Published online Dec 26, 2017. doi: 10.4330/wjc.v9.i12.830
Peer-review started: June 19, 2017
First decision: July 17, 2017
Revised: November 6, 2017
Accepted: November 22, 2017
Article in press: November 22, 2017
Published online: December 26, 2017
Processing time: 189 Days and 8 Hours
To assess safety and efficacy of early erythropoietin (Epo) administration in patients with out-of-hospital cardiac arrest (OHCA).
A systematic literature search was performed using PubMed, MEDLINE, EMBASE, EBSCO, CINAHL, Web of Science and Cochrane databases, of all studies published from the inception through October 10, 2016. Inclusion criteria included: (1) Adult humans with OHCA and successful sustained return of spontaneous circulation; and (2) studies including mortality/brain death, acute thrombotic events as their end points. Primary efficacy outcome was “brain death or Cerebral Performance Category (CPC) score of 5”. Secondary outcomes were “CPC score 1, and 2-4”, “overall thrombotic events” and “acute coronary stent thrombosis”.
We analyzed a total of 606 participants (n = 276 received Epo and n = 330 with standard of care alone) who experienced OHCA enrolled in 3 clinical trials. No significant difference was observed between the Epo and no Epo group in brain death or CPC score 5 (OR = 0.77; 95%CI: 0.42-1.39), CPC score 1 (OR = 1.16, 95%CI: 0.82-1.64), and CPC score 2-4 (OR = 0.77, 95%CI: 0.44-1.36). Epo group was associated with increased thrombotic complications (OR = 2.41, 95%CI: 1.26-4.62) and acute coronary stent thrombosis (OR = 8.16, 95%CI: 1.39-47.99). No publication bias was observed.
Our study demonstrates no improvement in neurological outcomes and increased incidence of thrombotic events and acute coronary stent thrombosis in OHCA patients who were treated with Epo in addition to standard therapy.
Core tip: This manuscript suggested that: (1) No improvement in neurological outcomes with erythropoietin (Epo) administration after out of hospital cardiac arrest; and (2) Epo administration was also associated with increased thrombotic events and acute stent thrombosis.
- Citation: Chaudhary R, Garg J, Krishnamoorthy P, Bliden K, Shah N, Agarwal N, Gupta R, Sharma A, Kern KB, Patel NC, Gurbel P. Erythropoietin therapy after out-of-hospital cardiac arrest: A systematic review and meta-analysis. World J Cardiol 2017; 9(12): 830-837
- URL: https://www.wjgnet.com/1949-8462/full/v9/i12/830.htm
- DOI: https://dx.doi.org/10.4330/wjc.v9.i12.830
Patients who undergo out-of-hospital cardiac arrest (OHCA) frequently have post-anoxic encephalopathy, even after successful initial resuscitation. This brain insult can be either transient or definitive, and is the major cause of mortality[1]. Even after successful resuscitation and restoration of cerebral perfusion, brain injury continues to progress due to reperfusion injury. At present, apart from targeted therapeutic hypothermia, no other modalities have demonstrated a reduction in cerebral anoxic brain ischemia after OHCA[2,3]. In recent years, pre-clinical studies have suggested tissue protective effects of erythropoietin (Epo) and its analogues especially after brain and myocardial damage from ischemia-reperfusion injury[4,5]. However, this did not translate into a significant clinical benefit in patients with either acute myocardial infarction or stroke[6-8]. In the setting of OHCA, there is whole body ischemia and clinical studies have shown conflicting results with 2 studies demonstrating mortality benefit with early Epo administration[9,10] and a recent randomized controlled trial with no significant benefit[11]. In view of these studies, we aim to perform a meta-analysis to assess for any significant mortality benefit of early Epo administration in patients with OHCA.
The present review was performed according to Cochrane Collaboration and Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statements[12].
