Copyright
©The Author(s) 2017.
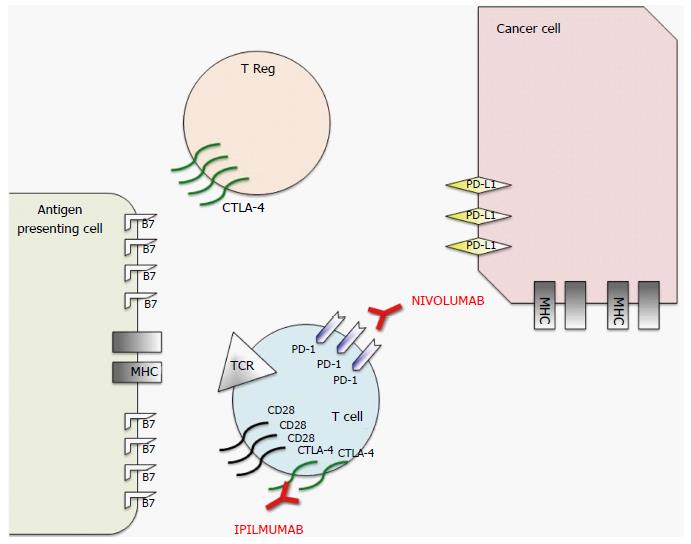
Figure 1 Schematic representation of mechanism of action of nivolumab and ipilimumab, two Food and Drug Administration approved immune checkpoint inhibitors.
To prevent autoimmunity, numerous checkpoint pathways regulate the activation of T cells at multiple steps (process known as peripheral tolerance). Central in this process are the cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) and programmed death 1 (PD-1) immune checkpoints pathways. CTLA-4 is potentially able to stop autoreactive T cells at the initial stage of naive T-cell activation, typically in lymph nodes, while PD-1 regulates previously activated T cells at the later stages of an immune response in peripheral tissues. The binding between T-cell receptor (TCR), which is expressed on T cell surface, with major histocompatibility complex (MHC) expressed on antigen presenting cells (APCs) provides specificity to T-cell activation. However, T cell activation requires more than one stimulatory signal. Among them a central role is played by the binding between B7 molecules (APC) with CD28 (T-Cell). CTLA-4 is a CD28 homolog which does not produce a stimulatory signal but inhibits TCR-MHC binding and thus the T-Cell activation. Different from T-cells in which the amount of CTLA-4 is low, T-Regs highly express CTLA-4. In these cells CTLA-4 might play a role in their suppressive functions. PD-1 is a member of the B7/CD38 family of protein, which is able to bind with two different ligands: Programmed death ligand 1 (PD-L1) and programmed death ligand 2 (PD-L2). PD-1 activation in a T-cell prevents the phosphorylation of key TCR signaling intermediates and thus T-cell activation, resulting in suboptimal control of infections and cancers. Therefore, even though they act at different phases of T-cell activation, the negative effect of PD-1 and CTLA-4 on T-cell activity is similar. Moreover, different from CTLA-4, PD-1 expression is not specific in T-cells, but can be observed also in B-cells and myeloid cells. The rationale for immune checkpoint inhibition (represented in red) for cancer treatment is that CTLA-4 and PD1 pathways are strictly related to cancer survival and thus targeting these molecules or their ligands with monoclonal antibodies permits to impact on cancer growth. Therefore, even if the exact mechanism of action of these monoclonal antibodies in the antitumor response remains unclear, research data suggest that it is at least partially related to an activation and proliferation of T-cells regardless of TCR specificity (due to the inhibition of the inhibitory activity of these checkpoints), which enhances the anti-cancer immune reaction.
- Citation: Bauckneht M, Piva R, Sambuceti G, Grossi F, Morbelli S. Evaluation of response to immune checkpoint inhibitors: Is there a role for positron emission tomography? World J Radiol 2017; 9(2): 27-33
- URL: https://www.wjgnet.com/1949-8470/full/v9/i2/27.htm
- DOI: https://dx.doi.org/10.4329/wjr.v9.i2.27