Published online Jul 26, 2014. doi: 10.4330/wjc.v6.i7.689
Revised: April 17, 2014
Accepted: May 16, 2014
Published online: July 26, 2014
Processing time: 232 Days and 15.1 Hours
Effective height, which represents the height difference between the central free margins and the aortic insertion lines can be easily determined by 2-D echocardiography and allows for identification of prolapse in the native cusps and assessment of prolapse correction after valve repair. Nonetheless, it allows to see only two of three aortic valve (AV) coaptation planes and this may lead to misunderstanding of the underlying pathophysiological mechanism for aortic regurgitation and hence in unsuccessful repair. In contrast, 3D transoesophageal echocardiography and multiple plane reconstruction lets visualize all the three coaptation planes between the AV cusps and it represents an invaluable tool in the assessment of aortic valve geometry. It is highly recommendable before AV repair to accurately study the complex three dimensional cusps anatomy and their geometric interrelation with aortic root.
Core tip: 3D transesophageal echocardiography and multiple plane reconstruction lets visualize all the three coaptation planes between the aortic valve (AV) cusps and overcomes the limits of 2-D echocardiography which allows to see only two of three AV coaptation planes and this may lead to misunderstanding of the underlying pathophysiological mechanism for aortic regurgitation and hence in unsuccessful repair. It is highly recommendable before AV repair to accurately study the complex three dimensional cusps anatomy and their geometric interrelation with aortic root.
- Citation: Nijs J, Gelsomino S, Kietselaer BB, Parise O, Lucà F, Maessen JG, Meir ML. 3D-echo in preoperative assessment of aortic cusps effective height. World J Cardiol 2014; 6(7): 689-691
- URL: https://www.wjgnet.com/1949-8462/full/v6/i7/689.htm
- DOI: https://dx.doi.org/10.4330/wjc.v6.i7.689
In the recent years aortic valve (AV) repair has gained increasing interest in the treatment of aortic root pathology[1] as a feasible alternative to aortic valve replacement[2].
Good results have been achieved with valve-preserving aortic replacement for patients in whom aortic regurgitation is solely caused by aortic root dilatation with morphologically preserved valve leaflets[3]. In contrast, cusp repair still remains a surgical challenge when prolapse of cusp tissue impairs coaptation[4].
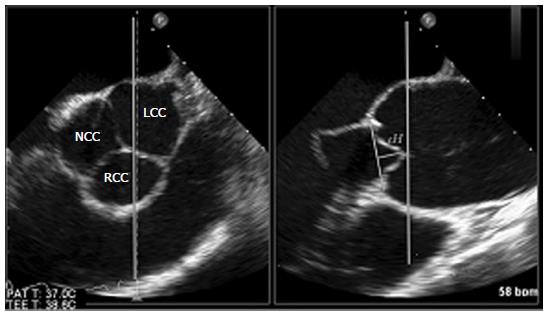
The most prominent echocardiographic phenomenon indicating cusps prolapse is a decreased effective height (eH) which represents the height difference between the central free margins and the aortic insertion lines[4]. This measurement, which depends on the complex relationship of root and cusp, can be easily determined by 2-D echocardiography and allows for identification of prolapse in the native cusps and assessment of prolapse correction after valve repair. Nonetheless, with 2-D transesophageal echocardiography (2D-TEE) only two of three AV coaptation planes can be seen and the eH, a unidimensional value, can be measured only between the right coronary cusp anteriorly and either the non- or left coronary cusp (depending on probe rotation) posteriorly (Figure 1).
As a result, pathology of the AV cusp not included in the view may go undetected and this may eventually result in misunderstanding of the underlying pathophysiological mechanism for aortic regurgitation and hence in unsuccessful repair.
