Published online Jun 26, 2014. doi: 10.4330/wjc.v6.i6.405
Revised: March 10, 2014
Accepted: April 17, 2014
Published online: June 26, 2014
Processing time: 179 Days and 13.5 Hours
Cardiovascular disease (CVD) is the leading cause of death in the western world and is becoming more important in the developing world. Recently, advances in monitoring, revascularisation and pharmacotherapy have resulted in a reduction in mortality. However, although mortality rates have declined, the burden of disease remains large resulting in high direct and indirect healthcare costs related to CVDs. In Australia, acute coronary syndrome (ACS) accounts for more than 300000 years of life lost due to premature death and a total cost exceeding eight billion dollars annually. It is also the main contributor towards the discrepancy in life expectancy between indigenous and non-indigenous Australians. The high prevalence of CVD along with its associated cost urgently requires a reliable but non-invasive and cost-effective imaging modality. The imaging modality of choice should be able to accelerate the diagnosis of ACS, aid in the risk stratification of de novo coronary artery disease and avail incremental information of prognostic value such as viability which cardiovascular magnetic resonance (CMR) allows. Despite its manifold benefits, there are limitations to its wider use in routine clinical assessment and more studies are required into assessing its cost-effectiveness. It is hoped that with greater development in the technology and imaging protocols, CMR could be made less cumbersome, its imaging protocols less lengthy, the technology more inexpensive and easily applied in routine clinical practice.
Core tip: This review focuses on cardiovascular magnetic resonance in achieving speedy diagnosis, risk stratification and prognostication in acute coronary syndrome. It discusses the modalities already available towards achieving this end and the incremental information availed by cardiac magnetic resonance. The paper also discusses new imaging techniques and their contribution towards the cardiac magnetic resonance imaging assessment of patients with acute coronary syndrome.
- Citation: Azarisman SM, Teo KS, Worthley MI, Worthley SG. Role of cardiovascular magnetic resonance in assessment of acute coronary syndrome. World J Cardiol 2014; 6(6): 405-414
- URL: https://www.wjgnet.com/1949-8462/full/v6/i6/405.htm
- DOI: https://dx.doi.org/10.4330/wjc.v6.i6.405
Cardiovascular disease (CVD) is the leading cause of death in the western world and is becoming more important in the developing world[1,2]. Recently, advances in monitoring, revascularisation and pharmacotherapy have resulted in a reduction in mortality. However, although mortality rates have declined, the burden of disease remains large resulting in high direct and indirect healthcare costs related to CVDs[3-5]. In Australia, acute coronary syndrome (ACS) accounts for more than 300000 years of life lost due to premature death and a total cost exceeding eight billion dollars annually. It is also the main contributor towards the discrepancy in life expectancy between indigenous and non-indigenous Australians[6]. In the United States and Europe, approximately 15 million patients are treated annually for chest pain and suspicion of myocardial infarction (MI) and upwards of 20% are eventually diagnosed to have ACS[2,7].
The high prevalence of CVD along with its associated cost urgently requires a reliable but non-invasive and cost-effective imaging modality. The imaging modality of choice should be able to accelerate the diagnosis of ACS, aid in the risk stratification of de novo coronary artery disease (CAD) and avail incremental information of prognostic value such as viability.
It is well established that ACS refers to a spectrum of clinical presentations ranging from unstable angina to non ST-elevation myocardial infarction and ST-elevation myocardial infarction. These presentations refer to clinical symptoms compatible with myocardial ischaemia resulting from acute thrombosis induced by a ruptured or eroded atherosclerotic coronary artery plaque[1-4].
The main management strategy for ACS is prompt diagnosis leading to early coronary reperfusion. The usual assessment sequence involves a detailed case history delineating the patient’s risk factor profile, appropriate physical examination, electrocardiography (ECG) and laboratory risk markers such as creatine kinase and troponin levels.
Early reperfusion limits the final infarct size, halts progression of myocardial necrosis and andoptimises myocardial salvage thereby improving both short and long term outcomes[8,9]. Pertaining to these established aims, several questions need to be answered. What is the regional and global ventricular function, what is the extent of myocardial necrosis, is there any viable myocardium and are the epicardial coronary arteries patent?[10-12].
Over the past two decades, noninvasive imaging has emerged as the investigative modality of choice for ACS. It allows comprehensive cardiac assessment of patients, risk stratification of patients with ACS at an early management time point and provides diverse and complimentary information regarding possible differential diagnoses and prognosis[13-15].
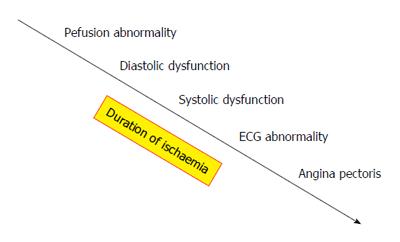
Early coronary reperfusion following diagnosis of ACS results in myocardial salvage and prevents irreversible injury[16,17]. Usual investigative tools such as ECG and Troponin assays are helpful but may be negative early. Echocardiography, although useful in establishing regional wall motion abnormalities and quantifying ventricular ejection fraction, can also be negative early as these abnormalities appear later in the temporal cascade of events following coronary artery occlusion (Figure 1). Furthermore, echocardiographic assessment lacks the tissue characterisation ability needed to rule out differentials such as myocarditis. Over the past two decades, computed tomography (CT) has emerged as a potentially useful imaging modality for ACS.
Positron emission tomography (PET) utilises several radionuclides namely 18F-Fluorodeoxyglucose (18FDG) for myocardial metabolism and 13N-Ammonia (13NH3) for myocardial perfusion assessment[18]. Myocardial segments with normal glucose metabolism and preserved myocardial flow indicate viable and adequately perfused myocardium. 18FDG allows differentiation between hibernating but viable, with infarcted and non-viable myocardium in regions with wall motion abnormalities when interpreted together with 13NH3[19,20]. Although clinically useful in identifying metabolism/perfusion mismatch in stable CAD, its utility in the setting of ACS is limited due to restricted availability, high costs, and limited data supporting its application[21].
