Published online Sep 26, 2017. doi: 10.4330/wjc.v9.i9.723
Peer-review started: February 20, 2017
First decision: March 27, 2017
Revised: May 10, 2017
Accepted: May 22, 2017
Article in press: May 24, 2017
Published online: September 26, 2017
Processing time: 218 Days and 14.9 Hours
Takotsubo cardiomyopathy (TC) is characterized by reversible ventricular dysfunction, not limited to the distribution of an epicardial coronary artery. A disease primarily afflicting post-menopausal women, it is frequently mistaken for acute anterior wall myocardial infarction. Alternatively called Stress Cardiomyopathy, physical or emotional triggers are identified in only three fourths of TC patients. Long considered a benign condition, recent findings suggest poor short term prognosis similar to acute coronary syndrome (ACS). Despite the widely recognized pathophysiological role of catecholamine excess, its diagnostic role is uncertain. TC is suspected based on typical wall motion abnormalities in ventriculogram or echocardiogram. Several additional electrocardiographic, laboratory and imaging parameters have been studied with the goal of clinical diagnosis of TC. While several clinical clues differentiate it from ACS, a clinical diagnosis is often elusive leading to avoidable cardiac catheterizations. Natriuretic peptides (NPs), a family of peptide hormones released primarily in response to myocardial stretch, play a significant role in pathophysiology, diagnosis as well as treatment of congestive heart failure. TC with its prominent ventricular dysfunction is associated with a significant elevation of NPs. NPs are elevated in ACS as well but the degree of elevation is typically lesser than in TC. Markers of myocardial injury such as troponin are usually elevated to a higher degree in ACS than in TC. This differential elevation of NPs and markers of myocardial injury may play a role in early clinical recognition of TC.
Core tip: Takotsubo cardiomyopathy (TC) characterized by reversible ventricular dysfunction is frequently mistaken for acute anterior wall myocardial infarction often leading to avoidable cardiac catheterizations. While several clinical clues differentiate TC and acute coronary syndrome (ACS), a clinical diagnosis still remains elusive. We review the pathophysiology and diagnosis of TC with a focus on role of cardiac biomarkers [natriuretic peptides - brain natriuretic peptide (BNP) and NT-proBNP and cardiac myonecrosis markers - Troponin, CKMB and Myoglobin]. We have done a review of several studies looking at diagnostic utility of cardiac biomarkers in differentiating TC and ACS.
- Citation: Gopalakrishnan P, Zaidi R, Sardar MR. Takotsubo cardiomyopathy: Pathophysiology and role of cardiac biomarkers in differential diagnosis. World J Cardiol 2017; 9(9): 723-730
- URL: https://www.wjgnet.com/1949-8462/full/v9/i9/723.htm
- DOI: https://dx.doi.org/10.4330/wjc.v9.i9.723
Takotsubo cardiomyopathy (TC), originally described by Sato et al[1] in 1990, is variably known as stress cardiomyopathy, broken heart syndrome, and apical ballooning syndrome. A disease process primarily affecting post-menopausal women, it is characterized by transient left ventricular (LV) dysfunction, not limited to distribution of an epicardial coronary artery. Clinical presentation of TC most often has a significant overlap with acute coronary syndrome (ACS) with symptoms, cardiac biomarker profiles and EKG changes suggesting myocardial ischemia or infarction. TC is estimated to occur in 1%-2% of patients presenting as ACS. With prevalence in 2008 reported as 0.02% of hospitalizations in United States[2], TC incidence has increased with a 3-fold increase in TC hospitalization in United States between 2007 and 2012[3]. The inability to confidently diagnose TC based on clinical presentation leads to almost universal use of cardiac catheterization in these patients. Several indicators including cardiac biomarker elevation have been studied with the goal of making a clinical diagnosis of TC (Table 1).
| History | Stressful stimulus | |
| Female sex | ||
| Age > 55 yr | ||
| Neuropsychiatric conditions | ||
| EKG findings | Absence or paucity of reciprocal ST depression | |
| Widespread T wave inversion | ||
| QTc prolongation | ||
| Laboratory findings | Catecholamine levels | Metanephrine, Normetanephrine |
| Natriuretic peptides | BNP, NT-proBNP | |
| Myonecrosis markers | Myoglobin, CK-MB, Troponin I, Troponin T | |
| Others | Copeptin, sST2, soluble lectin like oxidized LDL receptor-1 (sLOX-1), IMA | |
| Imaging | Echocardiogram | Reversible wall motion abnormalities > distribution of a epicardial coronary artery |
| Reversible mitral regurgitation, | ||
| Left ventricular outflow tract obstruction | ||
| Coronary angiogram | Absence of ruptured plaque | |
| Diminished flow | ||
| Coronary vasospasm | ||
| SPECT | Reduced Thallium uptake | |
| Reduced fatty acid metabolism in BMIPP imaging | ||
| Reduced myocardial MIBG uptake | ||
| PET | Reverse metabolism perfusion mismatch | |
| CMR | T2 hyperintensity; lack of first pass hypoperfusion; LGE (may be seen if MRI done early) |
Women have a 9-fold higher risk of TC compared to men[4]. Women > 55 years have about 5-fold higher risk than women < 55 years[4]. While a physical or emotional trigger is often identified, no specific triggers have been reported in little over a fourth of TC patients[4]. Reported stressors include surgery, critical illness, death of dear ones, dobutamine or ergonovine stress test, lightning strike, prolonged immobilization and thyrotoxicosis. TC recurrence has been reported in greater than 10% of the patients in the first four years[5]. Initially thought to have a benign course, recent data show short term prognosis for TC is similar to ACS. In the InterTAK registry, severe in-hospital complications, such as shock and death were similar in TC and ACS[4]. According to the SWEDEHEART study, prognosis of takotsubo syndrome is poor, with early and late mortality similar to STEMI and NSTEMI[6].