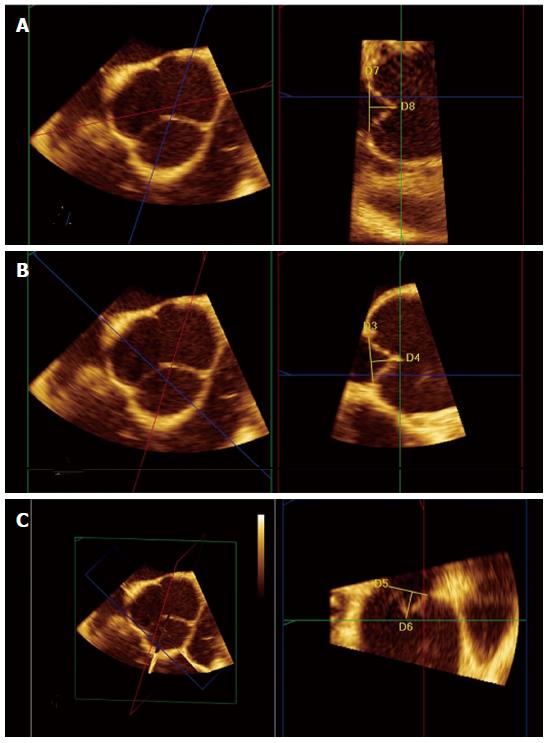
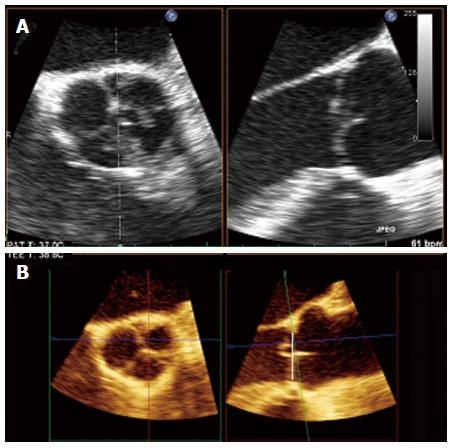
Recent development of real-time 3D transoesophageal echocardiography (3D-TEE) allows multiple plane reconstruction (MPR) which lets visualize all the three coaptation planes between the AV cusps. Using MPR is possible to adjust the orthogonal imaging planes for optimal visualization of all three aortic coaptation lines. By moving the planes is possible to identify the points where the cusps come together and to obtain, for each one, an accurate determination of the eH (Figure 2). 3D-echo multi-plane reconstruction is a valuable tool in the assessment of valve geometry and it is highly recommendable before AV repair to accurately study the complex three dimensional cusps anatomy and their geometric interrelation with aortic root as a functional unit (Figure 3). This is important since effective cusps height is a significant predictor of aortic valve repair failure. Indeed, an effective height > 8 mm is associated with 99.6% probability of clinically insignificant aortic regurgitation. Other significant predictors are residual regurgitation, a coaptation length < 4 mm and a level of cusp coaptation which is below the aortic annulus (type C)[5].
We acknowledge Dr. Judith Wilson for the English revision of the manuscript.
P- Reviewer: Grignola JC, Kettering K, Satoh H S- Editor: Wen LL L- Editor: A E- Editor: Wu HL
| 1. | Pettersson GB, Crucean AC, Savage R, Halley CM, Grimm RA, Svensson LG, Naficy S, Gillinov AM, Feng J, Blackstone EH. Toward predictable repair of regurgitant aortic valves: a systematic morphology-directed approach to bicommissural repair. J Am Coll Cardiol. 2008;52:40-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 2. | Kesavan S, Iqbal A, Khan Y, Hutter J, Pike K, Rogers C, Turner M, Townsend M, Baumbach A. Risk profile and outcomes of aortic valve replacement in octogenarians. World J Cardiol. 2011;3:359-366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | David TE, Ivanov J, Armstrong S, Feindel CM, Webb GD. Aortic valve-sparing operations in patients with aneurysms of the aortic root or ascending aorta. Ann Thorac Surg. 2002;74:S1758-61. [RCA] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 101] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 4. | Schäfers HJ, Bierbach B, Aicher D. A new approach to the assessment of aortic cusp geometry. J Thorac Cardiovasc Surg. 2006;132:436-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 227] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 5. | Augoustides JG, Szeto WY, Bavaria JE. Advances in aortic valve repair: focus on functional approach, clinical outcomes, and central role of echocardiography. J Cardiothorac Vasc Anesth. 2010;24:1016-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |