Published online Dec 26, 2017. doi: 10.4330/wjc.v9.i12.813
Peer-review started: July 27, 2017
First decision: September 5, 2017
Revised: September 29, 2017
Accepted: October 29, 2017
Article in press: October 29, 2017
Published online: December 26, 2017
Processing time: 148 Days and 12.9 Hours
To investigate the patient-outcomes of newly developed pressure drop coefficient (CDP) in diagnosing epicardial stenosis (ES) in the presence of concomitant microvascular disease (MVD).
Patients from our clinical trial were divided into two subgroups with: (1) cut-off of coronary flow reserve (CFR) < 2.0; and (2) diabetes. First, correlations were performed for both subgroups between CDP and hyperemic microvascular resistance (HMR), a diagnostic parameter for assessing the severity of MVD. Linear regression analysis was used for these correlations. Further, in each of the subgroups, comparisons were made between fractional flow reserve (FFR) < 0.75 and CDP > 27.9 groups for assessing major adverse cardiac events (MACE: Primary outcome). Comparisons were also made between the survival curves for FFR < 0.75 and CDP > 27.9 groups. Two tailed chi-squared and Fischer’s exact tests were performed for comparison of the primary outcomes, and the log-rank test was used to compare the Kaplan-Meier survival curves. P < 0.05 for all tests was considered statistically significant.
Significant linear correlations were observed between CDP and HMR for both CFR < 2.0 (r = 0.58, P < 0.001) and diabetic (r = 0.61, P < 0.001) patients. In the CFR < 2.0 subgroup, the %MACE (primary outcomes) for CDP > 27.9 group (7.7%, 2/26) was lower than FFR < 0.75 group (3/14, 21.4%); P = 0.21. Similarly, in the diabetic subgroup, the %MACE for CDP > 27.9 group (12.5%, 2/16) was lower than FFR < 0.75 group (18.2%, 2/11); P = 0.69. Survival analysis for CFR < 2.0 subgroup indicated better event-free survival for CDP > 27.9 group (n = 26) when compared with FFR < 0.75 group (n = 14); P = 0.10. Similarly, for the diabetic subgroup, CDP > 27.9 group (n = 16) showed higher survival times compared to FFR group (n = 11); P = 0.58.
CDP correlated significantly with HMR and resulted in better %MACE as well as survival rates in comparison to FFR. These positive trends demonstrate that CDP could be a potential diagnostic endpoint for delineating MVD with or without ES.
Core tip: Fractional flow reserve (FFR), a functional diagnostic index, is currently the gold standard for decision making in the catheterization laboratory. However, FFR can be confounded by concomitant microvascular disease (MVD). In this subgroup analysis study, pressure drop coefficient (CDP) showed improved clinical outcomes for patients with MVD compared to FFR, potentially making CDP a better diagnostic endpoint compared to FFR.
- Citation: Hebbar UU, Effat MA, Peelukhana SV, Arif I, Banerjee RK. Delineation of epicardial stenosis in patients with microvascular disease using pressure drop coefficient: A pilot outcome study. World J Cardiol 2017; 9(12): 813-821
- URL: https://www.wjgnet.com/1949-8462/full/v9/i12/813.htm
- DOI: https://dx.doi.org/10.4330/wjc.v9.i12.813
A persistent clinical challenge for interventional cardiologists today is the accurate assessment of intermediate coronary stenosis. While multiple quantitative anatomical methods were proposed, their applicability remains in question[1]. Functional diagnostic indices such as fractional flow reserve (FFR) and coronary flow reserve (CFR) agree well with non-invasive stress testing[2-4], although their efficacy is limited in the presence of significant microvascular disease (MVD) as FFR and CFR depend solely on either pressure or flow measurements[5,6].
FFR, the current gold standard for the functional evaluation of epicardial stenosis (ES) is defined as the ratio of distal and proximal pressures along an ES[7-9]. The parameter ranges from “0”, indicating a completely blocked vessel to “1” which indicates no obstruction. Earlier clinical outcome trials have established a cut-off value of 0.75[8] for significant coronary stenosis in the presence of single-vessel disease. However, FFR suffers from limitations, such as the zero-central venous pressure assumption as well as its dependence on the patient achieving maximal hyperemia. Also, constant minimum microvascular resistance may not be achieved in the case of sub-maximal hyperemia, leading to under-estimation of pressure drop and overestimation of FFR across the lesion[10].
CFR, the flow-derived parameter is defined as the ratio of hyperemic bood flow to basal (or resting) flow. The CFR values agreed well with non-invasive stress testing at a cut-off value of 2.0[2], and CFR < 2.0 was associated with reversible myocardial perfusion defects with high sensitivity and specificity[2]. It is worth noting that while CFR can provide the combined effect of ES and MVD, it cannot differentiate between the two conditions.