Four different morphotypes of TC have been described: (1) Classical - apical ballooning with basal hyperkinesis; (2) Mid-ventricular - basal hyperkinesis, mid-ventricular hypokinesis and normal or hyperkinetic apex; (3) Basal (inverted) - basal and mid-ventricular hypokinesis with apical hyperkinesis; and (4) Focal - hypokinesis of a focal myocardial segment[4]. TC predominantly affects the left ventricle but right ventricular (RV) involvement with a more malignant course has been described as well[7]. The classic type characterized by basal hyperkinesis is often associated with left ventricular outflow tract (LVOT) obstruction and shock[8]. Significant reversible mitral regurgitation (MR) and higher brain natriuretic peptide (BNP) levels related to the ventricular dilation have been described in the classic form. The inverted (basal) form seems to have higher levels of troponin and lower levels of BNP as well as lower incidence of LVOT obstruction and MR[8].
Several etiologies have been proposed including catecholamine excess, derangement of myocardial glucose and fatty acid metabolism, microcirculatory dysfunction, coronary vasospasm, estrogen deficiency etc. Norepinephrine may trigger α1-mediated coronary vasospasm and β1-mediated hyperdynamic basal contraction, as basal myocardium has higher density of sympathetic nerve endings and higher content of norepinephrine. The biased agonism of epinephrine and apical-basal gradient of β2-adrenergic receptor (β2AR) may explain the apical stunning. High level of epinephrine could trigger signal switching of β2AR from stimulatory G-protein to inhibitory G-protein. Apical myocardium with higher concentrations of β2AR may be more susceptible, compared to basal myocardium leading to apical stunning[9]. The histological changes of TC mirror catecholamine toxicity seen in pheochromocytoma. Loss of cardioprotective action of estrogen against catecholamine excess may explain higher incidence of TC in postmenopausal women. Positron emission tomography (PET) studies have suggested disturbances in glucose and fatty acid metabolism in TC patients[10]. Findings suggestive of coronary vasospasm as well as microcirculatory dysfunction have been described in coronary angiograms of TC patients.
ACS is the primary differential diagnosis as both disease states have significant overlap in their clinical presentation. TC is often mistook for acute anterior wall ST elevation myocardial infarction (occlusion of proximal left anterior descending artery). Other differential diagnoses include myocarditis, endogenous catecholamine excess (pheochromocytoma), exogenous catecholamine excess (Cocaine, Amphetamine), peripartum cardiomyopathy and cerebrovascular disease (Japanese guidelines have cerebrovascular disease as exclusionary criteria unlike the commonly used Mayo criteria). Other differential diagnosis for chest pain such as aortic dissection, pulmonary embolism should be considered as well.
Several diagnostic criteria including the Modified Mayo[11] and Japanese[12] criteria have been proposed underlining the difficulty in diagnosis of TC. As per the widely used Modified Mayo criteria, all of the following 4 criteria must be met for diagnosing TC: (1) transient hypokinesis, akinesis, or dyskinesis of the LV mid segments with or without apical involvement; the regional wall motion abnormalities extend beyond a single epicardial vascular distribution; a stressful trigger is often, but not always present; (2) absence of obstructive coronary disease or angiographic evidence of acute plaque rupture; (3) new electrocardiographic abnormalities (either ST-segment elevation and/or T-wave inversion) or modest elevation in cardiac troponin; and (4) absence of pheochromocytoma and myocarditis. Several approaches have been proposed to facilitate differentiating TC from ACS. They include use of laboratory findings [catecholamine levels, cardiac biomarkers, lipid levels and investigational markers such as soluble lectin like oxidized LDL receptor-1 (sLOX-1), Copeptin, ischemic modified albumin (IMA), sST2 (soluble suppression of tumorigenicity-2), etc.], imaging modalities [echocardiography, computed tomography (CT) coronary angiogram, invasive coronary angiogram, cardiac magnetic resonance imaging (CMR), single photon emission computed tomography (SPECT) and PET], EKG findings and risk scores.
