Revised: December 4, 2013
Accepted: January 13, 2014
Published online: February 26, 2014
Processing time: 110 Days and 19.1 Hours
Hypertrophic cardiomyopathy (HCM), the most variable cardiac disease in terms of phenotypic presentation and clinical outcome, represents the most common inherited cardiomyopathic process with an autosomal dominant trait of inheritance. To date, more than 1400 mutations of myofilament proteins associated with the disease have been identified, most of them “private” ones. This striking allelic and locus heterogeneity of the disease certainly complicates the establishment of phenotype-genotype correlations. Additionally, topics pertaining to patients’ everyday lives, such as sudden cardiac death (SCD) risk stratification and prevention, along with disease prognosis, are grossly related to the genetic variation of HCM. This review incorporates contemporary research findings and addresses major aspects of HCM, including preclinical diagnosis, genetic analysis, left ventricular outflow tract obstruction and SCD. More specifically, the spectrum of genetic analysis, the selection of the best method for obstruction alleviation and the need for a unique and accurate factor for SCD risk stratification are only some of the controversial HCM issues discussed. Additionally, future perspectives concerning HCM and myocardial ischemia, as well as atrial fibrillation, are discussed. Rather than enumerating clinical studies and guidelines, challenging problems concerning the disease are critically appraised by this review, highlighting current speculations and recommending future directions.
Core tip: Hypertrophic cardiomyopathy (HCM) represents the most common inherited cardiomyopathic process with an autosomal dominant trait of inheritance. This review incorporates contemporary research findings and addresses major and controversial aspects of HCM, including preclinical diagnosis, genetic analysis, left ventricular outflow tract obstruction, sudden cardiac death, myocardial ischemia and atrial fibrillation. Rather than enumerating clinical studies and guidelines, challenging problems concerning the disease are critically appraised by this review, highlighting current speculations and recommending future directions.
- Citation: Efthimiadis GK, Pagourelias ED, Gossios T, Zegkos T. Hypertrophic cardiomyopathy in 2013: Current speculations and future perspectives. World J Cardiol 2014; 6(2): 26-37
- URL: https://www.wjgnet.com/1949-8462/full/v6/i2/26.htm
- DOI: https://dx.doi.org/10.4330/wjc.v6.i2.26
Hypertrophic cardiomyopathy (HCM) represents the most common inherited cardiac disease, affecting 1 in every 500 people in the general population[1,2]. Classically, it is defined by the presence of a hypertrophied, non-dilated left ventricle (LV) in the absence of any cause capable of producing the magnitude of evident hypertrophy, such as pressure overload or storage/infiltrative diseases[3,4]. The main features of the disease are: (1) clinical and genetic heterogeneity, altering phenotypic expression and complicating both clinical and preclinical diagnosis; (2) obstruction, either in the left ventricular outflow tract (LVOTO) or in the midventricular level (MVO), and their pathophysiological significance; and (3) sudden cardiac death (SCD) and risk factors predisposing to it. Evaluation of the mentioned characteristics is essential in the assessment of every patient with HCM.
In this context, the aim of this review is to critically present current knowledge concerning the most controversial fields of HCM, including preclinical diagnosis, obstruction and SCD, and to briefly discuss treatment modalities that might prove useful, especially when applied in the preclinical level. Rather than enumerating clinical studies and guidelines, the authors have tried to appraise challenging problems concerning the disease, highlight current speculations and recommend future directions.
According to current guidelines, HCM diagnosis is mainly based on the detection [either by echocardiography or magnetic resonance imaging (MRI)] of a maximal wall thickness ≥ 15 mm or on the presence of a mild hypertrophy (13-14 mm) coexisting with a positive family history of HCM and/or an HCM compatible ECG[3-5]. Although diagnosis in cases of overt hypertrophy seems simplified, with the clinical distinction and differentiation of phenocopies being rather challenging, the real challenge in terms of HCM diagnostic evaluation today is preclinical diagnosis.
Preclinical diagnosis refers to the detection of subjects that carry any HCM-causing gene mutation, before or even without the development of LV hypertrophy [genotype (+)/phenotype (-) subjects]. The concept that HCM pathology may exist in the absence of LV hypertrophy is quite old[6], but the ability to recognize the presence of early myocardial changes is quite new. Although genetic testing may become the ultimate tool for assessing the risk of disease development, several issues complicate its use as a screening tool.
In 60% of cases, HCM is a familial disease with an autosomal dominant trait of inheritance. To date, more than 1400 HCM-related mutations in genes encoding different sarcomere or non-sarcomere proteins have been identified[4]. Among them, definitive HCM causative mutations are those implicating 8 sarcomere genes with approximately 80% of identified mutations concerning cardiac β-myosin heavy chain and cardiac myosin binding protein C[7-18]. Apart from the great number of mutations recognized up to now, some genetic defects, especially those concerning the cardiac myosin binding protein C gene, are founding mutations and referred to as homogeneous and closed concentrated populations. To further complicate things, the latest studies have documented that 5% of HCM families carry 2[19-21] or even 3 distinct causative mutations[22], including homozygous and double or compound heterozygous mutations. The “privacy” of many mutations (unique genetic defects inside specific families), the variable penetrance of recorded mutations allowing various phenotypic severity, the complexity of distinction between a genetic polymorphism and a causative mutation, and the involvement of multiple potential disease modifying variants[23] have led to a decrease of initial enthusiasm about the utility of genetic analysis in preclinical diagnosis. While still in search of the “Holy Grail” which is the phenotype-genotype correlation, the utility of genetic analysis is confined mostly to identify the proband’s relatives sharing the mutation and to diagnose HCM phenocopies, such as Anderson-Fabry’s disease and other glycogen or lysosomal storage diseases.