Computed tomography coronary angiogram (CTCA) is becoming a useful tool for evaluation of patients with ACS. It can be utilised both in the diagnosis and risk stratification of ACS[22,23]. Three recent trials affirmed the utility of employing CTCA for rapid triage via radiographic demonstration of the absence of coronary artery disease in low to intermediate risk patients[24-26]. Whilst all three trials reported more rapid and cost efficient discharge from the Emergency Department with the use of CTCA, the CT-STAT and ROMICAT II trials reported an increase in downstream testing and radiation exposure with no decrease in the overall costs of care[25,26]. Although the appropriate use criteria endorses its use in low to intermediate risk patients, it is primarily an exclusion tool with limited suitability for higher cardiac risk patients or pathological stress testing[27].
Coronary artery calcification can be evaluated by electron beam CT and multi-detector CT. It describes the extent of coronary arteriosclerosis and is correlated with increased cardiac risk. It has a high negatively predictive value, and can reliably exclude ACS in low to intermediate risk patients presenting with chest pain[28-30]. Unfortunately, its positive predictive value is unsatisfactory and a positive result usually warrants further downstream investigation. Moreover, conclusive evidence on its use in conjunction with other CT modalities such myocardial perfusion imaging (MPI) is still deficient[14,31].
Rest MPI becomes abnormal at the onset of impaired myocardial blood flow and therefore precedes other symptoms and signs of ACS. The non-invasive detection of a resting perfusion defect can be achieved with single-photon emission CT (SPECT), PET, cardiovascular magnetic resonance (CMR) and contrast enhanced echocardiography[32-34].
Resting myocardial perfusion is preserved with increasing severity of coronary stenosis through autoregulatory mechanisms in the microcirculation. This is exhausted when critical coronary artery stenosis develops and a resting myocardial perfusion abnormality will appear with complete occlusion of the coronary artery[35].
Cardiac CT based MPI has been utilised in animals since the late 1970s but its use in detection of MI only took off in mid-2000[36-38]. Resting MPI in addition to CTCA improves its diagnostic accuracy for detecting significant coronary artery disease. Studies have shown that in patients with chest pain, MPI with CTCA helps clarify the diagnosis of ACS[39-41]. Unfortunately rest MPI is not sensitive enough to identify the majority of ischaemic segments and vasodilator-induced hyperemia is required to detect significant disease[42-44].
Stress MPI detects the presence of a flow-limiting coronary stenosis by detecting regional variations in perfusion reserve. During vasodilator-induced hyperaemia, blood flow will not increase in already dilated arteriolar bed of stenosed coronary arteries. However, perfusion of normal coronaries will increase significantly and the resultant increase over resting blood flow is referred as the perfusion reserve. Consequently, the perfusion reserve of normal coronary territories will be greater than that of critically stenosed coronary territories and this regional discrepancy is detected by stress MPI[32-34].
Stress MPI is especially helpful in patients with coronary calcification and stents, with studies reporting a sensitivity and specificity of at least 95%[45,46]. Most studies however, report a sensitivity of between 50%-90% and specificity of 50%-98% when compared with either SPECT, CMR or invasive fractional-flow reserve (FFR) studies[32,45-50].
The major limitation to CT based rest and stress MPI, as with other CT based modalities, especially in research with comprehensive protocols remains exposure to ionizing radiation. Lack of long-term follow-up data of patients presenting to Emergency Department with chest pain and subsequently diagnosed with ACS is also compelling. Furthermore, although more recent studies have shown greater ability of different CT-based modalities in diagnosing and risk stratifying ACS, their utility remains only with those in the low to intermediate risk group. Cost effectiveness also becomes questionable with greater need for downstream investigation and greater overall cost of care especially in those with moderate to high risk of ACS.
In an Emergency setting, accurate early diagnosis of ACS along with efficacious institution of treatment is the main objective. As aforementioned, ECG and biomarkers are all helpful but may not be able to pick out early or equivocal ACS. Furthermore, these tests are presently unable to distinguish with certainty, ACS from other potential differentials, establish the extent of myocardial involvement, determine whether the damage is reversible, or even define the culprit artery with any reliability.
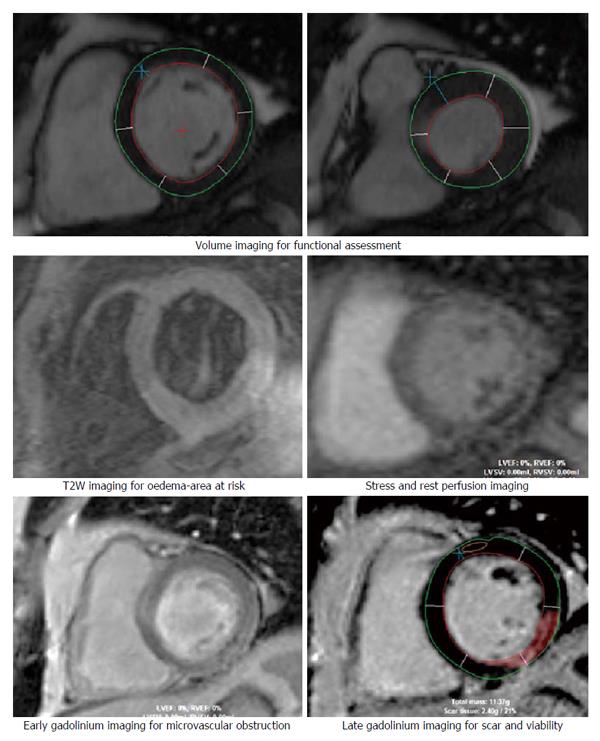
CMR offers high spatial resolution, accuracy and high reproducibility thereby allowing detailed volume and functional assessment, excellent tissue characterization in any tomographic plane and exceptional prognostic ability with late gadolinium enhancement (LGE) imaging (Figure 2). Radiation free examination also affords the CMR with the ability to incorporate extensive imaging protocols and repeated imaging necessary for both clinical and research imperatives.
