INTRODUCTION
Mediastinal radiotherapy (RT) has been successfully used to decrease mortality and recurrence of a number of thoracic malignancies for decades, particularly early Hodgkin’s lymphoma (HL) and breast cancer. Thanks to advances in chemotherapeutics and radiation oncology, HL is now eminently curable, with 20-year survival approaching 80%[1], while 15-year breast cancer survival is nearing the same threshold[2]. Increased longevity has unintended consequences, however, including radiation-induced cardiovascular disease (RICVD). Where the heart was once thought to be insensitive to radiation, RICVD is now known to be the chief non-malignant cause of death in these patients, responsible for between one-quarter and one-third of their mortality[1,3-5]. The intervening decades have witnessed significant decreases in the amount of radiation to which patients are exposed, but injury to the pericardium, myocardium, valvular architecture, and vasculature continue to impose significant challenges to patients and clinicians entrusted with their care. Here, we will briefly review the epidemiology and basic characteristics of the cardinal types of RICVD, focusing on emerging concepts in the pathophysiology, prevention, and treatment of this disease.
EPIDEMIOLOGY AND BASIC CHARACTERISTICS
The epidemiology of RICVD is complicated by the continual improvements in radiation dosimetry and shielding that tend to reduce cardiovascular exposure and the latent effects of radiation, which take years or decades to manifest. Thus, RICD is an inherently dynamic disease process, and while clinicians continue to cope with radiation-induced comorbidities afflicted by older and higher-dose radiation regimens, data derived from patients treated decades ago will tend to overestimate incidences and morbidities, etc., of newly evolving cases. Updated epidemiologic data is therefore of critical importance to inform both patients and clinicians. Several large studies have been published over the last few years that analyzed the outcomes of RT administered between one and four decades ago. In the following section, these data will be presented in reference to the four cardinal radiation-induced cardiovascular pathologies, as well as a brief overview of the gross anatomic and histopathologic derangements known to occur over the given timelines and at the described doses.
Acute and chronic pericarditis
Radiation-induced pericarditis is the earliest form of RICVD to occur following mediastinal radiation. It may occur in either of two forms, early and acute or delayed and chronic, which should be regarded from a histopathological standpoint as two distinct disease entities. As an early complication of very high dose radiation, early pericarditis is extremely rare today due to implementation of dose reducing techniques. It occurs either during RT or in the days or weeks after in response to irradiation in excess of the “tolerance dose” of the organ, which is variably described as a mean heart dose of greater than 36 or 40 Gy, or a > 50 Gy dose administered to > 30% of the heart[6-8]. The effect of these doses on histopathology is profound in the short-term. In the acute setting, the pericardium becomes porous, resulting in a neutrophilic infiltrate and collection of a high-protein exudate[9]. Nearly half of affected patients develop hemodynamically-significant effusions, although in most cases they are self-limited. The development of apparently benign pericardial effusions in the acute stage may predispose the patient to chronic pericarditis of delayed onset, however[10].
Chronic pericarditis is the most common cardiac complication of radiation therapy, observed in some 70%-90% of necropsy cases[11,12]. The effect is highly dose-dependent, with incidence increasing from < 10% to > 50% as the total dose is increased from 50 to 60 Gy[7]. The incidence of symptomatic chronic, delayed pericarditis has decreased dramatically since the 1970s, falling from 20% to 2.5% with the application of just a few of the radiation-sparing techniques that are used today[13]. Nevertheless, even the low-dose radiation to which contemporary cohorts are exposed increases the incidence of chronic pericarditis by a factor of 1.6 when comparing patients undergoing left- vs right-sided RT[14]. This finding suggests that through the early 2000s, breast cancer survivors were accruing excess risk of chronic pericardial disease despite modern dose-schedules.
The time to onset of symptoms in chronic pericarditis can range from three months to over a decade, with one year being the median[8]. In the months prior to presentation, these patients will experience fibrous thickening of the pericardium and replacement of pericardial fat by collagen[11]. In nearly 20% of cases, pericardial thickening is severe enough to cause a chronic constrictive pericarditis[15], which, when it becomes symptomatic, does so much later, requiring pericardiectomy at a median of 11 years after RT according to one recent study[16].
Radiation-induced cardiomyopathy
According to the latest epidemiologic data, radiation-induced cardiomyopathy (RICM) occurs at a 40-year cumulative incidence rate of 24.8%, though most of these cases evolve following a distinct cardiac insult such as valvular disease or myocardial infarction (MI)[17]. The risk of RICM increases after 5 years, but it can evolve decades after initial RT[18]. Higher doses of radiation exposure are required to instigate this level of injury; rat hearts display a tolerance dose of 15-20 Gy[19], whereas the tolerance dose of human myocardium is approximately 40 Gy[7]. That said, asymptomatic myocardial perfusion defects have been detected as soon as 6 mo following irradiation at the much lower mean heart radiation doses used in the contemporary treatment of breast cancer[20]. In the latter study, defects were observed in about 40% of patients within two years, suggesting that RICM will continue to be a significant late adverse effect of RT in the coming decades despite reductions in radiation exposure.
Pathologically, RICM is characterized by inflammation followed by the development of a diffuse, patchy interstitial fibrosis of the myocardium, and effacement of the peri-myocyte endothelium[21]. Perfusion defects can often be detected by nuclear medicine studies in the early years following RT. They lie in the irradiated regions and do not follow the major coronary artery distributions, reinforcing the view that microvascular injury is central to this pathology[22]. As the heart becomes fibrotic it loses compliance, resulting in diastolic dysfunction[23]. Wall-motion abnormalities follow, occurring in 18% and 29% of patients in their second and third decades after RT, respectively, vs 5% in non-irradiated age-matched subjects in the Framingham population[24]. In the same study, a decline in left ventricular mass and wall thickness was also noted, which runs contrary to the trend seen in normal aging. Impairment of systolic function occurs last and should be considered a sign of late RICVD.
Valvular heart disease
The natural history of valvular heart disease (VHD) varies with radiation dose and, by extension, the decade in which the patient was treated. A study of HL survivors irradiated under obsolete protocols between 1965 and 1995 revealed 13- and 30-year cumulative incidences of 10% and 20%, respectively. Prior history of RT increased the risk of VHD for these patients 7-fold[18]. Unfortunately, VHD progresses in more than 30% of irradiated HL survivors throughout the second and third decades following treatment in this dose range[25]. More recently, researchers at the Netherlands Cancer Institute found a stepwise decrease in 30-year cumulative incidence of VHD corresponding to diminishing doses of RT, from 12.4% at doses greater than 40 Gy to 3.0% at doses less than 30 Gy[26]. At the lower end of this steep dose-response curve, where most treatment regimens are dosed currently, the absolute difference in 30 year VHD risk in irradiated vs non-irradiated patients was estimated to be 1.4%. Nevertheless, patients treated in past decades will continue to experience higher rates of VHD in the coming decades, particularly those exposed to high doses of radiation in the remote past.
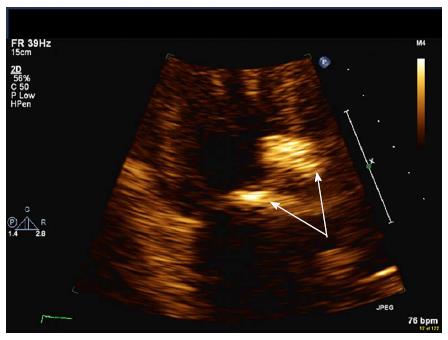
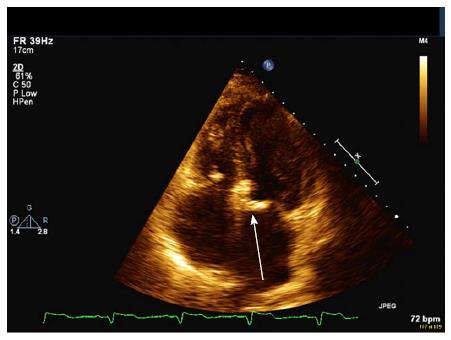
With respect to the gross pathology of VHD, the earliest change appears to be the formation of valvular retractions and accompanying regurgitation preferentially involving the mitral and aortic valves, occurring within the first 10 years. The progression to fibrotic thickening and calcification of the valves occurs much later, with stenosis often appearing 20 years after RT[25]. Mitral and aortic valve regurgitation are the most common defects, and when stenosis occurs, it most commonly afflicts the aortic valve (Figures 1 and 2).
Figure 1 Severe calcification of proximal aorta and aortic leaflets (arrows) resulting in moderate aortic regurgitation and stenosis.
Figure 2 Apical four chamber view of mitral annular calcification (arrow).
Radiation-induced coronary heart disease
Radiation-induced coronary heart disease (CHD) is currently the most active area of RICVD research. Until the 1990s, its existence was controversial, but it has been unmasked by longer survivorships and mass epidemiological studies in the ensuing decades. The disease burden it imposes is significant, in part because it can be induced by radiation doses that are well less than 10% of the tolerance dose of other cardiac tissues; thus, it more frequently complicates the course of breast cancer treatment than other forms of RICVD[7]. A large case control study of breast cancer survivors in Denmark and Sweden undertaken in 2013 found that the risk of a major CHD event begins to increase within the first 5 years post-treatment and continues to significantly exceed that of the general population through at least 20 years of follow-up[27]. These patients experienced increased risk of angina pectoris, MI, and sudden cardiac death despite having been treated with a modest mean heart dose of 3.6 Gy RT between 1958 and 2001. Patients receiving radiation doses of < 2 Gy, 2-4 Gy, 5-9 Gy and > 10 Gy experienced dose-dependent excess risks of 10%, 30%, 40% and 116%, respectively, vs carefully matched controls. Another large study of women in Denmark and Sweden (n = 35000) comparing incidences of MI in breast cancer survivors observed an incidence ratio of 1.22 in patients undergoing left-sided vs right-sided RT[14]. In that study, the mean heart dose in patients with right-sided tumors was 2.7 Gy (vs 6.3 Gy for left-sided tumors), so the incidence ratio likely underestimates the true excess risk of RT compared with the general population. Concerning higher-dose radiotherapy, a 2015 study from the Netherlands Cancer Institute found a 40-year cumulative CHD incidence of 22.9%, amounting to a 4- to 7-fold increase in risk and 475 excess cases per 10000 person-years as compared to the general population[18].
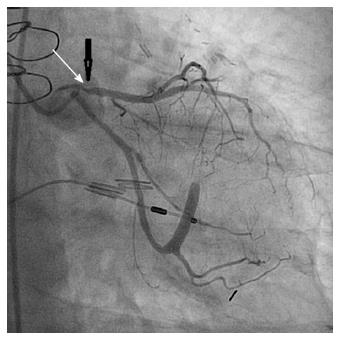
The gross pathology of radiation-induced CHD differs from that of ordinary CHD in certain key respects. Radiation-induced coronary artery lesions tend to be longer and to preferentially involve the ostium, and they are therefore more challenging to treat percutaneously[28-30]. The left anterior descending (LAD) coronary artery is often preferentially involved because of its proximity to the radiation field (Figure 3).
Figure 3 Severe proximal stenosis of the left anterior descending coronary artery (arrow).
This is particularly so in treatment of breast cancer where, while average heart doses are currently 1-5 Gy, the maximum LAD doses may exceed 20 Gy[31]. With respect to histopathology, these lesions tend to differ little from those of ordinary atherosclerosis and are characterized by intimal thickening, lipid accumulation, inflammation, and thrombosis[13]. They are often, however, somewhat more fibrous, with reduced lipid content, and the vessels involved tend to be more friable[23]. Other great vessels are likewise subject to radiation-induced friability, and the aorta and carotid artery have been known to rupture following RT on occasion[21]. Moreover, the carotid arteries have been noted to demonstrate early and rapid formation of unstable plaques following irradiation in rat models[7].
PATHOPHYSIOLOGY
Our basic understanding of the pathophysiology of RICVD has changed little since the seminal work of Fajardo et al[9,10] in the 1960s and 1970s. It has long been understood that irradiated pericardial, myocardial, endocardial, or endothelial tissue is prone to inflammation, which later results in tissue fibrosis and loss of capillaries at the microvascular level[10]. Until the early 2000s, studies in animal models and in vitro human tissues primarily focused on the mechanisms by which these changes occurred in the acute setting. Since the turn of the century, emphasis has shifted to the manner in which the acute inflammatory state gives way to chronic, pathological fibrosis. This section will begin with an overview of the inflammatory response, followed by a discussion of novel research into the mechanisms by which chronic and long-lasting profibrotic states become realized.
Acute inflammation
The mechanisms of tissue injury in the acute setting of radiation-induced pericarditis, valvular disease, cardiomyopathy and coronary disease are essentially the same and appear to be largely mediated by damage to the endothelium. Whether in the visceral pericardium, the highly vascular myocardium - which has a capillary density of 2800 capillaries/mm2 as compared to 350/mm2 in skeletal muscle[32] - or the small and medium-sized vessels that perfuse the heart, the endothelium is site of initial damage. Within minutes of irradiation, endothelial cells become hyperpermeable. By the passing of the second hour, the endothelium has begun to display membrane-bound molecules such as E- and P-selectin, which are involved in leukocyte cell rolling, and ICAM-1 and PECAM-1, which are involved in leukocyte arrest and transmigration[7].
These activities stimulate the neutrophilic response that predominates acutely, with these first-responders releasing pro-inflammatory cytokines such as tumor necrosis factor, monocyte chemotactic factor, and interleukin (IL)-8, resulting in recruitment of additional inflammatory cells[33]. While this pro-inflammatory activity of granulocytes and other immune cell types was once thought to be the chief, if not the sole cause of acute inflammation and fibrosis[34], inflammatory chemokine secretion by the endothelium itself has garnered much research interest in recent years. In vitro studies of cultured human microvascular endothelial cells have confirmed a radiation-induced increase in IL-6, IL-8, human fibroblast growth factor, and adhesion molecules such as ICAM-1, in the absence of immunologic cells. This suggests an immunologic and secretory functionality of the vascular endothelium that contributes to the pro-inflammatory state[35,36].
Finally, the contribution of coagulation to this acute endothelial inflammatory response merits consideration. The presence of early fibrin deposits in the capillary networks within radiation-exposed myocardium was noted in the initial studies of RICVD[37]. This is now known to result from impaired endogenous fibrinolysis, likely due in part to thrombomodulin inhibition by transforming growth factor-beta (TGF-β), and perhaps by RT itself[38]. The role of hyperacute coagulation in the eventual development of chronic fibrosis is as yet unknown. Certain coagulation factors such as thrombin, however, can induce endothelial secretion of chemokines such as IL-8 and monocyte chemoattractant peptide, which in turn promote chemotaxis of neutrophils and expression of adhesion molecules to upregulate inflammation[39,40].
Fibrosis
Fibrosis is the chief process by which chronic radiation damage occurs. At the biochemical level, fibrosis is the result of abnormal deposition of collagenous extracellular matrix (ECM) by activated myofibroblasts. The manner in which this comes about is still the subject of investigation. Cardiovascular fibrosis is a chronic but dynamic process that is propagated by pro-fibrotic cytokines, phenotypic alterations in various cell types, and the presence of chronic hypoxia and oxidative stress. Central to this process is the terminal differentiation of fibroblasts into myofibroblasts, which secrete more type I and III collagen, as well as alpha-smooth muscle actin, another ECM protein, than do their progenitors[41]. Stimuli that may lead to myofibroblast formation in radiation injury include pro-inflammatory cytokines, matricellular signals, and epigenetic reprogramming.
Pro-fibrotic cytokines such as platelet-derived growth factor (PDGF), IL-13, IL-4, and TGF-β are secreted in abundance by neutrophils and other immune cell types recruited to irradiated tissues. TGF-β in particular has many pro-fibrotic activities, including both the promulgation of myofibroblasts and the inhibition of collagenases[40,41]. IL-13 and IL-4 are chiefly secreted by Th2 lymphocytes and act at a variety of tissues to stimulate collagen deposition[34,42]. They have chiefly been studied in the context of hepatic and pulmonary fibrosis but are active in vascular tissues as well[43-45].
Matricellular signals also contribute to pro-fibrotic phenotypic changes. One such ECM protein that may constitute a future therapeutic target in RICVD is connective tissue growth factor (CTGF), which is induced by TGF-β and promotes differentiation of mesenchymal cells and resident fibroblasts into myofibroblasts[46-48]. Moreover, CTGF can continue to stimulate myofibroblasts to secrete ECM even after TGF-β levels have normalized, thus perpetuating fibrosis long after the initial insult has passed[49,50]. Indeed, knockdown of CTGF expression in human cardiac fibroblasts decreased fibroblast growth, and CTGF inhibition was shown to reverse fibrosis, decreasing vascular stiffness and myocardial dysfunction in rodent models, though this finding has not yet been replicated in irradiated models[51].
As terminally-differentiated cells, myofibroblasts are destined to undergo apoptosis rather than mitosis during normal wound healing. This typically results in a self-limited and acellular scar[52]. They persist in radiation-induced fibrosis, however, and a growing body of evidence links this to epigenetic reprogramming. DNA methylation is the most studied mode of epigenetic modification in radiation-induced fibrosis[53]. In murine fibroblasts, expression of the α-smooth muscle actin gene, a marker of myofibroblast differentiation, was reported to be regulated by methylation of CpG islands in the gene promoter[54]. Moreover, TGF-β-induced suppression of DNA methyltransferase expression contributed to induction of the α-smooth muscle actin gene and thus myofibroblast differentiation. In contrast, induction of α-smooth muscle actin expression during hypoxia was reported to be associated with DNA hypermethylation and upregulation of DNA methyltransferases[55]. This and other studies suggest that regulation of fibroblast differentiation via epigenetic DNA methylation is complex and context-specific[56,57].
Hypermethylation of genes involved in apoptosis has been observed following irradiation and is associated with decreased cell death, which could promote fibrosis[58]. Moreover, the patterns of DNA methylation predating irradiation may be a determinant of radiation fibrosis. Human dermal fibroblasts taken from patients who later developed radiation-induced fibrosis demonstrated decreased methylation of two intragenic sequences of the diacylglycerol kinase alpha gene, a regulator of fibrosis-associated signaling pathways[59]. Moreover, decreased DNA methylation at these sites correlated with future development of profibrotic fibroblast activation, highlighting the potential prognostic value of epigenetic modifications with respect to radiation-induced fibrosis. Methylation-inhibiting agents may hold promise in the treatment or prevention of RICVD and are currently in clinical trials. Aberrations in two other modes of epigenetic modulation - microRNA activity and histone modifications - have been linked to fibrosis in various tissues, including the heart (for a detailed review, see Weigel et al[53]), but we know very little about their contributions to RICVD at this time.
Oxidative stress
In addition to directly inflicting cellular injury, radiation-induced oxidative stress is thought to play a key role in the transition from acute inflammation to chronic inflammation and fibrosis[60]. Reactive oxygen species (ROS) are acutely generated by the direct action of radiation and subsequently produced by both macrophages and the inflamed endothelium, which are replete with ROS-generating enzymes[61]. Macrophages produce large quantities of superoxide and nitric oxide, the latter via inducible nitric oxide synthase[62,63]. Superoxide and nitric oxide react to form peroxynitrite, a toxic source of free radical injury[64]. The decreased availability of nitric oxide resulting from this conversion promotes vascular dysfunction and tissue hypoxia, which further exacerbates oxidative stress[63].
Once initiated, oxidative stress propagates inflammation through several mechanisms. For example, oxidative stress promotes chemotaxis by upregulating expression of adhesion molecules such as ICAM[65] and by increasing monocyte chemotactic protein-1 and TNF-α levels[66]. Moreover, ROS increase thrombin activity by inactivating thrombomodulin, potentially promoting inflammation as previously described[67]. Though a causal link between radiation-induced oxidative stress and inflammatory cytokine production is difficult to establish, anti-oxidant studies are informative. For example, administration of alpha-lipoic acid prior to irradiation was reported to decrease local levels of IL-1, IL-6, and metalloproteinases in mice[68], while melatonin decreased levels of IL-1, TNF-α, and TGF-β[69].
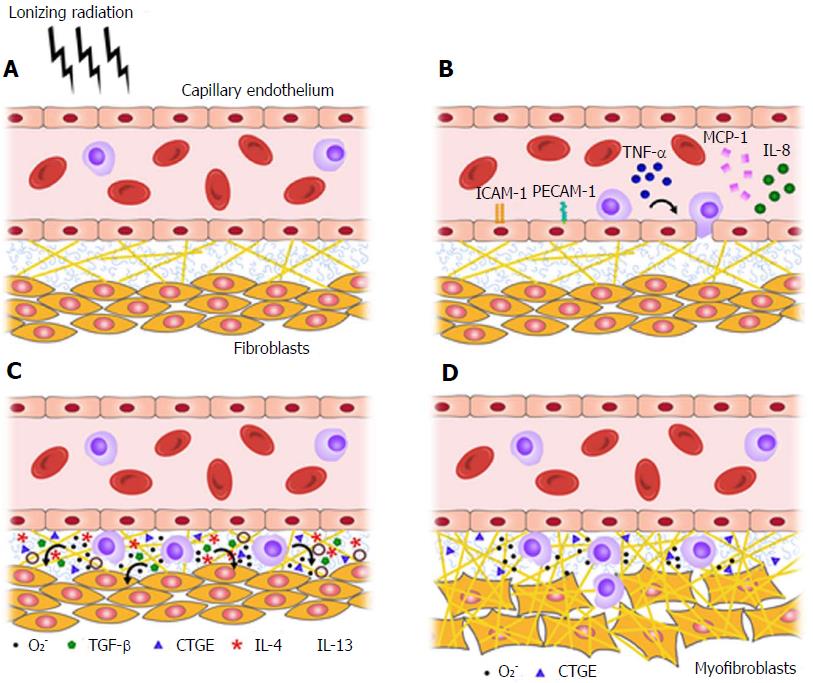
ROS also promote inflammation via their complex interaction with NF-κB, a transcription factor responsible for such critical functions as immune regulation and cell survival. In the setting of RICVD, NF-κB activation by ROS results in increased adhesion molecule, cytokine, and chemokine production[70]. An association with fibroblast stimulation and collagen deposition has also been demonstrated. Importantly, NF-κB upregulation was detected from week 4 through week 500 post-irradiation in small vessels of the neck in humans[71], suggesting that NF-κB might be a critical element in the transition from acute inflammation to chronic fibrosis (Figure 4).
Figure 4 Radiation injury and the transition from acute inflammation to chronic fibrosis, as mediated by pro-fibrotic cytokines and reactive oxygen species.
A-C: Normal tissue (A) becomes inflamed within hours of irradiation (B), and a pro-fibrotic cytokine profile predominates within days-to-weeks (C); D: Represents the chronic state of fibrosis characteristic of radiation injury. O2-: Reactive oxygen species; TNF-α: Tumor necrosis factor alpha; MCP-1: Monocyte chemotactic protein-1; CTGF: Connective tissue growth factor; TGF-β: Tumor growth factor beta; IL: Interleukin.
Free radicals produced by macrophages result in increased pro-fibrotic TGF-β production in irradiated animals[72]. This change is preceded by tissue hypoxia, which follows in RICVD from capillary effacement and diminished perfusion. Additionally, ROS have been reported to cleave TGF-β from its anchorage sites in the ECM, which in turn promotes myofibroblast differentiation and ECM deposition[61]. Lastly, free radicals establish a preference for the Th2 lymphocyte phenotype over the Th1 response[73,74], thus skewing the lymphocyte population towards those that preferentially secrete IL-4, IL-13, TGF-β, etc. These and other chemical signals act in concert to stimulate myofibroblast hyperactivity and disordered ECM deposition.
PREVENTION
The cardiovascular morbidity and mortality of RT can be forestalled through primary prevention, which consists of dose reduction and radioprotection, and secondary prevention, which consists of screening and radiomitigation. No pharmaceuticals are currently approved by the Food and Drug Administration for either purpose, although Amifostine, a scavenger of free radicals, was recently approved for the reduction of radiation-induced xerostomia[75]. This discussion will therefore focus on dose-reduction, screening, and risk modification, ending with a brief discussion of novel uses of existing pharmaceuticals, such as statins and ACE inhibitors.
The most important means of prevention is reduction in radiation exposure. Advances in radiation oncology have resulted in decreases in absolute 15-year cardiac mortality from 13% in the mid-1970s to 5.8% in the late 1980s for breast cancer survivors[76]. Meanwhile, the incidence of major CHD events in HL survivors has remained roughly unchanged during the same period despite dramatic increases in utilization of cardiotoxic chemotherapeutics[18]. Recent reviews have dealt with the techniques by which this has been achieved[77,78]. Some of these strategies involve manipulating the patient so as to exclude as much of the myocardium from the treatment field as possible; for instance, use of breast boards or prone positioning may reduce the volume of myocardium traversed by the radiation beam. Likewise, deep inspiration and inspiratory gating are two techniques by which radiation oncologists exploit the heart’s tendency to fall inferiorly and posteriorly out of the radiation field. Perhaps the most important advancement is the use of intensity-modulated RT, in which 3-D CT images are used in conjunction with multileaf collimators that can be manipulated to deliver radiation beams that conform closely to the shape of the tumor. With respect to HL, reductions in radiation exposure are mainly attributable to dose fractionation, and to the shift from mantle field radiation, which encompasses much of the neck, mediastinum, and axilla, to more limited, involved fields[79]. All of these techniques presuppose superior imaging and software technologies that deliver radiation more accurately - often to within several millimeters of the desired target - and with much smaller margins than were used in the in the previous century.
Despite these improvements, excess risk of morbidity and mortality persist. It is therefore imperative that cardio-oncologic care be coordinated prior to initiation of RT for the establishment of appropriate cardiac baselines and for continued surveillance throughout the patient’s lifetime. The younger the patient is at time of treatment, the more critical the need for surveillance, as both their relative risk of RICVD and their survivorship with respect to cancer are greater[18]. Cardio-oncologic care should begin with risk factor modification, as conventional CHD risk factors are particularly hazardous in this population. Indeed, traditional risk factors have been shown to more than double the relative risk of CHD events in these patients as compared to matched patients in the general population[80]. Thus, hypertension, hyperlipidemia, and diabetes mellitus should be managed aggressively, and patients should be counseled regarding smoking cessation, weight loss, and exercise where appropriate.
Screening and detection
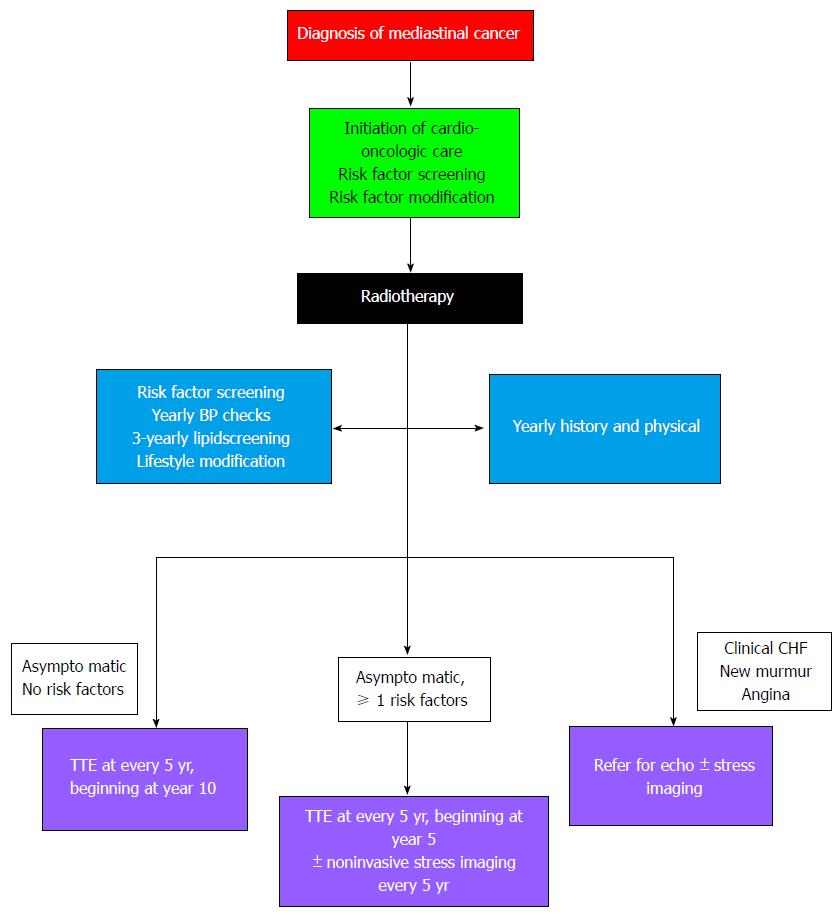
The cardiac morbidity and mortality associated with RT can be reduced if treated early, which justifies the need for screening and early detection of RICVD[81]. Prospective data regarding cost- and risk-benefit analyses with respect to screening are lacking, however. Although evidence-based guidelines are unavailable, several expert consensus statements have been derived based on the available randomized trials and epidemiological studies. In 2014, the American College of Radiology Appropriateness Criteria Report made a case for the importance of surveillance but stopped at recommending personalization[82]. The expert panel of the National Comprehensive Cancer Network (NCCN) called for aggressive management of cardiovascular risk factors with annual blood pressure and biannual lipid screening in their expert consensus statement released in 2015. They also recommended considering a baseline stress test or echocardiogram every 10 years after treatment[83]. Some experts have proposed that irradiation should be considered an additional CHD risk factor in the presence of hypertension, hyperlipidemia, or diabetes[81].
Finally, the most rigorous set of screening recommendations came from the European Association of Cardiovascular Imaging and the American Society of Echocardiography in 2013, which recommend aggressive risk factor modification and yearly physician visits. The statement went further, however, recommending baseline echocardiography prior to RT, followed by repeat echocardiography 10 years after treatment and every five years thereafter in heart-healthy patients[84]. For patients with one or more conventional risk factors, screening echocardiography was recommended in the fifth year after treatment, and noninvasive stress testing was recommended 5-10 years after treatment and at 5-year intervals, with a preference for stress echocardiography in these patients.
The prospective data supporting these statements was largely derived from a series of studies by Heidenreich et al[24], who screened asymptomatic HL survivors for RICVD. Their study of echocardiography in asymptomatic patients uncovered a 29% prevalence of significant valve disease in HL patients as compared to 3% in the general population[24]. Diastolic dysfunction was detected in 14% of the HL patients at a mean of 14 years post-RT[23]. While the cost-benefit ratio of screening for heart failure with preserved ejection fraction (EF) is uncertain due to the lack of effective treatment, a disproportionate number of patients thus afflicted also demonstrated stress-induce ischemia on subsequent stress echocardiogram or nuclear perfusion imaging (23%). Finally, Heidenreich et al[85] evaluated stress echocardiography and radionuclide perfusion imaging as screening tests for asymptomatic CHD after RT. They observed a 2.7% prevalence of severe, multivessel or proximal coronary stenosis, and a 7.5% prevalence of coronary stenosis greater than 50% at a mean 15 years after RT. The cohort overall had a documented 8% prevalence of coronary insufficiency or death. The generalizability of these data is limited by the high radiation doses employed in the cohort; the mean heart dose in the three trials was 43-44 Gy, which is much higher than most HL patients receive today. Nevertheless, these findings are likely pertinent to patients irradiated prior to the 1990s, or to more recently treated patients receiving mean heart doses greater than 35 Gy.
Thus, current literature supports use of transthoracic echocardiogram as the screening tool of choice to evaluate baseline left ventricular EF, diastolic function and VHD. Echocardiography is also important in the assessment of restrictive cardiomyopathy and constrictive pericarditis. Ultrasonographic technologies are constantly evolving, leading to improvements in the ability to detect subtle signs of RICVD disease via echocardiography. Using cardiac MRI as the gold standard, 3D echocardiography was reported to exhibit greater sensitivity than 2D echocardiography to detect left ventricular EFs less than 50% (53% vs 25%, respectively)[86]. Deformation imaging using speckle tracking or tissue Doppler velocities may be even more sensitive to detect subtle abnormalities in left ventricular function[87,88]. Reductions in systolic myocardial deformation were detected immediately and 2 mo after RT, in the absence of detectable reductions in EF[89]. Speckle tracking echocardiography demonstrated abnormal global longitudinal and global circumferential strain in 33% and 21.7%, respectively, of patients who underwent RT, while depressed EF was detected by 3D echocardiography in only 5.7% of patients at a median 22.6 years[90]. While no gold standard was applied, abnormal longitudinal strain was correlated with reduced quality of life and lower mean 6-min walk distances, even when it was the sole abnormal finding. Thus, while reduced EF is a late finding in RICVD, abnormal strain measurements may herald early onset disease and are increasingly being incorporated into screening protocols (Figure 5).
Figure 5 Proposed algorithm for cardio-oncologic screening following mediastinal radiotherapy[84].
CHF: Congestive heart failure; TTE: Transthoracic echocardiography.
Though it is not a first-line screening tool, cardiac MRI is helpful in evaluation of left ventricular EF and, with the addition of myocardial tagging, may be utilized for better evaluation of constrictive pericarditis. This modality is particularly well-suited to detection of the patchy fibrosis that may be associated with microvascular insufficiency even in the absence of classical ostial coronary stenosis, ischemia, or infarction[91]. The pattern of late gadolinium enhanced MRI images can help differentiate between MI, diffuse myocardial fibrosis, and constrictive pericarditis as the underlying mechanism of the cardiomyopathy[92,93].
Newer screening modalities for radiation-induced vascular disease have also been evaluated, including coronary artery calcium (CAC) imaging. In a cohort of 47 HL survivors who received a mean cardiac dose of 40.6 Gy, CAC imaging demonstrated a strong association between severity of CAC and the presence of coronary artery disease verified by angiography[94]. The proportion of patients with CAC scores of zero was much lower in the HL cohort than in the general population. Another study using CT angiography in HL survivors detected nearly twice as many atherosclerotic lesions in pre-cranial blood vessels contained within the radiation field as compared with a non-irradiated control group; the percentages of calcified vs non-calcified lesions were similar in the HL and control groups, suggesting that atherosclerosis, but not calcification, is a radiospecific finding[95]. Interestingly, this study also found that elevated total cholesterol, measured soon after RT, correlated strongly with later incidence of coronary artery disease. While these studies suggest a role for CT in screening for vascular disease in HL survivors, their generalization is limited by the high doses of radiation to which these older cohorts were exposed. Indeed, a larger study of CAC screening in 236, 12-year breast cancer survivors who had been exposed to lower doses of radiation did not find any excess CAC, though the duration of follow-up may have been too short to detect late occurrences[96]. Thus, CT imaging may be of greater utility in detection of CHD in cancer survivors who are in their second or third decade post-RT.
Laboratory monitoring is another important component of screening for RICVD. The importance of identification and management of hyperlipidemia in cancer survivors with a history of RT is emphasized by data showing a direct correlation between the presence of hypercholesterolemia soon after RT and atherosclerosis[95,96]. Chen et al[97] (2009) applied a decision-analytic model to perform a cost-benefit analysis in a hypothetical cohort of HL survivors to establish the cost-effectiveness of screening intervals for hyperlipidemia. Applying an assumed relative risk of cardiac mortality of 3.2 to a theoretical cohort that otherwise differed little from that of the general population, the optimal interval for screening for hyperlipidemia was determined to be every three years. With respect to biomarker screening, elevation of troponin-I and brain natriuretic peptide have been demonstrated in patients during and immediately following RT in a small cohort of breast or lung cancer patients[98]. A later study found subacute elevations of high-sensitivity troponin-T following RT that were dose-dependent[99]. This study also detected echocardiographic evidence of interventricular septal thickening and prolonged diastolic deceleration time in patients who experienced a greater than 30% increase in troponin levels from baseline, suggesting that elevated high-sensitivity troponin-T correlates with subtle abnormalities of cardiac function after RT. The implications for the utility of high-sensitivity troponins in screening for future cardiac disease are unknown but may become clearer after the planned follow-up with this cohort.
Radioprotection and radiomitigation
As to the role of pharmaceuticals, several commonly prescribed agents are currently being evaluated in primary and secondary prevention after promising results were observed in animal trials. Statins have been studied extensively in rodent and in vitro human models and have shown promise through several of the mechanisms discussed in the Pathophysiology section. Pravastatin has been found to inhibit the activity of CTGF by modulating associated proteins such as Ras-homologous (Rho) GTPases[100]. Rho-family proteins regulate cellular responses to pro-fibrotic cytokines such as TGF-β, and to oxidative stress[46]. They also increase cell adhesion and contribute to the reorganization of the ECM[100,101]. Some of the statins’ anti-fibrotic activities may also be related to attenuation of radiation-induced NF-κB activity, which depends upon activation by Rho-family GTPases[102]. Additionally, statins upregulate thrombomodulin expression in human endothelium, decreasing the pro-inflammatory activities of thrombin as described previously[103]. Lastly, atorvastatin has been found to reduce injury to and apoptosis of the vascular endothelium[104]. Notably, several trials of statins in young patients who were treated with RT are underway[105]. Although surrogate endpoints, such as detection of endothelial function and carotid intimal-medial thickness, will be employed, these studies nevertheless may begin to illuminate the role of early statin therapy in reducing the long-term risk of RICVD.
Angiotensin converting enzyme (ACE) inhibitors are another commonly prescribed class of drug with radioprotective and radiomitigating potential. Rats treated with captopril shortly after radiation of the lung demonstrated dramatically increased survival and improved vasoreactivity, as well as decreased perivascular fibrosis and inflammatory cell infiltration[106]. Similar findings with respect to the pulmonary vasculature have been reported in rats treated with ACE inhibitors two weeks after irradiation[107]. More recently, rats treated with captopril exhibited reduced diastolic dysfunction and perivascular necrosis in the left ventricle following radiation exposure[108]. Although these data are intriguing, prospective studies evaluating the efficacy of ACE inhibitors in patients undergoing RT have not been reported.
Lastly, antioxidant approaches are the subject of much investigation. Amifostine was previously mentioned in connection with its indication for treatment of xerostomia, but in a small rodent study of RICVD, this drug was found to reduce myocardial fibrosis and impairment of aortic and coronary blood flow[75]. Melatonin is also being evaluated for this use, as it is known to act both as a scavenger of free radicals and as a stimulant of antioxidants[109,110]. It was reported to reduce the development of vasculitis, myocyte necrosis, and fibrosis following high-dose radiation in a rat model[111]. These and several other antioxidant strategies such as the use of selenium[112] show promise and warrant further exploration in animal studies.
TREATMENT
Acute and chronic pericarditis
Acute pericarditis is extremely rare thanks to reductions in the mean heart dose during RT. The clinical presentation may occur during treatment or in the following weeks. In the former instance, pericarditis is typically the result of the presence of a heavy tumor burden adjacent to or extending into the pericardium, and the subsequent tumor lysis. In both instances, the presentation is similar to that of idiopathic acute pericarditis, characterized by fever, pleuritic chest pain, and a pericardial friction rub. ECG may demonstrate low QRS voltage and diffuse ST or T wave changes. Standard transthoracic echocardiography typically demonstrates a pericardial effusion. This syndrome is usually self-limited and responds to treatment with NSAIDs and colchicine, but it may progress to tamponade physiology[37]. Pericardiocentesis is indicated in the event of hemodynamic compromise.
Radiation-induced chronic pericarditis is a more unique disease entity, in that it frequently presents as fibrinous constrictive pericarditis. The most common presentation is as an incidentally discovered asymptomatic effusion, however. This type of pericarditis rarely progresses to tamponade because of its chronicity, and the presentation is similar to that of acute pericarditis. The imaging modality of choice is echocardiography in these patients, for reasons of cost, ease of use, and reproducibility. The latter advantage is critically important in the case of recurrent effusions and symptomatic constrictive pericarditis, which may help the clinician to make appropriate referral for invasive procedures[84]. Cardiac CT and MR, on the other hand, have proven to be more sensitive in the diagnosis of constrictive pericarditis owing to better visualization of pericardial thickening and calcifications. Moreover, cardiac MR is more specific in the diagnosis of constrictive pericarditis, distinguishing it from transient constriction due to active inflammation and effusive-constrictive pericarditis. This can be useful in assessing prognosis and in determining whether or not to proceed with a high-risk pericardiectomy[93].
Recurrent symptomatic effusions may require pericardiectomy, which is also the mainstay treatment of symptomatic constrictive pericarditis. While the procedure does provide benefit to irradiated patients, these patients have poor prognoses, with a 21% perioperative mortality and a 7-year survival rate of just 27%[16,113]. In one study, zero of five patients survived beyond five years[114]. Unfortunately, these mortality rates may reflect both the technical difficulties in operating on the irradiated heart and progression of other forms of RICVD that inevitably follow from large radiation exposures.
RICM
RICM may remain asymptomatic for years before presenting as a typical clinical heart failure syndrome with shortness of breath and other symptoms of volume overload. Diastolic dysfunction is typically the earliest imaging finding in RICM, followed by abnormalities pertaining to strain and strain rate such as discussed in the screening and detection section. Therefore, this new echocardiographic modality is optimal for establishing an early diagnosis of RICM. When a reduction in EF occurs, it is often a late finding. Cardiac MR is appropriate for use in patients with poor acoustic windows and not only detects reductions in EF, but also visualizes the inciting myocardial inflammation and fibrosis[84]. Once RICM has been confirmed, treatment should be initiated per the ACC/AHA guidelines, as there are no drugs specifically approved to treat radiation-induced myocardial inflammation or fibrosis. This is also the case with respect to implantable cardioverter-defibrillator (ICD) placement, as the indications for its use have not been specifically evaluated in RICM. As to the location of ICD implantation, it has been suggested that a sub-pectoral approach may be preferred in order to avoid instrumenting the irradiated superficial tissues[5].
In patients with biventricular heart failure due to radiation-induced restrictive cardiomyopathy, cardiac transplant may be performed as a last resort. Several case series have been published in recent years detailing the outcomes of these cases. The largest was a 2012 study of patients undergoing transplant for restrictive cardiomyopathy, which included a subgroup of 35 patients with RICM. This group demonstrated 1-, 5- and 10-year transplant survival rates of 71%, 47%, and 32%, respectively - the poorest survival rates amongst all of the subgroups[115]. A 12-subject cohort of patients transplanted for RICM reported a lower mortality, with a 5- and 10-year survival of 75% and 47%[116]. Of note, eight of these patients were transplanted for treatment of restrictive cardiomyopathy. Given the limitation in donor hearts eligible for transplantation, and the large numbers of patients currently on waiting lists, cardiac transplantation is likely to play a very limited role in patients with end-stage RICVD.
VHD
Little has been written about peculiarities of the clinical presentation of radiation-induced VHD. Echocardiographic studies have demonstrated that it typically begins as an asymptomatic regurgitation of the mitral and/or aortic valves, progressing to include aortic stenosis in 39% of patients[25]. Radiation-induced VHD is most commonly diagnosed after a long latent period[117] and in the context of clinical symptoms of heart failure[18], to which valvular insufficiency is either contributing or responsible. When VHD is suspected, i.e., on the basis of a new murmur, transthoracic Doppler echocardiography is the first line of investigation, with transesophageal echocardiography reserved for when the initial evaluation is non-diagnostic[84].
The frequency of radiation-induced VHD is significantly greater than seen in the general population. In a cohort of HL patients, the standardized incidence ratio for valve surgery was found to be 9.19 when compared to the estimated expected national incidence in the United States, though this may be an overestimate, as some of these patients were irradiated under older protocols[118]. Aortic valve replacement was the most common procedure in this cohort, though mitral and tricuspid valve disease may also require intervention. Crestanello et al[119] reported that 32% of previously irradiated patients who underwent mitral and/or tricuspid valve repair experienced severe valve deterioration, likely because of progression of radiation-induced tissue injury. In light of these findings and the known dangers of reoperation in this cohort, the authors concluded that mitral and tricuspid valve replacement may be superior to repair in patients with RICVD.
Over the past several years, transcutaneous aortic valve replacement (TAVR) has proven equal or superior to surgical valve replacement in high-risk patients[120,121]. As valve technology and techniques for TAVR have evolved, favorable outcomes are now also being observed in intermediate risk patients. Approximately 5% of patients enrolled in recently published TAVR trials have a history of prior chest wall radiation, with initial favorable results[122]. However, long-term results are unavailable. Nevertheless, TAVR is likely to play an increasingly prominent role in treatment of patients with radiation-induced aortic disease given the associated surgical morbidity/mortality in this high-risk population.
CHD
There is currently no basis of evidence to suggest specific deviations from treatment guidelines for the medical management CHD in patients with a history of mediastinal irradiation. The increased risk of CHD in patients with a history of RT may prompt a more aggressive approach where the etiology of chest pain is in question and/or diagnostic findings are ambiguous. As always, coronary angiography is the gold standard, and clinicians should have a lower threshold to consider it in this population. On the other hand, both percutaneous interventions (PCI) and surgical revascularization are often more challenging and less effective in this population, which must be taken into account. As noted previously, coronary artery lesions tend to be proximal or ostial in this population, and may not be readily amenable to PCI. A prospective study of bare metal stent placement in HL survivors was conducted between 1993 and 2003 and revealed in-stent restenosis in 86% of irradiated patients within the first six months, with an odds ratio for this event of 21.7[123]. Moreover, revascularization of the target vessel with balloon angioplasty was required in 67% of the RT cohort at six months per coronary angiography. However, most of these patients were treated with early generation stents and single antiplatelet therapy. A larger case control study in which 36% of patients received newer drug-eluting stents, and all patients received dual antiplatelet therapy, found no difference in the rate of in-stent restenosis requiring revascularization between irradiated and non-irradiated patients[124]. Drug-eluting stents did not outperform bare metal stents in this study; nevertheless, use of newer generation drug-eluting stents is usually preferred in this population.
Surgical revascularization of the irradiated heart is often necessary, but is not without complication. Operative mortality rates of 6% have been reported, and one- and five-year actuarial survival has been estimated to be 87% and 72%, respectively[125]. Sixty-two percent of patients in the latter cohort required valve surgery concomitantly or after the initial surgery, suggesting that valvular dysfunction is a significant contributor to mortality in this population. In another, larger study of cardiothoracic surgical outcomes in irradiated patients, a dose-dependence was observed with regard to post-operative and long-term mortality data[126]. At lower doses of radiation exposure, breast cancer patients undergoing open-heart surgery were found to approach, but not reach, the levels of 4-year survival expected of the general population, while the outlook for HL patients was much worse (73%, 64% and 57% survival at 1-, 2- and 4-years, respectively). Lastly, irradiated patients often exhibit friability of the left internal mammary artery, a well-known complication encountered when that vessel lies in the irradiated field, which compromises its use as a bypass conduit and diminishes the overall benefit of bypass surgery in patients with RT.
CONCLUSION
Despite advancements in radiation oncology, it appears that cancer survivors treated with breast and mediastinal radiotherapy will continue to present with complicated cardiovascular problems for the foreseeable future. Further research is needed to elucidate pro-fibrotic mechanisms and identify promising therapies that can be implemented early during the course of treatment. The phenotypic shift from fibroblast to myofibroblast is a result of the complex interplay of radiation-induced oxidative stress, inflammation, cell signaling, and epigenetic modifications, which requires further study in animal models. Medications such as ACE inhibitors and statins favorably impact many of these pathways and have shown promise in animal models of RICVD; these agents are just now beginning to be tested in patients who have undergone RT. Novel imaging approaches, such as 3D echocardiography, strain imaging, and CT/MRI scanning, are enabling the detection of early-stage RICVD, which will help to better evaluate risk and facilitate future interventional trials. Evolution of PCI (i.e., transcutaneous valve replacement and drug-eluting stents) holds great potential for improving treatment of patients with RICVD, and these techniques are rapidly gaining favor given their encouraging outcomes and lower complication rates as compared to surgical interventions. Although evidence-based guidelines with respect to screening, prevention and treatment of RICVD are lacking, algorithms have been developed by experts in the field that favor a more aggressive approach than was typically pursued in prior decades. Coordination of care between oncologists, cardiologists, and primary care physicians for the purpose of early detection, risk factor modification and treatment provides the best hope of reducing the morbidity and mortality associated with RICVD.