FFR and CFR are based solely on pressure measurements and flow measurements, respectively. Thus, both the indices can be misleading in the presence of extended MVD[5,6]. Hybrid parameters based on pressure and flow were proposed to overcome these limitations of FFR and CFR. However, such a parameter, e.g., hyperemic stenosis resistance index (HSR; ratio of pressure drop across the lesion to the distal velocity)[3] evaluates only ES. On a similar note, hyperemic microvascular resistance index (HMR; ratio of mean distal pressure and distal hyperemic velocity)[11] assesses MVD only.
To simultaneously detect ES and MVD using a single parameter, we recently introduced pressure drop coefficient (CDP), a functional diagnostic index which utilizes pressure as well as flow measurements. CDP was validated via in vitro[12,13] as well as in vivo animal studies[12-18], and could differentiate between ES and MVD. The CDP was recently employed to differentiate between degrees of stenotic severity in a patient population[19]. In order to make interventional decisions, an equivalent cut-off to FFR < 0.75 for single vessel disease was established for CDP (CDP > 27.9)[20,21] as a marker for significant ES.
Our earlier pilot clinical study[22] validated the proposed cut-off value for CDP with positive clinical outcomes associated with the CDP > 27.9 group when compared with the FFR < 0.75 group. The objective of the current study is to assess the efficacy of CDP in delineating ES within patient subgroups suffering from MVD only. Therefore, this follow-up pilot study compares the outcomes between CDP > 27.9 and FFR < 0.75 for MVD patient subgroups extracted from the complete patient data analyzed previously[22]. Accordingly, two subgroups having possible MVD were studied: One consisting of patients with abnormal CFR (CFR < 2.0), and the other consisting of patients suffering from diabetes. Both of these subgroups were correlated with possible microvascular dysfunction in literature[23-28]. Survival curves were also compared between the FFR < 0.75 and CDP > 27.9 groups for both subgroups. Additionally, CDP was correlated with HMR - another index that uses both pressure and flow measurements to evaluate MVD. This correlation was done in both subgroups to evaluate CDP’s ability to delineate MVD in the presence of ES.
The protocol[19] for the study was approved by the institutional review board at the University of Cincinnati (UC) and Cincinnati Veteran Affairs Medical Center (CVAMC), and informed consent was obtained from all the participants. Patients who underwent exercise testing and myocardial perfusion scans were consented based on the inclusion and exclusion criteria, which are reported in detail in our earlier study for the complete patient group[22]. The study population consisted of 86 patients enrolled at the UC and CVAMC. Table 1 summarizes the clinical characteristics of the enrolled patients in the complete patient group.
| Variable | Study/group |
| Sex (M/F) | 77/9 |
| Age (yr) | 61 ± 9 |
| Ejection fraction (%) | 58 ± 10 |
| Clinical history | |
| Diabetes | 42/86 |
| Hypertension | 70/86 |
| Dyslipidemia | 60/86 |
| Previous myocardial infarction | 21/86 |
| Smoking history | 52/86 |
| Family history of CAD | 23/86 |
| LV hypertrophy | 4/86 |
| Affected artery | |
| LAD | 43 |
| LCX | 17 |
| RCA | 26 |
Standard-of-care catheterization techniques[22] were utilized to obtain intra-coronary pressure and flow measurements across the stenosis. All signals were recorded continuously through rest, and induction as well as decline of maximal hyperemia.
CDP is defined as the ratio of trans-stenotic pressure drop to distal dynamic pressure.
CDP = Δp/(0.5 × r × APV2) (1)
where, is the pressure drop across the lesion; the distal dynamic pressure is the product of, blood density (assumed to be a constant value of 1.05 g/cm3), the square of average peak flow velocity (APV) and a constant value of 0.5.
The follow-up for the consented patients was performed through either chart review, a phone call, and/or a questionnaire. A minimum of 1-year follow-up was ensured. Over the follow-up period, the primary outcomes, which consisted of major adverse cardiac events (MACE) were determined. MACE was defined as the composite of all-cause mortality, myocardial infarction (MI), and repeat revascularization (Table 2).
| CFR < 2.0 | ||||
| FFR < 0.75 | FFR > 0.75 | CDP > 27.9 | CDP < 27.9 | |
| All-cause mortality | 3/14 | 2/34 | 2/26 | 3/22 |
| Myocardial infarction | ||||
| Revascularization | ||||
| Diabetic | ||||
| All-cause mortality | 1/11 | 3/31 | 2/16 | 2/26 |
| Myocardial infarction | ||||
| Revascularization | 1/11 | 1/26 | ||
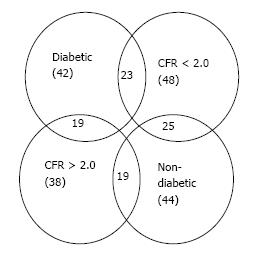
In order to perform the subgroup analysis for patients suffering from possible MVD, two subgroups were extracted from the complete patient group data: One subgroup was composed of patients who exhibited an abnormal CFR value (CFR < 2.0); the other subgroup was composed of patients suffering from diabetes. Figure 1 summarizes the patient data via a Venn diagram showing overlap of the various patient subgroups.