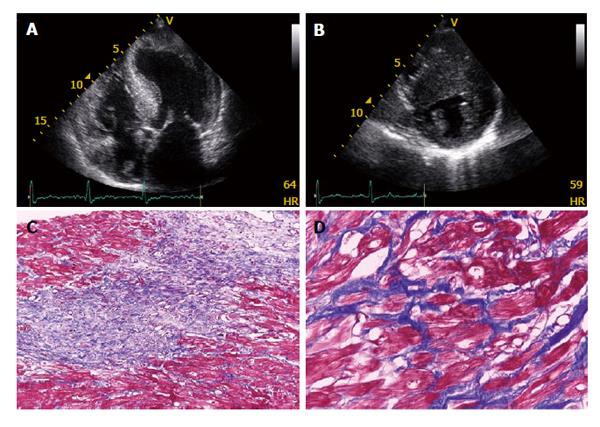
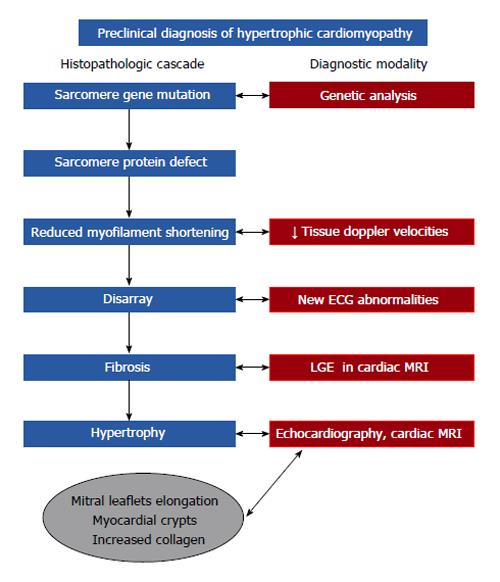
The complexity of genetic analysis has led to the adoption of other diagnostic approaches to unveil HCM in the preclinical stage, mainly by discovering disease features that precede the development of overt hypertrophy. In the excellent paper by Geisterfer-Lowrance et al[7], based on a mouse model of familial HCM, cardiac dysfunction preceded histopathological changes, myocyte disarray came next, while hypertrophy and fibrosis tended to increase with age (Figure 1). Reflecting on this experiment in clinical settings, cardiac dysfunction (detected by tissue Doppler imaging), myocyte disarray (encoded by new ECG abnormalities), hypertrophy (visualised by echocardiography or cardiac MRI) and fibrosis [detected by cardiac MRI Late Gadolinium Enhancement (LGE)] are early signs of HCM that should be properly searched for (Figure 2)[24-26]. Overall, several clinical reports have demonstrated that the majority of HCM genotype (+)/phenotype (-) subjects display “early” myocardial functional or histopathological changes, such as reduced tissue Doppler imaging-derived systolic and diastolic velocities, abnormal ECG, cardiac magnetic resonance (CMR)-visualized myocardial crypts, mitral leaflet elongation and evidence of a fibrotic state, such as increased type I procollagen synthesis, CMR-increased myocardial extracellular volume, and late gadolinium myocardial enhancement[24-27].
Preclinical diagnosis of HCM has many medical and social implications. At present, there is no evidence that early detection will change the course of the disease; however, early application of therapy may improve the lifelong management of these subjects. Experimental therapies in HCM-models using conventional medications have shown promising results on reversal or prevention of hypertrophy and fibrosis. Larger studies in clinical settings during the preclinical stage of HCM are necessary to demonstrate the potential benefit in prevention of HCM phenotypic changes or complications, including SD.
After a lasting controversy concerning its role in HCM, obstruction is evidenced to be related to severity of symptoms, especially by augmentation of gradient during exercise, in the context of diastolic dysfunction and myocardial ischemia[28]. Maron et al[29] in 2003 documented that obstruction at rest is a strong, independent predictor of progression to severe heart failure and death, while according to another study, 70% of patients are echocardiographically found to have obstruction at rest or during exercise[30]. Despite establishing a connection between LVOTO and progression to heart failure in HCM, a controversy concerning the potential impact of LVOTO on SCD survival is still ongoing. Two major studies have demonstrated that a resting gradient > 30 mmHg was associated with a 2.4-fold increase in the risk of SCD[29,31], presenting, however, a very low positive predictive value (< 10%) and a very low SCD annual rate (0.37%-1.5%)[29,31,32]. In our cohort of HCM patients, obstruction did not show a significant correlation with SCD incidence[33]. At present, obstruction at rest does not serve as a sole risk factor for SCD[34]. Probably, severe gradients (> 100 mmHg) may serve as SCD arbitrator in the context of other risk factors[4].
A minority of HCM patients present with a mid-LV level obstruction due to midventricular muscular apposition creating an hourglass-shaped LV[35-40]. MVO is associated with an unfavorable prognosis in terms of end stage HCM, SCD and lethal arrhythmic events[40,41]. A very challenging and distinct complication of midventricular obstruction is that of LV apical aneurysm formation associated with transmural myocardial scarring. Maron et al[42] and our team found a 2% prevalence of apical aneurysms in HCM patient cohorts. About 70% of patients with apical aneurysms had a midventricular, whereas the remaining 30% presented with an apical type of hypertrophy. More than 40% of patients with an apical aneurysm experienced cardiovascular complications, including SCD, appropriate implantable cardioverter-defibrillator (ICD) discharges, thromboembolic stroke and progressive heart failure-death, over a 4 years mean time of follow-up[42].
Concerning therapeutic approaches for obstruction, interventional procedures should be applied in patients who are severely symptomatic, with a maximal instantaneous gradient > 50 mmHg at rest or with physiological provocation despite optimal medical treatment (B-blockers, verapamil, disopyramide or combination there of)[4]. The major goal of pharmacological therapy in symptomatic patients with HCM is to alleviate symptoms of exertional dyspnea, palpitations and chest discomfort, which may reflect pathophysiological mechanisms such as LVOTO obstruction, reduced supply of myocardial oxygen, mitral regurgitation and impaired LV diastolic relaxation and compliance[3,43]. Beta blockers accomplish that through their negative inotropic and chronotropic effects[44] improving myocardial oxygen supply-demand relationships, prolonging the diastolic filling period, allowing for more efficient inactivation of myocardial contractile proteins and leading thus to LVOTO alleviation[45,46]. Negative inotropic and rate lowering effects are the mechanism of action for verapamil and diltiazem, whereas negative inotropic action is also the pharmacological pathway of disopyramide[4].
Surgical septal myectomy, accomplished through a transaortic approach and extended muscular resection (Morrow procedure), resulting in physical enlargement of the LV outflow has been fairly considered the gold standard of invasive therapies for relief of obstruction in severely symptomatic HCM patients[47-54]. In a major retrospective study from the Mayo Clinic, surgical myectomy performed in severely symptomatic patients with obstructive HCM was associated with a long term survival equivalent to that of the US general population and superior to survival observed in patients with obstructive HCM without operation[50]. In another study coming from the same center, surgical myectomy in patients carrying an ICD was associated with a significant reduction in the rate of appropriate ICD discharge and a reduction in the risk of SCD[52].
Alcohol septal ablation via a percutaneous intra-coronary approach uses administration of absolute ethanol to the septal perforator branch, inducing a localized infarction of the basal septum at the point of contact of the anterior mitral valve leaflet, reducing thereby the LV outflow tract gradient[53-55].
Selection of the best interventional treatment should depend on demographic, anatomic-electrical and hemodynamic criteria. More specifically, the age of the patient, operator and institutional experience on each specific method, the presence of comorbidities (chronic kidney disease, coronary artery disease, chronic pulmonary or hepatic impairment, etc.) and, last but not least, patient’s preference, are among the most crucial demographic factors influencing implementation of an interventional strategy. Additionally, the magnitude and extent of ventricular hypertrophy, dislocation of papillary muscles and their functionality, the presence of intrinsic mitral valve disease potentially demanding the need of additional surgical approaches, the complexity of coronary vasculature along with the existence or not of conduction abnormalities may influence final decisions based on an electro-anatomic and hemodynamic basis[4].
Although long-term outcome studies comparing the effectiveness and mortality after alcohol septal ablation or septal myectomy are lacking, a recent meta-analysis reviewing 12 studies comparing the two interventional techniques found no significant differences concerning short and post-adjustment long term mortality, post-intervention functional status, improvement in New York Heart Association functional class, ventricular arrhythmia occurrence, re-interventions performed and post-procedure mitral regurgitation[56]. However, septal ablation was connected to a higher post-procedure incidence of complete heart block requiring a permanent pacemaker (10%-20% vs 2% after surgery)[4], while it was found to increase the risk of right bundle branch block (RBBB). Patients with left bundle branch block and RBBB are more likely to develop complete heart block with surgery and alcohol septal ablation, respectively[4]. Finally, the percentage of patients showing a higher residual gradient was also deemed to be higher among patients having undergone septal ablation[56].
Current evidence suggests that any attempt to conduct a double blind randomized study comparing the long term effects of the 2 main therapeutic options for LVOTO in HCM would be complicated, if not impossible. Furthermore, septal ablation and septal myectomy are 2 very different techniques; the former causing ischemia and generating a scar and the latter leading to myocardial resection. The myocardial scar caused by septal ablation has aroused concern of a potentially increased risk of malignant arrhythmias. Ventricular arrhythmias have been reported as an effect of ischemia in the early post procedural phase[57,58]. However, no increased risk of malignant arrhythmias has been shown in patients who already had an ICD implanted because of a previously estimated high risk of SCD[59,60]. In a recent report, various factors, including age ≥ 65 years, gradient < 100 mmHg, septal hypertrophy ≤ 18 mm and left anterior descending artery diameter < 4.0 mm, were the strongest patient characteristics that predicted clinical success after septal alcohol ablation[61]. The increasing experience of involved tertiary centers and proper training of physicians providing both interventional treatments will diminish the rate of complications in future and significantly alter the natural course of the disease, especially among those patients presenting with more symptoms and eventually higher mortality.
SCD is the most dramatic complication of HCM. Even although primary estimates of the SCD rate emanating from tertiary center based cohorts have been as high as 6% per year, true prevalence based on data coming from large scale community registries is significantly lower, approximately 0.7% annually[3,4]. It is evident that the prevalence of SCD is higher in younger people, approximately before 35 years of age, even although according to other studies, longevity is not synonymous with immunity[62,63]. HCM related SCD is the leading mortality cause among competitive athletes following different sport disciplines[64,65]. The vast majority of SD (85%) occurs during daily activities (walking, rest, driving or during sleep), while 70% of patients dying suddenly are asymptomatic or have few symptoms (functional class I or II)[62]. Despite the fact that SCD objectively affects a small minority of HCM patients, early recognition of predisposing factors and concomitant prevention still remains a major clinical challenge since SCD and associated lethal arrhythmic events may be fully prevented, either primarily or secondarily, by means of implantable ICDs.
Apart from personal history of ventricular fibrillation (VF), sustained ventricular tachycardia (SVT) or resuscitated cardiac arrest which has been found to represent the highest risk predisposing to new potentially lethal arrhythmic events (secondary prevention)[66-68], 5 non interventional clinical factors have been identified up to now to represent risk markers for SCD in HCM: (1) Family history of SCD affecting at least one first degree relative < 40 years; (2) Syncope, without a known causal factor occurring in the recent past (< 6 mo); (3) Extreme left ventricular hypertrophy as this is represented by a maximum wall thickness of any myocardial segment > 30 mm; (4) Abnormal blood pressure response (ABPR) to exercise, defined as either a failure of systolic blood pressure to increase by at least 20 mmHg or a drop below baseline resting values during effort and even a drop of systolic pressure during maximal exercise; and (5) Non SVT (NSVT), defined as recording on ambulatory 24-h Holter of ≥ 3 consecutive ventricular ectopic beats at a rate of ≥ 120 beats lasting < 30 s[69-73]. NSVT is considered a risk factor for SCD, primarily in patients under the age of 30[74].
Recent HCM guidelines have suggested an escalation in risk stratification, suggesting that personal history of SVT or VF is Class I indication for ICD implantation[4]. Existing literature suggests that these patients have 33% mortality in 7 years[66] and that in 5 years 41% will experience SD or ICD-discharge[67]. The presence of a family history of SCD, syncope or a maximal wall thickness > 30 mm confers a Class IIa indication for ICDs, whereas NSVT or ABPR alone probably could not justify ICD implantation needing reassessment of risk profile based on the rest of risk factors or potential arbitrators[4]. Several clinical or laboratory aspects of HCM have been studied as potential risk modifiers for SCD, as shown in Table 1. Among them, 3 certain features of HCM may affect our decision in favor of ICD implantation based on evidence from published trials[4,69]: the presence of LGE on MRI[75]; certain mutations, especially coexistence of more than 1 sarcomere mutation[22]; and marked LV outflow tract obstruction at rest[4,29,31].
| Established risk markers | Risk modifiers or novel risk factors |
| Prior resuscitated cardiac arrest (VF, sustained VT) | LGE in MRI |
| MWT > 30 mm | Marked LVOTO |
| FH of SCD | Severe or multiple sarcomeric mutations |
| Syncope | Certain phenotypic expressions: Apical aneurysms, midventricular obstruction |
| NSVT | Severe systolic or diastolic impairment, e.g., burnt out HCM, restrictive pattern |
| ABPR | CAD |
| Arrhythmic substrate: Atrial fibrillation |
All of the above mentioned factors describe the very same phenomenon from a different point of view: extent of replacement and interstitial fibrosis leading to different conduction pathways in the myocardium, thus facilitating reentry events and finally malignant ventricular tachyarrhythmias[76,77]. Based on the previous assumptions, detection of LGE by MRI could be the main pillar of SCD risk stratification since it reflects the extent of fibrosis, the main determinant of malignant arrhythmias. However, a recent meta analysis concluded that LGE showed a trend towards significance for predicting SCD/aborted SCD (pooled OR = 2.39; 95%CI: 0.87-6.58, P = 0.091), failing to accurately define individual patients with HCM reaching this end point[75]. To date, there is no compelling published evidence that the extent is more important than just the presence of LGE for risk-prediction. Moreover, the 2011 current guidelines emphasize that it is the presence and not the extent of LGE that relates to adverse CV events. However, this is an interesting, controversial topic that should be addressed by future research [an ongoing multicenter trial with over 1000 HCM patients will probably show that the extent of LGE is also relevant (Martin Maron, ACC 2013)].
ICDs have proved to be effective in terminating life-threatening ventricular tachyarrhythmias in HCM, altering the natural course of the disease and prolonging life. ICDs should be offered after detailed discussion with the patient and his/her family and after benefits are anticipated to outweigh the potential risks. Data from retrospective analysis of sizeable cohorts of recipients have demonstrated that the number of risk factors prior to implantation for primary prophylaxis is disproportionate to the number and frequency of appropriate shocks delivered, while the time interval from ICD implant to first appropriate device discharge is quite variable in length since some patients have survived over 10 years after an initial episode of cardiac arrest without receiving appropriate ICD discharges[78,79]. A careful evaluation of data coming from American and European registries could easily reveal that the annual rate of ICD adverse events (including inappropriate shocks and lead complications) may range between 8.6% and 25%, at least 2-fold higher than the rate of patients receiving appropriate shocks per year[78-80]. The rate of inappropriate shocks and lead dislodgment/fractures seems to be higher among younger populations (children, adolescents), mainly due to their increased activity levels and body growth[81].
For much of the past 50 years, HCM progression was mainly connected to LVOTO and diastolic dysfunction, under appreciating (or even worse, under recognizing) myocardial ischemia as an important pathophysiological component of the disease. Even now, assessment of myocardial ischemia is currently not part of routine clinical diagnostic or management strategies in HCM[4].
Initial evidence that myocardial ischemia participates in the pathophysiological mechanism of HCM was based on post mortem studies of patients who had died suddenly and presented with extensive areas of myocardial damage. A spectrum of ischemic injury was observed, from an acute phase with coagulative necrosis and neutrophilic infiltrate to a chronic post-necrotic replacement-type fibrosis, always in the absence of atherosclerotic epicardial coronary artery disease[82]. In addition to gross pathological evidence of myocardial scarring, autopsy studies in HCM patients have shown structural abnormalities of intramural coronary arterioles, characterized by thickening of the intima and/or medial layers of the vessel wall associated with a decreased luminal cross-sectional area. These morphological changes are the main substrate for functional decompensation, which translates to blunted myocardial blood flow during stress[83-85].
Among contemporary non invasive imaging modalities that have been used for revealing impaired myocardial blood flow in HCM, PET with either 13N labeled ammonia or 15O-labeled water is the most reliable[86]. The measurement of myocardial blood flow under basal conditions and in conditions of near-maximal vasodilatation (after intravenous adenosine or dipyridamole) permits the calculation of coronary flow reserve, that is, the ratio of maximum to basal blood flow. On the other hand, although SPECT myocardial perfusion imaging is widely available, this technique is limited by allowing only the assessment of relative changes in regional perfusion and byan inability to quantify absolute MBF[87].
Similarly to PET, recent stress CMR studies in HCM showed blunted myocardial blood flow in response to stress. Importantly, areas of the myocardium in which fibrosis was present (as determined by LGE) were most often associated with reduced myocardial blood flow[88], even although a small proportion of patients had LGE in the absence of perfusion abnormalities[89]. Taken together, these CMR observations show an association between ischemia, myocardial fibrosis and LV remodeling, providing further support for the principle that abnormal blood flow caused by microvascular dysfunction is responsible for myocardial ischemia-mediated myocyte death and ultimately repair in the form of replacement fibrosis[87]. Traditional, noninvasive methods for detecting myocardial ischemia clinically, including ST-segment changes on 12-lead and ambulatory Holter electrocardiographic monitoring or exercise testing, have proved to be insufficiently sensitive or specific for detecting ischemia in HCM[87].
Verapamil and beta-blockers may improve symptoms of chest pain and exertional dyspnea in HCM. This probably occurs via reduction in heart rate and oxygen consumption and possibly because of direct effects on the microvasculature and diastolic filling leading to improved perfusion, especially in the mostly “stressed” subendocardial regions[87]. Also, since there is now evidence showing an improvement in myocardial perfusion after septal reduction therapy[90], consideration should be given to these procedures to relieve severe chest pain refractory to drug therapy[87].
Concerning the importance of revealing blunted myocardial blood flow in HCM, a previous study has shown that severe abnormalities in myocardial blood flow caused by microvascular dysfunction seemed to be a powerful determinant of impaired systolic function, whereas preserved myocardial blood flow identified the low-risk subgroup[91]. Therefore, an impaired myocardial blood flow could possibly differentiate individuals with a higher risk for progression towards a “burnt out” phenotype (dilatation and severe systolic impairment). This has important clinical implications since HCM patients in the end stage experience a high rate of unfavorable disease consequences, including progressive heart failure (often requiring heart transplantation) and SCD (prompting consideration for prophylactic ICD).
In conclusion, blunted myocardial blood flow seems to be an important component of HCM physiology. However, many current controversies need to be clarified by future research. First of all, the dynamic interaction between fibrosis and ischemia needs further study so as to define which phenomenon precedes the other in the vicious circle set. This is extremely important since identifying the stage when active myocardial ischemia begins (with respect to the development of the HCM phenotype) answers the question of whether impaired myocardial blood flow could be considered an early therapeutic target. Secondly, future research should highlight optimal non-invasive imaging modalities as well as biomarkers with sufficient sensitivity and specificity to reveal HCM patients with ischemia predisposing to disease progression. Finally, future studies should not only discover novel therapies targeting myocardial ischemia in HCM (especially for those patients presenting refractory angina to common medication), but also define the groups of patients who should mostly benefit from anti-ischemic treatment.
Patients with HCM are at increased risk of AF compared with age-matched cohorts, while AF is an important cause of symptoms, morbidity and even mortality in patients with HCM[4,62]. The 2011 ACC/AHA guidelines for diagnosis and treatment of HCM recognize the importance of AF for HCM prognosis, extrapolating, however, AF diagnostic and therapeutic options recommended for the general population in HCM patients[92].
The risk of systemic embolization is high in HCM patients with AF but does not seem to be related to the severity of symptoms[4,62]. Risk scores that seem to be efficient and therapy guiding in the general population (like CHADS2-VASc) might be less effective in HCM where other risk factors may also play an important role in predisposing to embolic events. LVOTO, SAM and of course the magnitude of LA enlargement (a common morphological feature in many HCM patients) seem to be additional factors that increase the risk for stroke[4,62]. Even although paroxysmal, persistent or chronic AF followed by a CHADS2-VASc score > 2 is a strong indication for anticoagulation with a vitamin K antagonist[92], the threshold for AF that warrants anticoagulation remains unresolved. For example, should HCM patients with a sole AF episode receive anticoagulant treatment given the high risk of thromboembolism in HCM? Is a large LA volume or volume index sufficient as a risk factor for a vitamin K antagonist prescription in HCM patients prior to AF occurrence or in AF without the presence of other risk factors? Finally, could aspirin prevent embolic episodes in HCM patients with AF and low CHADS2-VASc score?
Contemporary developments in anticoagulation and rhythm control management in AF warrant a cautious assessment before their application in HCM patients. Unfortunately, few data exist concerning the safety and efficacy of dabigatran or activated X factor inhibitors in HCM. Accordingly, the long-term benefits of radiofrequency ablation vs antiarrhythmic drugs in patients with HCM remain to be established. Furthermore, there are no data regarding the efficacy of other class I antiarrhythmic agents, sotalol or dronedarone, in HCM[4,93]. Overall, AF is an important feature of HCM pathophysiology and disease progression necessitating further research efforts to optimize existing treatment options.
Five decades following the original description of HCM, there is still a dismal paucity of data supporting pharmacological treatment strategies for this complex disease. By comparison, device-based, percutaneous and surgical treatments of LVOTO obstruction have received significantly greater attention, although rarely in a double blind randomized fashion. This can be regarded as a paradox as only a minority of patients requires surgery or a device, whereas the large majority is treated pharmacologically[94]. Additionally, few data exist concerning the therapeutic approach of HCM patients without obstruction (1/3 of the HCM population)[94].
Treatment application in the preclinical phase of HCM may have a beneficial effect, whereas treatment during the mature phase of the disease could be rather problematic since a possible regression of hypertrophy may lead to LV dilation and reduced EF[95]. Taking into consideration the pathophysiological cascade of HCM progression, early treatment options could be simply divided into 3 categories: therapies targeting impaired calcium homeostasis and related disorders; drugs blocking the results of neurohumoral response secondary to sarcomere dysfunction; and anti-fibrotic agents.