We carried out a literature search using PubMed, MEDLINE, EMBASE, EBSCO, CINAHL, Web of Science and Cochrane databases, of all studies published from the inception through October 10, 2016 comparing early Epo administration in addition to standard care in patients with OHCA with standard care alone. We combined the terms (“out of hospital cardiac arrest” OR “cardiac arrest” OR “OHCA”) AND (“erythropoietin” OR “EPO”) as keywords or medical subject heading terms in different combinations. All references of the retrieved articles were reviewed for further identification of potentially relevant studies. The identified studies were systematically assessed using the inclusion and exclusion criteria described below.
The studies had to fulfill the following criteria to be included in the analysis: (1) adult human subjects with OHCA and successful sustained return of spontaneous circulation (ROSC); and (2) studies including mortality/brain death, acute thrombotic events as their end points. All studies with retrospective design, abstracts, case reports, conference presentations, editorials, reviews, and expert opinions were excluded from our analysis. Longest available follow-up data from individual studies was used for our analysis.
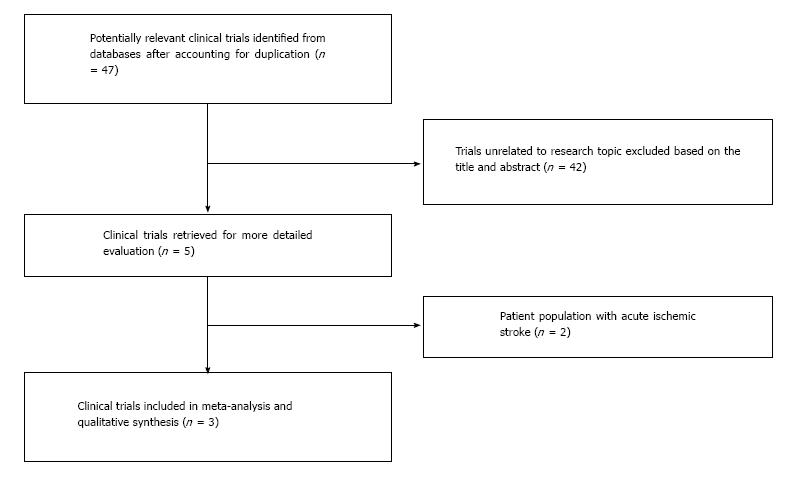
Two authors (Rahul Chaudhary and Jalaj Garg) searched the studies and extracted the data independently and in duplicate. The abstractors (Jalaj Garg and Rahul Chaudhary) independently assessed the quality items, and any discrepancies were resolved by discussion and consensus with the third author (PK). Final results were reviewed by senior investigator (NP) (Figure 1).
Assessment of risk of bias for each selected study was performed according to PRISMA 2009 guidelines. Qualitative evaluation of bias using the following key parameters were performed for each study: (1) clear definition of study population; (2) clear definition of outcomes and outcome assessment; (3) independent assessment of outcome parameters; (4) sufficient duration of follow-up; (5) selective loss during follow-up; and (6) important confounders and prognostic factors identified. The quality of non-randomized studies were evaluated using the Newcastle-Ottawa quality assessment scale[13] and randomized controlled trials were evaluated using Cochrane Risk of Bias tool.
The primary efficacy outcome in our study was “brain death or Cerebral Performance Category (CPC) score of 5”. Briefly, the CPC scale ranges between 1 and 5. A score of 1 represents good cerebral performance or minor disability, 2 moderate disability, 3 severe disability, 4 coma or vegetative state, and 5 represent brain death[14]. Secondary outcome assessed in our study were “CPC score 1 and 2-4”, “overall thrombotic events” (defined as a combination of venous thrombosis, acute coronary stent thrombosis and other arterial thrombosis) and “acute coronary stent thrombosis”.
