Published online Nov 26, 2015. doi: 10.4330/wjc.v7.i11.817
Peer-review started: January 29, 2015
First decision: May 13, 2015
Revised: August 27, 2015
Accepted: October 16, 2015
Article in press: October 19, 2015
Published online: November 26, 2015
Processing time: 304 Days and 15.4 Hours
Many patients with left ventricular systolic dysfunction have concomitant mitral regurgitation (MR). Their symptoms and prognosis worsen with increasing severity of MR. Percutaneous MitraClip® can be used safely to reduce the severity of MR even in patients with advanced heart failure and is associated with improved symptoms, quality of life and exercise tolerance. However, a few patients with very poor left ventricular systolic function may experience significant haemodynamic disturbance in the peri-procedural period. We present three such patients, highlighting some of the potential problems encountered and discuss their possible pathophysiological mechanisms and safety measures.
Core tip: We described three patients with severe mitral regurgitation and very poor left ventricular systolic function undergoing percutaneous MitraClip treatment. These patients experienced haemodynamic instability peri-procedurally immediately upon reduction of their mitral regurgitation. These patients shared a few features such as right ventricular dysfunction and pulmonary hypertension which may help to identify those who may develop such peri-procedural haemodynamic compromise. Our cases highlight that although MitraClip is generally a safe procedure, there should not be complacency especially when treating patients with very poor left ventricular function. Extreme caution and vigilance should be exercised when treating such patients.
- Citation: Loh PH, Bourantas CV, Chan PH, Ihlemann N, Gustafsson F, Clark AL, Price S, Mario CD, Moat N, Alamgir F, Estevez-Loureiro R, Søndergaard L, Franzen O. Catheter-based intervention for symptomatic patient with severe mitral regurgitation and very poor left ventricular systolic function - Safe but no room for complacency. World J Cardiol 2015; 7(11): 817-821
- URL: https://www.wjgnet.com/1949-8462/full/v7/i11/817.htm
- DOI: https://dx.doi.org/10.4330/wjc.v7.i11.817
Approximately 35%-50% of patients with left ventricular systolic dysfunction (LVSD) develop significant mitral regurgitation (MR)[1]. As the severity of MR increases, their symptom and prognosis worsen with a 5-year mortality up to 60% in those with severe MR[2]. Whether treating MR in these patients alters the prognosis or progression of LVSD is not known. Percutaneous intervention for mitral regurgitation is now possible and can reduce the severity of MR even in patients with advanced heart failure with associated improvement in symptoms, quality of life and exercise tolerance[3,4]. However, special consideration should be given to patients with very poor left ventricular (LV) systolic function who may have very fragile hemodynamics that can be easily disturbed. We present a series of cases which outline some potential concerns.
A 72-year-old man presented with NYHA class III symptoms of heart failure and was found to have a dilated LV with severe LVSD and moderate-to-severe MR due to ischemic heart disease (IHD) (Table 1). He had previously undergone coronary artery bypass grafting, and had a history of cerebrovascular accident. Echocardiography demonstrated significant right ventricular (RV) impairment and moderately raised pulmonary arterial systolic pressure (PASP) (Table 1).
| LVEF(%) | LVEDD(mm) | Severity of MR | Cause of MR | Mid RV diameter (mm) | RV systolic dysfunction | PASP(mmHg) | |
| Case 1 | 20 | 64 | Moderate-to-severe | Secondary | 51 | Severe | 50 |
| Case 2 | 15 | 68 | Severe | Secondary | 42 | Moderate | 65 |
| Case 3 | 20 | 70 | Severe | Secondary | 45 | Mild | 65 |
A centrally placed MitraClip reduced the severity of MR to mild. Although there was no change in blood pressure (BP) which was maintained in the region of 96/50 mmHg, trans-esophageal echocardiogram (TEE) showed worsening of LV systolic function (LV ejection fraction < 10%) and spontaneous echo contrast in the LV. After reversal of general anaesthesia, he had an episode of ventricular fibrillation that was successfully defibrillated with a single biphasic DC shock. Adrenaline and dobutamine support had to be administered for 48 h. At discharge, echocardiography demonstrated mild residual MR with LV ejection fraction (LVEF) returned to 20%. His symptoms improved to NYHA II.
A 78-year-old lady with severe MR, a dilated LV and severe LVSD secondary to IHD had persistent NYHA class III symptoms despite optimal medication and cardiac resynchronisation therapy (CRT) (Table 1). She had moderate aortic stenosis with a valve area of 1.4 cm2, moderate RV impairment and raised PASP (Table 1).
A centrally placed MitraClip successfully reduced the severity of her MR to mild. However, there was acute deterioration in her LV systolic function on TEE and a reduction in her cardiac output from 3.5 to 3.1 L/min. Her BP dropped from 104/60 to 88/54 mmHg. The MitraClip was re-positioned and deployed more medially which left her with moderate MR but her LV function, cardiac output and BP recovered. At discharge, echocardiography showed residual moderate MR and LVEF 15%. Her symptoms improved to NYHA class II which was sustained at 1-mo follow-up.
A 68-year-old man with severe LVSD due to idiopathic dilated cardiomyopathy had severe MR and persistent NYHA III-IV breathlessness despite optimal medication and CRT. He had RV dilation with mild RV systolic impairment and raised PASP (Table 1).
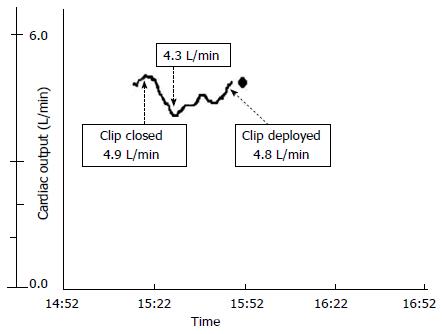
Initial placement of the MitraClip reduced the severity of his MR to trivial. However, there was worsening of his LV systolic function on TEE with reduction in cardiac output from 4.9 to 4.3 L/min (Figure 1) and BP from 116/55 to 97/42 mmHg. These gradually improved to baseline after a period of observation and the clip was deployed. Invasive hemodynamic study showed an immediate reduction in his pulmonary arterial systolic pressure from 57 to 46 mmHg and mean left atrial pressure from 32 to 24 mmHg. At discharge, his symptom improved to NYHA II with trivial MR and LVEF of 20% on TTE.
These cases suggest that although percutaneous intervention for MR can be safely performed in patients with poor LV systolic function[3,4], significant clinical deterioration can occur in those with very poor LV function. The exact mechanism is unclear but likely to be due to a complex interaction of various cardiac mechanics and haemodynamic factors. In clinical setting, the haemodynamic effects following acute reduction of MR has been poorly understood as most were extrapolated from surgical patients and can be confounded by factors such as cardioplegia and cardiopulmonary bypass. Siegel et al[5] evaluated the acute haemodynamic effects of MitraClip therapy in 107 patients and found that successful MitraClip treatment was associated with an increase in cardiac output, cardiac index and forward stroke volume with a reduction in LV end diastolic pressure and calculated peripheral vascular resistance. These haemodynamic changes were thought to reduce the risk of low cardiac output state following the reduction of MR. However, the mean LVEF in their study was approximately 60% and patients with significant LVSD was not represented in their study[5]. In a predominantly secondary MR cohort of 50 patients with overall lower normal range of cardiac index (CI) and mean LVEF approximately 50%, Gaemperli et al[6] reported an improvement in CI following MitraClip. However, recent report suggests that afterload mismatch can manifest as early transient worsening of LV function following Mitraclip in patients with poorer LV function (mean LVEF 26%)[7].
It is likely that complex haemodynamic factors may be involved in patients with severe MR and significant LVSD, especially in those with very low LVEF[8,9]. The LV contractility is regulated by the Frank-Starling mechanism, force-frequency relationship and neurohormonal control. The effectiveness of the later two mechanisms is impaired in failing human heart but the Frank-Starling mechanism can be preserved even in end-stage heart failure[10]. In the failing ventricles, the maximum LV performance is achieved at much larger LV volumes than that of the normal ventricles. This shows the importance of the Frank-Starling mechanism as a vital compensatory mechanism to maintain LV systolic contraction in the presence of significant LVSD[11]. In an experimental model using dogs with chronic heart failure, a reduction of LV end-diastolic diameter to normal level by abrupt inferior vena caval occlusion led to further reduction in LV systolic function indicating that the increase in LV preload is crucial to maintain LV systolic function[12]. Therefore, an acute and significant reduction in MR may lead to a decrease in LV end-diastolic volume (preload)[5], compromising the Frank-Starling compensatory mechanism and causes further deterioration in the LV systolic performance. In a way, this may be similar to that observed in the Fontan circulation where preload is known to be the most important determinant of cardiac output especially in the presence of a dilated or impaired ventricle[13].
Another potential factor is the poorly understood “pop-off valve” concept[14]. It was suggested that in patients with poor LV function, there is a need for the ventricle to offload into the low-impedance left atrium. However, the improvement in the outcome of mitral valve surgery in patients with poor LV function and understanding in the importance of chordal preservation has disputed this concept[15-17]. Nevertheless, it may be relevant in patients with very poor LV systolic function experiencing acute and significant reduction in MR. In experimental canine model where an external LV-to-left atrial shunt was created to mimic the effect of MR, acute shunt closure led to an increase in the LV mean systolic pressure and wall stress (afterload)[18,19]. Closure of the shunt was associated with an improvement in the haemodynamic state with reduction in LV end-diastolic pressure (LVEDP) and increased forward cardiac output. However, in the presence of severe LV systolic dysfunction, LVEDP increased (preload) despite a reduction in LV diastolic filling and the forward flow decreased[18]. The increase in LV systolic and diastolic wall stress leads to an increase in myocardial oxygen consumption; whilst increased LVEDP may reduce coronary blood flow leading to relative subendocardial ischaemia[20]. This represents an experimental model which is not confounded by the effect of cardioplegia and cardiopulmonary bypass and may partly explain the adverse events in our patients. Since percutaneous MitraClip can be performed without cardiopulmonary bypass, the real-time beat-to-beat hemodynamic variables, LV function and MR severity can be assessed. This may help understand the pathophysiology of haemodynamic disturbance following acute MR reduction in patients with significant LVSD.
Our patients shared some common features[7] which may have contributed to the acute adverse events. They had dilated LV with advanced LVSD (LVEF ≤ 20%), pulmonary hypertension, RV systolic impairment and significant MR with substantial reduction following intervention. A cautious approach in managing the patients with very poor LV systolic function is obligatory, especially in the light of the surgical experience[1].
Every patient with significant LVSD should have individualised management within a multi-disciplinary team approach involving the interventional cardiologists, cardiac surgeons, cardiac anaesthetists and intensive care physicians, heart failure and imaging specialists and other paramedical members. Treatment strategies and options in case of procedural failure should be discussed. The patients should be pre-assessed thoroughly whilst receiving optimal heart failure therapy. TEE is mandatory to determine the anatomical suitability for the procedure and cardiac catheterisation can provide accurate haemodynamic evaluation. The assessment of LV contractile reserve and RV function may be helpful[16].
Pre-procedurally, some patients may need stabilisation of their clinical state with intravenous diuretic and/or nitrate infusion. In patients at risk of significant haemodynamic disturbance during the procedure, pre-procedural infusion of inodilators or insertion of intra-aortic balloon pump (IABP) may be considered with close liaison with all who are involved in the care of the patient including the heart failure and intensive care teams.
During the procedure, LVEF may decrease immediately after the reduction of MR in some patients. Since LVEF represents a combination of forward stroke volume (SV) and regurgitant volume (RVol) of MR, reduction in the RVol may lead to a net increase in SV even if the LVEF reduces[5,9]. Without any significant changes in the heart rate, an increase in cardiac output and systolic blood pressure are signs of positive acute haemodynamic response. Conversely, a reduction in cardiac output suggests serious haemodynamic compromise. Therefore, cardiac output monitoring is important during the peri-procedural period. Surrogate findings such as lower central venous saturation, reduction in systolic blood pressure and the appearance of spontaneous echo contrast in the LV may indicate a decrease in cardiac output. Other information, such as mitral valve opening area, left atrial or pulmonary capillary wedge pressure, left ventricular pressure[9] and if available, contractility derived from a conductance catheter[21] might help assess the situation. Continuous observation of the haemodynamic response following provisional clip deployment but before final release allows the situation to be assessed and as in patient 2, the clip to be repositioned if there is a persistent unfavourable hemodynamic response.
Adverse events may still develop some time after the procedure and so the patients should be monitored continuously until they have fully recovered from the acute effect of the procedure and general anaesthesia.
Patients with poor LV function and significant MR can benefit from percutaneous MitraClip treatment. However, some of them may experience significant acute hemodynamic disturbance peri-procedurally and caution is necessary when treating these patients.
Peri-procedural haemodynamic instability during percutaneous MitraClip in three patients.
Decreased cardiac output and blood pressure on invasive haemodynamic study.
Afterload mismatch, access site bleeding, cardiac tamponde.
Invasive hemodynamics and echocardiographic are the main diagnostic tools.
Trans-esophageal echocardiogram confirmed deteriorating left ventricular (LV) function and often with appearance of spontaneous echo contrast in the LV.
Cautious and intensive haemodynamic support and monitoring before MitraClip deployment is effective in most cases; but rare cases may require repositioning of MitraClip to avoid substantial acute reduction of mitral regurgitation.
Prior hemodynamic studies mainly limited to patients with better left ventricular ejection fraction. However, similar to this case series, Melisurgo et al reported a small group of patients with poor LV function experiencing afterload mismatch peri-procedurally.
“Pop-off valve” concept proposed that mitral regurgitation (MR) allows a degree of afterload reduction in the LV with poor systolic function and this is abolished upon acute reduction in the regurgitant volume. The resultant increase in LV afterload casues afterload mismatch leading to worsening LV function and haemodynamic instability.
Although MitraClip is beneficial, a minority of patients with very poor LV function and significant MR may experience significant acute hemodynamic disturbance peri-procedurally, especially in those with co-existing right ventricular dysfunction and pulmonary hypertension. Hence caution is necessary when treating these patients.
The article describes a group of patients with very poor LV function and severe secondary MR who may experience significant haemodynamic disturbance during percutaneous MitraClip treatment. Although it is a relatively safe procedure, there is no room for complacency and extreme caution needs to be taken when treating those patients with very poor LV function.
P- Reviewer: Li YL, Sangiorgi GM S- Editor: Tian YL L- Editor: A E- Editor: Wu HL
| 1. | Di Salvo TG, Acker MA, Dec GW, Byrne JG. Mitral valve surgery in advanced heart failure. J Am Coll Cardiol. 2010;55:271-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 90] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 2. | Trichon BH, Felker GM, Shaw LK, Cabell CH, O’Connor CM. Relation of frequency and severity of mitral regurgitation to survival among patients with left ventricular systolic dysfunction and heart failure. Am J Cardiol. 2003;91:538-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 507] [Cited by in RCA: 508] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 3. | Franzen O, Baldus S, Rudolph V, Meyer S, Knap M, Koschyk D, Treede H, Barmeyer A, Schofer J, Costard-Jäckle A. Acute outcomes of MitraClip therapy for mitral regurgitation in high-surgical-risk patients: emphasis on adverse valve morphology and severe left ventricular dysfunction. Eur Heart J. 2010;31:1373-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 237] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 4. | Franzen O, van der Heyden J, Baldus S, Schlüter M, Schillinger W, Butter C, Hoffmann R, Corti R, Pedrazzini G, Swaans MJ. MitraClip® therapy in patients with end-stage systolic heart failure. Eur J Heart Fail. 2011;13:569-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 279] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 5. | Siegel RJ, Biner S, Rafique AM, Rinaldi M, Lim S, Fail P, Hermiller J, Smalling R, Whitlow PL, Herrmann HC. The acute hemodynamic effects of MitraClip therapy. J Am Coll Cardiol. 2011;57:1658-1665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 152] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 6. | Gaemperli O, Moccetti M, Surder D, Biaggi P, Hurlimann D, Kretschmar O, Buehler I, Bettex D, Felix C, Luscher TF. Acute haemodynamic changes after percutaneous mitral valve repair: relation to mid-term outcomes. Heart. 2012;98:126-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 7. | Melisurgo G, Ajello S, Pappalardo F, Guidotti A, Agricola E, Kawaguchi M, Latib A, Covello RD, Denti P, Zangrillo A. Afterload mismatch after MitraClip insertion for functional mitral regurgitation. Am J Cardiol. 2014;113:1844-1850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 8. | Loh PH, Veien K, Gaemperli O, Corti R. Hemodynamic Perspective of Edge-to-edge Mitral Valve Repair. Atlas of Percutaenous Edge-to-edge Mitral Valve Repair. London: Springer-Verlag 2013; 147-162. [DOI] [Full Text] |
| 9. | Jilaihawi H, Makkar R, Hussaini A, Trento A, Kar S. Contemporary application of cardiovascular hemodynamics: transcatheter mitral valve interventions. Cardiol Clin. 2011;29:201-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Weil J, Eschenhagen T, Hirt S, Magnussen O, Mittmann C, Remmers U, Scholz H. Preserved Frank-Starling mechanism in human end stage heart failure. Cardiovasc Res. 1998;37:541-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 43] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Holubarsch C, Ruf T, Goldstein DJ, Ashton RC, Nickl W, Pieske B, Pioch K, Lüdemann J, Wiesner S, Hasenfuss G. Existence of the Frank-Starling mechanism in the failing human heart. Investigations on the organ, tissue, and sarcomere levels. Circulation. 1996;94:683-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 148] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 12. | Gill RM, Jones BD, Corbly AK, Ohad DG, Smith GD, Sandusky GE, Christe ME, Wang J, Shen W. Exhaustion of the Frank-Starling mechanism in conscious dogs with heart failure induced by chronic coronary microembolization. Life Sci. 2006;79:536-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Gewillig M, Brown SC, Eyskens B, Heying R, Ganame J, Budts W, La Gerche A, Gorenflo M. The Fontan circulation: who controls cardiac output? Interact Cardiovasc Thorac Surg. 2010;10:428-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 209] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 14. | Kirklin JW. Replacement of the mitral valve for mitral incompetence. Surgery. 1972;72:827-836. [PubMed] |
| 15. | Rozich JD, Carabello BA, Usher BW, Kratz JM, Bell AE, Zile MR. Mitral valve replacement with and without chordal preservation in patients with chronic mitral regurgitation. Mechanisms for differences in postoperative ejection performance. Circulation. 1992;86:1718-1726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 125] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Acker MA. Should moderate or greater mitral regurgitation be repaired in all patients with LVEF & lt; 30%? Mitral valve repair in patients with advanced heart failure and severe functional mitral insufficiency reverses left ventricular remodeling and improves symptoms. Circ Heart Fail. 2008;1:281-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Yun KL, Rayhill SC, Niczyporuk MA, Fann JI, Zipkin RE, Derby GC, Handen CE, Daughters GT, Ingels NB, Bolger AF. Mitral valve replacement in dilated canine hearts with chronic mitral regurgitation. Importance of the mitral subvalvular apparatus. Circulation. 1991;84:III112-III124. [PubMed] |
| 18. | Rankin JS, Nicholas LM, Kouchoukos NT. Experimental mitral regurgitation: effects on left ventricular function before and after elimination of chronic regurgitation in the dog. J Thorac Cardiovasc Surg. 1975;70:478-488. [PubMed] |
| 19. | Spratt JA, Olsen CO, Tyson GS, Glower DD, Davis JW, Rankin JS. Experimental mitral regurgitation. Physiological effects of correction on left ventricular dynamics. J Thorac Cardiovasc Surg. 1983;86:479-489. [PubMed] |
| 20. | Buckberg GD, Fixler DE, Archie JP, Hoffman JI. Experimental subendocardial ischemia in dogs with normal coronary arteries. Circ Res. 1972;30:67-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 680] [Cited by in RCA: 635] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 21. | Baan J, van der Velde ET, de Bruin HG, Smeenk GJ, Koops J, van Dijk AD, Temmerman D, Senden J, Buis B. Continuous measurement of left ventricular volume in animals and humans by conductance catheter. Circulation. 1984;70:812-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 697] [Cited by in RCA: 620] [Article Influence: 15.1] [Reference Citation Analysis (0)] |