The authors had sufficient prior biostatistics background, as evidenced by previous publications[19-22]. First, correlations were performed between CDP and HMR in the diabetic and CFR < 2.0 subgroups using linear regression to evaluate the agreement of CDP with HMR, a parameter reported to identify the severity of MVD.
The patient data for each subgroup was then divided per the cut-off value of FFR < 0.75 for significant ES. On a similar note, CDP > 27.9[20,21] was used as an equivalent cut-off for significant stenosis. In the primary outcome study, for each subgroup (CFR < 2.0 and diabetic), the %MACE in the FFR < 0.75 group was quantified and compared with the %MACE in the corresponding CDP > 27.9 group. All comparisons were performed using the two-tailed χ2 test and further evaluated using the Fischer’s exact test.
Kaplan-Meier survival curves were generated to compare the long-term event free survival of the FFR < 0.75 patient group and the CDP > 27.9 patient group. This analysis was performed for both subgroups in the study. The duration between the index procedure, and the time when the patient was last followed-up was recorded. Any patient who reached the primary outcome (MACE) was recorded as positive. Patients lost to follow-up or who did not reach the primary outcome were considered as censored data. The generated survival curves were compared using the log-rank test for statistically significant difference.
All statistical analyses were performed using MedCalc (V10.2, Mariakerke, Belgium). All results obtained were considered statistically significant if P < 0.05.
CDP was first correlated with HMR to identify its efficacy in evaluating MVD in patients suffering from concomitant ES. Further, to test the efficacy of CDP cut-off values (CDP > 27.9) as a guide for decisions on clinical intervention in patients with ES in presence of microvascular impairment, the %MACE outcomes for a CDP based strategy were statistically compared with those for a FFR based strategy (FFR < 0.75) using the two-tailed χ2 test. The results for the Fischer’s exact test were also computed to account for the lower sample size. Comparisons were performed for both subgroup methodologies: The diabetic subgroup, and the CFR < 2.0 subgroup. Further, Kaplan-Meier survival curves were generated and comparisons were made for both subgroups.
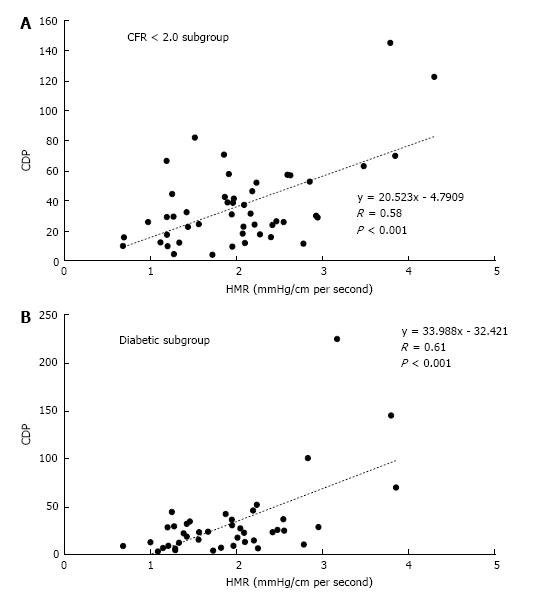
The results for the correlation between CDP and HMR are presented in Figure 2. For the CFR < 2.0 subgroup, CDP showed a moderate but significant correlation (Figure 2A) with HMR (r = 0.58, P < 0.001). In the diabetic subgroup (Figure 2B), CDP again correlated moderately with HMR and the correlation was statistically significant (r = 0.61, P < 0.001). These results further highlight the ability of CDP to delineate severity of MVD in patients who suffer from concomitant epicardial lesions.