More studies have focused on laboratory findings due to their universal availability at the time of presentation as well as availability of repeat measurements. Several markers including Copeptin[13], lipid profile[14], sLOX-1[15], IMA[16], sST-2[17] have been proposed for differentiating TC from ACS. High HDL-C and lower levels of LDL and triglycerides have been reported in TC compared to MI[14]. Forty percent of TC pts had hyperalphalipoproteinemia or hypotriglyceridemia. sLOX-1 elevation has been found comparable to troponin rise in ACS and is lower in non-ACS patients including TC[15]. Changes in level of sST2 have additional predictive value for TC in patients with normal Troponin I[17]. The most studied laboratory findings though are natriuretic peptides (NP), markers of cardiomyonecrosis (troponin I and T, creatine kinase and myoglobin) and catecholamines.
NP belong to a family of peptide hormones with natriuretic and vasodilatory properties in addition to other pleotropic effects[18]. Atrial natriuretic peptide (ANP), BNP and C-type natriuretic peptide (CNP) constitute the natriuretic peptide family. Under normal conditions ANP is primarily released from atria, BNP from both atria and ventricles (ventricles more than atria) and CNP from nervous tissue and vascular endothelium[19,20]. The NPs act via the natriuretic peptide receptors (NPR) NPR-A, NPR-B and NPR-C[18]. ANP and BNP act primarily through NPR-A leading to natriuresis, vasodilation, inhibition of aldosterone synthesis, thirst suppression, sympatholysis and inhibition of release of vasopressin and adrenocorticotropic hormone[18,20]. Additional effects on pulmonary vasculature and airway smooth muscle cells have been described[20]. CNP which has less potent natriuretic effect, acts primarily via NPR-B and modulates vascular tone, cardiac remodeling and proliferation of vascular smooth muscle cells. Primary mechanism of NP clearance is by NPR-C mediated internalization and lysosomal degradation[21]. While ANP was discovered earlier in the 1980s, BNP and amino terminal proBNP (NT-proBNP) - an inactive by-product of BNP formation, have been more widely studied for their role in pathophysiology, diagnosis as well as treatment of heart failure.
BNP is initially produced in the form of preproBNP a 134 amino acid (AA) peptide. Cleavage of the 26 AA signal peptide forms the proBNP which is further cleaved by enzyme Corin into active 32 AA BNP and inactive 76 AA amino terminal proBNP (NT-proBNP). BNP has a short half-life (about 20 min) and is cleared by neutral endopeptidase (Neprilysin) and by NPR-C mediated clearance. NT-proBNP has a longer half-life (120 min) and is cleared renally[22]. Use of neprilysin inhibitor (sacubitril) increases BNP levels by inhibiting its clearance but does not affect clearance of NT-proBNP[23]. Upper limit of normal in the non-acute setting is 35 pg/mL for BNP and 125 pg/mL for NT-proBNP[24]. In acute setting, higher cut-off values are recommended (BNP < 100 pg/mL and NT-proBNP < 300 pg/mL)[24]. In the Breathing Not Properly trial, BNP < 100 pg/mL had a high diagnostic accuracy of 83.4% to distinguish other causes of dyspnea from heart failure[25]. The PRIDE (ProBNP Investigation of DyspnEa) study proposed an age based cut-off for NT-proBNP (> 450 pg/mL for age < 50, > 900 pg/mL for age > 50) for diagnosing HF and < 300 pg/mL for ruling out CHF[26]. International Collaborative of NT-proBNP (ICON) study, a pooled analysis recommended a cut off of > 1800 pg/mL for age > 75[27]. Asians and african americans have higher levels compared to caucasians and hispanics[28]. Obese patients tend to have lower levels and heart failure with preserved ejection fraction (HfpEF) patients have levels lower than heart failure with reduced ejection fraction (HfrEF) patients[29,30]. Causes of BNP and NT-proBNP elevation include cardiac causes such as heart failure, ACS, valvular heart disease, pericardial diseases, atrial fibrillation, myocarditis, and cardioversion and non-cardiac causes such as advancing age, anemia, renal failure, pulmonary diseases, critical illness, sepsis, burns, etc[24].
Reversible LV dysfunction without significant myocardial ischemia and or necrosis is the hallmark of TC, leading to significant elevation of NP. Among TC patients, the classic form of TC with basal hyperkinesis and apical ballooning appears to have higher degree of NP elevation compared to the basal (inverted) variant[8]. BNP has been correlated with the degree of basal hyperkinesis, measured by δBase (difference between end systolic and end diastolic dimension of the LV base measured 10 mm below aortic valve)[31]. NT-proBNP levels rise within first 24 h after the onset of symptoms with slow and incomplete resolution during the 3 mo thereafter[32]. NT-proBNP levels have been shown to correlate with plasma catecholamine levels and the severity of LV dysfunction, as measured by the wall motion score index and LV ejection fraction[32].
With lack of significant myonecrosis, TC patients usually have lesser degree of elevation of cardiac myonecrosis markers such as myoglobin, creatine kinase and troponin when compared to ACS patients. Studies comparing TC with anterior ST elevation myocardial infarction (STEMI) showed significantly lower mean peak troponin T levels in TC patients[33]. Some studies suggested threshold values for troponin to rule out TC while other studies contradicted it. Ramaraj et al[34] found troponin T > 6 ng/mL or troponin I > 15 ng/mL were unlikely in TC but Song et al[8] found about 20% of patients included in their study of TC patients had troponin I > 15 ng/mL. Among TC patients, inverted (basal) type TC patients tend to have higher elevation of myonecrosis markers[8].