Studies have already shown that CMR techniques such as myocardial function, perfusion imaging and LGE is able to provide a more accurate diagnosis of ACS compared with standard clinical assessment that includes ECG and biomarkers[51]. The use of new imaging techniques such as T2-weighted sequences for oedema detection also increases its diagnostic performance[52]. Moreover, unlike CT-based imaging, CMR utility can be extended to patients with intermediate to high risk for ACS but without ECG or biomarker evidence of MI[53].
In essence, CMR represents a “one-stop-shop” for early and comprehensive assessment towards accurate and reliable diagnosis, risk stratification and prognostication of patients with ACS.
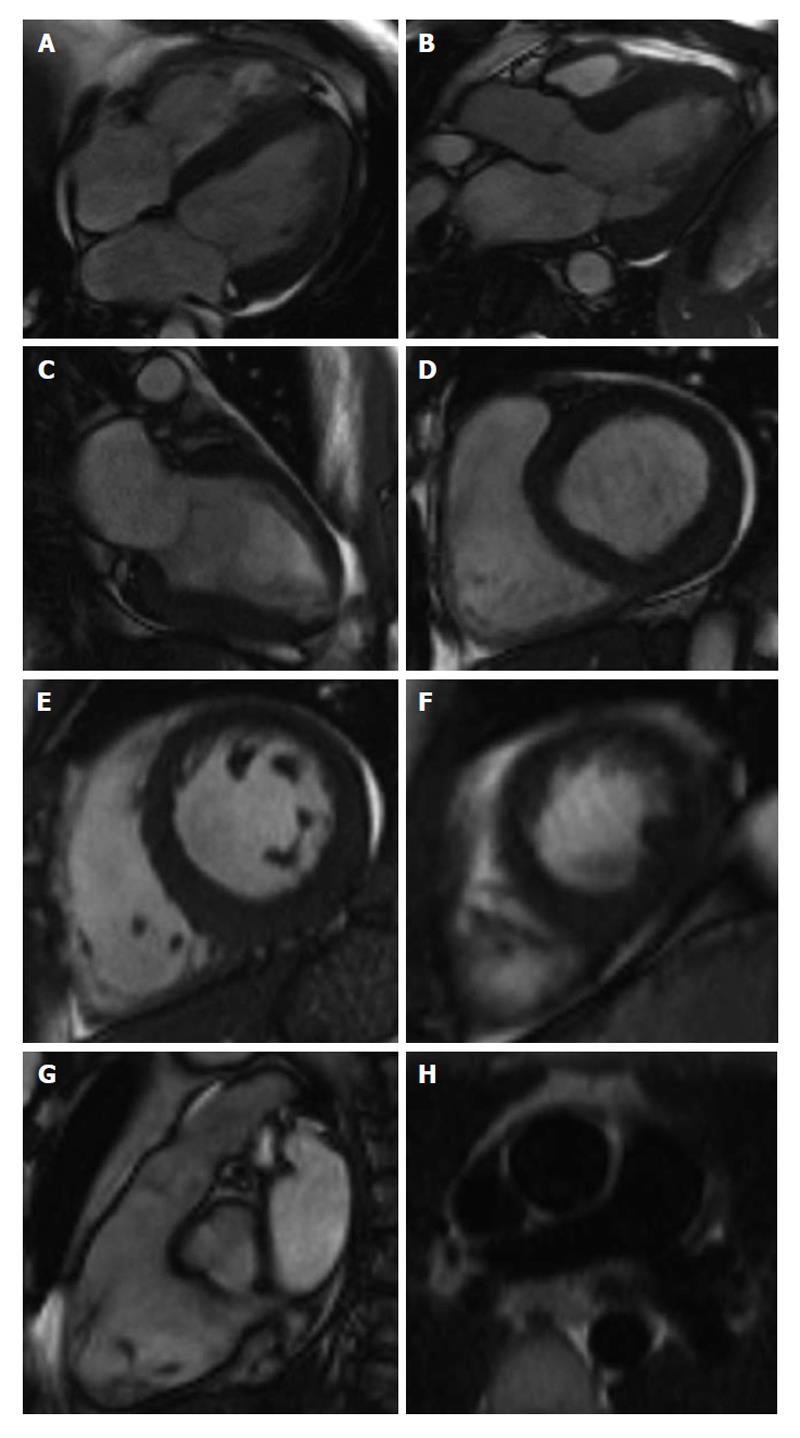
Rest cine magnetic resonance imaging utilises steady-state free precession sequences to acquire a series of consecutive, breath-hold, long and short-axis slices (Figure 3). The excellent spatial resolution, coupled with the high contrast between blood and myocardium allows the endocardial border to be detected easily. This allows easy assessment of ventricular wall motion, ventricular volumes, ejection fraction, myocardial mass and anatomy of the extracardiac structures. These CMR assessments are accurate, reproducible and well validated[54,55].
In the Emergency Department, these initial CMR imaging sequences can also be utilized to detect diseases of the aorta that may mimic ACS such as dissection or penetrating ulcer[56]. Findings typical of myocarditis and Takotsubo cardiomyopathy can also be seen and confirmed by LGE[57-60]. Initial review of the right ventricle and ventricular outflow tract, interventricular septum and pulmonary vasculature may also yield signs characteristic of acute pulmonary embolism which can then be subsequently confirmed with MR angiography[61].
T2-weighted (T2W) imaging with short tau inversion recovery (STIR) sequences is used to detect myocardial oedema which has increased signal intensity. The presence of oedematous myocardial segments on T2W imaging is a sign of ischaemic myocardium and a negative prognostic indicator for cardiovascular events[62]. Oedematous segments also allow acute-on-chronic differentiation of myocardial segments in established CAD patients[63]. Acutely, T2W imaging also identifies the area-at-risk (AAR) which is defined as an area of potentially reversible myocardial injury but at risk of infarction. The extent of the AAR has been validated against histopathological and angiographic measurements and is predictive of the risk of further cardiovascular event or death[62,64-66].
Perfusion imaging is performed both at rest and stress (with Adenosine infusion) and assesses myocardial blood flow by capturing the transit of contrast medium through the myocardium. It is a well established tool for assessing acute impairment in myocardial blood flow, patency of microvasculature, myocardial perfusion reserve and viability[51,67]. In patients with chest pain with intermediate to high probability of ACS and a paucity of ischaemic signs, stress perfusion has a high negative predictive value with high diagnostic and prognostic value[53,68].
CMR perfusion imaging is a potential alternative to CT-based perfusion imaging due to improved subendocardial resolution, lack of ionizing radiation and cost effectiveness with reduced downstream investigation. Comparison with SPECT, PET and/or coronary angiography have shown good sensitivity and specificity of CMR in detecting perfusion defects of 87%-90% and 85%, respectively[69,70]. Rest and stress perfusion imaging is well complemented by LGE and adds to a comprehensive assessment of patients with ACS. Its utility, reliability and accuracy in patients with intermediate to high risk of ACS also puts it ahead of CT-based perfusion studies.
Gadolinium based contrast is an extracellular contrast agent that accumulates in the interstitial space following myocardial death and replacement with fibrosis. Increased signal intensity denotes myocardial injury and scarring[71]. Positive gadolinium enhancement coupled with CMRs high spatial resolution allows accurate and reliable quantification of the volume of injury and the transmural extent of the scarring[72,73]. This is crucial in estimating the extent of the scar as a percentage of wall thickness with ramifications towards viability and therefore, reversibility of the underlying myocardial dysfunction[74].
LGE essentially differentiates between irreversibly damaged (and thus non-viable) myocardium, from stunned myocardium which is ischaemic but viable. Acutely ischaemic but viable myocardium will have high signal intensity on T2W imaging but will be LGE negative. Generally, in a patient with MI, a transmural extent of scarring greater than 50% will signal a poor likelihood of functional recovery following revascularization[75]. This has an important clinical ramification, as the prevalence of non-viable myocardial segments subtending the occluded epicardial artery will negate the need for immediate revascularization in an emergency setting.
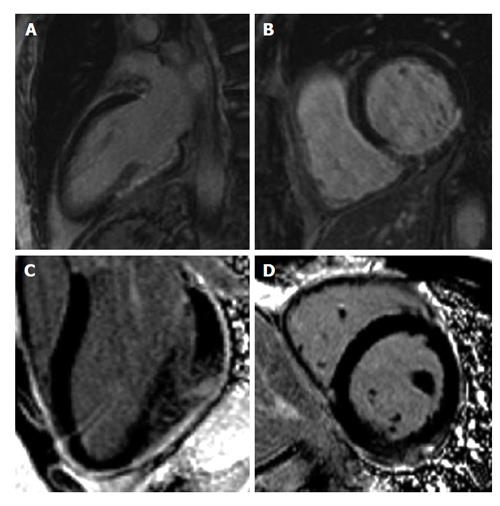
LGE also has a role earlier in the diagnostic milieu of ACS by differentiating between ischaemic and non-ischaemic causes of chest pain with biomarker rise. Differentials such as myocarditis and cardiomyopathy will have a different pattern of hyperenhancement. Ischaemia typically causes a more coalescent and subendocardial distribution of gadolinium enhancement confined to a particular vascular territory. Myocarditis has a typically epicardial or mid-myocardial distribution and cardiomyopathy has a patchy, mid-wall distribution (Figure 4).
LGE is also used in identifying microvascular obstruction (MVO) which is known angiographically as the “no reflow” phenomenon. Pathologically it is caused by failure of reperfusion at a microvascular level despite patent coronary arteries following revascularization. It is seen as a hypoenhanced core surrounded by hyperenhanced, scarred myocardium. MVO is well established as a negative prognostic marker and have been shown to be predictors of adverse remodeling following myocardial infarction[76-78].
On another note, LGE is also of use for the detection of left ventricular (LV) thrombus which is a serious complication post-MI. It has a higher sensitivity and specificity than echocardiography for the detection of LV thrombus especially laminar, mural and apical thrombi[79,80].
CMR is already the gold-standard imaging modality for assessing left ventricular volumes, ventricular function and tissue characterization in cardiomyopathies. These factors along with infarct size and MVO are common surrogate end-points in many clinical trials and strong predictors of clinical outcome. Other imaging sequences coming to the fore include T1 relaxation times with modified look-locker imaging, myocardial tagging and phase contrast imaging for flow assessment. These sequences are especially pertinent in assessing diastolic function which is becoming more routinely assessed and thus gaining greater importance in post-MI imaging.
The main obstruction to incorporating CMR as a routine assessment for ACS in Emergency Department is the high capital outlay required both in terms of hardware and human resource. This limits the CMRs ability to accommodate emergency studies in an Emergency Department setting despite the manifold benefits that it offers. Likewise, newer imaging protocols introduced as part of clinical studies may lengthen the scan time beyond what is acceptable for revascularization targets and thus rule out its relevance in the Emergency setting. Having a strong magnetic field also negates its use in patients with metallic implants, aside from those who are claustrophobic. It is also not as mobile and easy to use as an echocardiogram and thus may not be usable in an intensive care unit setting for those who may gain the most from its use. More research is required into establishing the cost-effectiveness of CMR in routine clinical practice.
CMR allows comprehensive assessment of patients presenting to the Emergency department with chest pain. Its ability to accurately and reliably diagnose, risk stratify and prognosticate ACS puts it ahead of other imaging modalities currently available. Despite its manifold benefits, there are limitations to its wider use in routine clinical assessment and more studies are required into assessing its cost-effectiveness. It is hoped that with greater development in the technology and imaging protocols, CMR could be made less cumbersome, its imaging protocols less lengthy, the technology more inexpensive and easily applied in routine clinical practice.
P- Reviewers: Cavaliere F, Ma JY S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
| 1. | Hamm CW, Bassand JP, Agewall S, Bax J, Boersma E, Bueno H, Caso P, Dudek D, Gielen S, Huber K. [ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. The Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC)]. G Ital Cardiol (Rome). 2012;13:171-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 2. | Wright RS, Anderson JL, Adams CD, Bridges CR, Casey DE, Ettinger SM, Fesmire FM, Ganiats TG, Jneid H, Lincoff AM. 2011 ACCF/AHA focused update incorporated into the ACC/AHA 2007 Guidelines for the Management of Patients with Unstable Angina/Non-ST-Elevation Myocardial Infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in collaboration with the American Academy of Family Physicians, Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons. J Am Coll Cardiol. 2011;57:e215-e367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 305] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 3. | Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2011;123:e18-e209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3702] [Cited by in RCA: 3715] [Article Influence: 265.4] [Reference Citation Analysis (0)] |
| 4. | Fox KA, Birkhead J, Wilcox R, Knight C, Barth J; British Cardiac Society Working Group. British Cardiac Society Working Group on the definition of myocardial infarction. Heart. 2004;90:603-609. [PubMed] |
| 5. | Fox KA, Steg PG, Eagle KA, Goodman SG, Anderson FA, Granger CB, Flather MD, Budaj A, Quill A, Gore JM. Decline in rates of death and heart failure in acute coronary syndromes, 1999-2006. JAMA. 2007;297:1892-1900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 639] [Cited by in RCA: 633] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 6. | Brieger DB, Redfern J. Contemporary themes in acute coronary syndrome management: from acute illness to secondary prevention. Med J Aust. 2013;199:174-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Nawar EW, Niska RW, Xu J. National Hospital Ambulatory Medical Care Survey: 2005 emergency department summary. Adv Data. 2007;1-32. [PubMed] |
| 8. | Fox KA, Carruthers KF, Dunbar DR, Graham C, Manning JR, De Raedt H, Buysschaert I, Lambrechts D, Van de Werf F. Underestimated and under-recognized: the late consequences of acute coronary syndrome (GRACE UK-Belgian Study). Eur Heart J. 2010;31:2755-2764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 319] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 9. | Reimer KA, Lowe JE, Rasmussen MM, Jennings RB. The wavefront phenomenon of ischemic cell death. 1. Myocardial infarct size vs duration of coronary occlusion in dogs. Circulation. 1977;56:786-794. [PubMed] |
| 10. | Ahmed N, Carrick D, Layland J, Oldroyd KG, Berry C. The role of cardiac magnetic resonance imaging (MRI) in acute myocardial infarction (AMI). Heart Lung Circ. 2013;22:243-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Raj V, Agrawal SK. Ischaemic heart disease assessment by cardiovascular magnetic resonance imaging. Postgrad Med J. 2010;86:532-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Schwitter J, Arai AE. Assessment of cardiac ischaemia and viability: role of cardiovascular magnetic resonance. Eur Heart J. 2011;32:799-809. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 13. | Gersh BJ. Noninvasive imaging in acute coronary disease. A clinical perspective. Circulation. 1991;84:I140-I147. [PubMed] |
| 14. | Gani F, Jain D, Lahiri A. The role of cardiovascular imaging techniques in the assessment of patients with acute chest pain. Nucl Med Commun. 2007;28:441-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Gruettner J, Henzler T, Sueselbeck T, Fink C, Borggrefe M, Walter T. Clinical assessment of chest pain and guidelines for imaging. Eur J Radiol. 2012;81:3663-3668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Rathore SS, Curtis JP, Chen J, Wang Y, Nallamothu BK, Epstein AJ, Krumholz HM. Association of door-to-balloon time and mortality in patients admitted to hospital with ST elevation myocardial infarction: national cohort study. BMJ. 2009;338:b1807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 355] [Cited by in RCA: 346] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 17. | Kushner FG, Hand M, Smith SC, King SB, Anderson JL, Antman EM, Bailey SR, Bates ER, Blankenship JC, Casey DE. 2009 Focused Updates: ACC/AHA Guidelines for the Management of Patients With ST-Elevation Myocardial Infarction (updating the 2004 Guideline and 2007 Focused Update) and ACC/AHA/SCAI Guidelines on Percutaneous Coronary Intervention (updating the 2005 Guideline and 2007 Focused Update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2009;120:2271-2306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 770] [Cited by in RCA: 730] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 18. | Camici P, Ferrannini E, Opie LH. Myocardial metabolism in ischemic heart disease: basic principles and application to imaging by positron emission tomography. Prog Cardiovasc Dis. 1989;32:217-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 167] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 19. | Camici PG, Rimoldi OE. Myocardial blood flow in patients with hibernating myocardium. Cardiovasc Res. 2003;57:302-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Bonow RO, Maurer G, Lee KL, Holly TA, Binkley PF, Desvigne-Nickens P, Drozdz J, Farsky PS, Feldman AM, Doenst T. Myocardial viability and survival in ischemic left ventricular dysfunction. N Engl J Med. 2011;364:1617-1625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 696] [Cited by in RCA: 624] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 21. | Galiuto L, Paraggio L, De Caterina AR, Fedele E, Locorotondo G, Leccisotti L, Giordano A, Rebuzzi AG, Crea F. Positron emission tomography in acute coronary syndromes. J Cardiovasc Transl Res. 2012;5:11-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 22. | Miller JM, Rochitte CE, Dewey M, Arbab-Zadeh A, Niinuma H, Gottlieb I, Paul N, Clouse ME, Shapiro EP, Hoe J. Diagnostic performance of coronary angiography by 64-row CT. N Engl J Med. 2008;359:2324-2336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1383] [Cited by in RCA: 1340] [Article Influence: 78.8] [Reference Citation Analysis (0)] |
| 23. | Stein PD, Yaekoub AY, Matta F, Sostman HD. 64-slice CT for diagnosis of coronary artery disease: a systematic review. Am J Med. 2008;121:715-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 128] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 24. | Litt HI, Gatsonis C, Snyder B, Singh H, Miller CD, Entrikin DW, Leaming JM, Gavin LJ, Pacella CB, Hollander JE. CT angiography for safe discharge of patients with possible acute coronary syndromes. N Engl J Med. 2012;366:1393-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 538] [Cited by in RCA: 513] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 25. | Goldstein JA, Chinnaiyan KM, Abidov A, Achenbach S, Berman DS, Hayes SW, Hoffmann U, Lesser JR, Mikati IA, O’Neil BJ, Shaw LJ, Shen MY, Valeti US, Raff GL. The CT-STAT (Coronary Computed Tomographic Angiography for Systematic Triage of Acute Chest Pain Patients to Treatment) trial. J Am Coll Cardiol. 2011;58:1414-1422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 428] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 26. | Hoffmann U, Truong QA, Schoenfeld DA, Chou ET, Woodard PK, Nagurney JT, Pope JH, Hauser TH, White CS, Weiner SG. Coronary CT angiography versus standard evaluation in acute chest pain. N Engl J Med. 2012;367:299-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 645] [Cited by in RCA: 654] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 27. | Taylor AJ, Cerqueira M, Hodgson JM, Mark D, Min J, O’Gara P, Rubin GD, Kramer CM, Berman D, Brown A. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 appropriate use criteria for cardiac computed tomography. A report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the North American Society for Cardiovascular Imaging, the Society for Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. J Am Coll Cardiol. 2010;56:1864-1894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 727] [Cited by in RCA: 704] [Article Influence: 46.9] [Reference Citation Analysis (0)] |
| 28. | Budoff MJ, Achenbach S, Blumenthal RS, Carr JJ, Goldin JG, Greenland P, Guerci AD, Lima JA, Rader DJ, Rubin GD. Assessment of coronary artery disease by cardiac computed tomography: a scientific statement from the American Heart Association Committee on Cardiovascular Imaging and Intervention, Council on Cardiovascular Radiology and Intervention, and Committee on Cardiac Imaging, Council on Clinical Cardiology. Circulation. 2006;114:1761-1791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1033] [Cited by in RCA: 1020] [Article Influence: 53.7] [Reference Citation Analysis (0)] |
| 29. | Hamon M, Morello R, Riddell JW, Hamon M. Coronary arteries: diagnostic performance of 16- versus 64-section spiral CT compared with invasive coronary angiography--meta-analysis. Radiology. 2007;245:720-731. [PubMed] |
| 30. | Vanhoenacker PK, Heijenbrok-Kal MH, Van Heste R, Decramer I, Van Hoe LR, Wijns W, Hunink MG. Diagnostic performance of multidetector CT angiography for assessment of coronary artery disease: meta-analysis. Radiology. 2007;244:419-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 305] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 31. | Almoudi M, Sun ZH. A head-to-head comparison of the coronary calcium score by computed tomography with myocardial perfusion imaging in predicting coronary artery disease. J Geriatr Cardiol. 2012;9:349-354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 32. | Patel AR, Bhave NM, Mor-Avi V. Myocardial perfusion imaging with cardiac computed tomography: state of the art. J Cardiovasc Transl Res. 2013;6:695-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 33. | Coelho-Filho OR, Rickers C, Kwong RY, Jerosch-Herold M. MR myocardial perfusion imaging. Radiology. 2013;266:701-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 34. | Becker A, Becker C. CT imaging of myocardial perfusion: possibilities and perspectives. J Nucl Cardiol. 2013;20:289-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 35. | Gould KL, Lipscomb K. Effects of coronary stenoses on coronary flow reserve and resistance. Am J Cardiol. 1974;34:48-55. [PubMed] |
| 36. | Siemers PT, Higgins CB, Schmidt W, Ashburn W, Hagan P. Detection, quantitation and contrast enhancement of myocardial infarction utilizing computerized axial tomography: comparison with histochemical staining and 99mTc-pyrophosphate imaging. Invest Radiol. 1978;13:103-109. [PubMed] |
| 37. | Hoffmann U, Millea R, Enzweiler C, Ferencik M, Gulick S, Titus J, Achenbach S, Kwait D, Sosnovik D, Brady TJ. Acute myocardial infarction: contrast-enhanced multi-detector row CT in a porcine model. Radiology. 2004;231:697-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 103] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 38. | Nikolaou K, Sanz J, Poon M, Wintersperger BJ, Ohnesorge B, Rius T, Fayad ZA, Reiser MF, Becker CR. Assessment of myocardial perfusion and viability from routine contrast-enhanced 16-detector-row computed tomography of the heart: preliminary results. Eur Radiol. 2005;15:864-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 101] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 39. | Schepis T, Achenbach S, Marwan M, Muschiol G, Ropers D, Daniel WG, Pflederer T. Prevalence of first-pass myocardial perfusion defects detected by contrast-enhanced dual-source CT in patients with non-ST segment elevation acute coronary syndromes. Eur Radiol. 2010;20:1607-1614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 40. | Bezerra HG, Loureiro R, Irlbeck T, Bamberg F, Schlett CL, Rogers I, Blankstein R, Truong QA, Brady TJ, Cury RC. Incremental value of myocardial perfusion over regional left ventricular function and coronary stenosis by cardiac CT for the detection of acute coronary syndromes in high-risk patients: a subgroup analysis of the ROMICAT trial. J Cardiovasc Comput Tomogr. 2011;5:382-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 41. | Feuchtner GM, Plank F, Pena C, Battle J, Min J, Leipsic J, Labounty T, Janowitz W, Katzen B, Ziffer J. Evaluation of myocardial CT perfusion in patients presenting with acute chest pain to the emergency department: comparison with SPECT-myocardial perfusion imaging. Heart. 2012;98:1510-1517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 42. | Spiro AJ, Haramati LB, Jain VR, Godelman A, Travin MI, Levsky JM. Resting cardiac 64-MDCT does not reliably detect myocardial ischemia identified by radionuclide imaging. AJR Am J Roentgenol. 2013;200:337-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 43. | Mor-Avi V, Lodato JA, Kachenoura N, Chandra S, Freed BH, Newby B, Lang RM, Patel AR. Quantitative three-dimensional evaluation of myocardial perfusion during regadenoson stress using multidetector computed tomography. J Comput Assist Tomogr. 2012;36:443-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 44. | Tarroni G, Corsi C, Antkowiak PF, Veronesi F, Kramer CM, Epstein FH, Walter J, Lamberti C, Lang RM, Mor-Avi V. Myocardial perfusion: near-automated evaluation from contrast-enhanced MR images obtained at rest and during vasodilator stress. Radiology. 2012;265:576-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 45. | Ko BS, Cameron JD, Leung M, Meredith IT, Leong DP, Antonis PR, Crossett M, Troupis J, Harper R, Malaiapan Y. Combined CT coronary angiography and stress myocardial perfusion imaging for hemodynamically significant stenoses in patients with suspected coronary artery disease: a comparison with fractional flow reserve. JACC Cardiovasc Imaging. 2012;5:1097-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 113] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 46. | Ko BS, Cameron JD, Meredith IT, Leung M, Antonis PR, Nasis A, Crossett M, Hope SA, Lehman SJ, Troupis J. Computed tomography stress myocardial perfusion imaging in patients considered for revascularization: a comparison with fractional flow reserve. Eur Heart J. 2012;33:67-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 154] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 47. | Bettencourt N, Rocha J, Ferreira N, Pires-Morais G, Carvalho M, Leite D, Melica B, Santos L, Rodrigues A, Braga P. Incremental value of an integrated adenosine stress-rest MDCT perfusion protocol for detection of obstructive coronary artery disease. J Cardiovasc Comput Tomogr. 2011;5:392-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 48. | Bettencourt N, Chiribiri A, Schuster A, Ferreira N, Sampaio F, Pires-Morais G, Santos L, Melica B, Rodrigues A, Braga P. Direct comparison of cardiac magnetic resonance and multidetector computed tomography stress-rest perfusion imaging for detection of coronary artery disease. J Am Coll Cardiol. 2013;61:1099-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 128] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 49. | George RT, Arbab-Zadeh A, Miller JM, Vavere AL, Bengel FM, Lardo AC, Lima JA. Computed tomography myocardial perfusion imaging with 320-row detector computed tomography accurately detects myocardial ischemia in patients with obstructive coronary artery disease. Circ Cardiovasc Imaging. 2012;5:333-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 134] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 50. | George RT, Arbab-Zadeh A, Miller JM, Kitagawa K, Chang HJ, Bluemke DA, Becker L, Yousuf O, Texter J, Lardo AC. Adenosine stress 64- and 256-row detector computed tomography angiography and perfusion imaging: a pilot study evaluating the transmural extent of perfusion abnormalities to predict atherosclerosis causing myocardial ischemia. Circ Cardiovasc Imaging. 2009;2:174-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 248] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 51. | Kwong RY, Schussheim AE, Rekhraj S, Aletras AH, Geller N, Davis J, Christian TF, Balaban RS, Arai AE. Detecting acute coronary syndrome in the emergency department with cardiac magnetic resonance imaging. Circulation. 2003;107:531-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 226] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 52. | Cury RC, Shash K, Nagurney JT, Rosito G, Shapiro MD, Nomura CH, Abbara S, Bamberg F, Ferencik M, Schmidt EJ. Cardiac magnetic resonance with T2-weighted imaging improves detection of patients with acute coronary syndrome in the emergency department. Circulation. 2008;118:837-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 171] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 53. | Miller CD, Hwang W, Hoekstra JW, Case D, Lefebvre C, Blumstein H, Hiestand B, Diercks DB, Hamilton CA, Harper EN. Stress cardiac magnetic resonance imaging with observation unit care reduces cost for patients with emergent chest pain: a randomized trial. Ann Emerg Med. 2010;56:209-219.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 54. | Pennell DJ, Sechtem UP, Higgins CB, Manning WJ, Pohost GM, Rademakers FE, van Rossum AC, Shaw LJ, Yucel EK. Clinical indications for cardiovascular magnetic resonance (CMR): Consensus Panel report. Eur Heart J. 2004;25:1940-1965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 545] [Cited by in RCA: 481] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 55. | Grothues F, Moon JC, Bellenger NG, Smith GS, Klein HU, Pennell DJ. Interstudy reproducibility of right ventricular volumes, function, and mass with cardiovascular magnetic resonance. Am Heart J. 2004;147:218-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 566] [Cited by in RCA: 541] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 56. | Schwitter J. MRI and MRA of the thoracic aorta. Appl Radiol. 2006;Suppl:6-13. |
| 57. | Laissy JP, Hyafil F, Feldman LJ, Juliard JM, Schouman-Claeys E, Steg PG, Faraggi M. Differentiating acute myocardial infarction from myocarditis: diagnostic value of early- and delayed-perfusion cardiac MR imaging. Radiology. 2005;237:75-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 149] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 58. | Mahrholdt H, Wagner A, Deluigi CC, Kispert E, Hager S, Meinhardt G, Vogelsberg H, Fritz P, Dippon J, Bock CT. Presentation, patterns of myocardial damage, and clinical course of viral myocarditis. Circulation. 2006;114:1581-1590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 661] [Cited by in RCA: 589] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 59. | Haghi D, Fluechter S, Suselbeck T, Kaden JJ, Borggrefe M, Papavassiliu T. Cardiovascular magnetic resonance findings in typical versus atypical forms of the acute apical ballooning syndrome (Takotsubo cardiomyopathy). Int J Cardiol. 2007;120:205-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 89] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 60. | Mitchell JH, Hadden TB, Wilson JM, Achari A, Muthupillai R, Flamm SD. Clinical features and usefulness of cardiac magnetic resonance imaging in assessing myocardial viability and prognosis in Takotsubo cardiomyopathy (transient left ventricular apical ballooning syndrome). Am J Cardiol. 2007;100:296-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 61. | Hochhegger B, Ley-Zaporozhan J, Marchiori E, Irion K, Souza AS, Moreira J, Kauczor HU, Ley S. Magnetic resonance imaging findings in acute pulmonary embolism. Br J Radiol. 2011;84:282-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 62. | Raman SV, Simonetti OP, Winner MW, Dickerson JA, He X, Mazzaferri EL, Ambrosio G. Cardiac magnetic resonance with edema imaging identifies myocardium at risk and predicts worse outcome in patients with non-ST-segment elevation acute coronary syndrome. J Am Coll Cardiol. 2010;55:2480-2488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 99] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 63. | Abdel-Aty H, Zagrosek A, Schulz-Menger J, Taylor AJ, Messroghli D, Kumar A, Gross M, Dietz R, Friedrich MG. Delayed enhancement and T2-weighted cardiovascular magnetic resonance imaging differentiate acute from chronic myocardial infarction. Circulation. 2004;109:2411-2416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 406] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 64. | Aletras AH, Tilak GS, Natanzon A, Hsu LY, Gonzalez FM, Hoyt RF, Arai AE. Retrospective determination of the area at risk for reperfused acute myocardial infarction with T2-weighted cardiac magnetic resonance imaging: histopathological and displacement encoding with stimulated echoes (DENSE) functional validations. Circulation. 2006;113:1865-1870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 419] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 65. | Wright J, Adriaenssens T, Dymarkowski S, Desmet W, Bogaert J. Quantification of myocardial area at risk with T2-weighted CMR: comparison with contrast-enhanced CMR and coronary angiography. JACC Cardiovasc Imaging. 2009;2:825-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 120] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 66. | Carlsson M, Ubachs JF, Hedström E, Heiberg E, Jovinge S, Arheden H. Myocardium at risk after acute infarction in humans on cardiac magnetic resonance: quantitative assessment during follow-up and validation with single-photon emission computed tomography. JACC Cardiovasc Imaging. 2009;2:569-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 160] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 67. | Hamon M, Fau G, Née G, Ehtisham J, Morello R, Hamon M. Meta-analysis of the diagnostic performance of stress perfusion cardiovascular magnetic resonance for detection of coronary artery disease. J Cardiovasc Magn Reson. 2010;12:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 212] [Cited by in RCA: 185] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 68. | Ingkanisorn WP, Kwong RY, Bohme NS, Geller NL, Rhoads KL, Dyke CK, Paterson DI, Syed MA, Aletras AH, Arai AE. Prognosis of negative adenosine stress magnetic resonance in patients presenting to an emergency department with chest pain. J Am Coll Cardiol. 2006;47:1427-1432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 195] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 69. | Schwitter J, Nanz D, Kneifel S, Bertschinger K, Büchi M, Knüsel PR, Marincek B, Lüscher TF, von Schulthess GK. Assessment of Myocardial Perfusion in Coronary Artery Disease by Magnetic Resonance: A Comparison With Positron Emission Tomography and Coronary Angiography. Circulation. 2001;103:2230e5. |
| 70. | Al-Saadi N, Nagel E, Gross M, Bornstedt A, Schnackenburg B, Klein C, Klimek W, Oswald H, Fleck E. Noninvasive detection of myocardial ischemia from perfusion reserve based on cardiovascular magnetic resonance. Circulation. 2000;101:1379-1383. |
| 71. | Kim RJ, Fieno DS, Parrish TB, Harris K, Chen EL, Simonetti O, Bundy J, Finn JP, Klocke FJ, Judd RM. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation. 1999;100:1992-2002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1774] [Cited by in RCA: 1744] [Article Influence: 67.1] [Reference Citation Analysis (0)] |
| 72. | Ricciardi MJ, Wu E, Davidson CJ, Choi KM, Klocke FJ, Bonow RO, Judd RM, Kim RJ. Visualization of discrete microinfarction after percutaneous coronary intervention associated with mild creatine kinase-MB elevation. Circulation. 2001;103:2780-2783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 295] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 73. | Nassenstein K, Breuckmann F, Bucher C, Kaiser G, Konorza T, Schäfer L, Konietzka I, de Greiff A, Heusch G, Erbel R. How much myocardial damage is necessary to enable detection of focal late gadolinium enhancement at cardiac MR imaging? Radiology. 2008;249:829-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 74. | Kim RJ, Wu E, Rafael A, Chen EL, Parker MA, Simonetti O, Klocke FJ, Bonow RO, Judd RM. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000;343:1445-1453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2376] [Cited by in RCA: 2211] [Article Influence: 88.4] [Reference Citation Analysis (0)] |
| 75. | Gerber BL, Garot J, Bluemke DA, Wu KC, Lima JA. Accuracy of contrast-enhanced magnetic resonance imaging in predicting improvement of regional myocardial function in patients after acute myocardial infarction. Circulation. 2002;106:1083-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 282] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 76. | Hombach V, Grebe O, Merkle N, Waldenmaier S, Höher M, Kochs M, Wöhrle J, Kestler HA. Sequelae of acute myocardial infarction regarding cardiac structure and function and their prognostic significance as assessed by magnetic resonance imaging. Eur Heart J. 2005;26:549-557. [PubMed] |
| 77. | Wu KC, Kim RJ, Bluemke DA, Rochitte CE, Zerhouni EA, Becker LC, Lima JA. Quantification and time course of microvascular obstruction by contrast-enhanced echocardiography and magnetic resonance imaging following acute myocardial infarction and reperfusion. J Am Coll Cardiol. 1998;32:1756-1764. [RCA] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 229] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 78. | Wu KC, Zerhouni EA, Judd RM, Lugo-Olivieri CH, Barouch LA, Schulman SP, Blumenthal RS, Lima JA. Prognostic significance of microvascular obstruction by magnetic resonance imaging in patients with acute myocardial infarction. Circulation. 1998;97:765-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 985] [Cited by in RCA: 924] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 79. | Srichai MB, Junor C, Rodriguez LL, Stillman AE, Grimm RA, Lieber ML, Weaver JA, Smedira NG, White RD. Clinical, imaging, and pathological characteristics of left ventricular thrombus: a comparison of contrast-enhanced magnetic resonance imaging, transthoracic echocardiography, and transesophageal echocardiography with surgical or pathological validation. Am Heart J. 2006;152:75-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 302] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 80. | Weinsaft JW, Kim RJ, Ross M, Krauser D, Manoushagian S, LaBounty TM, Cham MD, Min JK, Healy K, Wang Y. Contrast-enhanced anatomic imaging as compared to contrast-enhanced tissue characterization for detection of left ventricular thrombus. JACC Cardiovasc Imaging. 2009;2:969-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 171] [Article Influence: 10.7] [Reference Citation Analysis (0)] |