Descriptive statistics are presented as means and SDs for continuous variables and as number of cases and percentages for dichotomous and categorical variables. Data were summarized across treatment arms using the Mantel-Haenszel odds ratio (OR) fixed effects model. Between-study heterogeneity was analyzed by means of Higgins I2 statistic[15]. In cases of heterogeneity (defined as I2 > 25%), random effects models of DerSimonian and Laird were used[16]. Funnel plot were evaluated visually to assess for any publication bias[17]. If any bias was observed, further bias quantification was measured using the Begg-Mazumdar Test[18], Egger Test[19] and Duval-Tweedie test[20]. The statistical analysis was performed using the Cochrane Collaborative software, RevMan 5.3.
A total of 47 studies were identified after exclusion of duplicate or irrelevant references (Figure 1). After detailed evaluation, 3 clinical trials (2 case-controlled studies and 1 randomized controlled study) with a total of 606 patients (276 patients received Epo in conjunction to standard of care compared to 330 patients with standard of care alone) were included in our analysis[9-11]. The characteristics of these trials and mean follow-up periods are described in Table 1.
| Name of study | Cariou et al[9], 2008 | Grmec et al[10], 2009 | Cariou et al[11], 2016 |
| Study design | Single center, case-control | Single center, case-control | Multicenter, single blind RCT |
| Total dose of Epo administered | 200000 IU | 90000 IU | 200000 IU |
| Timing of Epo administration | Immediately after ROSC | Within 1 or 2 min of physician assisted CPR | Immediately after ROSC |
| No. of participants, n (intervention/control) | 18/40 | 24/48 | 234/242 |
| Mean age, yr (intervention/control) | 59/58 | 59/60 | 60.5/58.6 |
| Male gender, n (intervention/control) | 16/39 | 16/34 | 192/184 |
| Initial rhythm PEA/asystole, n (intervention/control) | 2/8 | 12/17 | 94/100 |
| Initial rhythm shockable (VF/VT), n (intervention/control) | 16/32 | 12/31 | 115/110 |
| Perfusing rhythm after bystander defibrillation, n (intervention/control) | 0/0 | 0/0 | 24/31 |
| Unknown rhythm, n (intervention/control) | 0/0 | 0/0 | 1/3 |
| Follow-up duration | 28 d | Till hospital discharge | 60 d |
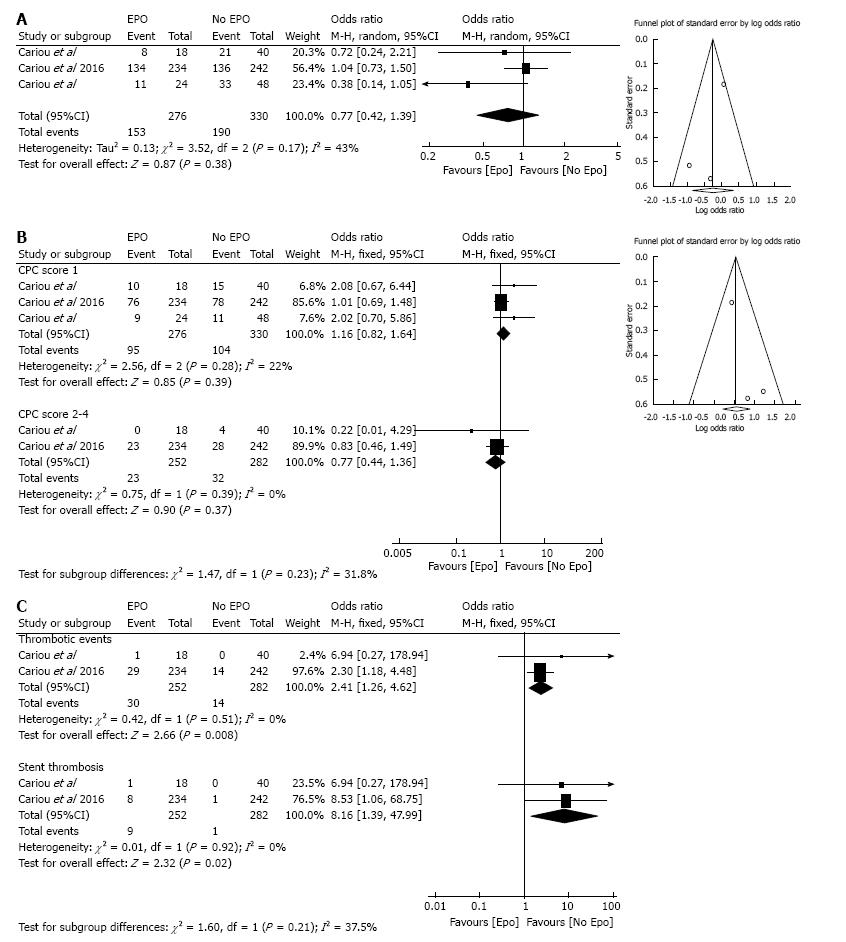
Overall, there were clear definitions of the study population, outcomes, and assessment in the component studies. The quality assessment of individual trials is listed in Table 2. Funnel plots did not reveal publication bias for comparison of CPC score 5 and CPC score 1-4 (Figure 2).
| Newcastle-Ottawa scale for bias assessment for case-controlled studies | ||
| Newcastle-Ottawa scale for bias assessment | Cariou et al[9], 2008 | Grmec et al[10], 2009 |
| Selection | 3 | 2 |
| Comparability | 2 | 2 |
| Exposure | 3 | 3 |
| Cochrane Risk of Bias tool for the Randomized controlled study (Cariou et al[11]) | ||
| Entry | Judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Patients were randomly assigned in a 1:1 ratio to the intervention or the control group” |
| Allocation concealment (selection bias) | Low risk | Quote: “Randomization was performed centrally with the use of a computer-generated assignment sequence Intervention assignments were made in permuted blocks of varying size and were stratified according to site” |
| Blinding of participants and personnel (performance bias) | High risk | Comment: Probably done |
| Quote: “Single-blinded”; “physicians performing neurological follow-up and final outcome measurement, as well as study administrators and statisticians, were unaware of the intervention assignments” | ||
| Comment: Probably done. However, only single blinding performed | ||
| Blinding of outcome assessment (detection bias) (patient-reported outcomes) | Low risk | Quote: “Single-blinded” |
| Comment: Probably done | ||
| Blinding of outcome assessment (detection bias) (mortality) | Low risk | Obtained from medical records; Quote “CPC was assessed by face-to-face contact with patients still hospitalized, and through phone interviews in discharged patients using a standardized protocol” |
| Review authors do not believe this will introduce bias | ||
| Incomplete outcome data addressed (attrition bias) (Longer-term outcomes, > 6 wk) | Low risk | 60 d: 1/234 missing from intervention group (“lost to follow-up”); 0/242 missing from control group |
| Selective reporting (reporting bias) | Low risk | A single scale to assess neurological outcomes was used and reported (CPC score) |
In the participant studies, there were no significant differences between the two groups in terms of age, gender, and initial rhythm (pulseless electrical activity or asystole and ventricular fibrillation or ventricular tachycardia). No significant heterogeneity was observed (Table 3). The mean age of our study population was 59.1 years (range 58 to 60.5 years) with 80% males.
| Baseline characteristic | Epo | No Epo | n | Studies (n) | P for overall effect |
| Age, yr | 59.5 | 58.9 | 606 | 3 | 0.22 |
| Males, % | 79.2 | 81.4 | 606 | 3 | 0.93 |
| Initial rhythm PEA/asystole, % | 33.8 | 32.2 | 606 | 3 | 0.85 |
| Initial rhythm VF/VT, % | 62.7 | 63.3 | 606 | 3 | 0.45 |
In the component studies, standard of care included use of therapeutic hypothermia immediately upon ICU admission (or continued if initiated pre-hospital) using external or internal cooling during the first 24 h in order to obtain a target temperature between 32 °C and 34 °C. Normothermia between 37 °C and 37.5 °C was then achieved using passive rewarming and maintained for the next 48 h. In patients with a high suspicion of acute coronary syndrome as the cause of OHCA, coronary angiograms were performed at hospital admission and followed by immediate percutaneous coronary interventions (PCIs) when indicated. Vasopressor agents were used, when indicated to keep the mean arterial blood pressure above 65 mmHg.
In the first clinical trial evaluating use of Epo, in addition to standard therapy, for patients with OHCA, Cariou et al[9] showed a non-significant improvement in survival rates (55% vs 47.5%, P = 0.17) and rates of full neurological recovery (55% vs 37.5%, P > 0.05) in a case-control study of 58 patients (n = 18 in Epo group and n = 40 in control group)[9]. In 2009, Grmec et al[10] showed an association of early Epo administration in patients with OHCA with higher incidence of return of spontaneous circulation (92% vs 71%, P = 0.06), 24-h survival (83% vs 52%, P = 0.01) and hospital survival (54% vs 31%, P = 0.06) in a study of 72 patients (n = 24 in Epo group and n = 48 in control group). After adjustment for pretreatment covariates all the above-mentioned outcomes were statistically significant[7]. In 2016, Cariou et al[11] performed a large-scale multicenter, single blind, randomized controlled trial (RCT), of 476 patients followed for a period of 60 d. They demonstrated no improvement in neurological outcomes (CPC score 1 in patients in Epo group 32.4% vs 43.1% in no Epo group; OR = 1.01, 95%CI: 0.68-1.48) and reported no differences between the mortality rate and proportion of patients in each CPC level between the two groups at any time points. Additionally, they observed a higher incidence of more serious adverse events with Epo administration compared to controls (22.6% vs 14.9%; P = 0.03), particularly thrombotic complications (12.4% vs 5.8%; P = 0.01)[11].
Brain death or CPC score of 5 was observed in 55% (153/276) of patients in Epo group compared to 57% (190/330) in control with no significant difference between the two groups (OR = 0.77; 95%CI: 0.42-1.39; I2 = 43%) (Figure 2A).
No significant differences were observed between the Epo and No Epo group with CPC scores 1 (34% vs 31% respectively, OR = 1.16, 95%CI: 0.82-1.64; I2 = 22%), and CPC score 2-4 (9% vs 11% respectively; OR = 0.77, 95%CI: 0.44-1.36; I2 = 0%) (Figure 2B).
Erythropoietin therapy was associated with a significant increase in overall thrombotic events (12% vs 5% for Epo and control group respectively; OR = 2.41, 95%CI: 1.26-4.62; I2 = 0%) and acute coronary stent thrombosis (3% vs 0.3% for Epo and control group respectively; OR = 8.16, 95%CI: 1.39-47.99; I2 = 0%) (Figure 2C).
To best of our knowledge, this is the first meta-analysis comparing early use of Epo in conjunction to standard therapy with standard therapy alone in patients with out-of-hospital cardiac arrest. The major findings in our study are as follows: (1) Epo plus standard therapy was not associated with any improved neurologic recovery (brain death, i.e., CPC score 5, CPC score 1-4); and (2) Epo plus standard therapy was significantly associated with increased incidence of overall thrombotic complications and acute coronary stent thrombosis.
Use of Epo as a neuroprotective agent emerged from animal models demonstrating Epo induced neuronal and vascular protection from ischemia-reperfusion injury[4,21]. Although promising, these results did not translate into improvement in clinical outcomes in patients with ischemic stroke or acute MI[6-8]. In 2008, Cariou et al[9] reported the first clinical study evaluating early use of Epo plus standard therapy in patients with OHCA. This study demonstrated encouraging results with a higher rate of full neurological recovery in Epo treated patients (55% vs 37.5%) with no significant difference mortality benefit. Similarly, Ehrenreich et al[7], in 2009 observed higher incidence of return of spontaneous circulation, 24-h survival and hospital survival with early administration of Epo in patients with OHCA. However, both these studies were case-control, single centered and non-randomized with a small patient population. Recently, Cariou et al[11] performed a large-scale multicenter, single blind, randomized controlled trial (RCT), which did not show any improvement in neurological outcomes with early administration of Epo. The results in our study are consistent with this recent RCT and other major RCTs evaluating the role of Epo in a similar setting, i.e., acute MI and acute ischemic stroke[6-8]. The discrepancy between animal and human studies could be due to inter-species variability in action of Epo and mechanism of neurological injury[11].
In addition, our study demonstrated an increased incidence of overall thrombotic events and acute coronary stent thrombosis. In prior studies, Epo has been associated with increased thrombotic events including stent thrombosis in patients treated for cancer associated anemia[22] and acute myocardial infarction[6]. The underlying mechanisms involved with increased thrombogenicity with Epo in patients with OHCA remains unclear. Several mechanism have been proposed to explain stent thrombosis in patients undergoing therapeutic hypothermia - impaired drug metabolism and reduced bioavailability[23,24], increased platelet activation[25], ineffective platelet inhibition, hypothermia induced mast cell degranulation[26]. In a recently published article from our group, we demonstrated no statistical significant difference in stent thrombosis in patients undergoing therapeutic hypothermia[27]. Also, Epo or its analogues have not been shown to enhance platelet activation[28] or activation of coagulation factors[29]. Thus it is possible increased thrombotic events in the Epo arm may be due to additional factors that were not accounted in our study (i.e., erythropoietin induced increase in blood viscosity, vasoconstriction and elevated blood pressure[22,30], timing of dual antiplatelet therapy, hemodynamic circulatory support, presence of congestive heart failure, cardiogenic shock and number of stents)[27].
A major limitation of the current meta-analysis includes paucity of data from RCT’s - with data from 2 case-controlled studies and only 1 RCT with a small patient population. Despite, differences in trials design, no significant heterogeneity was observed.
In conclusion, this study demonstrates no improvement in neurological outcomes and increased incidence of thrombotic events and acute coronary stent thrombosis in OHCA patients who were treated with Epo in addition to standard therapy.
Patients with out-of-hospital cardiac arrest (OHCA) frequently have post-anoxic encephalopathy, even after successful initial resuscitation. This brain insult can be either transient or definitive, and is the major cause of mortality. Even after successful resuscitation and restoration of cerebral perfusion, brain injury continues to progress due to reperfusion injury.
In the setting of OHCA, there is whole body ischemia and clinical studies have shown conflicting results with 2 studies demonstrating mortality benefit with early Erythropoietin (Epo) administration and a recent randomized controlled trial with no significant benefit. In view of these studies, the authors aim to perform a meta-analysis to assess for any significant mortality benefit of early Epo administration in patients with OHCA.
The primary efficacy outcome in this study was “brain death or Cerebral Performance Category (CPC) score of 5”. Secondary outcomes assessed in this study were “CPC scores 1 and 2-4”, “overall thrombotic events” and “acute coronary stent thrombosis”.
A systematic literature search was performed using PubMed, MEDLINE, EMBASE, EBSCO, CINAHL, Web of Science and Cochrane databases, of all studies published from the inception through October 10, 2016. The included trials were evaluated for publication bias and data summarized across treatment arms using the random effects model as odds ratio (OR).
No significant differences were observed between the two groups in brain death or CPC score of 5 (OR = 0.77; 95%CI: 0.42-1.39; I2 = 43%), CPC score 1 (OR = 1.16, 95%CI: 0.82-1.64; I2 = 22%), and CPC score 2-4 (OR = 0.77, 95%CI: 0.44-1.36; I2 = 0%). Epo therapy was associated with a significant increase in overall thrombotic events (OR = 2.41, 95%CI: 1.26-4.62; I2 = 0%) and acute coronary stent thrombosis (OR = 8.16, 95%CI: 1.39-47.99; I2 = 0%).
This study demonstrates no improvement in neurological outcomes and increased incidence of thrombotic events and acute coronary stent thrombosis in OHCA patients who were treated with Epo in addition to standard therapy.
Epo administration in patients with OHCA demonstrated an increase in adverse events with no mortality benefit in addition to current standard of care. Based on the currently available literature and this systematic review, further studies are needed in order to assess the safety and efficacy of Epo in Out-Of-Cardiac-Arrest patients.
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Caceres-Loriga FM, Qi X S- Editor: Kong JX L- Editor: A E- Editor: Lu YJ
| 1. | Lemiale V, Dumas F, Mongardon N, Giovanetti O, Charpentier J, Chiche JD, Carli P, Mira JP, Nolan J, Cariou A. Intensive care unit mortality after cardiac arrest: the relative contribution of shock and brain injury in a large cohort. Intensive Care Med. 2013;39:1972-1980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 473] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 2. | Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4038] [Cited by in RCA: 3812] [Article Influence: 165.7] [Reference Citation Analysis (0)] |
| 3. | Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3961] [Cited by in RCA: 3781] [Article Influence: 164.4] [Reference Citation Analysis (0)] |
| 4. | Sirén AL, Fratelli M, Brines M, Goemans C, Casagrande S, Lewczuk P, Keenan S, Gleiter C, Pasquali C, Capobianco A. Erythropoietin prevents neuronal apoptosis after cerebral ischemia and metabolic stress. Proc Natl Acad Sci USA. 2001;98:4044-4049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 776] [Cited by in RCA: 747] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 5. | Maiese K, Li F, Chong ZZ. New avenues of exploration for erythropoietin. JAMA. 2005;293:90-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 407] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 6. | Najjar SS, Rao SV, Melloni C, Raman SV, Povsic TJ, Melton L, Barsness GW, Prather K, Heitner JF, Kilaru R. Intravenous erythropoietin in patients with ST-segment elevation myocardial infarction: REVEAL: a randomized controlled trial. JAMA. 2011;305:1863-1872. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 176] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 7. | Ehrenreich H, Hasselblatt M, Dembowski C, Cepek L, Lewczuk P, Stiefel M, Rustenbeck HH, Breiter N, Jacob S, Knerlich F. Erythropoietin therapy for acute stroke is both safe and beneficial. Mol Med. 2002;8:495-505. [PubMed] |
| 8. | Ehrenreich H, Weissenborn K, Prange H, Schneider D, Weimar C, Wartenberg K, Schellinger PD, Bohn M, Becker H, Wegrzyn M. Recombinant human erythropoietin in the treatment of acute ischemic stroke. Stroke. 2009;40:e647-e656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 435] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 9. | Cariou A, Claessens YE, Pène F, Marx JS, Spaulding C, Hababou C, Casadevall N, Mira JP, Carli P, Hermine O. Early high-dose erythropoietin therapy and hypothermia after out-of-hospital cardiac arrest: a matched control study. Resuscitation. 2008;76:397-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 70] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 10. | Grmec S, Strnad M, Kupnik D, Sinkovic A, Gazmuri RJ. Erythropoietin facilitates the return of spontaneous circulation and survival in victims of out-of-hospital cardiac arrest. Resuscitation. 2009;80:631-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Cariou A, Deye N, Vivien B, Richard O, Pichon N, Bourg A, Huet L, Buleon C, Frey J, Asfar P. Early High-Dose Erythropoietin Therapy After Out-of-Hospital Cardiac Arrest: A Multicenter, Randomized Controlled Trial. J Am Coll Cardiol. 2016;68:40-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 12. | Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151:W65-W94. [PubMed] |
| 13. | Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8858] [Cited by in RCA: 12666] [Article Influence: 844.4] [Reference Citation Analysis (0)] |
| 14. | Nielsen N, Wetterslev J, Cronberg T, Erlinge D, Gasche Y, Hassager C, Horn J, Hovdenes J, Kjaergaard J, Kuiper M. Targeted temperature management at 33°C versus 36°C after cardiac arrest. N Engl J Med. 2013;369:2197-2206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1992] [Cited by in RCA: 2091] [Article Influence: 174.3] [Reference Citation Analysis (0)] |
| 15. | Higgins JPT GS, eds . Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. The Cochrane Collaboration. [accessed. 2016;Oct 31] Available from: http://training.cochrane. org/handbook. |
| 16. | DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26739] [Cited by in RCA: 30428] [Article Influence: 780.2] [Reference Citation Analysis (0)] |
| 17. | Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, Carpenter J, Rücker G, Harbord RM, Schmid CH. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3796] [Cited by in RCA: 4933] [Article Influence: 352.4] [Reference Citation Analysis (0)] |
| 18. | Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088-1101. [PubMed] |
| 19. | Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629-634. [PubMed] |
| 20. | Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7948] [Cited by in RCA: 9100] [Article Influence: 364.0] [Reference Citation Analysis (0)] |
| 21. | Junk AK, Mammis A, Savitz SI, Singh M, Roth S, Malhotra S, Rosenbaum PS, Cerami A, Brines M, Rosenbaum DM. Erythropoietin administration protects retinal neurons from acute ischemia-reperfusion injury. Proc Natl Acad Sci USA. 2002;99:10659-10664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 313] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 22. | Smith KJ, Bleyer AJ, Little WC, Sane DC. The cardiovascular effects of erythropoietin. Cardiovasc Res. 2003;59:538-548. [PubMed] |
| 23. | Součková L, Opatřilová R, Suk P, Čundrle I Jr, Pavlík M, Zvoníček V, Hlinomaz O, Šrámek V. Impaired bioavailability and antiplatelet effect of high-dose clopidogrel in patients after cardiopulmonary resuscitation (CPR). Eur J Clin Pharmacol. 2013;69:309-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 24. | Ibrahim K, Christoph M, Schmeinck S, Schmieder K, Steiding K, Schoener L, Pfluecke C, Quick S, Mues C, Jellinghaus S. High rates of prasugrel and ticagrelor non-responder in patients treated with therapeutic hypothermia after cardiac arrest. Resuscitation. 2014;85:649-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 101] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 25. | Straub A, Breuer M, Wendel HP, Peter K, Dietz K, Ziemer G. Critical temperature ranges of hypothermia-induced platelet activation: possible implications for cooling patients in cardiac surgery. Thromb Haemost. 2007;97:608-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Kounis NG, Kounis GN, Soufras GD. Therapeutic hypothermia associated with stent thrombosis: is Kounis mast cell activation-associated syndrome the culprit? Resuscitation. 2015;87:e3-e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 27. | Shah N, Chaudhary R, Mehta K, Agarwal V, Garg J, Freudenberger R, Jacobs L, Cox D, Kern KB, Patel N. Therapeutic Hypothermia and Stent Thrombosis: A Nationwide Analysis. JACC Cardiovasc Interv. 2016;9:1801-1811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 28. | Demetz G, Laux M, Scherhag A, Hoekstra T, Suttorp MM, Dekker F, Roest M, Marcus-Kalish M, Mittelman M, Ott I. The influence of Erythropoietin on platelet activation, thrombin generation and FVII/active FVII in patients with AMI. Thromb J. 2014;12:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Pawlak K, Pawlak D, Mysliwiec M. Long-term erythropoietin therapy does not affect endothelial markers, coagulation activation and oxidative stress in haemodialyzed patients. Thromb Res. 2007;120:797-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Besarab A, Bolton WK, Browne JK, Egrie JC, Nissenson AR, Okamoto DM, Schwab SJ, Goodkin DA. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med. 1998;339:584-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1528] [Cited by in RCA: 1486] [Article Influence: 55.0] [Reference Citation Analysis (0)] |