Comparing TC to STEMI, Madhavan et al[35] found lower troponin (0.62 ng/mL vs 3.8 ng/mL), higher BNP (944 pg/mL vs 206 pg/mL) but no significant differences in plasma normetanephrine, metanephrine, cortisol or hs-CRP levels. Fröhlich et al[36] found NT-proBNP (ng/L)/myoglobin (μg/L) ratio of 3.8, distinguished TC from STEMI, while a NT-proBNP (ng/L)/myoglobin (μg/L) ratio of 14, distinguished TC from NSTEMI. NT-proBNP (ng/L)/TnT (μg/L) ratio of 2889, distinguished TC from STEMI, while a NT-proBNP (ng/L)/TnT (μg/L) ratio of 5000 distinguished TC from NSTEMI. NT-proBNP levels usually peaked 22 to 26 h after a cardiac event, whereas TnT levels peaked 8 to 13 h after the first manifestation of chest pain. In a study of 52 patients with TC, Lahoti et al[37] found higher NT-proBNP/troponin T in TC than in ACS patients (5154 vs 183). Peak BNP/peak troponin ratio > 2500 yielded a 90% sensitivity and specificity for TC.
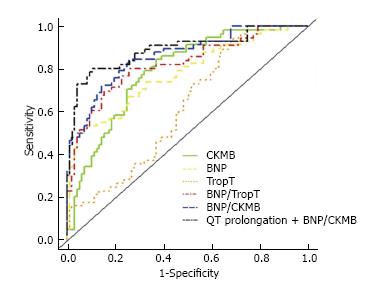
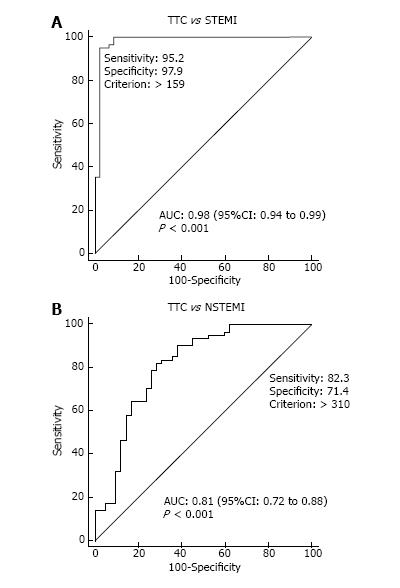
Randhawa et al[38] compared 58 patients and 97 acute myocardial infarction patients and found early BNP/TnT and BNP/CKMB ratios help to differentiate TC from AMI with greater accuracy than BNP alone. Median BNP/TnT and BNP/CKMB ratios were, respectively, 1292 and 28.44 in the TC group and 226.9 and 3.63 in the AMI group. TC was distinguished from AMI with 95% specificity with the use of BNP/TnT ratio of ≥ 1272 (sensitivity 52%) with area under the curve (AUC) of 0.822 and BNP/CKMB ratio ≥ 29.9 (sensitivity 50%) with AUC of 0.862. When QT prolongation was combined with BNP/CKMB, the AUC was even higher (Figure 1). Doyen et al[39] found TnI elevations in TC comparable to anterior NSTEMI but lower than anterior STEMI, earlier peaking of troponin in TC than ACS (6 h vs 12 h) and higher BNP/TnI ratio (642) than anterior NSTEMI (184.5) or anterior STEMI (7.5). BNP/TnI ratio showed high area under the curve (AUC) in receiver operating characteristic (ROC) analysis. The AUC for TC vs STEMI was 0.98 (0.94 to 0.99) and TC vs NSTEMI was 0.81 (0.72 to 0.88) (Figure 2). The InterTAK registry study group[40] compared matched cohorts of 455 TC (out of 1750 TC patients in InterTAK registry) and 455 ACS patients. Median troponin levels in TC were not significantly different from ACS but CK and BNP levels were significantly different.
InterTAK Diagnostic Score[39] was developed using a derivation cohort with TC patients recruited from the International Takotsubo Registry and ACS patients from a Zurich hospital (TC, n = 218; ACS, n = 436). The score has seven variables each with an assigned score value: Female sex 25, emotional trigger 24, physical trigger 13, absence of ST-segment depression (except in lead aVR) 12, psychiatric disorders 11, neurologic disorders 9, and QTc prolongation 6 points. A cut-off value of 40 score points yielded a sensitivity of 89% and specificity 91%. With a score of ≥ 50, nearly 95% of TC patients were correctly diagnosed and with a score ≤ 31, approximately 95% of ACS patients were diagnosed correctly[39]. The score was subsequently validated in an independent validation cohort (TTS, n = 173; ACS, n = 226)[39].