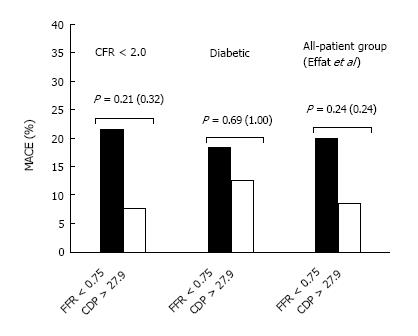
The comparison of %MACE outcomes between the FFR and CDP based cut-offs for the two subgroup analyses are summarized in Figure 3. Further, the comparison performed in our earlier outcome study[22] for the complete patient group is also presented. For the CFR < 2.0 subgroup, the %MACE outcomes in the FFR < 0.75 group (3 out of 14, 21.4%) were higher than the corresponding values for the CDP > 27.9 group (7.7%, 2 out of 26) although the results were not statistically significant as per the chi-squared test (P = 0.21) and the Fischer’s exact test (P = 0.32). On a similar note, for the diabetic subgroup, the %MACE in the FFR < 0.75 group (18.2%, 2 out of 11) was higher than the %MACE seen in the CDP > 27.9 group (12.5%, 2 out of 16), but the results were not statistically significant as per the chi-squared test (P = 0.69). The Fischer’s exact test resulted in a P-value of 1 due to the smaller sample size. Similar to the findings in this study, the analysis performed in our earlier study[22] for the complete patient group yielded a lower %MACE outcome for the CDP > 27.9 group though the results were not significant (P = 0.24) for both the chi-squared test and the Fischer’s exact test. These initial results suggest that if a CDP-based strategy were to be implemented, reduced %MACE outcomes would be observed when compared with the FFR-based strategy for the patient group with lower CFR values, as well as diabetic patients, who are known to suffer from MVD.
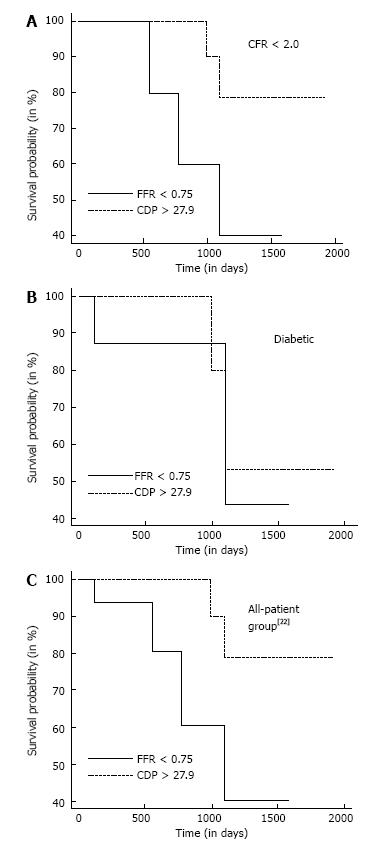
Figure 4A summarizes the Kaplan-Meier survival analysis performed for the CFR < 2.0 subgroup. The hazard ratio was computed to be 0.26 (95%CI: 0.04-1.82) implying that the survival probability in the CDP > 27.9 group is 3.85 times the corresponding probability in the FFR < 0.75 group. The difference in survival time for the FFR < 0.75 group (n = 14) compared with the CDP > 27.9 group (n = 26) was borderline significant (P = 0.10). On a similar note, Figure 4B summarizes the survival analysis performed for the diabetic subgroup. The computed hazard ratio was 0.60 (95%CI: 0.08-4.57), indicating higher survival probability for the CDP > 27.9 group. The survival time for the FFR < 0.75 group (n = 11) was not statistically different (P = 0.58) compared to the CDP > 27.9 group (n = 16). Figure 4C shows the survival analysis performed for the complete patient group in our previous study[22]. The hazard ratio was computed to be 0.22 (95%CI: 0.06-1.24), again indicating higher survival probability for the CDP > 27.9 group. In this comparison, a statistically significant improvement in survival time for the CDP > 27.9 group was observed when compared with the FFR < 0.75 group (P = 0.048). In summary, the survival analysis indicates better survival times for the CDP > 27.9 group when compared with the FFR < 0.75 group for the complete patient group as well as for patients suffering from MVD.
The advantages of using CDP, a combined pressure-flow diagnostic endpoint, have been reported in earlier studies[14,20-22]. However, the applicability of such a parameter in clinically relevant scenarios, particularly in the presence of MVD needs further assessment.
HMR is a dimensional diagnostic index which utilizes pressure and flow measurements to specifically evaluate the severity of MVD in patients. In contrast, CDP is a unique non-dimensional parameter developed from fluid dynamics principles that combines pressure and flow measurements to evaluate the severity of both ES and MVD. CDP showed a moderate but significant correlation with HMR for both CFR < 2.0 and diabetic patient-subgroups, further strengthening the hypothesis that CDP can be used to evaluate the severity of MVD, with or without the presence of concomitant ES.
One of the significant contributors to improved quality of life is reduced incidence of MACE. Therefore, the comparison of %MACE in the FFR-based group and the CDP-based group was one of the primary results of this study. The first methodology used in the subgroup analysis was based on CFR values. It is well documented that abnormally low CFR (< 2.0) values is associated with possible MVD[23] and an inability to achieve peak hyperemia. Under this scenario, constant minimal microvascular resistance is not assured, leading to an underestimation of the pressure drop which in turn results in an overestimation of FFR values[24]. It is worth noting that in the presence of MVD and submaximal hyperemia, both blood flow as well as pressure drop over the stenosis are affected in a similar manner. Physiologically, the reduction seen in the peak hyperemic blood flow due to MVD dominates over the corresponding reduction effected by ES[14]. The formulation of CDP accounts for this effect though the square of the maximal hyperemic flow in the denominator, thus providing improved resolution for accurate evaluation of the status of the stenosis. The results for the %MACE comparisons between the FFR-based strategy and the CDP-based strategy in the study show the improved resolving power for the CDP-based patient group via the lower %MACE. However, considering the low rates of MACE and the relatively lower sample size in this study, a prospective randomized trial with larger patient population is required to confirm the outcomes of this pilot study.
Similar to patients with abnormal CFR, patients with diabetes are also associated with potential MVD. Previous studies of the arterioles and small arteries of diabetic patients have indicated functional microvasculature damage evidenced by reduced vasodilation of the coronary arterioles. This could be the result of a decrease in activity of ATP sensitive potassium channels[25-28]. As an additional confirmation of our hypothesis, a subsequent analysis was performed on a subgroup consisting of diabetic patients by evaluating and comparing the %MACE outcomes between the FFR-based strategy and the CDP-based strategy. The results indicated a similar trend as in the case of the CFR (< 2.0) based subgroup analysis. Additionally, the comparisons performed for the complete patient group in our previous study[22] report similar results of reduced %MACE outcomes for the CDP group, thereby strengthening the argument that CDP can accurately delineate the status of ES, particularly in the presence of concomitant MVD. Again, these results require further assessment using a prospective randomized clinical trial with larger sample size.
Another significant measure which affects the quality of life for patients suffering from cardiovascular disease is long-term event-free survival. Comparisons of the Kaplan-Meier survival curves using the log-rank test were performed between the FFR based group and the CDP based group for both subgroup methodologies discussed above. The results indicated improved long-term event free survival for the CDP-based groups in both the subgroup analyses. Furthermore, the survival curves comparison performed for the complete patient group in our previous study[22] indicated a significant improvement in long-term event free survival for the CDP group, lending further strength to the resolving power of CDP in diagnosing the status of ES with concomitant MVD.
CDP, a non-dimensional parameter based on fundamental fluid dynamics is defined as the ratio of coronary trans-lesional pressure drop (Δp) to the distal dynamic pressure (0.5 blood density APV2) where APV (average peak blood flow velocity) is measured during peak hyperemia. In the presence of increased microvascular resistance, FFR and CFR along an ES are affected in opposite directions. Therefore, ischemic assessment performed by measuring FFR and CFR in such a coronary artery with concomitant diseases might potentially lead to discordant results in up to 40% of the cases[29]. A possible explanation would be the presence of diffuse epicardial lesions wherein lower CFR would be observed without notable changes in FFR values. On the other hand, healthy microvasculature and auto-regulatory function could allow for normal CFR values while leading to abnormal FFR values. The complex interaction of pressure and flow seen in such scenarios may not be captured adequately by FFR or CFR alone, since these parameters depend solely on pressure and flow, respectively. In contrast, CDP is a combined physiological parameter derived from fundamental fluid dynamics principles involving both pressure and flow measurements, and can adequately distinguish between ES and MVD[20].
Considering the numerous advantages afforded by CDP, we believe that this parameter has a potentially significant role in modern clinical practice. However, it is worth mentioning that the dual-sensor wires necessary for computing CDP has not become prevalent in catheterization laboratories. Nevertheless, the use of these guidewires is expected to increase with: (1) technological advancement; and (2) mounting evidence of better clinical outcomes. This would make the measurement of functional diagnostic indices such as CDP standard-of-care with reduced complexities. Several prior studies have validated the clinical application of functional measures such as FFR in treatment of coronary stenosis. These include the DEFER study[24], the FAME trial[30] and the FAME 2 trial[31], which confirmed the role of FFR as a guide to management of coronary artery disease. This study proposes CDP as an improved measure over FFR for accurate prediction of major ischemic events as well as long-term event-free survival in the presence of confounding scenarios such as MVD. While statistical significance was not reached, consistent improved outcomes were observed over all the measures. Significant statistical significance in the comparisons may be observed on repeating the analysis for a larger patient group and longer follow-up periods.
All the clinical decisions in this study were made based on FFR values alone. Thus, a larger sample size and a prospective randomized clinical trial is needed. This will allow improved evaluation of the performance of CDP compared to FFR under clinical settings and confirm the patient outcomes of this current cohort study.
In this follow-up pilot study to our earlier clinical trial[22], a subgroup analysis was performed with two subgroups: one consisting of patients exhibiting CFR < 2.0, and the other consisting of diabetic patients. CDP showed moderate but significant correlation with HMR in both the diabetic and CFR < 2.0 subgroups. Comparison of primary (%MACE) outcomes led to lower %MACE in the CDP > 27.9 groups in comparison to the FFR < 0.75 groups for both subgroups, although statistical significance was not reached. Further, event-free survival rates in the CDP > 27.9 group were higher when compared with the FFR < 0.75 group for both subgroups, with the difference being borderline significant for the CFR < 2.0 subgroup. Further clinical trials with a larger patient population and longer follow-up periods could validate the positive trends seen for the CDP group in this study, while proving the efficacy of CDP as a useful clinical endpoint for decision making in the cardiac catheterization laboratory.
Accurate assessment of coronary stenosis is an important aspect of interventional cardiology. Although existing functional diagnostic indices such as fractional flow reserve (FFR) and coronary flow reserve (CFR) have been validated extensively via clinical trials, their efficacy is limited in the presence of concomitant microvascular disease (MVD) as they depend solely on pressure or flow measurements. This pilot study explores the efficacy of a combined pressure-flow diagnostic endpoint, pressure drop coefficient (CDP) compared to pressure-based FFR. It was hypothesized that CDP would show better clinical outcomes compared to FFR for patient subgroups with MVD.
Diagnosis of epicardial stenosis (ES) with concomitant MVD is a challenge with existing diagnostic indices (such as FFR, CFR) as they depend solely on pressure or flow. Pressure-based FFR can be overestimated in the presence of concomitant MVD, leading to possible misdiagnosis of severity of the stenosis, while CFR cannot differentiate between the effects of the stenosis and MVD. There is a need for combined pressure-flow diagnostic endpoints (such as CDP) to better diagnose coronary stenosis, particularly in the presence of MVD.
The primary objective of this research was to compare the clinical outcomes of patients with stenosis and possible MVD evaluated using FFR and CDP. Secondly, CDP was correlated with an existing index (HMR) used to evaluate the severity of MVD. CDP showed better clinical outcomes compared to FFR, as well as longer survival times for the patients. Also, CDP showed significant correlation with HMR, validating its efficacy at evaluation of MVD. It is to be noted that larger sample sizes and a randomized clinical trial is required to further confirm the results of this exploratory pilot study.
Patients from our clinical trial was divided into two subgroups with: (1) cut-off of CFR < 2.0; and (2) diabetes. First, correlations were performed for both subgroups between CDP and HMR, a diagnostic parameter for assessing the severity of MVD. Linear regression analysis was used for these correlations. Further, in each of the subgroups, comparisons were made between FFR < 0.75 and CDP > 27.9 groups for assessing major adverse cardiac events (MACE: primary outcome). Comparisons were also made between the survival curves for FFR < 0.75 and CDP > 27.9 groups. Two tailed chi-squared and Fischer’s exact tests were performed for comparison of the primary outcomes, and the log-rank test was used to compare the Kaplan-Meier survival curves. P < 0.05 for all tests was considered statistically significant.
Significant linear correlations were observed between CDP and HMR for both CFR < 2.0 (r = 0.58, P < 0.001) and diabetic (r = 0.61, P < 0.001) patients. In the CFR < 2.0 subgroup, the %MACE (primary outcomes) for CDP > 27.9 group (7.7%, 2/26) was lower than FFR < 0.75 group (3/14, 21.4%); P = 0.21. Similarly, in the diabetic subgroup, the %MACE for CDP > 27.9 group (12.5%, 2/16) was lower than FFR < 0.75 group (18.2%, 2/11); P = 0.69. Survival analysis for CFR < 2.0 subgroup indicated better event-free survival for CDP > 27.9 group (n = 26) when compared with FFR < 0.75 group (n = 14); P = 0.10. Similarly, for the diabetic subgroup, CDP > 27.9 group (n = 16) showed higher survival times compared to FFR group (n = 11); P = 0.58.
CDP correlated significantly with HMR and resulted in better %MACE as well as survival rates in comparison to FFR. These positive trends demonstrate that CDP could be a potential diagnostic endpoint for delineating MVD with or without ES.
This study highlights the ability of CDP in delineating MVD in patients with or without ES. In this patient subgroup analysis, CDP showed better clinical outcomes and higher survival rates compared to FFR, which is the current gold standard in functional diagnosis of coronary artery disease. There is a clear need for functional diagnostic endpoints which can better evaluate ES with concomitant MVD. In future, a large scale randomized clinical trial comparing the outcomes of CDP and FFR is required.
The authors would like to acknowledge Dr. Kranthi Kolli’s work in initial data assimilation, Dr. Jason Meunier, Rachel Mardis, and Ginger Conway for their assistance in data assimilation. Further, the authors would like to acknowledge Drs. Leesar and Helmy for the clinical catheterization procedure and data acquisition, as reported in previous publications.
