currently, there remain many unanswered questions related to imaging and anticoagulation strategies. Maintaining a high index of suspicion and use of imaging for early diagnosis of VTE without universal screening appears to be the most logical method in managing this issue until further research can be completed and validated.
Manuscript source: Invited manuscript
Specialty type: Respiratory system
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Alberca RW S-Editor: Zhang H L-Editor: A P-Editor: Yuan YY
| 1. | COVID-19 Map - Johns Hopkins Coronavirus Resource Center. [cited January 21, 2021]. Available from: https://coronavirus.jhu.edu. [Cited in This Article: ] |
| 2. | Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094-1099. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2447] [Cited by in F6Publishing: 2408] [Article Influence: 602.0] [Reference Citation Analysis (0)] |
| 3. | Chi G, Lee JJ, Jamil A, Gunnam V, Najafi H, Memar Montazerin S, Shojaei F, Marszalek J. Venous Thromboembolism among Hospitalized Patients with COVID-19 Undergoing Thromboprophylaxis: A Systematic Review and Meta-Analysis. J Clin Med. 2020;9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 68] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 4. | Poggiali E, Bastoni D, Ioannilli E, Vercelli A, Magnacavallo A. Deep Vein Thrombosis and Pulmonary Embolism: Two Complications of COVID-19 Pneumonia? Eur J Case Rep Intern Med. 2020;7:001646. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 5. | Smilowitz NR, Subashchandran V, Yuriditsky E, Horowitz JM, Reynolds HR, Hochman JS, Berger JS. Thrombosis in hospitalized patients with viral respiratory infections versus COVID-19. Am Heart J. 2021;231:93-95. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 6. | Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers DAMPJ, Kant KM, Kaptein FHJ, van Paassen J, Stals MAM, Huisman MV, Endeman H. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145-147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2925] [Cited by in F6Publishing: 3225] [Article Influence: 806.3] [Reference Citation Analysis (0)] |
| 7. | Cui S, Chen S, Li X, Liu S, Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18:1421-1424. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1298] [Cited by in F6Publishing: 1277] [Article Influence: 319.3] [Reference Citation Analysis (0)] |
| 8. | Ooi MWX, Rajai A, Patel R, Gerova N, Godhamgaonkar V, Liong SY. Pulmonary thromboembolic disease in COVID-19 patients on CT pulmonary angiography - Prevalence, pattern of disease and relationship to D-dimer. Eur J Radiol. 2020;132:109336. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 9. | Alonso-Fernández A, Toledo-Pons N, Cosío BG, Millán A, Calvo N, Ramón L, de Mendoza SH, Morell-García D, Bauça-Rossello JM, Núñez B, Pons J, Palmer JA, Martín L, Peñaranda M, Pou JA, Sauleda J, Sala-Llinas E. Prevalence of pulmonary embolism in patients with COVID-19 pneumonia and high D-dimer values: A prospective study. PLoS One. 2020;15:e0238216. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 10. | Liao SC, Shao SC, Chen YT, Chen YC, Hung MJ. Incidence and mortality of pulmonary embolism in COVID-19: a systematic review and meta-analysis. Crit Care. 2020;24:464. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 11. | Wichmann D, Sperhake JP, Lütgehetmann M, Steurer S, Edler C, Heinemann A, Heinrich F, Mushumba H, Kniep I, Schröder AS, Burdelski C, de Heer G, Nierhaus A, Frings D, Pfefferle S, Becker H, Bredereke-Wiedling H, de Weerth A, Paschen HR, Sheikhzadeh-Eggers S, Stang A, Schmiedel S, Bokemeyer C, Addo MM, Aepfelbacher M, Püschel K, Kluge S. Autopsy Findings and Venous Thromboembolism in Patients With COVID-19: A Prospective Cohort Study. Ann Intern Med. 2020;173:268-277. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1577] [Cited by in F6Publishing: 1648] [Article Influence: 412.0] [Reference Citation Analysis (0)] |
| 12. | Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, Vanstapel A, Werlein C, Stark H, Tzankov A, Li WW, Li VW, Mentzer SJ, Jonigk D. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N Engl J Med. 2020;383:120-128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4103] [Cited by in F6Publishing: 3739] [Article Influence: 934.8] [Reference Citation Analysis (0)] |
| 13. | McFadyen JD, Stevens H, Peter K. The Emerging Threat of (Micro)Thrombosis in COVID-19 and Its Therapeutic Implications. Circ Res. 2020;127:571-587. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 428] [Cited by in F6Publishing: 388] [Article Influence: 97.0] [Reference Citation Analysis (0)] |
| 14. | Longchamp A, Longchamp J, Manzocchi-Besson S, Whiting L, Haller C, Jeanneret S, Godio M, Garcia Martinez JJ, Bonjour T, Caillat M, Maitre G, Thaler JM, Pantet R, Donner V, Dumoulin A, Emonet S, Greub G, Friolet R, Robert-Ebadi H, Righini M, Sanchez B, Delaloye J. Venous thromboembolism in critically Ill patients with COVID-19: Results of a screening study for deep vein thrombosis. Res Pract Thromb Haemost. 2020;4:842-847. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 73] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 15. | Skeik N, Smith JE, Patel L, Mirza AK, Manunga JM, Beddow D. Risk and Management of Venous Thromboembolism in Patients with COVID-19. Ann Vasc Surg. 2021;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Duffett L, Castellucci LA, Forgie MA. Pulmonary embolism: update on management and controversies. BMJ. 2020;370:m2177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 17. | Ceriani E, Combescure C, Le Gal G, Nendaz M, Perneger T, Bounameaux H, Perrier A, Righini M. Clinical prediction rules for pulmonary embolism: a systematic review and meta-analysis. J Thromb Haemost. 2010;8:957-970. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 18. | Zotzmann V, Lang CN, Wengenmayer T, Bemtgen X, Schmid B, Mueller-Peltzer K, Supady A, Bode C, Duerschmied D, Staudacher DL. Combining lung ultrasound and Wells score for diagnosing pulmonary embolism in critically ill COVID-19 patients. J Thromb Thrombolysis. 2020;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Weitz JI, Fredenburgh JC, Eikelboom JW. A Test in Context: D-Dimer. J Am Coll Cardiol. 2017;70:2411-2420. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 202] [Cited by in F6Publishing: 268] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 20. | Johnson ED, Schell JC, Rodgers GM. The D-dimer assay. Am J Hematol. 2019;94:833-839. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 21. | Crawford F, Andras A, Welch K, Sheares K, Keeling D, Chappell FM. D-dimer test for excluding the diagnosis of pulmonary embolism. Cochrane Database Syst Rev. 2016: CD010864. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 22. | Wells PS, Anderson DR, Rodger M, Forgie M, Kearon C, Dreyer J, Kovacs G, Mitchell M, Lewandowski B, Kovacs MJ. Evaluation of D-dimer in the diagnosis of suspected deep-vein thrombosis. N Engl J Med. 2003;349:1227-1235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 999] [Cited by in F6Publishing: 836] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 23. | Zhang JJ, Dong X, Cao YY, Yuan YD, Yang YB, Yan YQ, Akdis CA, Gao YD. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75:1730-1741. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2139] [Cited by in F6Publishing: 2250] [Article Influence: 562.5] [Reference Citation Analysis (0)] |
| 24. | Zhang L, Yan X, Fan Q, Liu H, Liu X, Liu Z, Zhang Z. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J Thromb Haemost. 2020;18:1324-1329. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 837] [Cited by in F6Publishing: 790] [Article Influence: 197.5] [Reference Citation Analysis (0)] |
| 25. | Rodriguez-Sevilla JJ, Rodó-Pin A, Espallargas I, Villar-García J, Molina L, Pérez Terán P, Vázquez Sanchez A, Masclans JR, Jiménez C, Millan Segovia R, Zuccarino F, Salar A, Rodriguez-Chiaradía DA. Pulmonary Embolism in Patients With Covid-19 Pneumonia: The Utility of D-dimer. Arch Bronconeumol. 2020;56:758-759. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Artifoni M, Danic G, Gautier G, Gicquel P, Boutoille D, Raffi F, Néel A, Lecomte R. Systematic assessment of venous thromboembolism in COVID-19 patients receiving thromboprophylaxis: incidence and role of D-dimer as predictive factors. J Thromb Thrombolysis. 2020;50:211-216. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 176] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 27. | Garcia-Olivé I, Sintes H, Radua J, Abad Capa J, Rosell A. D-dimer in patients infected with COVID-19 and suspected pulmonary embolism. Respir Med. 2020;169:106023. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 59] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 28. | Mos IC, Klok FA, Kroft LJ, DE Roos A, Dekkers OM, Huisman MV. Safety of ruling out acute pulmonary embolism by normal computed tomography pulmonary angiography in patients with an indication for computed tomography: systematic review and meta-analysis. J Thromb Haemost. 2009;7:1491-1498. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 29. | Ai T, Yang Z, Hou H, Zhan C, Chen C, Lv W, Tao Q, Sun Z, Xia L. Correlation of Chest CT and RT-PCR Testing for Coronavirus Disease 2019 (COVID-19) in China: A Report of 1014 Cases. Radiology. 2020;296:E32-E40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3542] [Cited by in F6Publishing: 3193] [Article Influence: 798.3] [Reference Citation Analysis (0)] |
| 30. | Gandhi D, Jain N, Khanna K, Li S, Patel L, Gupta N. Current role of imaging in COVID-19 infection with recent recommendations of point of care ultrasound in the contagion: a narrative review. Ann Transl Med. 2020;8:1094. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 31. | Birk R, Shaw D, Kennedy C, Higashi Y, Patel R, Gupta A, Au-Yong I. Low Detection Rate of Pulmonary Embolism in Patients Presenting to the Emergency Department With Suspected Coronavirus Disease 2019 (COVID-19): A Single-Centre UK Study. Curr Probl Diagn Radiol. 2020;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Gervaise A, Bouzad C, Peroux E, Helissey C. Acute pulmonary embolism in non-hospitalized COVID-19 patients referred to CTPA by emergency department. Eur Radiol. 2020;30:6170-6177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 78] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 33. | Poissy J, Goutay J, Caplan M, Parmentier E, Duburcq T, Lassalle F, Jeanpierre E, Rauch A, Labreuche J, Susen S; Lille ICU Haemostasis COVID-19 Group. Pulmonary Embolism in Patients With COVID-19: Awareness of an Increased Prevalence. Circulation. 2020;B: 184-186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 724] [Cited by in F6Publishing: 837] [Article Influence: 209.3] [Reference Citation Analysis (0)] |
| 34. | Helms J, Tacquard C, Severac F, Leonard-Lorant I, Ohana M, Delabranche X, Merdji H, Clere-Jehl R, Schenck M, Fagot Gandet F, Fafi-Kremer S, Castelain V, Schneider F, Grunebaum L, Anglés-Cano E, Sattler L, Mertes PM, Meziani F; CRICS TRIGGERSEP Group (Clinical Research in Intensive Care and Sepsis Trial Group for Global Evaluation and Research in Sepsis). High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089-1098. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1669] [Cited by in F6Publishing: 1931] [Article Influence: 482.8] [Reference Citation Analysis (0)] |
| 35. | Bompard F, Monnier H, Saab I, Tordjman M, Abdoul H, Fournier L, Sanchez O, Lorut C, Chassagnon G, Revel MP. Pulmonary embolism in patients with COVID-19 pneumonia. Eur Respir J. 2020;56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 214] [Cited by in F6Publishing: 252] [Article Influence: 63.0] [Reference Citation Analysis (0)] |
| 36. | Ambesh P, Obiagwu C, Shetty V. Homan's sign for deep vein thrombosis: A grain of salt? Indian Heart J. 2017;69:418-419. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 37. | PROTECT Investigators for the Canadian Critical Care Trials Group and the Australian and New Zealand Intensive Care Society Clinical Trials Group; Cook D, Meade M, Guyatt G, Walter S, Heels-Ansdell D, Warkentin TE, Zytaruk N, Crowther M, Geerts W, Cooper DJ, Vallance S, Qushmaq I, Rocha M, Berwanger O, Vlahakis NE. Dalteparin versus unfractionated heparin in critically ill patients. N Engl J Med. 2011;364:1305-1314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 304] [Cited by in F6Publishing: 286] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 38. | Arabi YM, Burns KEA, Alsolamy SJ, Alshahrani MS, Al-Hameed FM, Arshad Z, Almaani M, Hawa H, Mandourah Y, Almekhlafi GA, Al Aithan A, Khalid I, Rifai J, Rasool G, Abdukahil SAI, Jose J, Afesh LY, Al-Dawood A; Saudi Critical Care Trials Group. Surveillance or no surveillance ultrasonography for deep vein thrombosis and outcomes of critically ill patients: a pre-planned sub-study of the PREVENT trial. Intensive Care Med. 2020;46:737-746. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 39. | Llitjos JF, Leclerc M, Chochois C, Monsallier JM, Ramakers M, Auvray M, Merouani K. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J Thromb Haemost. 2020;18:1743-1746. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 850] [Cited by in F6Publishing: 883] [Article Influence: 220.8] [Reference Citation Analysis (0)] |
| 40. | McBane RD 2nd, Torres Roldan VD, Niven AS, Pruthi RK, Franco PM, Linderbaum JA, Casanegra AI, Oyen LJ, Houghton DE, Marshall AL, Ou NN, Siegel JL, Wysokinski WE, Padrnos LJ, Rivera CE, Flo GL, Shamoun FE, Silvers SM, Nayfeh T, Urtecho M, Shah S, Benkhadra R, Saadi SM, Firwana M, Jawaid T, Amin M, Prokop LJ, Murad MH. Anticoagulation in COVID-19: A Systematic Review, Meta-analysis, and Rapid Guidance From Mayo Clinic. Mayo Clin Proc. 2020;95:2467-2486. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 72] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 41. | Hussain A, Via G, Melniker L, Goffi A, Tavazzi G, Neri L, Villen T, Hoppmann R, Mojoli F, Noble V, Zieleskiewicz L, Blanco P, Ma IWY, Wahab MA, Alsaawi A, Al Salamah M, Balik M, Barca D, Bendjelid K, Bouhemad B, Bravo-Figueroa P, Breitkreutz R, Calderon J, Connolly J, Copetti R, Corradi F, Dean AJ, Denault A, Govil D, Graci C, Ha YR, Hurtado L, Kameda T, Lanspa M, Laursen CB, Lee F, Liu R, Meineri M, Montorfano M, Nazerian P, Nelson BP, Neskovic AN, Nogue R, Osman A, Pazeli J, Pereira-Junior E, Petrovic T, Pivetta E, Poelaert J, Price S, Prosen G, Rodriguez S, Rola P, Royse C, Chen YT, Wells M, Wong A, Xiaoting W, Zhen W, Arabi Y. Multi-organ point-of-care ultrasound for COVID-19 (PoCUS4COVID): international expert consensus. Crit Care. 2020;24:702. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 74] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 42. | The European Society for Cardiology. ESC Guidance for the Diagnosis and Management of CV Disease during the COVID-19 Pandemic. [cited January 21, 2021]. Available from: https://www.escardio.org/Education/COVID-19-and-Cardiology/ESCCOVID-19-Guidance. [Cited in This Article: ] |
| 43. | Revel MP, Parkar AP, Prosch H, Silva M, Sverzellati N, Gleeson F, Brady A; European Society of Radiology (ESR) and the European Society of Thoracic Imaging (ESTI). COVID-19 patients and the radiology department - advice from the European Society of Radiology (ESR) and the European Society of Thoracic Imaging (ESTI). Eur Radiol. 2020;30:4903-4909. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 212] [Cited by in F6Publishing: 244] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 44. | Lemos ACB, do Espírito Santo DA, Salvetti MC, Gilio RN, Agra LB, Pazin-Filho A, Miranda CH. Therapeutic versus prophylactic anticoagulation for severe COVID-19: A randomized phase II clinical trial (HESACOVID). Thromb Res. 2020;196:359-366. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 181] [Cited by in F6Publishing: 175] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 45. | Al-Samkari H, Karp Leaf RS, Dzik WH, Carlson JCT, Fogerty AE, Waheed A, Goodarzi K, Bendapudi PK, Bornikova L, Gupta S, Leaf DE, Kuter DJ, Rosovsky RP. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood. 2020;136:489-500. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 740] [Cited by in F6Publishing: 863] [Article Influence: 215.8] [Reference Citation Analysis (0)] |
| 46. | NIH. COVID-19 Treatment Guidelines. [cited January 21, 2021]. Available from: http://www.covid19treatmentguidelines.nih.gov. [Cited in This Article: ] |
| 47. | Obi AT, Barnes GD, Wakefield TW, Brown S, Eliason JL, Arndt E, Henke PK. Practical diagnosis and treatment of suspected venous thromboembolism during COVID-19 pandemic. J Vasc Surg Venous Lymphat Disord. 2020;8:526-534. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 66] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 48. | Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, Nigoghossian C, Ageno W, Madjid M, Guo Y, Tang LV, Hu Y, Giri J, Cushman M, Quéré I, Dimakakos EP, Gibson CM, Lippi G, Favaloro EJ, Fareed J, Caprini JA, Tafur AJ, Burton JR, Francese DP, Wang EY, Falanga A, McLintock C, Hunt BJ, Spyropoulos AC, Barnes GD, Eikelboom JW, Weinberg I, Schulman S, Carrier M, Piazza G, Beckman JA, Steg PG, Stone GW, Rosenkranz S, Goldhaber SZ, Parikh SA, Monreal M, Krumholz HM, Konstantinides SV, Weitz JI, Lip GYH; Global COVID-19 Thrombosis Collaborative Group; Endorsed by the ISTH, NATF, ESVM, and the IUA; Supported by the ESC Working Group on Pulmonary Circulation and Right Ventricular Function. COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-Up: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;75:2950-2973. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2080] [Cited by in F6Publishing: 2065] [Article Influence: 516.3] [Reference Citation Analysis (0)] |
| 49. | Talasaz AH, Sadeghipour P, Kakavand H, Aghakouchakzadeh M, Kordzadeh-Kermani E, Van Tassell BW, Gheymati A, Ariannejad, Hosseini SH, Jamalkhani S, Sholzberg M, Monreal M, Jimenez D, Piazza G, Parikh SA, Kirtane A, Eikelboom JW, Connors JM, Hunt BJ, Konstantinides SV, Cushman M, Weitz JI, Stone GW, Krumholz PM, Lip GYH, Goldhaber SZ, Bikdeli B. Antithrombotic Therapy in COVID-19: Systematic Summary of Ongoing or Completed Randomized Trials. medRxiv 2021.01.04. 21249227;. [DOI] [Cited in This Article: ] |
| 50. | Cook D, Crowther M, Meade M, Rabbat C, Griffith L, Schiff D, Geerts W, Guyatt G. Deep venous thrombosis in medical-surgical critically ill patients: prevalence, incidence, and risk factors. Crit Care Med. 2005;33: 1565-1571. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 274] [Cited by in F6Publishing: 272] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 51. | Samama MM, Cohen AT, Darmon JY, Desjardins L, Eldor A, Janbon C, Leizorovicz A, Nguyen H, Olsson CG, Turpie AG, Weisslinger N. A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in Medical Patients with Enoxaparin Study Group. N Engl J Med. 1999;341:793-800. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1159] [Cited by in F6Publishing: 1130] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 52. | Khouli H, Shapiro J, Pham VP, Arfaei A, Esan O, Jean R, Homel P. Efficacy of deep venous thrombosis prophylaxis in the medical intensive care unit. J Intensive Care Med. 2006;21:352-358. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 53. | Leizorovicz A, Cohen AT, Turpie AG, Olsson CG, Vaitkus PT, Goldhaber SZ; PREVENT Medical Thromboprophylaxis Study Group. Randomized, placebo-controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients. Circulation. 2004;110:874-879. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 662] [Cited by in F6Publishing: 697] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 54. | Cohen AT, Davidson BL, Gallus AS, Lassen MR, Prins MH, Tomkowski W, Turpie AG, Egberts JF, Lensing AW; ARTEMIS Investigators. Efficacy and safety of fondaparinux for the prevention of venous thromboembolism in older acute medical patients: randomised placebo controlled trial. BMJ. 2006;332:325-329. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 601] [Cited by in F6Publishing: 619] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 55. | Goldhaber SZ, Leizorovicz A, Kakkar AK, Haas SK, Merli G, Knabb RM, Weitz JI; ADOPT Trial Investigators. Apixaban versus enoxaparin for thromboprophylaxis in medically ill patients. N Engl J Med. 2011;365:2167-2177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 419] [Cited by in F6Publishing: 409] [Article Influence: 31.5] [Reference Citation Analysis (0)] |