While several studies have reported higher levels of NPs in TC and higher troponin in ACS, utilizing ratio of NP to troponin, CKMB or myoglobin to differentiate TC from ACS in clinical practice is more complicated. As discussed earlier the cut off values used in different studies varied widely (Table 2). In general the ratio is higher for TC than ACS and among ACS the ratio is higher for NSTEMI compared to STEMI. The use of different markers for myonecrosis - troponin I and T, CKMB or myoglobin as well as ventricular stretch - BNP or NT-proBNP in different studies affects the wider applicability. Also, most of the studies used peak troponin and or NP levels instead of levels at presentation, which limits the utility of this ratio in avoiding cardiac catheterizations in acute settings. In addition, all these studies were retrospective. The InterTAK score derived from a large cohort study did not include cardiac biomarkers. In the derivation cohort, while the CK was higher in ACS patients and BNP higher in TC patients, the troponin levels were surprisingly higher in TC patients (6.67 × ULN) compared to ACS patients (3.75).
| Ref. | Biomarker and time of collection | Takotsubo cardiomyopathy | Acute coronary syndrome | ||
| Frölich et al[36] | NT-proBNP (peak) | Cutoff NT-proBNP/TnT to differentiate TC and NSTEMI | Cutoff NT-proBNP/TnT to differentiate TC and STEMI | ||
| TnT (peak) | 5000 | 2889 | |||
| Lahoti et al[37] | NT-proBNP (mean) | NT-proBNP/TnT | NT-proBNP/TnT (STEMI) | ||
| TnT (peak) | 5154 ± 1891.2 | 183 ± 128.9 | |||
| Randhawa et al[38] | First simultaneous BNP and TnT | BNP/TnT | BNP/CKMB | BNP/TnT (AMI) | BNP/CKMB (AMI) |
| 1292 (443.4-2657.9) | 28.44 (13.7-94.8) | 226.9 (69.9-426.3) | 3.63 (1.1-10.0) | ||
| Doyen et al[39] | BNP (admission) | BNP/TnI | BNP/TnI (NSTEMI) | BNP/TnI (STEMI) | |
| TnI (peak) | 642 (331.8-1226.5) | 184.5 (50.5-372.3) | 7.5 (2.0-29.6) | ||
With catecholamine excess thought to underlie the pathogenesis of TC, several studies have looked at catecholamine measurements with mixed results. Nguyen et al[32] reported correlation of peak NT-proBNP levels in TC patients with simultaneous plasma normetanephrine levels as well as LV ejection fraction. On the contrary Madhavan et al[35] found significantly higher elevation of BNP in TC patients compared to STEMI patients but similar plasma normetanephrine, metanephrine and cortisol levels. In their study majority of TC patients had normal 24-h urine metanephrines, catecholamines and cortisol.
Echocardiographic findings in TC include reversible wall motion abnormalities extending beyond distribution of an epicardial coronary artery, basal hyperkinesis, LVOT obstruction, reversible MR and RV dysfunction. Reverse Mcconnell’s sign with RV basal hyperkinesis and hypokinesis of RV apex has been described in TC[41]. Common coronary angiogram findings include absence of ruptured plaque or obstructive coronary artery disease. Coronary vasospasm with provocative maneuvers as well as delayed filling has been reported in TC patients. Ventriculogram often demonstrates the typical takotsubo-like shape. Microcirculatory dysfunction has been demonstrated in TC using index of microvascular resistance[42]. CMR findings include enhancement in T2-weighted images representing myocardial edema in the hypocontractile segments during acute phase and absence of first-pass perfusion hypoenhancement[43]. Evidence on late gadolinium enhancement (LGE) findings in TC are conflicting. Some studies suggest absence of LGE differentiates TC from ACS and myocarditis while other studies have reported reversible LGE in TC, if CMR is done in acute phase (< 72 h)[44,45]. Reduction of fatty acid metabolism during acute phase has been reported using 123I-β-methyliodophenylpentadecanoic acid (BMIPP) imaging[46]. Reduced intramyocardial uptake during 123I-metaiodobenzylguanidine (MIBG) imaging suggests sympathetic denervation[46]. A reverse perfusion metabolism mismatch in PET with normal perfusion and reduced 18F-fluoro deoxyglucose (FDG) uptake has been described in TC patients[43].
Several EKG criteria have been proposed to help differentiate TC from ACS. These include lack or rarity of reciprocal ST depression, widespread T wave inversion, low QRS voltage on presentation, attenuation of QRS voltage in serial EKGs, QTc prolongation, frontal plane ST vector, ST segment elevation (STE) in aVR without STE in V1, lower rate of Q-waves, more frequent STE in the inferior leads, higher ratio of the sums of STEs in leads V4-V6 to the sums of STEs in leads in V1-V3, lower amplitude of STE (< 1.5 mm) and a summated amplitude of the S-wave in V1 plus the R-wave in V6 < 1.5 mV[47,48]. While these EKG findings could have additive value in diagnosis of TC, their diagnostic accuracy for TC diagnosis have been found wanting in some studies[49,50].
TC presents a diagnostic challenge by virtue of its similarity in clinical presentation with anterior wall STEMI. The different pathophysiology underlying these two processes leads to a differential degree of elevation in NP and troponin with NP relatively higher in TC and troponin relatively higher in STEMI. While conceptually sound, the use of various assays (BNP vs NT-proBNP, Troponin I vs Troponin T) and wide range in elevation of NPs and Troponin with significant overlap in these two conditions, limits the diagnostic utility of ratio of NPs and troponin. Use of uniform assays for NP and myonecrosis markers and larger trials could pave the way for wider use of NP/troponin ratio in clinical decision making in future.
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Fett JD, Said SAM S- Editor: Song XX L- Editor: A E- Editor: Lu YJ
| 1. | Sato H, Tateishi H, Uchida T. Takotsubo-type cardiomyopathy due to multivessel spasm. Clinical Aspect of Myocardial Injury: From Ischemia to Heart Failure. Tokyo, Japan: Kagakuhyouronsha 1990; 56-64. |
| 2. | Deshmukh A, Kumar G, Pant S, Rihal C, Murugiah K, Mehta JL. Prevalence of Takotsubo cardiomyopathy in the United States. Am Heart J. 2012;164:66-71.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 299] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 3. | Khera R, Light-McGroary K, Zahr F, Horwitz PA, Girotra S. Trends in hospitalization for takotsubo cardiomyopathy in the United States. Am Heart J. 2016;172:53-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 4. | Templin C, Ghadri JR, Diekmann J, Napp LC, Bataiosu DR, Jaguszewski M, Cammann VL, Sarcon A, Geyer V, Neumann CA. Clinical Features and Outcomes of Takotsubo (Stress) Cardiomyopathy. N Engl J Med. 2015;373:929-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1401] [Cited by in RCA: 1714] [Article Influence: 171.4] [Reference Citation Analysis (1)] |
| 5. | Elesber AA, Prasad A, Lennon RJ, Wright RS, Lerman A, Rihal CS. Four-year recurrence rate and prognosis of the apical ballooning syndrome. J Am Coll Cardiol. 2007;50:448-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 404] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 6. | Redfors B, Vedad R, Angerås O, Råmunddal T, Petursson P, Haraldsson I, Ali A, Dworeck C, Odenstedt J, Ioaness D. Mortality in takotsubo syndrome is similar to mortality in myocardial infarction - A report from the SWEDEHEART registry. Int J Cardiol. 2015;185:282-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 245] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 7. | Elesber AA, Prasad A, Bybee KA, Valeti U, Motiei A, Lerman A, Chandrasekaran K, Rihal CS. Transient cardiac apical ballooning syndrome: prevalence and clinical implications of right ventricular involvement. J Am Coll Cardiol. 2006;47:1082-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 168] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 8. | Song BG, Chun WJ, Park YH, Kang GH, Oh J, Lee SC, Park SW, Oh JK. The clinical characteristics, laboratory parameters, electrocardiographic, and echocardiographic findings of reverse or inverted takotsubo cardiomyopathy: comparison with mid or apical variant. Clin Cardiol. 2011;34:693-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 9. | Lyon AR, Rees PS, Prasad S, Poole-Wilson PA, Harding SE. Stress (Takotsubo) cardiomyopathy--a novel pathophysiological hypothesis to explain catecholamine-induced acute myocardial stunning. Nat Clin Pract Cardiovasc Med. 2008;5:22-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 700] [Cited by in RCA: 643] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 10. | Obunai K, Misra D, Van Tosh A, Bergmann SR. Metabolic evidence of myocardial stunning in takotsubo cardiomyopathy: a positron emission tomography study. J Nucl Cardiol. 2005;12:742-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Prasad A, Lerman A, Rihal CS. Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): a mimic of acute myocardial infarction. Am Heart J. 2008;155:408-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1193] [Cited by in RCA: 1296] [Article Influence: 76.2] [Reference Citation Analysis (0)] |
| 12. | Kawai S, Kitabatake A, Tomoike H; Takotsubo Cardiomyopathy Group. Guidelines for diagnosis of takotsubo (ampulla) cardiomyopathy. Circ J. 2007;71:990-992. [PubMed] |
| 13. | Yalta K. Serum copeptin/NT-proBNP ratio: a more reliable index of absolute endogenous stress and prognosis during the course of Tako-tsubo cardiomyopathy? Int J Cardiol. 2012;154:376-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Gaddam S, Nimmagadda KC, Nagrani T, Naqi M, Wetz RV, Weiserbs KF, McCord D, Ghavami F, Gala B, Lafferty JC. Serum lipoprotein levels in takotsubo cardiomyopathy vs. myocardial infarction. Int Arch Med. 2011;4:14. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Kobayashi N, Hata N, Kume N, Shinada T, Tomita K, Shirakabe A, Kitamura M, Nozaki A, Inami T, Seino Y. Soluble lectin-like oxidized LDL receptor-1 and high-sensitivity troponin T as diagnostic biomarkers for acute coronary syndrome. Improved values with combination usage in emergency rooms. Circ J. 2011;75:2862-2871. [PubMed] |
| 16. | Zhong Y, Wang N. Other diagnostic methods with high sensitivity can be used to differentiate Takotsubo cardiomyopathy from acute coronary syndrome. Int J Cardiol. 2016;222:1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 17. | Yang HS, Kim HJ, Shim HJ, Kim SJ, Hur M, Di Somma S; GREAT Network. Soluble ST2 and troponin I combination: Useful biomarker for predicting development of stress cardiomyopathy in patients admitted to the medical intensive care unit. Heart Lung. 2015;44:282-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Wilkins MR, Redondo J, Brown LA. The natriuretic-peptide family. Lancet. 1997;349:1307-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 150] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 19. | Pucci A, Wharton J, Arbustini E, Grasso M, Diegoli M, Needleman P, Viganò M, Moscoso G, Polak JM. Localization of brain and atrial natriuretic peptide in human and porcine heart. Int J Cardiol. 1992;34:237-247. [PubMed] |
| 20. | Calzetta L, Orlandi A, Page C, Rogliani P, Rinaldi B, Rosano G, Cazzola M, Matera MG. Brain natriuretic peptide: Much more than a biomarker. Int J Cardiol. 2016;221:1031-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 21. | Potter LR. Natriuretic peptide metabolism, clearance and degradation. FEBS J. 2011;278:1808-1817. [DOI] [Full Text] |
| 22. | Woodard GE, Rosado JA. Recent advances in natriuretic peptide research. J Cell Mol Med. 2007;11:1263-1271. [DOI] [Full Text] |
| 23. | Zile MR, Claggett BL, Prescott MF, McMurray JJ, Packer M, Rouleau JL, Swedberg K, Desai AS, Gong J, Shi VC. Prognostic Implications of Changes in N-Terminal Pro-B-Type Natriuretic Peptide in Patients With Heart Failure. J Am Coll Cardiol. 2016;68:2425-2436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 283] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 24. | Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129-2200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10348] [Cited by in RCA: 9339] [Article Influence: 1037.7] [Reference Citation Analysis (3)] |
| 25. | Maisel AS, Krishnaswamy P, Nowak RM, McCord J, Hollander JE, Duc P, Omland T, Storrow AB, Abraham WT, Wu AH. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002;347:161-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2401] [Cited by in RCA: 2312] [Article Influence: 100.5] [Reference Citation Analysis (0)] |
| 26. | Januzzi JL, Camargo CA, Anwaruddin S, Baggish AL, Chen AA, Krauser DG, Tung R, Cameron R, Nagurney JT, Chae CU. The N-terminal Pro-BNP investigation of dyspnea in the emergency department (PRIDE) study. Am J Cardiol. 2005;95:948-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 835] [Cited by in RCA: 867] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 27. | Januzzi JL, van Kimmenade R, Lainchbury J, Bayes-Genis A, Ordonez-Llanos J, Santalo-Bel M, Pinto YM, Richards M. NT-proBNP testing for diagnosis and short-term prognosis in acute destabilized heart failure: an international pooled analysis of 1256 patients: the International Collaborative of NT-proBNP Study. Eur Heart J. 2006;27:330-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 861] [Cited by in RCA: 868] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 28. | Krim SR, Vivo RP, Krim NR, Qian F, Cox M, Ventura H, Hernandez AF, Bhatt DL, Fonarow GC. Racial/Ethnic differences in B-type natriuretic peptide levels and their association with care and outcomes among patients hospitalized with heart failure: findings from Get With The Guidelines-Heart Failure. JACC Heart Fail. 2013;1:345-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 29. | Maisel A, Mueller C, Adams K, Anker SD, Aspromonte N, Cleland JG, Cohen-Solal A, Dahlstrom U, DeMaria A, Di Somma S. State of the art: using natriuretic peptide levels in clinical practice. Eur J Heart Fail. 2008;10:824-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 547] [Cited by in RCA: 594] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 30. | Maisel AS, McCord J, Nowak RM, Hollander JE, Wu AH, Duc P, Omland T, Storrow AB, Krishnaswamy P, Abraham WT. Bedside B-Type natriuretic peptide in the emergency diagnosis of heart failure with reduced or preserved ejection fraction. Results from the Breathing Not Properly Multinational Study. J Am Coll Cardiol. 2003;41:2010-2017. [PubMed] |
| 31. | Akashi YJ, Musha H, Nakazawa K, Miyake F. Plasma brain natriuretic peptide in takotsubo cardiomyopathy. QJM. 2004;97:599-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 32. | Nguyen TH, Neil CJ, Sverdlov AL, Mahadavan G, Chirkov YY, Kucia AM, Stansborough J, Beltrame JF, Selvanayagam JB, Zeitz CJ. N-terminal pro-brain natriuretic protein levels in takotsubo cardiomyopathy. Am J Cardiol. 2011;108:1316-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 105] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 33. | Sharkey SW, Lesser JR, Menon M, Parpart M, Maron MS, Maron BJ. Spectrum and significance of electrocardiographic patterns, troponin levels, and thrombolysis in myocardial infarction frame count in patients with stress (tako-tsubo) cardiomyopathy and comparison to those in patients with ST-elevation anterior wall myocardial infarction. Am J Cardiol. 2008;101:1723-1728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 122] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 34. | Ramaraj R, Sorrell VL, Movahed MR. Levels of troponin release can aid in the early exclusion of stress-induced (takotsubo) cardiomyopathy. Exp Clin Cardiol. 2009;14:6-8. [PubMed] |
| 35. | Madhavan M, Borlaug BA, Lerman A, Rihal CS, Prasad A. Stress hormone and circulating biomarker profile of apical ballooning syndrome (Takotsubo cardiomyopathy): insights into the clinical significance of B-type natriuretic peptide and troponin levels. Heart. 2009;95:1436-1441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 139] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 36. | Fröhlich GM, Schoch B, Schmid F, Keller P, Sudano I, Lüscher TF, Noll G, Ruschitzka F, Enseleit F. Takotsubo cardiomyopathy has a unique cardiac biomarker profile: NT-proBNP/myoglobin and NT-proBNP/troponin T ratios for the differential diagnosis of acute coronary syndromes and stress induced cardiomyopathy. Int J Cardiol. 2012;154:328-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 37. | Lahoti A, Badri M, Iqbal M, Mohammed KS, Saeed W, Gnall E, Zolty R, Sardar MR. The Role of Cardiac Biomarkers in Takotsubo Cardiomyopathy. J Heart Lung Transpl. 2012;31:S152. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 38. | Randhawa MS, Dhillon AS, Taylor HC, Sun Z, Desai MY. Diagnostic utility of cardiac biomarkers in discriminating Takotsubo cardiomyopathy from acute myocardial infarction. J Card Fail. 2014;20:377.e25-377.e31. [PubMed] |
| 39. | Doyen D, Moceri P, Chiche O, Schouver E, Cerboni P, Chaussade C, Mansencal N, Ferrari E. Cardiac biomarkers in Takotsubo cardiomyopathy. Int J Cardiol. 2014;174:798-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 40. | Ghadri JR, Cammann VL, Jurisic S, Seifert B, Napp LC, Diekmann J, Bataiosu DR, D’Ascenzo F, Ding KJ, Sarcon A. A novel clinical score (InterTAK Diagnostic Score) to differentiate takotsubo syndrome from acute coronary syndrome: results from the International Takotsubo Registry. Eur J Heart Fail. 2016;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 155] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 41. | Liu K, Carhart R. “Reverse McConnell’s sign?”: a unique right ventricular feature of Takotsubo cardiomyopathy. Am J Cardiol. 2013;111:1232-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 42. | Warisawa T, Naganuma T, Nakamura S. Reversible Microvascular Dysfunction in Takotsubo Syndrome Shown Using Index of Microcirculatory Resistance. Circ J. 2016;80:750-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 43. | Ono R, Falcão LM. Takotsubo cardiomyopathy systematic review: Pathophysiologic process, clinical presentation and diagnostic approach to Takotsubo cardiomyopathy. Int J Cardiol. 2016;209:196-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 98] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 44. | Laraudogoitia Zaldumbide E, Pérez-David E, Larena JA, Velasco del Castillo S, Rumoroso Cuevas JR, Onaindía JJ, Lekuona Goya I, García-Fernández MA. The value of cardiac magnetic resonance in patients with acute coronary syndrome and normal coronary arteries. Rev Esp Cardiol. 2009;62:976-983. [PubMed] |
| 45. | Avegliano G, Huguet M, Costabel JP, Ronderos R, Bijnens B, Kuschnir P, Thierer J, Tobón-Gomez C, Martinez GO, Frangi A. Morphologic pattern of late gadolinium enhancement in Takotsubo cardiomyopathy detected by early cardiovascular magnetic resonance. Clin Cardiol. 2011;34:178-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 46. | Yoshikawa T. Takotsubo cardiomyopathy, a new concept of cardiomyopathy: clinical features and pathophysiology. Int J Cardiol. 2015;182:297-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 47. | Looi JL, Wong CW, Lee M, Khan A, Webster M, Kerr AJ. Usefulness of ECG to differentiate Takotsubo cardiomyopathy from acute coronary syndrome. Int J Cardiol. 2015;199:132-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 48. | Madias JE. Transient attenuation of the amplitude of the QRS complexes in the diagnosis of Takotsubo syndrome. Eur Heart J Acute Cardiovasc Care. 2014;3:28-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 148] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 49. | Vervaat FE, Christensen TE, Smeijers L, Holmvang L, Hasbak P, Szabó BM, Widdershoven JW, Wagner GS, Bang LE, Gorgels AP. Is it possible to differentiate between Takotsubo cardiomyopathy and acute anterior ST-elevation myocardial infarction? J Electrocardiol. 2015;48:512-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 50. | Johnson NP, Chavez JF, Mosley WJ, Flaherty JD, Fox JM. Performance of electrocardiographic criteria to differentiate Takotsubo cardiomyopathy from acute anterior ST elevation myocardial infarction. Int J Cardiol. 2013;164:345-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |