Published online Feb 26, 2016. doi: 10.4330/wjc.v8.i2.120
Peer-review started: May 6, 2015
First decision: July 22, 2015
Revised: October 8, 2015
Accepted: December 17, 2015
Article in press: December 18, 2015
Published online: February 26, 2016
Processing time: 299 Days and 14.6 Hours
Psoriasis is a chronic inflammatory immune-mediated skin disease, frequently associated with systemic comorbidities. According to recent data, patients with psoriasis show a greater prevalence of metabolic syndrome, which confers a higher cardiovascular risk. The link between these pathological conditions appears to be a chronic low-grade inflammatory status. The aim of this review is to focus on the multiple epidemiological and physio-pathogenetic aspects linking non-alcoholic fatty liver disease, psoriasis, and cardiovascular disease.
Core tip: The review focuses on recent scientific data regarding the multiple physio-pathogenetic aspects of the possible link between psoriasis, non-alcoholic fatty liver disease, and cardiovascular disease. The multidisciplinary approach to psoriatic patients appears mandatory to treat concomitant psoriasis-related comorbidity, and the risk/benefit of both biologic and non-biologic therapies should be evaluated.
- Citation: Ganzetti G, Campanati A, Molinelli E, Offidani A. Psoriasis, non-alcoholic fatty liver disease, and cardiovascular disease: Three different diseases on a unique background. World J Cardiol 2016; 8(2): 120-131
- URL: https://www.wjgnet.com/1949-8462/full/v8/i2/120.htm
- DOI: https://dx.doi.org/10.4330/wjc.v8.i2.120
Psoriasis is a chronic inflammatory relapsing disease affecting 1%-4% of the general population[1].
Despite that psoriasis is a skin disorder clinically characterized by red scaly plaques, it is no more limited to the skin surface and has been identified as a complex clinical entity with a systemic involvement. Many comorbidities have been associated with psoriatic disease, such as psoriatic arthritis (PsA), metabolic syndrome (MetS), cardiovascular disease (CVD), non-alcoholic fatty liver disease (NAFLD), inflammatory bowel disease, uveitis, depression and malignancy[2-5].
An higher prevalence of cardiovascular risk factors, such as dyslipidemia and obesity, has been reported in psoriatic patients[4,6].
NAFLD is one of the most frequent cause of chronic liver disease with a prevalence of 10%-25% in the general population[7].
NAFLD is now considered the hepatic manifestation of the MetS and a prospective cohort study has evidenced that MetS and its components may independently predict the risk of NAFLD[8,9].
NAFLD itself represents a further independent cardiovascular risk factor for atherosclerosis which is likely linked to arterial stiffness[10,11].
The aim of the present review is to focus on the association of psoriasis, NAFLD and CVD, focusing on epidemiologic data and the underlying common pathogenic process.
The prevalence of the MetS has been estimated to be about 15%-25% in the general population, appearing significantly higher (an increase by about 3-fold) in psoriatic patients, as documented by many case-controls studies[12-16].
The association between psoriasis and MetS is directly correlated with the severity of psoriasis, independent from the presence of obesity in psoriatics[17-19].
NAFLD is defined as a spectrum of hepatic pathologies ranging from fatty liver disease (steatosis) to steatohepatitis (NASH) with the risk of evolution to cirrhosis and hepatocellular carcinoma[20].
NAFLD is more prevalent in psoriatic patients than in the general population. Roberts et al[21] enrolled a cohort of 103 psoriatic patients and found that NAFLD affected about 47% of patients and one of five of them showed NASH.
A large prospective population-based cohort study had been conducted by van der Voort et al[22] in patients older than 55 years. Among 2292 participants, 5.1% of the population were affected by psoriasis with a prevalence of NAFLD of about 46.2% in psoriatic patients vs 33.3% in subjects without psoriasis.
Furthermore, a recent meta-analysis has documented that patients with PsA and patients with moderate to severe psoriasis showed a significantly greater risk of NAFLD compared with those with mild psoriasis[23].
Aspartate transaminase (AST)/alanine aminotransferase (ALT) ratio is considered an independent predictive factor for liver fibrosis in patients with NAFLD; significantly higher AST/ALT ratio and higher non-invasive fibrosis scores have been detected in patients with both psoriasis and NAFLD compared to controls with only NAFLD[24].
MetS confers an increased risk of cardiovascular events and mortality due to CVDs[25,26]. Psoriatic patients show a higher prevalence of cardiovascular risk factors, which are shared by NAFLD and CVD, thus representing the trade union between these pathologies. Obesity represents a great burden in global individual’s health, significantly increasing morbidity and mortality[27].
Data from large cohort studies have shown that among 163517 enrolled individuals, 17% were obese (11465 men and 16612 women). Thus, obesity represents a great public health problem reaching worrying proportions both in pediatric and adult populations[27].
As demonstrated by recent observational studies, psoriatic population may have a higher risk of over-weight and obesity with the consequent higher risk of components of MetS[28].
Danielsen et al[29] have conducted a recent population-based study confirming an increased prevalence of MetS in patients affected by psoriasis compared to controls. Interestingly, a different trend was emphasized between genders: A 3.8-times higher odds of MetS was found in young women (< 30 years) with an odds ratio reduction with increasing age. Conversely, men showed a 1.35-times higher odds ratio of MetS, independently from age.
Moreover, a direct correlation between severity of psoriasis and obesity has been evidenced in a recent meta-analysis: An odds ratio of 1.46 was found in mild psoriasis and an odds ratio of 2.23 in severe psoriasis[22,30].
Dyslipidemia is a further risk factor, which is shared by NAFLD, psoriasis and CVD. Observational studies have detected a lipid metabolism alteration in psoriatic patients contributing to a dyslipidemic profile and conferring a significant cardiovascular risk[31].
Psoriatic children present high plasma levels of total cholesterol, high content of total cholesterol and high cholesterol/protein ratio in LDL and in HDL[32].
Moreover, an increased odds of hypertriglyceridemia, significantly reduced levels of HDL cholesterol (< 40 mg/dL), hyperlipoproteinemia and hypercholesterolemia have been identified in psoriatic populations[31,33,34].
As for obesity, a positive correlation was found between dyslipidemia and severity of psoriasis with an increased odds of 1.10-3.38 in mild psoriasis and 1.36-5.55 in severe psoriasis[35,36].
The dyslipidemic profile appears extremely relevant; in fact, it is known that hypercholesterolemia can lead to atherosclerosis and coronary heart disease. In animal models, adipocyte differentiation and maturation can be altered by cholesterol accumulation in preadipocytes, leading to adipocyte hypertrophy and adipose tissue inflammation. In humans, it has been demonstrated that hypercholesterolemia leads to an imbalance in the pro- and anti-inflammatory adipocytokine production by adipose tissue[37].
CVDs include atherosclerosis, hypertension, ischemic heart disease, myocardial infarction, stroke and arrhythmias[4].
An increased incidence of cardiovascular risk factors and major cardiovascular events has been found in psoriasis[4,5,15].
Gelfand et al[38] performed a cohort study on patients affected by severe psoriasis, evidencing a further 6.2% absolute risk of a 10-year rate of major cardiovascular events and suggesting the possible role of severity of the disease in the pathogenesis of CVD.
In particular, a 6-year reduction in life expectancy has been evidenced in patients with severe psoriasis[39].
Although the role of the extent of psoriasis-involved body sites has not been completely elucidated, studies showed that a wide skin involvement and the presence of inter-gluteal lesions may represent independent predictor factors of CVD in psoriatics[40].
A prospective, population-based cohort study had been conducted by Gelfand et al[41] in 2006, evaluating the risk of myocardial infarction (MI) in psoriatic patients. The authors found that psoriatics had a higher incidence of MI which was positively correlated with disease severity: 4.04 per 1000 person-years (95%CI: 3.88-4.21) in mild psoriasis and 5.13 per 1000 person-years (95%CI: 4.22-6.17) in severe psoriasis. Moreover, the risk of MI was higher in young 30-year-old psoriatic patients, and this risk persisted higher after adjustment for major risk factors for MI, suggesting that psoriasis itself confers an independent risk of MI.
This aspect was also confirmed by Brauchli et al[42], who found the highest incidence rate of MI in psoriatic patients aged 30-39 years with severe skin disease.
The concomitant presence of PsA seems to lead to an increased risk of non-fatal MI; a risk up to 10% of CVD disease within 10 years of PsA incidence has been identified in most of newly diagnosed PsA patients[43,44].
A retrospective study has shown that the concomitant presence of arterial hypertension (AH) and diabetes mellitus (DM) enhances the risk of CVD in PsA patients. The prevalence of AH and DM was significantly greater in PsA patients who have had CVD compared to those without CVD; the prevalence of AH was 95% vs 45% and the prevalence of DM was 60% vs 19%. These aspects have important repercussions on early recognition and targeted treatment of comorbidities in psoriatic patients in order to reduce morbidity and mortality[45].
An association between psoriasis and atherosclerotic disease has been recognized. A cross-sectional study conducted by Yiu et al[46] evaluated the prevalence and the extent of coronary and carotid atherosclerosis in 70 psoriatic patients compared to age- and gender-matched healthy controls. Psoriatic patients showed a 10-fold increased risk of subclinical coronary atherosclerosis and premature diffuse coronary and carotid atherosclerosis.
The subclinical vascular atherosclerosis in psoriasis has been also studied by Balci et al[47] on 43 psoriatic patients without cardiovascular risk factors and 43 healthy controls matched for sex and age. Significantly higher mean intima-media thickness values of the right, left and averaged common carotid arteries had been detected in psoriatics than in controls (0.607 ± 0.144 mm vs 0.532 ± 0.101 mm, 0.611 ± 0.157 mm vs 0.521 ± 0.117 mm, and 0.609 ± 0.146 mm vs 0.526 ± 0.104 mm, respectively). Conversely, the mean flow-mediated dilatation and nitroglycerin-induced dilatation values were significantly lower in patients with psoriasis than in controls (13.36 ± 6.39 mm vs 19.60 ± 11.23 mm and 21.08 ± 8.38 mm vs 26.85 ± 12.38 mm; P = 0.002 and P = 0.013, respectively).
It is well documented that calcium exerts an important role in atherosclerosis, and it is an important index of subclinical atherosclerosis and greatly impacts on the atherosclerotic plaque burden[48].
A recent case-control study was conducted on 40 patients with psoriasis and 42 controls matched for age, sex, and cardiovascular risk profile in order to examine the prevalence of coronary calcification. The same prevalence of calcified and non-calcified atherosclerotic coronary lesions was evidenced in both groups[49]. Conversely, emerging data show that patients with psoriasis have a higher coronary calcium score (CAC), which was directly correlated with psoriasis severity[50].
A cross-sectional study was conducted on Mediterranean population, aiming to determine the prevalence of ischemic CAD in patients with psoriasis establishing a significant independent association between psoriasis and CAD[51].
The coronary microvascular function has been evaluated in psoriatic patients by echocardiographic examination to emphasize the coronary flow reserve (CFR). A coronary impairment was shown with a reduction in CFR and with a positive inverse correlation between CFR and PASI score, disease duration and C-reactive protein[52].
Interestingly, it has been recently documented that psoriasis and coronary artery disease share similarities in coronary function and myocardial deformation with a subclinical left ventricular deformation. This aspect may contribute to vascular dysfunction in psoriatic patients, increasing the risk of coronary artery disease[53].
The early detection of specific inflammatory biomarkers implicated in CVDs and in subclinical atherosclerosis remains a fundamental item to promptly identify the cardiovascular risk in this population[54].
New data have emerged from studies on this topic. In particular, N-terminal pro B-type natriuretic peptide (NT-proBNP) is a molecule secreted by the ventricular myocardium in response to increased ventricular stretch and it plays an important role as a predictor of cardiovascular mortality, of negative outcome in stroke and of left ventricular systolic dysfunction[54].
Significantly higher serum levels of NT-proBNP were found in 73 male psoriatic patients compared to controls with a direct correlation with disease duration[55].
These results appear relevant in the light of echocardiographic abnormalities found by Biyik et al[56], who showed left ventricle hypertrophy, diastolic dysfunction and wall motion alterations in patients affected by psoriasis. Moreover, a higher frequency of mitral and tricuspid valve prolapse had been diagnosed in psoriatics.
Another useful biomarker is homocysteine, which is considered an independent risk factor for CVD by promoting oxidative stress, lipoperoxidation and endothelial cell dysfunction. Moreover, hyperomocysteinemia is considered an independent risk factor for CVD, conferring an elevated risk of atherosclerosis, stroke and peripheral occlusive vascular diseases[57].
Homocysteine plasma levels have been evaluated in psoriasis, and significantly higher levels were found in psoriasis patients compared to healthy subjects, with a positive correlation with disease severity. No correlation was found between homocysteine serum levels and disease duration or the presence of arthritis[57,58].
Furthermore, high homocysteine plasma levels and reduced folic acid plasma levels in psoriatic patients seem to be implicated in a pro-thrombotic state[59].
A new interesting biomarker of vascular damage, YKL-40, has been recently studied in psoriatic patients. YKL-40 belongs to the chitinase family and it has been detected in atherosclerotic plaque, contributing to endothelial dysfunction (ED) and predicting early vascular damage in diseases with high cardiovascular risk. Increased levels of YKL-40 have been found in inflammatory conditions, such as rheumatoid arthritis, osteoarthritis, systemic lupus erythematosus and Crohn’s disease[60,61].
A case-control study on 48 psoriatic patients has emphasized a statistically significant elevation of YKL-40 levels. These data had been confirmed by Erfan et al[60], who had also performed ultrasonography in order to identify ED. Psoriatic patients with ED showed higher YKL-40 serum levels than healthy controls without ED; moreover, psoriatic patients with concomitant cardiovascular risk factors, such as smoking, obesity and diabetes, showed higher YKL-40 levels than those without[62].
The higher cardiovascular risk in psoriasis appears also linked to the increased prevalence and incidence of hypertension. In fact, hypertension is a well-established risk factor for CVDs and cardiovascular mortality[63].
The association between psoriasis and hypertension has been evaluated in a recent meta-analysis conducted by Armstrong et al[63], who documented a higher odds of hypertension of 1.58 times in psoriatics compared to the general population. Moreover, hypertension and psoriasis severity were positively correlated with a hazard ratio (HR) of 1.17 in patients with severe psoriasis and HR of 1.07 in those with mild psoriasis. PsA patients showed a higher odds ratio of 2.07 compared to patients with only psoriasis.
The increased detection of hypertension in psoriatic patients could explain the increased risk of atrial fibrillation in this population. Atrial fibrillation is the most frequent cardiac arrhythmia, accounting for 0.4%-1% of the general population and strictly linking to cardiovascular morbidity and mortality[64].
Emerging data have focused on the potential association between psoriasis and atrial fibrillation and documented that psoriasis may be independently associated with a higher risk of new onset atrial fibrillation[64].
A Danish nationwide cohort study evaluated 36765 mild psoriasis patients and 2793 severe psoriasis patients vs 4478926 controls: An increased risk of atrial fibrillation was found in psoriatics with a direct correlation with skin disease severity. Furthermore, a strong association between atrial fibrillation and early onset psoriasis had emerged[65].
Conversely, Armstrong et al[66] had considered a cohort of 2078 psoriatic patients matched to 6234 healthy subjects and evidenced no statistically significant difference in a 5-year atrial fibrillation incidence between the two groups (2.5% vs 3.3%) and no association between incident atrial fibrillation and psoriasis severity.
Psoriasis, NAFLD and CVD are considered multifactorial and multi-step diseases with not completely fully elucidated interactions between genetic, immunological and environmental factors[67,68].
Psoriasis is an immune-mediated disorder sustained and maintained by a Th1-Th17-Th22 cell immune response. The Th1-Th17-Th22 downstream pro-inflammatory cytokines contribute to creating a cytokine milieu participating in a systemic chronic inflammation process[69,70].
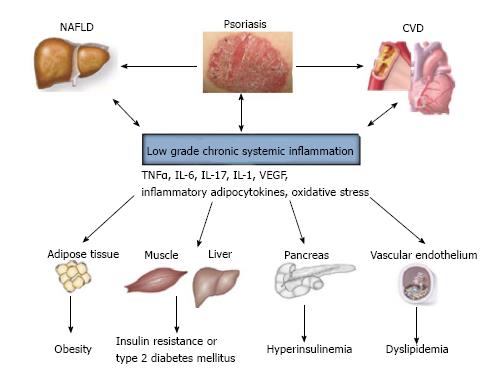
In fact, the low-grade chronic inflammatory process seems to represent the major component linking psoriasis to its comorbidities and leading to insulin resistance, to dysmetabolic profile and to ED and thus predisposing psoriatic patients to atherosclerosis and higher cardiovascular risk[2,4].
Both innate and adaptive immunity participates in physio-pathologic mechanism underlying psoriasis and atherosclerosis. Hansson et al[71] in 2012 have interestingly proposed the concept of “two plaques for one syndrome”. In fact, the development of both psoriatic and atherosclerotic plaques is strictly dependent on T cells, monocytes, macrophages and pro-inflammatory cytokines. It is know that Th1 hyperactivity and the overexpression of Th1-related cytokines represent the basis for ED being associated with atherosclerotic plaque instability and with an increased risk of athero-thrombotic events[71-74].
Most of inflammatory cytokines are produced by the adipose tissue[75,76] (Table 1). It is known that the adipose tissue is a real endocrine organ able to synthetize adipocytokines, bioactive molecules deeply involved in the inflammation and in the development of MetS and its components, such as dyslipidemia and insulin resistance[75,76].
| Psoriasis | CVD | NAFLD | |
| TNF-alpha | ↑Keratinocyte proliferation ↑Pro-inflammatory cytokine production ↑Expression of vascular endothelial cell adhesion molecules ↑Angiogenesis | ↑LDL transcytosis | ↓IRS-1 phosphorylation ↑Insulin-resistance ↑Hepatic fibrogenesis |
| IL-1 | ↑Keratinocyte proliferation ↑Pro-inflammatory cytokine production ↑Expression of vascular endothelial cell adhesion molecules | ↑Synthesis of IL-6, fibrinogen, RCP ↑Expression of adhesion molecules (ICAM, VCAM) | ↑Activation of MAP and ERK pathways |
| IL-6 | ↑Pro-inflammatory cytokines (TNF-alpha, IL-1, IL-17) ↑Dermal and epidermal cell growth and differentiation ↑T cell migration into the epidermis | ↑Pro-inflammatory cytokine production | ↑Insulin-resistance ↓Hepatic cytokine signaling-3 |
| Leptin | ↑Keratinocyte proliferation ↑Promotes Th1 responses ↑Angiogenesis | ↑Vascular smooth muscle cell migration and proliferation ↑Synthesis of TNF-alpha | ↑Activation of JAK-2/IRS-2/PI3-K/Akt pathways ↑Leptin resistance ↑Hepatic fibrogenesis |
| Adiponectin | ↑Anti-inflammatory cytokine production (Reduced levels in PsO) | ↑Endothelial NO production ↑Endothelial dysfunction (Reduced levels in CVD) | ↑Insulin sensitivity (Reduced levels in NAFLD) |
| Resistin | ↑Pro-inflammatory cytokine production | ↑Arterial inflammation ↑Vascular smooth muscle cell proliferation ↑Endothelial dysfunction | ↑Insulin resistance (controversial data on NAFLD) |
| Visfatin | ↑ | ↑ | ↑Protection against liver injury (not altered in the early stage) |
| IL-17 | ↑Pro-inflammatory cytokine production ↑Expression of vascular endothelial cell adhesion molecules | ↑Atherosclerotic plaque vulnerability | ↑Hepatic steatosis ↑Synthesis of pro-inflammatory cytokines |
| VEGF | ↑Keratinocyte proliferation ↑Angiogenesis | ↑ | ↑Microvascular changes implicated in the hepatic disease (fibrosis to cirrhosis) |
Among Th1 pro-inflammatory cytokines, tumor necrosis factor (TNF)-α is considered one of the most representative cytokines in psoriasis; elevated serum levels of TNF-α have been detected in psoriatics with a positive correlation with disease severity[77] (Table 1).
In psoriasis, TNF-α promotes keratinocyte proliferation, pro-inflammatory cytokine production, expression of vascular endothelial cell adhesion molecules and angiogenesis[78].
Although the role of TNF-α in the pathogenesis of atherosclerosis remains not completely elucidated, it seems to increase the LDL transcytosis across endothelial cells and to facilitate LDL retention in the vascular wall[79] (Table 1).
Furthermore, TNF-α interferes with insulin metabolism, thus reducing the auto-phosphorylation of tyrosine residues of insulin receptor and phosphorylation of insulin receptor substrate 1 (IRS-1) and contributing to the first hit of NAFLD[80] (Table 1).
Interleukin (IL)-1 is another important pro-inflammatory cytokine exerting both autocrine and paracrine effects on keratinocytes, lymphocytes and vascular endothelium. In particular, it stimulates the synthesis of inflammatory cardiovascular mediators such as IL-6, fibrinogen, C-reactive protein, and increases the expression of adhesion molecules ICAM and VCAM-1 by dermal endothelial cells, leading to the skin recruitment of immune cells[81] (Table 1).
Human atherosclerotic plaques show elevated levels of IL-1β mRNA. This element could suggest that the synthesis of growth factors and other cytokines leading to local inflammatory cascades may be activated by locally synthesized IL-1 protein[82] (Table 1).
IL-1 also participates in pancreatic β-cell activity by stimulating mitogen-activated protein kinases (MAPK) and extracellular signal-regulated kinase (ERK), by affecting the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and by activating the inducible nitric oxide synthase (iNOS)[83].
IL-6 is an inflammatory cytokine, which amplifies inflammatory responses by synergizing with other pro-inflammatory cytokines, such as TNF-α, IL-1 and IL-17. IL-6 is responsible for dermal and epidermal cell growth and differentiation and for T cell migration into the epidermis[84] (Table 1).
Although the role of IL-6 is contradictory in NAFLD, recent data have evidenced that it may suppress hepatic cytokine signaling-3 leading to insulin resistance[85] (Table 1).
In addition to the above cytokines, adipose tissue produces leptin, adiponectin, resistin and visfatin, which are impaired in psoriasis and NAFLD and contribute to the ED[86].
ED is considered an early manifestation of vascular alterations which precede the development of hypertension and atherosclerosis in obese people[86].
In psoriasis, increased serum levels of leptin, resistin and visfatin and reduced serum levels of adiponectin have been detected[7].
Leptin is a pro-inflammatory adipocytokine which interacts with its specific receptor on endothelial cells, leading to the activation of JAK-2/IRS-2/PI3-K/Akt pathways and nuclear translocation of STAT (signal transducer and activator of transcription) proteins[87].
Moreover, leptin is considered a pro-atherogenic factor by promoting vascular smooth muscle cell migration and proliferation and by stimulating the synthesis of TNF-α with the consequent amplification of inflammatory TNF-α related pathways. Recently, hyperleptinemia has been found as a possible risk factor for acute myocardial infarction[88,89] (Table 1).
Resistin seems to support atherosclerosis by favoring ED, vascular smooth muscle cell proliferation, arterial inflammation and foam cell formation. Serum levels of resistin were higher in patients with acute myocardial infarction compared to patients with stable angina. As another pro-inflammatory adipocytokine, resistin may be involved in the pathogenesis of MetS in psoriatic patients, despite its role in NAFLD remains uncertain[90,91] (Table 1).
Adiponectin is an anti-inflammatory adipocytokine that increases nitric oxide production in endothelial cells by the activation of phosphotidylinositol-3 (PI-3) kinase/Akt signalling pathway. Serum level of adiponectin appears reduced both in psoriasis and NAFLD and it may be associated to the decreased endothelial production of NO, which is in turn considered a marker of ED[87,91] (Table 1).
Visfatin is pro-inflammatory adipocytokine contributing to insulin resistance and to atherosclerotic plaque destabilization. Serum levels of visfatin had been found higher in patients with ischemic cerebrovascular disease and myocardial infarction[92,93] (Table 1).
Th17 is implied in the pathogenesis of psoriasis and of other immune-mediated inflammatory diseases by modulating immune cell trafficking and initiating inflammation and cytokine production[94].
Th17 had been found overexpressed both in psoriatics’ serum and plaque with a positive correlation with disease severity; IL-17A levels were significantly higher in moderate to severe psoriasis than in mild psoriasis[95,96] (Table 1).
Although the precise role of Th17 in atherosclerosis remains controversial, recent data have hypothesized a putative role in the atherosclerotic plaque vulnerability, which represents the initial step of plaque rupture leading to vessel occlusion, myocardial infarction and stroke. In fact, increased expression of IL-17A has been observed in human carotid artery plaques of symptomatic patients with stroke or transient ischemic attack[97] (Table 1).
In mice, Th17 and IL-17 may be implicated in the progression from steatosis to steatohepatitis[98] (Table 1).
Angiogenesis is a physio-pathologic process characterized by the new blood vessel formation from the pre-existing vasculature and appears important in inflammatory, autoimmune and neoplastic diseases. Therefore, angiogenesis may represent a further link between psoriasis and psoriasis-related comorbidities[99].
Vascular endothelial growth factor (VEGF) is the pivotal angiogenic factor participating in the regulation of metabolism, gene expression, cell proliferation, migration, and survival[100] (Table 1).
VEGF participates in the pathogenesis of psoriasis either in an autocrine manner by directly stimulating keratinocyte proliferation and in a paracrine manner by inducing angiogenesis and by providing the fundamental elements to support epidermal proliferation. VEGF is upregulated in serum and lesional psoriatic skin with a correlation with disease severity[101] (Table 1).
Coulon et al[102] tested the TNF-α, IL-6 and VEGF serum concentrations in an obese population with NAFLD and found higher levels than those of controls, thus indicating the role of pro-inflammatory and pro-angiogenic factors in this pathology.
This aspect appears relevant; in fact, angiogenesis participates in the microvascular changes which are implicated in the hepatic disease progression from fibrosis to cirrhosis[103].
A further mechanism shared by psoriasis, NAFLD and CVD may be oxidative stress. Oxidative stress results from disequilibrium between the reduced antioxidant systems and abnormal excessive production of reactive oxygen species (ROS) or reactive nitrogen species. ROS are produced mainly by mitochondria and their production is regulated by the redox state of the respiratory chain[104,105].
The pathogenesis and progression of psoriasis are strictly linked to the redox sensitive cellular signaling pathways, such as mitogen-activated protein kinase/activator protein 1 (MAPK/AP1), NFκB, and Janus kinase-signal transducers (JAK) and transcription activators[106].
Many studies have been conducted to investigate the role of oxidative stress in psoriasis and have evidenced that psoriatics show an imbalance between biomarkers of oxidative stress and the antioxidant system. Ferretti et al[107] have shown an impairment of oxidant/antioxidant system; significantly higher serum levels of lipoprotein a [Lp(a)] and lipid hydroperoxides have been found in psoriatics compared to controls. Conversely, paraoxonase-1 (PON1), an anti-inflammatory and anti-oxidant enzyme, was lower than in healthy subjects. A positive correlation was found between serum levels of Lp(a), markers of lipid peroxidation and the severity of the disease, whereas PON1 activity and Lp(a) were negatively correlated[107,108].
Emre et al[109] have investigated the relation between oxidative status and smoking in psoriasis, demonstrating the increased serum levels of triglycerides and reduced levels of HDL cholesterol and arylesterase activity in smoker compared to non-smoker psoriatic patients. Therefore, smoking could be considered a risk factor for psoriasis severity by increasing oxidative stress and thus predisposing psoriatic patients to a higher risk of cardiovascular comorbidities.
A reduction in total antioxidant capacity and in antioxidant vitamins A and E has been found by Rocha-Pereira et al[110], who had also confirmed a pro-atherogenic lipid profile in psoriatic patients with an increase of cholesterol, triglycerides, low density lipoprotein cholesterol (LDL), very low density lipoprotein cholesterol (VLDL), apolipoprotein B (apo B), Lp(a) and lipoperoxidation products. These data tend to underline an increased cardiovascular risk in psoriatic patients, particularly in those with severe disease.
It is known that oxidative stress participates in the second hit of the pathogenesis of NAFLD and it may be implicated in the NAFLD progression by interfering with normal cell division. In murine models, alterations of the polyploidization process were found in fatty liver with a large proportion of highly polyploid mononuclear cells, which were only rarely observed in normal hepatic parenchyma. Moreover, in humans, alterations in hepatocyte ploidy have been documented in liver biopsies from patients with NASH[111].
Oxidative stress participates in the mild chronic vascular inflammation in CVD. In fact, oxygen metabolites are able to interfere with LDL metabolism and promote the formation of oxidized low-density lipoprotein (Ox-LDL), which plays a representative role in atherosclerotic plaque development and in endothelial damage favoring inflammatory vascular cell infiltration[112,113] (Figure 1).
As seen above, the inflammatory process represents the mainstay linking the pathogenesis of psoriasis, NAFLD and CVD. Therefore, anti-inflammatory drugs may represent important therapeutic options in the treatment and prevention of these pathologies. Data in the literature on the effect of both conventional and biological psoriatic therapies have shown discordant results on their possible action on NAFLD and cardiovascular risk factors[114].
Conventional treatments for moderate to severe psoriasis include cyclosporine A, methotrexate and retinoids. Although effective, their safety profile should be evaluated in their long-term use, with psoriasis-related comorbidities considered[115].
In fact, it is well known that methotrexate can mediate liver toxicity and patients with liver dysfunction, such as NAFLD/NASH patients, could present impaired drug metabolism with consequent liver accumulation and increased susceptibility to liver toxicity[116].
Methotrexate exerts opposite effects on cardiovascular risk in psoriatic patients. In 1989, Refsum et al[117] investigated the effect of methotrexate 25 mg weekly on plasma homocysteine levels and found a significant and transient increase within 48 h after administration.
Conversely, a lower risk of CVD has been found in psoriatic patient treated with MTX compared to patients without MTX[118].
Elevated serum levels of cholesterol and tryglicerides can occur during treatment with retinoids and cyclosporine, although no evidence of an increased cardiovascular risk has been stated with long-term use of etetrinate[118,119].
Moreover, as demonstrated in a prospective non-randomized study on patients affected by PsA, cyclosporine has been associated with a significant elevation of blood pressure values[119].
TNF-α inhibitors, IL12/23 inhibitors and IL-17 inhibitors represent three new classes of drugs used in moderate to severe psoriasis with a good efficacy and safety profile. Among biologics, metabolic effects of TNF-α blockers are most widely studied[120,121].
A cross-sectional study evaluated epicardial fat thickness (EAT), an emerging marker of cardiometabolic risk, in patients with rheumatoid arthritis (RA) treated with TNF-α inhibitors compared to RA patients treated with non-biological disease-modifying anti-rheumatic drugs (DMARDs). A significantly lower EAT thickness was detected in patients treated with TNF-α inhibitors than in those treated with DMARDs (8.56 ± 1.90 mm and 9.71 ± 1.45 mm, respectively)[122].
Jókai et al[123] evaluated the positive effect of TNF-α inhibitors on carotid and brachial intima-media thickness in patients with psoriasis.
Although data are few, TNF-α blockers seem to act on lipid and glucose metabolism by exerting a potential action on cardiovascular risk factors. An improvement of insulin-sensitivity in psoriatic patients treated with Etanercept and Infliximab has been evidenced[124,125].
Conversely, long-term use of TNF-α inhibitors in patients with rheumatoid arthritis seem not to influence insulin resistance parameters[126].
With regard to the lipid profile, although no statistically significant difference, raised values of total cholesterol, LDL-C and triglycerides were found after 24 wk of treatment with Etanercept in psoriatic patients[127].
Adipocytokine levels and fat distribution have been assessed in patients with RA and ankylosing spondilitis during long-term treatment with TNF-α blockers. A fat mass gain with a tendency to visceral fat accumulation, a reduction of resistin serum levels and no significant modification in leptin, total adiponectin or visfatin serum levels have been evidenced[128]. Another recent study focused on the influence of TNF-α inhibitors on serum levels of adipocytokines, showing a partial rebalancing between pro- and anti-inflammatory adipocytokines after 24 wk of anti-TNF-α treatment with a reduction of leptin, visfatin and resistin and a mild adiponectin increase[76].
A body weight increment has been identified after 6-mo treatment with Etanercept compared to psoriatic patients treated with methotrexate[129].
These data have been confirmed by Campanati et al[24] who showed an increase in waist-hip-ratio and BMI during treatment with Etanercept. The authors documented a possible preventive effect of Etanercept on liver fibrosis, evidencing a significant reduction of AST/ALT ratio and an improvement of insulin-sensitivity parameters. These elements confirm the strong relation between the alteration of glucose metabolism and NAFLD[24].
Although further larger studies are needed to confirm these data, this hypothetic preventive role may be linked to anti-inflammatory properties of TNF-α inhibitors and their action on glucose homeostasis[24].
The favorable effect of TNF-α blockers on the risk of MI has been identified in a retrospective study monitoring patients affected by only psoriasis, by only PsA and by both psoriasis and PsA. Patients with only psoriasis had a significant MI risk reduction (HR = 0.26; 95%CI: 0.12-0.56), whereas a non-significant MI risk reduction was detected in those with only PsA (HR = 0.86; 95%CI: 0.28-2.70) and in those with both psoriasis and PsA. The duration of TNF-α inhibitor treatment did not seem to influence the risk of MI[130,131].
Psoriasis is a complex and already partially unknown disease whose skin manifestations represent only the edge of an iceberg, which is widely submerged and unknown. Psoriasis and psoriasis-related comorbidities significantly impact on patient’s health and quality of life and negatively interfere in physical-psychic well-being with important repercussion in working daily life. As a multi-organ pathology, psoriasis needs a multidisciplinary approach and clinicians should evaluate this holistic vision in order to promptly identify and manage psoriasis-related comorbidities influencing patients’ morbidity and mortality. The underlying inflammatory process is the leitmotiv shared by psoriasis, NAFLD and CVD and overlaps both the common genetic predisposition and modifiable risk factors, such as sedentary lifestyle, smoking and alcohol consumption.
Therefore, the therapeutic strategy for psoriasis should be multifaceted and should specifically tailor outcome tools and disease-related items by a patient-based evaluation and by selectively verifying the risk/benefit of each single therapeutic option.
P- Reviewer: Castillo RL S- Editor: Ji FF L- Editor: Wang TQ E- Editor: Lu YJ
| 1. | Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet. 2007;370:263-271. [PubMed] |
| 2. | Boehncke WH, Boehncke S. More than skin-deep: the many dimensions of the psoriatic disease. Swiss Med Wkly. 2014;144:w13968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Campanati A, Orciani M, Gorbi S, Regoli F, Di Primio R, Offidani A. Effect of biologic therapies targeting tumour necrosis factor-α on cutaneous mesenchymal stem cells in psoriasis. Br J Dermatol. 2012;167:68-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 4. | Ryan C, Kirby B. Psoriasis is a systemic disease with multiple cardiovascular and metabolic comorbidities. Dermatol Clin. 2015;33:41-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 120] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 5. | Ni C, Chiu MW. Psoriasis and comorbidities: links and risks. Clin Cosmet Investig Dermatol. 2014;7:119-132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 6. | Sales R, Torres T. Psoriasis and metabolic syndrome. Acta Dermatovenerol Croat. 2014;22:169-174. [PubMed] |
| 7. | Ganzetti G, Campanati A, Offidani A. Non-alcoholic fatty liver disease and psoriasis: So far, so near. World J Hepatol. 2015;7:315-326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (1)] |
| 8. | Zhang T, Zhang C, Zhang Y, Tang F, Li H, Zhang Q, Lin H, Wu S, Liu Y, Xue F. Metabolic syndrome and its components as predictors of nonalcoholic fatty liver disease in a northern urban Han Chinese population: a prospective cohort study. Atherosclerosis. 2015;240:144-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 9. | Kotronen A, Yki-Järvinen H. Fatty liver: a novel component of the metabolic syndrome. Arterioscler Thromb Vasc Biol. 2008;28:27-38. [PubMed] |
| 10. | Ozturk K, Uygun A, Guler AK, Demirci H, Ozdemir C, Cakir M, Sakin YS, Turker T, Sari S, Demirbas S. Nonalcoholic fatty liver disease is an independent risk factor for atherosclerosis in young adult men. Atherosclerosis. 2015;240:380-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 11. | Chung GE, Choi SY, Kim D, Kwak MS, Park HE, Kim MK, Yim JY. Nonalcoholic fatty liver disease as a risk factor of arterial stiffness measured by the cardioankle vascular index. Medicine (Baltimore). 2015;94:e654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Gisondi P, Tessari G, Conti A, Piaserico S, Schianchi S, Peserico A, Giannetti A, Girolomoni G. Prevalence of metabolic syndrome in patients with psoriasis: a hospital-based case-control study. Br J Dermatol. 2007;157:68-73. [PubMed] |
| 13. | Armstrong AW, Harskamp CT, Armstrong EJ. Psoriasis and metabolic syndrome: a systematic review and meta-analysis of observational studies. J Am Acad Dermatol. 2013;68:654-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 285] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 14. | Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356-359. [PubMed] |
| 15. | Hu G, Qiao Q, Tuomilehto J, Balkau B, Borch-Johnsen K, Pyorala K. Prevalence of the metabolic syndrome and its relation to all-cause and cardiovascular mortality in nondiabetic European men and women. Arch Intern Med. 2004;164:1066-1076. [PubMed] |
| 16. | Zindancı I, Albayrak O, Kavala M, Kocaturk E, Can B, Sudogan S, Koç M. Prevalence of metabolic syndrome in patients with psoriasis. ScientificWorldJournal. 2012;2012:312463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 17. | Mallbris L, Ritchlin CT, Ståhle M. Metabolic disorders in patients with psoriasis and psoriatic arthritis. Curr Rheumatol Rep. 2006;8:355-363. [PubMed] |
| 18. | Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB, Gelfand JM. Prevalence of cardiovascular risk factors in patients with psoriasis. J Am Acad Dermatol. 2006;55:829-835. [PubMed] |
| 19. | Sommer DM, Jenisch S, Suchan M, Christophers E, Weichenthal M. Increased prevalence of the metabolic syndrome in patients with moderate to severe psoriasis. Arch Dermatol Res. 2006;298:321-328. [PubMed] |
| 20. | Adams LA, Angulo P, Lindor KD. Nonalcoholic fatty liver disease. CMAJ. 2005;172:899-905. [PubMed] |
| 21. | Roberts KK, Cochet AE, Lamb PB, Brown PJ, Battafarano DF, Brunt EM, Harrison SA. The prevalence of NAFLD and NASH among patients with psoriasis in a tertiary care dermatology and rheumatology clinic. Aliment Pharmacol Ther. 2015;41:293-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 22. | van der Voort EA, Koehler EM, Dowlatshahi EA, Hofman A, Stricker BH, Janssen HL, Schouten JN, Nijsten T. Psoriasis is independently associated with nonalcoholic fatty liver disease in patients 55 years old or older: Results from a population-based study. J Am Acad Dermatol. 2014;70:517-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 113] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 23. | Candia R, Ruiz A, Torres-Robles R, Chávez-Tapia N, Méndez-Sánchez N, Arrese M. Risk of non-alcoholic fatty liver disease in patients with psoriasis: a systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2015;29:656-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 110] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 24. | Campanati A, Ganzetti G, Di Sario A, Damiani A, Sandroni L, Rosa L, Benedetti A, Offidani A. The effect of etanercept on hepatic fibrosis risk in patients with non-alcoholic fatty liver disease, metabolic syndrome, and psoriasis. J Gastroenterol. 2013;48:839-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (1)] |
| 25. | Lee YA, Kang SG, Song SW, Rho JS, Kim EK. Association between metabolic syndrome, smoking status and coronary artery calcification. PLoS One. 2015;10:e0122430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Cohen JB, Cohen DL. Cardiovascular and renal effects of weight reduction in obesity and the metabolic syndrome. Curr Hypertens Rep. 2015;17:34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | van Vliet-Ostaptchouk JV, Nuotio ML, Slagter SN, Doiron D, Fischer K, Foco L, Gaye A, Gögele M, Heier M, Hiekkalinna T. The prevalence of metabolic syndrome and metabolically healthy obesity in Europe: a collaborative analysis of ten large cohort studies. BMC Endocr Disord. 2014;14:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 353] [Cited by in RCA: 399] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 28. | Gutmark-Little I, Shah KN. Obesity and the metabolic syndrome in pediatric psoriasis. Clin Dermatol. 2015;33:305-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 29. | Danielsen K, Wilsgaard T, Olsen AO, Eggen AE, Olsen K, Cassano PA, Furberg AS. Elevated odds of metabolic syndrome in psoriasis: a population-based study of age and sex differences. Br J Dermatol. 2015;172:419-427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 30. | Carrascosa JM, Rocamora V, Fernandez-Torres RM, Jimenez-Puya R, Moreno JC, Coll-Puigserver N, Fonseca E. Obesity and psoriasis: inflammatory nature of obesity, relationship between psoriasis and obesity, and therapeutic implications. Actas Dermosifiliogr. 2014;105:31-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 91] [Article Influence: 7.0] [Reference Citation Analysis (1)] |
| 31. | Ma C, Schupp CW, Armstrong EJ, Armstrong AW. Psoriasis and dyslipidemia: a population-based study analyzing the National Health and Nutrition Examination Survey (NHANES). J Eur Acad Dermatol Venereol. 2014;28:1109-1112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 32. | Offidani AM, Ferretti G, Taus M, Simonetti O, Dousset N, Valdiguie P, Curatola G, Bossi G. Lipoprotein peroxidation in adult psoriatic patients. Acta Derm Venereol Suppl (Stockh). 1994;186:38-40. [PubMed] |
| 33. | Driessen RJ, Boezeman JB, Van De Kerkhof PC, De Jong EM. Cardiovascular risk factors in high-need psoriasis patients and its implications for biological therapies. J Dermatolog Treat. 2009;20:42-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 34. | Cohen AD, Gilutz H, Henkin Y, Zahger D, Shapiro J, Bonneh DY, Vardy DA. Psoriasis and the metabolic syndrome. Acta Derm Venereol. 2007;87:506-509. [PubMed] |
| 35. | Al-Mutairi N, Al-Farag S, Al-Mutairi A, Al-Shiltawy M. Comorbidities associated with psoriasis: an experience from the Middle East. J Dermatol. 2010;37:146-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 115] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 36. | Langan SM, Seminara NM, Shin DB, Troxel AB, Kimmel SE, Mehta NN, Margolis DJ, Gelfand JM. Prevalence of metabolic syndrome in patients with psoriasis: a population-based study in the United Kingdom. J Invest Dermatol. 2012;132:556-562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 288] [Cited by in RCA: 343] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 37. | Aguilar D, Fernandez ML. Hypercholesterolemia induces adipose dysfunction in conditions of obesity and nonobesity. Adv Nutr. 2014;5:497-502. [PubMed] |
| 38. | Gelfand JM, Mehta NN, Langan SM. Psoriasis and cardiovascular risk: strength in numbers, part II. J Invest Dermatol. 2011;131:1007-1010. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 39. | Torres T, Bettencourt N. Psoriasis: the visible killer. Rev Port Cardiol. 2014;33:95-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 40. | Maradit-Kremers H, Icen M, Ernste FC, Dierkhising RA, McEvoy MT. Disease severity and therapy as predictors of cardiovascular risk in psoriasis: a population-based cohort study. J Eur Acad Dermatol Venereol. 2012;26:336-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 41. | Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735-1741. [PubMed] |
| 42. | Brauchli YB, Jick SS, Miret M, Meier CR. Psoriasis and risk of incident myocardial infarction, stroke or transient ischaemic attack: an inception cohort study with a nested case-control analysis. Br J Dermatol. 2009;160:1048-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 232] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 43. | Li WQ, Han JL, Manson JE, Rimm EB, Rexrode KM, Curhan GC, Qureshi AA. Psoriasis and risk of nonfatal cardiovascular disease in U.S. women: a cohort study. Br J Dermatol. 2012;166:811-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 44. | Ernste FC, Sánchez-Menéndez M, Wilton KM, Crowson CS, Matteson EL, Maradit Kremers H. Cardiovascular risk profile at the onset of psoriatic arthritis: a population-based cohort study. Arthritis Care Res (Hoboken). 2015;67:1015-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 45. | Favarato MH, Mease P, Gonçalves CR, Gonçalves Saad C, Sampaio-Barros PD, Goldenstein-Schainberg C. Hypertension and diabetes significantly enhance the risk of cardiovascular disease in patients with psoriatic arthritis. Clin Exp Rheumatol. 2014;32:182-187. [PubMed] |
| 46. | Yiu KH, Yeung CK, Zhao CT, Chan JC, Siu CW, Tam S, Wong CS, Yan GH, Yue WS, Khong PL. Prevalence and extent of subclinical atherosclerosis in patients with psoriasis. J Intern Med. 2013;273:273-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 47. | Balci DD, Balci A, Karazincir S, Ucar E, Iyigun U, Yalcin F, Seyfeli E, Inandi T, Egilmez E. Increased carotid artery intima-media thickness and impaired endothelial function in psoriasis. J Eur Acad Dermatol Venereol. 2009;23:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 146] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 48. | Mintz GS. Intravascular imaging of coronary calcification and its clinical implications. JACC Cardiovasc Imaging. 2015;8:461-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 157] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 49. | Seremet S, Genc B, Tastan A, Akyildiz ZI, Nazli C, Ozcelik S, Afsar FS, Solak A, Emren V. Are all patients with psoriasis at increased risk for coronary artery disease? Int J Dermatol. 2015;54:355-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 50. | Staniak HL, Bittencourt MS, de Souza Santos I, Sharovsky R, Sabbag C, Goulart AC, Lotufo PA, Benseñor IM. Association between psoriasis and coronary calcium score. Atherosclerosis. 2014;237:847-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 51. | Mahiques-Santos L, Soriano-Navarro CJ, Perez-Pastor G, Tomas-Cabedo G, Pitarch-Bort G, Valcuende-Cavero F. Psoriasis and ischemic coronary artery disease. Actas Dermosifiliogr. 2015;106:112-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 52. | Gullu H, Caliskan M, Dursun R, Ciftci O, Guven A, Muderrisoglu H. Impaired coronary microvascular function and its association with disease duration and inflammation in patients with psoriasis. Echocardiography. 2013;30:912-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 53. | Ikonomidis I, Makavos G, Papadavid E, Varoudi M, Andreadou I, Gravanis K, Theodoropoulos K, Pavlidis G, Triantafyllidi H, Parissis J. Similarities in coronary function and myocardial deformation between psoriasis and coronary artery disease: the role of oxidative stress and inflammation. Can J Cardiol. 2015;31:287-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 54. | Lee SJ, Lee DG, Lim DS, Hong S, Park JS. Difference in the prognostic significance of N-terminal pro-B-type natriuretic peptide between cardioembolic and noncardioembolic ischemic strokes. Dis Markers. 2015;2015:597570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 55. | Pietrzak A, Bartosinska J, Blaszczyk R, Chodorowska G, Brzozowski W, Hercogova J, Donica H, Lotti T. Increased serum level of N-terminal Pro-B-type natriuretic peptide as a possible biomarker of cardiovascular risk in psoriatic patients. J Eur Acad Dermatol Venereol. 2015;29:1010-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 56. | Biyik I, Narin A, Bozok MA, Ergene O. Echocardiographic and clinical abnormalities in patients with psoriasis. J Int Med Res. 2006;34:632-639. [PubMed] |
| 57. | Bilgiç Ö, Altınyazar HC, Baran H, Ünlü A. Serum homocysteine, asymmetric dimethyl arginine (ADMA) and other arginine-NO pathway metabolite levels in patients with psoriasis. Arch Dermatol Res. 2015;307:439-444. [PubMed] |
| 58. | Giannoni M, Consales V, Campanati A, Ganzetti G, Giuliodori K, Postacchini V, Liberati G, Azzaretto L, Vichi S, Guanciarossa F. Homocysteine plasma levels in psoriasis patients: our experience and review of the literature. J Eur Acad Dermatol Venereol. 2015;29:1781-1785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 59. | Erturan I, Köroğlu BK, Adiloğlu A, Ceyhan AM, Akkaya VB, Tamer N, Başak PY, Korkmaz S, Ersoy IH, Kilinç O. Evaluation of serum sCD40L and homocysteine levels with subclinical atherosclerosis indicators in patients with psoriasis: a pilot study. Int J Dermatol. 2014;53:503-509. [PubMed] |
| 60. | Erfan G, Guzel S, Alpsoy S, Rifaioglu EN, Kaya S, Kucukyalcın V, Topcu B, Kulac M. Serum YKL-40: a potential biomarker for psoriasis or endothelial dysfunction in psoriasis? Mol Cell Biochem. 2015;400:207-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 61. | Łata E, Gisterek I, Matkowski R, Szelachowska J, Kornafel J. [The importance of determining the prognostic marker YKL-40 in serum and tissues]. Pol Merkur Lekarski. 2010;28:505-508. [PubMed] |
| 62. | Ahmed SF, Attia EA, Saad AA, Sharara M, Fawzy H, El Nahrery EM. Serum YKL-40 in psoriasis with and without arthritis; correlation with disease activity and high-resolution power Doppler ultrasonographic joint findings. J Eur Acad Dermatol Venereol. 2015;29:682-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 63. | Armstrong AW, Harskamp CT, Armstrong EJ. The association between psoriasis and hypertension: a systematic review and meta-analysis of observational studies. J Hypertens. 2013;31:433-442; discussion 442-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 163] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 64. | Zhang Z, Li G, Liu T. Psoriasis and risk of atrial fibrillation. Int J Cardiol. 2015;185:301-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 65. | Ahlehoff O, Gislason GH, Jørgensen CH, Lindhardsen J, Charlot M, Olesen JB, Abildstrøm SZ, Skov L, Torp-Pedersen C, Hansen PR. Psoriasis and risk of atrial fibrillation and ischaemic stroke: a Danish Nationwide Cohort Study. Eur Heart J. 2012;33:2054-2064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 171] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 66. | Armstrong AW, Azizi S, Wu J, Harskamp CT, Farrow J, Johnson MA, Klem K, Anderson D, Armstrong EJ. Psoriasis, electrocardiographic characteristics, and incidence of atrial fibrillation. Arch Dermatol Res. 2013;305:891-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 67. | Ganzetti G, Campanati A, Santarelli A, Pozzi V, Molinelli E, Minnetti I, Brisigotti V, Procaccini M, Emanuelli M, Offidani A. Involvement of the oral cavity in psoriasis: results of a clinical study. Br J Dermatol. 2015;172:282-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 68. | Kouris A, Pistiki A, Katoulis A, Georgitsi M, Giatrakou S, Papadavid E, Netea MG, Stavrianeas N, Giamarellos-Bourboulis EJ. Proinflammatory cytokine responses in patients with psoriasis. Eur Cytokine Netw. 2014;25:63-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 69. | Boehncke S, Boehncke WH. ‘Upgrading’ psoriasis responsibly. Exp Dermatol. 2014;23:710-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 70. | Mrowietz U, Steinz K, Gerdes S. Psoriasis: to treat or to manage? Exp Dermatol. 2014;23:705-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 71. | Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685-1695. [PubMed] |
| 72. | Nilsson J, Hansson GK, Shah PK. Immunomodulation of atherosclerosis: implications for vaccine development. Arterioscler Thromb Vasc Biol. 2005;25:18-28. [PubMed] |
| 73. | Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006;6:508-519. [PubMed] |
| 74. | Flammer AJ, Ruschitzka F. Psoriasis and atherosclerosis: two plaques, one syndrome? Eur Heart J. 2012;33:1989-1991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 75. | Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548-2556. [PubMed] |
| 76. | Campanati A, Ganzetti G, Giuliodori K, Marra M, Bonfigli A, Testa R, Offidani A. Serum levels of adipocytokines in psoriasis patients receiving tumor necrosis factor-α inhibitors: results of a retrospective analysis. Int J Dermatol. 2015;54:839-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 77. | Pietrzak AT, Zalewska A, Chodorowska G, Krasowska D, Michalak-Stoma A, Nockowski P, Osemlak P, Paszkowski T, Roliński JM. Cytokines and anticytokines in psoriasis. Clin Chim Acta. 2008;394:7-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 112] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 78. | Hui E, Xu A, Bo Yang H, Lam KS. Obesity as the common soil of non-alcoholic fatty liver disease and diabetes: Role of adipokines. J Diabetes Investig. 2013;4:413-425. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 79. | Zhang Y, Yang X, Bian F, Wu P, Xing S, Xu G, Li W, Chi J, Ouyang C, Zheng T. TNF-α promotes early atherosclerosis by increasing transcytosis of LDL across endothelial cells: crosstalk between NF-κB and PPAR-γ. J Mol Cell Cardiol. 2014;72:85-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 148] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 80. | Gottlieb AB, Dann F, Menter A. Psoriasis and the metabolic syndrome. J Drugs Dermatol. 2008;7:563-572. [PubMed] |
| 81. | Foster AM, Baliwag J, Chen CS, Guzman AM, Stoll SW, Gudjonsson JE, Ward NL, Johnston A. IL-36 promotes myeloid cell infiltration, activation, and inflammatory activity in skin. J Immunol. 2014;192:6053-6061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 241] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 82. | Matsuura E, Atzeni F, Sarzi-Puttini P, Turiel M, Lopez LR, Nurmohamed MT. Is atherosclerosis an autoimmune disease? BMC Med. 2014;12:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 117] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 83. | Cieślak M, Wojtczak A, Cieślak M. Role of pro-inflammatory cytokines of pancreatic islets and prospects of elaboration of new methods for the diabetes treatment. Acta Biochim Pol. 2015;62:15-21. [PubMed] |
| 84. | Wang Y, Chen J, Zhao Y, Geng L, Song F, Chen HD. Psoriasis is associated with increased levels of serum leptin. Br J Dermatol. 2008;158:1134-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 85. | Pearce SG, Thosani NC, Pan JJ. Noninvasive biomarkers for the diagnosis of steatohepatitis and advanced fibrosis in NAFLD. Biomark Res. 2013;1:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 86. | DeMarco VG, Aroor AR, Sowers JR. The pathophysiology of hypertension in patients with obesity. Nat Rev Endocrinol. 2014;10:364-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 347] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 87. | Adya R, Tan BK, Randeva HS. Differential effects of leptin and adiponectin in endothelial angiogenesis. J Diabetes Res. 2015;2015:648239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 80] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 88. | Nalini D, Karthick R, Shirin V, Manohar G, Malathi R. “Role of the adipocyte hormone leptin in cardiovascular diseases - a study from Chennai based Population”. Thromb J. 2015;13:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 89. | Chiu CZ, Wang BW, Shyu KG. Molecular regulation of the expression of leptin by hypoxia in human coronary artery smooth muscle cells. J Biomed Sci. 2015;22:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 90. | Muse ED, Feldman DI, Blaha MJ, Dardari ZA, Blumenthal RS, Budoff MJ, Nasir K, Criqui MH, Cushman M, McClelland RL. The association of resistin with cardiovascular disease in the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2015;239:101-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 91. | Ronti T, Lupattelli G, Mannarino E. The endocrine function of adipose tissue: an update. Clin Endocrinol (Oxf). 2006;64:355-365. [PubMed] |
| 92. | Kong Q, Xia M, Liang R, Li L, Cu X, Sun Z, Hu J. Increased serum visfatin as a risk factor for atherosclerosis in patients with ischaemic cerebrovascular disease. Singapore Med J. 2014;55:383-387. [PubMed] |
| 93. | Mazaherioun M, Hosseinzadeh-Attar MJ, Janani L, Vasheghani Farahani A, Rezvan N, Karbaschian Z, Hossein-Nezhad A. Elevated serum visfatin levels in patients with acute myocardial infarction. Arch Iran Med. 2012;15:688-692. [PubMed] |
| 94. | Golden JB, McCormick TS, Ward NL. IL-17 in psoriasis: implications for therapy and cardiovascular co-morbidities. Cytokine. 2013;62:195-201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 95. | Zhang L, Yang XQ, Cheng J, Hui RS, Gao TW. Increased Th17 cells are accompanied by FoxP3(+) Treg cell accumulation and correlated with psoriasis disease severity. Clin Immunol. 2010;135:108-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 166] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 96. | Kagami S, Rizzo HL, Lee JJ, Koguchi Y, Blauvelt A. Circulating Th17, Th22, and Th1 cells are increased in psoriasis. J Invest Dermatol. 2010;130:1373-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 481] [Cited by in RCA: 482] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 97. | Erbel C, Akhavanpoor M, Okuyucu D, Wangler S, Dietz A, Zhao L, Stellos K, Little KM, Lasitschka F, Doesch A. IL-17A influences essential functions of the monocyte/macrophage lineage and is involved in advanced murine and human atherosclerosis. J Immunol. 2014;193:4344-4355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 123] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 98. | Tang Y, Bian Z, Zhao L, Liu Y, Liang S, Wang Q, Han X, Peng Y, Chen X, Shen L. Interleukin-17 exacerbates hepatic steatosis and inflammation in non-alcoholic fatty liver disease. Clin Exp Immunol. 2011;166:281-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 241] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 99. | Campanati A, Goteri G, Simonetti O, Ganzetti G, Giuliodori K, Giuliano A, Sabato S, Stramazzotti D, Gulini E, Dusi D. Angiogenesis in psoriatic skin and its modifications after administration of etanercept: videocapillaroscopic, histological and immunohistochemical evaluation. Int J Immunopathol Pharmacol. 2009;22:371-377. [PubMed] |
| 100. | Smith GA, Fearnley GW, Harrison MA, Tomlinson DC, Wheatcroft SB, Ponnambalam S. Vascular endothelial growth factors: multitasking functionality in metabolism, health and disease. J Inherit Metab Dis. 2015;38:753-763. [PubMed] |
| 101. | Li W, Man XY, Chen JQ, Zhou J, Cai SQ, Zheng M. Targeting VEGF/VEGFR in the treatment of psoriasis. Discov Med. 2014;18:97-104. [PubMed] |
| 102. | Coulon S, Francque S, Colle I, Verrijken A, Blomme B, Heindryckx F, De Munter S, Prawitt J, Caron S, Staels B. Evaluation of inflammatory and angiogenic factors in patients with non-alcoholic fatty liver disease. Cytokine. 2012;59:442-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 103. | Coulon S, Heindryckx F, Geerts A, Van Steenkiste C, Colle I, Van Vlierberghe H. Angiogenesis in chronic liver disease and its complications. Liver Int. 2011;31:146-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 221] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 104. | Skulachev VP. Role of uncoupled and non-coupled oxidations in maintenance of safely low levels of oxygen and its one-electron reductants. Q Rev Biophys. 1996;29:169-202. [PubMed] |
| 105. | Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1-13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6484] [Cited by in RCA: 6062] [Article Influence: 378.9] [Reference Citation Analysis (0)] |
| 106. | Zhou Q, Mrowietz U, Rostami-Yazdi M. Oxidative stress in the pathogenesis of psoriasis. Free Radic Biol Med. 2009;47:891-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 182] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 107. | Nemati H, Khodarahmi R, Sadeghi M, Ebrahimi A, Rezaei M, Vaisi-Raygani A. Antioxidant status in patients with psoriasis. Cell Biochem Funct. 2014;32:268-273. [PubMed] |
| 108. | Ferretti G, Bacchetti T, Campanati A, Simonetti O, Liberati G, Offidani A. Correlation between lipoprotein(a) and lipid peroxidation in psoriasis: role of the enzyme paraoxonase-1. Br J Dermatol. 2012;166:204-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 109. | Emre S, Metin A, Demirseren DD, Kilic S, Isikoglu S, Erel O. The relationship between oxidative stress, smoking and the clinical severity of psoriasis. J Eur Acad Dermatol Venereol. 2013;27:e370-e375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 110. | Rocha-Pereira P, Santos-Silva A, Rebelo I, Figueiredo A, Quintanilha A, Teixeira F. Dislipidemia and oxidative stress in mild and in severe psoriasis as a risk for cardiovascular disease. Clin Chim Acta. 2001;303:33-39. [PubMed] |
| 111. | Gentric G, Maillet V, Paradis V, Couton D, L’Hermitte A, Panasyuk G, Fromenty B, Celton-Morizur S, Desdouets C. Oxidative stress promotes pathologic polyploidization in nonalcoholic fatty liver disease. J Clin Invest. 2015;125:981-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 186] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 112. | Gradinaru D, Borsa C, Ionescu C, Prada GI. Oxidized LDL and NO synthesis-Biomarkers of endothelial dysfunction and ageing. Mech Ageing Dev. 2015;151:101-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 120] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 113. | Perrotta I, Aquila S. The role of oxidative stress and autophagy in atherosclerosis. Oxid Med Cell Longev. 2015;2015:130315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 110] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 114. | Grine L, Dejager L, Libert C, Vandenbroucke RE. An inflammatory triangle in psoriasis: TNF, type I IFNs and IL-17. Cytokine Growth Factor Rev. 2015;26:25-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 145] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 115. | Busard C, Zweegers J, Limpens J, Langendam M, Spuls PI. Combined use of systemic agents for psoriasis: a systematic review. JAMA Dermatol. 2014;150:1213-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 116. | Hardwick RN, Clarke JD, Lake AD, Canet MJ, Anumol T, Street SM, Merrell MD, Goedken MJ, Snyder SA, Cherrington NJ. Increased susceptibility to methotrexate-induced toxicity in nonalcoholic steatohepatitis. Toxicol Sci. 2014;142:45-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 117. | Refsum H, Helland S, Ueland PM. Fasting plasma homocysteine as a sensitive parameter of antifolate effect: a study of psoriasis patients receiving low-dose methotrexate treatment. Clin Pharmacol Ther. 1989;46:510-520. [PubMed] |
| 118. | Horreau C, Pouplard C, Brenaut E, Barnetche T, Misery L, Cribier B, Jullien D, Aractingi S, Aubin F, Joly P. Cardiovascular morbidity and mortality in psoriasis and psoriatic arthritis: a systematic literature review. J Eur Acad Dermatol Venereol. 2013;27 Suppl 3:12-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 220] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 119. | Mosca S, Gargiulo P, Balato N, Di Costanzo L, Parente A, Paolillo S, Ayala F, Trimarco B, Crea F, Perrone-Filardi P. Ischemic cardiovascular involvement in psoriasis: a systematic review. Int J Cardiol. 2015;178:191-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 120. | Lebwohl M. Biologics for psoriasis: a translational research success story. J Invest Dermatol. 2015;135:1205-1207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 121. | Campanati A, Giuliodori K, Ganzetti G, Liberati G, Offidani AM. A patient with psoriasis and vitiligo treated with etanercept. Am J Clin Dermatol. 2010;11 Suppl 1:46-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 122. | Lima-Martínez MM, Campo E, Salazar J, Paoli M, Maldonado I, Acosta C, Rodney M, Contreras M, Cabrera-Rego JO, Iacobellis G. Epicardial fat thickness as cardiovascular risk factor and therapeutic target in patients with rheumatoid arthritis treated with biological and nonbiological therapies. Arthritis. 2014;2014:782850. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 123. | Jókai H, Szakonyi J, Kontár O, Marschalkó M, Szalai K, Kárpáti S, Holló P. Impact of effective tumor necrosis factor-alfa inhibitor treatment on arterial intima-media thickness in psoriasis: results of a pilot study. J Am Acad Dermatol. 2013;69:523-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 124. | Puig L, Strohal R, Fuiman J, Pedersen R, Szumski A, Koenig AS, Robertson D, Drexel H. Cardiometabolic biomarkers in chronic plaque psoriasis before and after etanercept treatment. J Dermatolog Treat. 2014;25:470-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 125. | Marra M, Campanati A, Testa R, Sirolla C, Bonfigli AR, Franceschi C, Marchegiani F, Offidani A. Effect of etanercept on insulin sensitivity in nine patients with psoriasis. Int J Immunopathol Pharmacol. 2007;20:731-736. [PubMed] |
| 126. | Ferraz-Amaro I, Arce-Franco M, Muñiz J, López-Fernández J, Hernández-Hernández V, Franco A, Quevedo J, Martínez-Martín J, Díaz-González F. Systemic blockade of TNF-α does not improve insulin resistance in humans. Horm Metab Res. 2011;43:801-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 127. | Lestre S, Diamantino F, Veloso L, Fidalgo A, Ferreira A. Effects of etanercept treatment on lipid profile in patients with moderate-to-severe chronic plaque psoriasis: a retrospective cohort study. Eur J Dermatol. 2011;21:916-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 128. | Toussirot É, Mourot L, Dehecq B, Wendling D, Grandclément É, Dumoulin G. TNFα blockade for inflammatory rheumatic diseases is associated with a significant gain in android fat mass and has varying effects on adipokines: a 2-year prospective study. Eur J Nutr. 2014;53:951-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 129. | Gisondi P, Cotena C, Tessari G, Girolomoni G. Anti-tumour necrosis factor-alpha therapy increases body weight in patients with chronic plaque psoriasis: a retrospective cohort study. J Eur Acad Dermatol Venereol. 2008;22:341-344. [PubMed] |
| 130. | Wu JJ, Poon KY. Tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis, psoriatic arthritis, or both. J Drugs Dermatol. 2014;13:932-934. [PubMed] |
| 131. | Wu JJ, Poon KY, Bebchuk JD. Association between the type and length of tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. J Drugs Dermatol. 2013;12:899-903. [PubMed] |