Manuscript source: Unsolicited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Vermeersch P S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Smith SC Jr, Feldman TE, Hirshfeld JW Jr, Jacobs AK, Kern MJ, King SB 3rd, Morrison DA, O’Neill WW, Schaff HV, Whitlow PL, Williams DO, Antman EM, Adams CD, Anderson JL, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Hunt SA, Nishimura R, Ornato JP, Page RL, Riegel B; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; American College of Cardiology/American Heart Association/Society for Cardiovascular Angiography and Interventions Writting Committee to Update the 2001 Guidelines for Percutaneous Coronary Intervention. ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention--summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/SCAI Writing Committee to Update the 2001 Guidelines for Percutaneous Coronary Intervention). Circulation. 2006;113:156-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 331] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 2. | Kern MJ, Lerman A, Bech JW, De Bruyne B, Eeckhout E, Fearon WF, Higano ST, Lim MJ, Meuwissen M, Piek JJ. Physiological assessment of coronary artery disease in the cardiac catheterization laboratory: a scientific statement from the American Heart Association Committee on Diagnostic and Interventional Cardiac Catheterization, Council on Clinical Cardiology. Circulation. 2006;114:1321-1341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 358] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 3. | Meuwissen M, Siebes M, Chamuleau SA, van Eck-Smit BL, Koch KT, de Winter RJ, Tijssen JG, Spaan JA, Piek JJ. Hyperemic stenosis resistance index for evaluation of functional coronary lesion severity. Circulation. 2002;106:441-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 142] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 4. | Kern MJ. Coronary physiology revisited: practical insights from the cardiac catheterization laboratory. Circulation. 2000;101:1344-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 145] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 5. | Hoffman JI. Problems of coronary flow reserve. Ann Biomed Eng. 2000;28:884-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 79] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 6. | van de Hoef TP, Nolte F, Rolandi MC, Piek JJ, van den Wijngaard JP, Spaan JA, Siebes M. Coronary pressure-flow relations as basis for the understanding of coronary physiology. J Mol Cell Cardiol. 2012;52:786-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 7. | Pijls NH, Fearon WF, Tonino PA, Siebert U, Ikeno F, Bornschein B, van’t Veer M, Klauss V, Manoharan G, Engstrøm T. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention in patients with multivessel coronary artery disease: 2-year follow-up of the FAME (Fractional Flow Reserve Versus Angiography for Multivessel Evaluation) study. J Am Coll Cardiol. 2010;56:177-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 807] [Cited by in RCA: 837] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 8. | Pijls NH, van Son JA, Kirkeeide RL, De Bruyne B, Gould KL. Experimental basis of determining maximum coronary, myocardial, and collateral blood flow by pressure measurements for assessing functional stenosis severity before and after percutaneous transluminal coronary angioplasty. Circulation. 1993;87:1354-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 807] [Cited by in RCA: 802] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 9. | Tonino PA, Fearon WF, De Bruyne B, Oldroyd KG, Leesar MA, Ver Lee PN, Maccarthy PA, Van’t Veer M, Pijls NH. Angiographic versus functional severity of coronary artery stenoses in the FAME study fractional flow reserve versus angiography in multivessel evaluation. J Am Coll Cardiol. 2010;55:2816-2821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 893] [Cited by in RCA: 928] [Article Influence: 61.9] [Reference Citation Analysis (0)] |
| 10. | Pijls NH, Kern MJ, Yock PG, De Bruyne B. Practice and potential pitfalls of coronary pressure measurement. Catheter Cardiovasc Interv. 2000;49:1-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Siebes M, Verhoeff BJ, Meuwissen M, de Winter RJ, Spaan JA, Piek JJ. Single-wire pressure and flow velocity measurement to quantify coronary stenosis hemodynamics and effects of percutaneous interventions. Circulation. 2004;109:756-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 136] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 12. | Sinha Roy A, Back MR, Khoury SF, Schneeberger EW, Back LH, Velury VV, Millard RW, Banerjee RK. Functional and anatomical diagnosis of coronary artery stenoses. J Surg Res. 2008;150:24-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Peelukhana SV, Back LH, Banerjee RK. Influence of coronary collateral flow on coronary diagnostic parameters: an in vitro study. J Biomech. 2009;42:2753-2759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Banerjee RK, Ashtekar KD, Effat MA, Helmy TA, Kim E, Schneeberger EW, Sinha RA, Gottliebson WM, Back LH. Concurrent assessment of epicardial coronary artery stenosis and microvascular dysfunction using diagnostic endpoints derived from fundamental fluid dynamics principles. J Invasive Cardiol. 2009;21:511-517. [PubMed] |
| 15. | Kolli KK, Banerjee RK, Peelukhana SV, Helmy TA, Leesar MA, Arif I, Schneeberger EW, Hand D, Succop P, Gottliebson WM. Influence of heart rate on fractional flow reserve, pressure drop coefficient, and lesion flow coefficient for epicardial coronary stenosis in a porcine model. Am J Physiol Heart Circ Physiol. 2011;300:H382-H387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Kolli KK, Banerjee RK, Peelukhana SV, Effat MA, Leesar MA, Arif I, Schneeberger EW, Succop P, Gottliebson WM, Helmy TA. Effect of changes in contractility on pressure drop coefficient and fractional flow reserve in a porcine model. J Invasive Cardiol. 2012;24:6-12. [PubMed] |
| 17. | Peelukhana SV, Banerjee RK, Kolli KK, Effat MA, Helmy TA, Leesar MA, Schneeberger EW, Succop P, Gottliebson W, Irif A. Effect of heart rate on hemodynamic endpoints under concomitant microvascular disease in a porcine model. Am J Physiol Heart Circ Physiol. 2012;302:H1563-H1573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Peelukhana SV, Kolli KK, Leesar MA, Effat MA, Helmy TA, Arif I, Schneeberger EW, Succop P, Banerjee RK. Effect of myocardial contractility on hemodynamic end points under concomitant microvascular disease in a porcine model. Heart Vessels. 2014;29:97-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Kolli KK, Helmy TA, Peelukhana SV, Arif I, Leesar MA, Back LH, Banerjee RK, Effat MA. Functional diagnosis of coronary stenoses using pressure drop coefficient: a pilot study in humans. Catheter Cardiovasc Interv. 2014;83:377-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Kolli KK, Arif I, Peelukhana SV, Succop P, Back LH, Helmy TA, Leesar MA, Effat MA, Banerjee RK. Diagnostic performance of pressure drop coefficient in relation to fractional flow reserve and coronary flow reserve. J Invasive Cardiol. 2014;26:188-195. [PubMed] |
| 21. | Kolli KK, van de Hoef TP, Effat MA, Banerjee RK, Peelukhana SV, Succop P, Leesar MA, Imran A, Piek JJ, Helmy TA. Diagnostic cutoff for pressure drop coefficient in relation to fractional flow reserve and coronary flow reserve: A patient-level analysis. Catheter Cardiovasc Interv. 2016;87:273-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Effat MA, Peelukhana SV, Banerjee RK. Clinical outcomes of combined flow-pressure drop measurements using newly developed diagnostic endpoint: Pressure drop coefficient in patients with coronary artery dysfunction. World J Cardiol. 2016;8:283-292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Feigl EO. Coronary physiology. Physiol Rev. 1983;63:1-205. [PubMed] |
| 24. | Pijls NH, van Schaardenburgh P, Manoharan G, Boersma E, Bech JW, van’t Veer M, Bär F, Hoorntje J, Koolen J, Wijns W. Percutaneous coronary intervention of functionally nonsignificant stenosis: 5-year follow-up of the DEFER Study. J Am Coll Cardiol. 2007;49:2105-2111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1099] [Cited by in RCA: 1166] [Article Influence: 64.8] [Reference Citation Analysis (0)] |
| 25. | Picchi A, Capobianco S, Qiu T, Focardi M, Zou X, Cao JM, Zhang C. Coronary microvascular dysfunction in diabetes mellitus: A review. World J Cardiol. 2010;2:377-390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 26. | Muller JM, Davis MJ, Chilian WM. Integrated regulation of pressure and flow in the coronary microcirculation. Cardiovasc Res. 1996;32:668-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 27. | Bagi Z, Toth E, Koller A, Kaley G. Microvascular dysfunction after transient high glucose is caused by superoxide-dependent reduction in the bioavailability of NO and BH(4). Am J Physiol Heart Circ Physiol. 2004;287:H626-H633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Miura H, Wachtel RE, Loberiza FR Jr, Saito T, Miura M, Nicolosi AC, Gutterman DD. Diabetes mellitus impairs vasodilation to hypoxia in human coronary arterioles: reduced activity of ATP-sensitive potassium channels. Circ Res. 2003;92:151-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 139] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 29. | Johnson NP, Kirkeeide RL, Gould KL. Is discordance of coronary flow reserve and fractional flow reserve due to methodology or clinically relevant coronary pathophysiology? JACC Cardiovasc Imaging. 2012;5:193-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 246] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 30. | Tonino PA, De Bruyne B, Pijls NH, Siebert U, Ikeno F, van’ t Veer M, Klauss V, Manoharan G, Engstrøm T, Oldroyd KG. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360:213-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2974] [Cited by in RCA: 3058] [Article Influence: 191.1] [Reference Citation Analysis (0)] |
| 31. | De Bruyne B, Pijls NH, Kalesan B, Barbato E, Tonino PA, Piroth Z, Jagic N, Möbius-Winkler S, Rioufol G, Witt N. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med. 2012;367:991-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1837] [Cited by in RCA: 1992] [Article Influence: 153.2] [Reference Citation Analysis (0)] |