With the knowledge that altered intracellular Ca2+ handling occurs early in disease pathogenesis, diltiazem, an L-type calcium channel blocker, inhibited the development of HCM phenotype when administered to young (pre-hypertrophic) mice carrying a pathogenic myosin heavy chain mutation (αMHC403/+)[96]. Importantly, treatment initiated after the development of LV hypertrophy was unable to reverse the established phenotype in these animals[96]. In an observational study enrolling a small number (6 patients) of genotype (+)/phenotype (-) HCM patients, oral daily administration of 240 mg of diltiazem led to normalization of early diastolic and systolic velocities about 8 wk after treatment initiation[97]. Obviously, data on the actions of diltiazem in preclinical HCM patients are lacking, while an ongoing trial which is expected to terminate in December 2013 is testing the effects of diltiazem in preventing phenotypes in preclinical HCM, i.e., in subjects with identified sarcomere mutation with no overt LVH[98]. In a similar way, ranolazine is another factor that could possibly interrupt the vicious circle of calcium in preclinical HCM, deterring the establishment of an overt HCM phenotype. The drug is currently used as a metabolic modulator in ischemic heart disease[99] but further insights suggested the role of this drug as a selective inhibitor of INaL in cardiomyocytes. Tomberli et al[100] demonstrated the potential role of intracellular Na+ overload in inducing an altered Ca2+ homeostasis in HCM myocardial samples. This mechanism can play an important role in cardiac remodeling in HCM.
Statins, 3-hydroxy-3-methyl-glutaryl-CoA reductase inhibitors and angiotensin II receptor blockers (ARBs) are demonstrated to inhibit angiotensin II-mediated cardiac hypertrophy[101,102]. Senthil et al[103] treated 15 pre-hypertrophic βMHC-Q403 rabbits with Atorvastatin, 2.5 mg/kg per day, vs a placebo group for 1 year. Rabbits treated with statins did not develop hypertrophy and showed a reduction in both the myocyte cross-sectional area and collagen volume fraction. Similarly, Teekakirikul et al[104] treated pre-hypertrophic α-MHC719/+ mice with Losartan for 2 wk prior and during Cyclosporine A induction of hypertrophy which prevented the emergence of hypertrophy, non-myocyte proliferation and fibrosis. Although statins and ARBs seemed to be able to reverse hypertrophy and fibrosis and to prevent the development of the phenotype in HCM animal models, these results were not replicated in clinical trials[105,106].
The rational for using N-acetylcysteine and spironolactone in HCM comes from the demonstration of the anti-fibrotic effects of the drugs in several human tissues and animal models[107-113]. However, there are no demonstrations of efficacy of the long term treatment on humans yet.
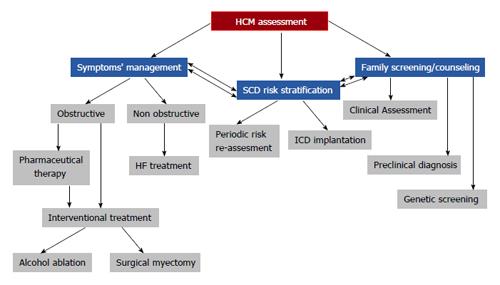
HCM assessment is based on a multilevel approach taking into consideration symptom (obstruction) management, SCD risk stratification and preclinical diagnosis/genetic screening/counseling (Figure 3). Could diagnostic evaluation and therapeutic approach be substantially improved over the next few years?
There has been a remarkable evolution during the last few years driven by the discovery of new mutations connected with the disease, expanding its known genetic database. Widespread adoption of genetic analysis, at least from tertiary referral centers, involving newer techniques such as next-generation sequencing along with the progress of bio-informatics, will help to better organize genetic bases by faster and more cost effective approaches of the responsible exons, thus bypassing the striking allelic and locus heterogeneity of the disease[114]. Based on these achievements, differentiating no disease causing polymorphisms from disease causing mutations will become significantly easier, permitting genotype-phenotype correlations from thoroughly followed up patient cohorts. The introduction of proteomics will hopefully facilitate better definition of the molecular mechanisms of the disease, identifying the pathophysiological pathways from genetic mutations to phenotypic presentation and clinical course. All the above developments will certainly highlight new therapeutic targets, which may impede genotypic expression and disease progression, and may provide a more accurate risk assessment for SCD prevention based on an individual clinical-genetic assessment.
P- Reviewers: Bravo PE, Yang Q S- Editor: Zhai HH L- Editor: Roemmele A E- Editor: Liu SQ
| 1. | Maron BJ. Hypertrophic cardiomyopathy: an important global disease. Am J Med. 2004;116:63-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 117] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 2. | Maron BJ, Gardin JM, Flack JM, Gidding SS, Kurosaki TT, Bild DE. Prevalence of hypertrophic cardiomyopathy in a general population of young adults. Echocardiographic analysis of 4111 subjects in the CARDIA Study. Coronary Artery Risk Development in (Young) Adults. Circulation. 1995;92:785-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1307] [Cited by in RCA: 1431] [Article Influence: 47.7] [Reference Citation Analysis (0)] |
| 3. | Maron BJ. Hypertrophic cardiomyopathy: a systematic review. JAMA. 2002;287:1308-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1394] [Cited by in RCA: 1421] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 4. | Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS, Naidu SS, Nishimura RA, Ommen SR, Rakowski H. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2011;58:2703-2738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 208] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 5. | Efthimiadis GK, Parcharidou D, Pagourelias ED, Meditskou S, Spanos G, Hadjimiltiades S, Pliakos C, Gavrielides S, Karvounis H, Styliadis IH. Prevalence and clinical outcomes of incidentally diagnosed hypertrophic cardiomyopathy. Am J Cardiol. 2010;105:1445-1450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | McKenna WJ, Stewart JT, Nihoyannopoulos P, McGinty F, Davies MJ. Hypertrophic cardiomyopathy without hypertrophy: two families with myocardial disarray in the absence of increased myocardial mass. Br Heart J. 1990;63:287-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 119] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Geisterfer-Lowrance AA, Kass S, Tanigawa G, Vosberg HP, McKenna W, Seidman CE, Seidman JG. A molecular basis for familial hypertrophic cardiomyopathy: a beta cardiac myosin heavy chain gene missense mutation. Cell. 1990;62:999-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 963] [Cited by in RCA: 945] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 8. | Morita H, Rehm HL, Menesses A, McDonough B, Roberts AE, Kucherlapati R, Towbin JA, Seidman JG, Seidman CE. Shared genetic causes of cardiac hypertrophy in children and adults. N Engl J Med. 2008;358:1899-1908. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 330] [Cited by in RCA: 305] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 9. | Morita H, Larson MG, Barr SC, Vasan RS, O’Donnell CJ, Hirschhorn JN, Levy D, Corey D, Seidman CE, Seidman JG. Single-gene mutations and increased left ventricular wall thickness in the community: the Framingham Heart Study. Circulation. 2006;113:2697-2705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 94] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 10. | Niimura H, Bachinski LL, Sangwatanaroj S, Watkins H, Chudley AE, McKenna W, Kristinsson A, Roberts R, Sole M, Maron BJ. Mutations in the gene for cardiac myosin-binding protein C and late-onset familial hypertrophic cardiomyopathy. N Engl J Med. 1998;338:1248-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 552] [Cited by in RCA: 495] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 11. | Niimura H, Patton KK, McKenna WJ, Soults J, Maron BJ, Seidman JG, Seidman CE. Sarcomere protein gene mutations in hypertrophic cardiomyopathy of the elderly. Circulation. 2002;105:446-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 219] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 12. | Ackerman MJ, VanDriest SL, Ommen SR, Will ML, Nishimura RA, Tajik AJ, Gersh BJ. Prevalence and age-dependence of malignant mutations in the beta-myosin heavy chain and troponin T genes in hypertrophic cardiomyopathy: a comprehensive outpatient perspective. J Am Coll Cardiol. 2002;39:2042-2048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 159] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 13. | Anan R, Greve G, Thierfelder L, Watkins H, McKenna WJ, Solomon S, Vecchio C, Shono H, Nakao S, Tanaka H. Prognostic implications of novel beta cardiac myosin heavy chain gene mutations that cause familial hypertrophic cardiomyopathy. J Clin Invest. 1994;93:280-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 181] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 14. | Van Driest SL, Ackerman MJ, Ommen SR, Shakur R, Will ML, Nishimura RA, Tajik AJ, Gersh BJ. Prevalence and severity of “benign” mutations in the beta-myosin heavy chain, cardiac troponin T, and alpha-tropomyosin genes in hypertrophic cardiomyopathy. Circulation. 2002;106:3085-3090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 117] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 15. | Watkins H, McKenna WJ, Thierfelder L, Suk HJ, Anan R, O’Donoghue A, Spirito P, Matsumori A, Moravec CS, Seidman JG. Mutations in the genes for cardiac troponin T and alpha-tropomyosin in hypertrophic cardiomyopathy. N Engl J Med. 1995;332:1058-1064. [PubMed] [DOI] [Full Text] |
| 16. | Watkins H, Rosenzweig A, Hwang DS, Levi T, McKenna W, Seidman CE, Seidman JG. Characteristics and prognostic implications of myosin missense mutations in familial hypertrophic cardiomyopathy. N Engl J Med. 1992;326:1108-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 511] [Cited by in RCA: 485] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 17. | Moolman JC, Corfield VA, Posen B, Ngumbela K, Seidman C, Brink PA, Watkins H. Sudden death due to troponin T mutations. J Am Coll Cardiol. 1997;29:549-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 240] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 18. | Available from: http: //www.cardiogenomics.med.harvard.edu. |
| 19. | Van Driest SL, Vasile VC, Ommen SR, Will ML, Tajik AJ, Gersh BJ, Ackerman MJ. Myosin binding protein C mutations and compound heterozygosity in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2004;44:1903-1910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 326] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 20. | Richard P, Charron P, Carrier L, Ledeuil C, Cheav T, Pichereau C, Benaiche A, Isnard R, Dubourg O, Burban M. Hypertrophic cardiomyopathy: distribution of disease genes, spectrum of mutations, and implications for a molecular diagnosis strategy. Circulation. 2003;107:2227-2232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 891] [Cited by in RCA: 903] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 21. | Ingles J, Doolan A, Chiu C, Seidman J, Seidman C, Semsarian C. Compound and double mutations in patients with hypertrophic cardiomyopathy: implications for genetic testing and counselling. J Med Genet. 2005;42:e59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 303] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 22. | Girolami F, Ho CY, Semsarian C, Baldi M, Will ML, Baldini K, Torricelli F, Yeates L, Cecchi F, Ackerman MJ. Clinical features and outcome of hypertrophic cardiomyopathy associated with triple sarcomere protein gene mutations. J Am Coll Cardiol. 2010;55:1444-1453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 216] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 23. | Lopes LR, Zekavati A, Syrris P, Hubank M, Giambartolomei C, Dalageorgou C, Jenkins S, McKenna W; Uk10k Consortium, Plagnol V, Elliott PM. Genetic complexity in hypertrophic cardiomyopathy revealed by high-throughput sequencing. J Med Genet. 2013;50:228-239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 165] [Cited by in RCA: 169] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 24. | Nagueh SF, Bachinski LL, Meyer D, Hill R, Zoghbi WA, Tam JW, Quiñones MA, Roberts R, Marian AJ. Tissue Doppler imaging consistently detects myocardial abnormalities in patients with hypertrophic cardiomyopathy and provides a novel means for an early diagnosis before and independently of hypertrophy. Circulation. 2001;104:128-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 398] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 25. | Lakdawala NK, Thune JJ, Maron BJ, Cirino AL, Havndrup O, Bundgaard H, Christiansen M, Carlsen CM, Dorval JF, Kwong RY. Electrocardiographic features of sarcomere mutation carriers with and without clinically overt hypertrophic cardiomyopathy. Am J Cardiol. 2011;108:1606-1613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 26. | Efthimiadis GK, Meditskou S, Parcharidis GE. Athletes with repolarization abnormalities. N Engl J Med. 2008;358:2296; author reply 2297-2298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 27. | Ho CY, López B, Coelho-Filho OR, Lakdawala NK, Cirino AL, Jarolim P, Kwong R, González A, Colan SD, Seidman JG. Myocardial fibrosis as an early manifestation of hypertrophic cardiomyopathy. N Engl J Med. 2010;363:552-563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 550] [Cited by in RCA: 521] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 28. | Maron BJ, Maron MS, Wigle ED, Braunwald E. The 50-year history, controversy, and clinical implications of left ventricular outflow tract obstruction in hypertrophic cardiomyopathy from idiopathic hypertrophic subaortic stenosis to hypertrophic cardiomyopathy: from idiopathic hypertrophic subaortic stenosis to hypertrophic cardiomyopathy. J Am Coll Cardiol. 2009;54:191-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 173] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 29. | Maron MS, Olivotto I, Betocchi S, Casey SA, Lesser JR, Losi MA, Cecchi F, Maron BJ. Effect of left ventricular outflow tract obstruction on clinical outcome in hypertrophic cardiomyopathy. N Engl J Med. 2003;348:295-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 939] [Cited by in RCA: 1005] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 30. | Maron MS, Olivotto I, Zenovich AG, Link MS, Pandian NG, Kuvin JT, Nistri S, Cecchi F, Udelson JE, Maron BJ. Hypertrophic cardiomyopathy is predominantly a disease of left ventricular outflow tract obstruction. Circulation. 2006;114:2232-2239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 636] [Cited by in RCA: 730] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 31. | Elliott PM, Gimeno JR, Tomé MT, Shah J, Ward D, Thaman R, Mogensen J, McKenna WJ. Left ventricular outflow tract obstruction and sudden death risk in patients with hypertrophic cardiomyopathy. Eur Heart J. 2006;27:1933-1941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 303] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 32. | Efthimiadis GK, Pliakos C, Pagourelias ED, Parcharidou DG, Giannakoulas G, Kamperidis V, Hadjimiltiades S, Karvounis C, Gavrielidis S, Styliadis IH. Identification of high risk patients with hypertrophic cardiomyopathy in a northern Greek population. Cardiovasc Ultrasound. 2009;7:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 33. | Efthimiadis GK, Parcharidou DG, Giannakoulas G, Pagourelias ED, Charalampidis P, Savvopoulos G, Ziakas A, Karvounis H, Styliadis IH, Parcharidis GE. Left ventricular outflow tract obstruction as a risk factor for sudden cardiac death in hypertrophic cardiomyopathy. Am J Cardiol. 2009;104:695-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 34. | Maron BJ, Olivotto I, Maron MS. The dilemma of left ventricular outflow tract obstruction and sudden death in hypertrophic cardiomyopathy: do patients with gradients really deserve prophylactic defibrillators? Eur Heart J. 2006;27:1895-1897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 35. | Falicov RE, Resnekov L, Bharati S, Lev M. Mid-ventricular obstruction: a variant of obstructive cardiomyopathy. Am J Cardiol. 1976;37:432-437. [PubMed] |
| 36. | Falicov RE, Resnekov L. Mid ventricular obstruction in hypertrophic obstructive cardiomyopathy. New diagnostic and therapeutic challenge. Br Heart J. 1977;39:701-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 45] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 37. | Fighali S, Krajcer Z, Edelman S, Leachman RD. Progression of hypertrophic cardiomyopathy into a hypokinetic left ventricle: higher incidence in patients with midventricular obstruction. J Am Coll Cardiol. 1987;9:288-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 109] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 38. | Efthimiadis GK, Pliakos C, Pagourelias ED, Parcharidou DG, Spanos G, Paraskevaidis S, Styliadis IH, Parcharidis G. Hypertrophic cardiomyopathy with midventricular obstruction and apical aneurysm formation in a single family: case report. Cardiovasc Ultrasound. 2009;7:26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 39. | Shah A, Duncan K, Winson G, Chaudhry FA, Sherrid MV. Severe symptoms in mid and apical hypertrophic cardiomyopathy. Echocardiography. 2009;26:922-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 40. | Minami Y, Kajimoto K, Terajima Y, Yashiro B, Okayama D, Haruki S, Nakajima T, Kawashiro N, Kawana M, Hagiwara N. Clinical implications of midventricular obstruction in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2011;57:2346-2355. [PubMed] |
| 41. | Efthimiadis GK, Pagourelias ED, Parcharidou D, Gossios T, Kamperidis V, Theofilogiannakos EK, Pappa Z, Meditskou S, Hadjimiltiades S, Pliakos C. Clinical characteristics and natural history of hypertrophic cardiomyopathy with midventricular obstruction. Circ J. 2013;77:2366-2374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 42. | Maron MS, Finley JJ, Bos JM, Hauser TH, Manning WJ, Haas TS, Lesser JR, Udelson JE, Ackerman MJ, Maron BJ. Prevalence, clinical significance, and natural history of left ventricular apical aneurysms in hypertrophic cardiomyopathy. Circulation. 2008;118:1541-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 383] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 43. | Spirito P, Seidman CE, McKenna WJ, Maron BJ. The management of hypertrophic cardiomyopathy. N Engl J Med. 1997;336:775-785. [PubMed] |
| 44. | Sherrid MV, Pearle G, Gunsburg DZ. Mechanism of benefit of negative inotropes in obstructive hypertrophic cardiomyopathy. Circulation. 1998;97:41-47. [PubMed] |
| 45. | Alvares RF, Goodwin JF. Non-invasive assessment of diastolic function in hypertrophic cardiomyopathy on and off beta adrenergic blocking drugs. Br Heart J. 1982;48:204-212. [PubMed] |
| 46. | Bourmayan C, Razavi A, Fournier C, Dussaule JC, Baragan J, Gerbaux A, Gay J. Effect of propranolol on left ventricular relaxation in hypertrophic cardiomyopathy: an echographic study. Am Heart J. 1985;109:1311-1316. [PubMed] |
| 47. | Maron BJ, Yacoub M, Dearani JA. Controversies in cardiovascular medicine. Benefits of surgery in obstructive hypertrophic cardiomyopathy: bring septal myectomy back for European patients. Eur Heart J. 2011;32:1055-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 48. | Maron BJ, Dearani JA, Ommen SR, Maron MS, Schaff HV, Gersh BJ, Nishimura RA. The case for surgery in obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol. 2004;44:2044-2053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 126] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 49. | Maron BJ. Controversies in cardiovascular medicine. Surgical myectomy remains the primary treatment option for severely symptomatic patients with obstructive hypertrophic cardiomyopathy. Circulation. 2007;116:196-206; discussion 206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 121] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 50. | Ommen SR, Maron BJ, Olivotto I, Maron MS, Cecchi F, Betocchi S, Gersh BJ, Ackerman MJ, McCully RB, Dearani JA. Long-term effects of surgical septal myectomy on survival in patients with obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol. 2005;46:470-476. [PubMed] |
| 51. | Morrow AG. Hypertrophic subaortic stenosis. Operative methods utilized to relieve left ventricular outflow obstruction. J Thorac Cardiovasc Surg. 1978;76:423-430. [PubMed] |
| 52. | McLeod CJ, Ommen SR, Ackerman MJ, Weivoda PL, Shen WK, Dearani JA, Schaff HV, Tajik AJ, Gersh BJ. Surgical septal myectomy decreases the risk for appropriate implantable cardioverter defibrillator discharge in obstructive hypertrophic cardiomyopathy. Eur Heart J. 2007;28:2583-2588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 147] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 53. | Fifer MA, Sigwart U. Controversies in cardiovascular medicine. Hypertrophic obstructive cardiomyopathy: alcohol septal ablation. Eur Heart J. 2011;32:1059-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 54. | Sigwart U. Catheter treatment for hypertrophic obstructive cardiomyopathy: for seniors only? Circulation. 2008;118:107-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 55. | Sorajja P, Valeti U, Nishimura RA, Ommen SR, Rihal CS, Gersh BJ, Hodge DO, Schaff HV, Holmes DR. Outcome of alcohol septal ablation for obstructive hypertrophic cardiomyopathy. Circulation. 2008;118:131-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 193] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 56. | Agarwal S, Tuzcu EM, Desai MY, Smedira N, Lever HM, Lytle BW, Kapadia SR. Updated meta-analysis of septal alcohol ablation versus myectomy for hypertrophic cardiomyopathy. J Am Coll Cardiol. 2010;55:823-834. [PubMed] |
| 57. | Alam M, Dokainish H, Lakkis N. Alcohol septal ablation for hypertrophic obstructive cardiomyopathy: a systematic review of published studies. J Interv Cardiol. 2006;19:319-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 133] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 58. | Boltwood CM, Chien W, Ports T. Ventricular tachycardia complicating alcohol septal ablation. N Engl J Med. 2004;351:1914-1915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 44] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 59. | Lawrenz T, Obergassel L, Lieder F, Leuner C, Strunk-Mueller C, Meyer Zu Vilsendorf D, Beer G, Kuhn H. Transcoronary ablation of septal hypertrophy does not alter ICD intervention rates in high risk patients with hypertrophic obstructive cardiomyopathy. Pacing Clin Electrophysiol. 2005;28:295-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 60. | Cuoco FA, Spencer WH, Fernandes VL, Nielsen CD, Nagueh S, Sturdivant JL, Leman RB, Wharton JM, Gold MR. Implantable cardioverter-defibrillator therapy for primary prevention of sudden death after alcohol septal ablation of hypertrophic cardiomyopathy. J Am Coll Cardiol. 2008;52:1718-1723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 61. | Sorajja P, Binder J, Nishimura RA, Holmes DR, Rihal CS, Gersh BJ, Bresnahan JF, Ommen SR. Predictors of an optimal clinical outcome with alcohol septal ablation for obstructive hypertrophic cardiomyopathy. Catheter Cardiovasc Interv. 2013;81:E58-E67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 62. | Maron BJ, Olivotto I, Spirito P, Casey SA, Bellone P, Gohman TE, Graham KJ, Burton DA, Cecchi F. Epidemiology of hypertrophic cardiomyopathy-related death: revisited in a large non-referral-based patient population. Circulation. 2000;102:858-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 557] [Cited by in RCA: 555] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 63. | Colan SD, Lipshultz SE, Lowe AM, Sleeper LA, Messere J, Cox GF, Lurie PR, Orav EJ, Towbin JA. Epidemiology and cause-specific outcome of hypertrophic cardiomyopathy in children: findings from the Pediatric Cardiomyopathy Registry. Circulation. 2007;115:773-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 318] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 64. | Maron BJ, Pelliccia A. The heart of trained athletes: cardiac remodeling and the risks of sports, including sudden death. Circulation. 2006;114:1633-1644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 460] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 65. | Pagourelias ED, Efthimiadis GK, Kouidi E, Fragakis N, Athyros VG, Geleris P. Athlete’s heart or hypertrophic cardiomyopathy: the dilemma is still there. Am J Cardiol. 2011;108:1841-1842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 66. | Cecchi F, Maron BJ, Epstein SE. Long-term outcome of patients with hypertrophic cardiomyopathy successfully resuscitated after cardiac arrest. J Am Coll Cardiol. 1989;13:1283-1288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 78] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 67. | Elliott PM, Sharma S, Varnava A, Poloniecki J, Rowland E, McKenna WJ. Survival after cardiac arrest or sustained ventricular tachycardia in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 1999;33:1596-1601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 155] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 68. | Maron BJ, Haas TS, Shannon KM, Almquist AK, Hodges JS. Long-term survival after cardiac arrest in hypertrophic cardiomyopathy. Heart Rhythm. 2009;6:993-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 69. | Maron BJ. Contemporary insights and strategies for risk stratification and prevention of sudden death in hypertrophic cardiomyopathy. Circulation. 2010;121:445-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 206] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 70. | Elliott PM, Poloniecki J, Dickie S, Sharma S, Monserrat L, Varnava A, Mahon NG, McKenna WJ. Sudden death in hypertrophic cardiomyopathy: identification of high risk patients. J Am Coll Cardiol. 2000;36:2212-2218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 664] [Cited by in RCA: 611] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 71. | Spirito P, Rapezzi C, Autore C, Bruzzi P, Bellone P, Ortolani P, Fragola PV, Chiarella F, Zoni-Berisso M, Branzi A. Prognosis of asymptomatic patients with hypertrophic cardiomyopathy and nonsustained ventricular tachycardia. Circulation. 1994;90:2743-2747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 101] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 72. | Gimeno JR, Tomé-Esteban M, Lofiego C, Hurtado J, Pantazis A, Mist B, Lambiase P, McKenna WJ, Elliott PM. Exercise-induced ventricular arrhythmias and risk of sudden cardiac death in patients with hypertrophic cardiomyopathy. Eur Heart J. 2009;30:2599-2605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 127] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 73. | Olivotto I, Gistri R, Petrone P, Pedemonte E, Vargiu D, Cecchi F. Maximum left ventricular thickness and risk of sudden death in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2003;41:315-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 112] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 74. | Monserrat L, Elliott PM, Gimeno JR, Sharma S, Penas-Lado M, McKenna WJ. Non-sustained ventricular tachycardia in hypertrophic cardiomyopathy: an independent marker of sudden death risk in young patients. J Am Coll Cardiol. 2003;42:873-879. [PubMed] |
| 75. | Green JJ, Berger JS, Kramer CM, Salerno M. Prognostic value of late gadolinium enhancement in clinical outcomes for hypertrophic cardiomyopathy. JACC Cardiovasc Imaging. 2012;5:370-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 331] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 76. | Marian AJ, Roberts R. The molecular genetic basis for hypertrophic cardiomyopathy. J Mol Cell Cardiol. 2001;33:655-670. [PubMed] |
| 77. | Frey N, Luedde M, Katus HA. Mechanisms of disease: hypertrophic cardiomyopathy. Nat Rev Cardiol. 2012;9:91-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 170] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 78. | Maron BJ, Shen WK, Link MS, Epstein AE, Almquist AK, Daubert JP, Bardy GH, Favale S, Rea RF, Boriani G. Efficacy of implantable cardioverter-defibrillators for the prevention of sudden death in patients with hypertrophic cardiomyopathy. N Engl J Med. 2000;342:365-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 698] [Cited by in RCA: 626] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 79. | Maron BJ, Spirito P, Shen WK, Haas TS, Formisano F, Link MS, Epstein AE, Almquist AK, Daubert JP, Lawrenz T. Implantable cardioverter-defibrillators and prevention of sudden cardiac death in hypertrophic cardiomyopathy. JAMA. 2007;298:405-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 532] [Cited by in RCA: 522] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 80. | O’Mahony C, Lambiase PD, Quarta G, Cardona M, Calcagnino M, Tsovolas K, Al-Shaikh S, Rahman SM, Arnous S, Jones S. The long-term survival and the risks and benefits of implantable cardioverter defibrillators in patients with hypertrophic cardiomyopathy. Heart. 2012;98:116-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 122] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 81. | Hauser RG, Maron BJ, Marine JE, Lampert R, Kadish AH, Winters SL, Scher DL, Biria M, Kalia A. Safety and efficacy of transvenous high-voltage implantable cardioverter-defibrillator leads in high-risk hypertrophic cardiomyopathy patients. Heart Rhythm. 2008;5:1517-1522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 82. | Basso C, Thiene G, Corrado D, Buja G, Melacini P, Nava A. Hypertrophic cardiomyopathy and sudden death in the young: pathologic evidence of myocardial ischemia. Hum Pathol. 2000;31:988-998. [PubMed] |
| 83. | Kaul S, Ito H. Microvasculature in acute myocardial ischemia: part I: evolving concepts in pathophysiology, diagnosis, and treatment. Circulation. 2004;109:146-149. [PubMed] |
| 84. | Krams R, Kofflard MJ, Duncker DJ, Von Birgelen C, Carlier S, Kliffen M, ten Cate FJ, Serruys PW. Decreased coronary flow reserve in hypertrophic cardiomyopathy is related to remodeling of the coronary microcirculation. Circulation. 1998;97:230-233. [PubMed] |
| 85. | Schwartzkopff B, Mundhenke M, Strauer BE. Alterations of the architecture of subendocardial arterioles in patients with hypertrophic cardiomyopathy and impaired coronary vasodilator reserve: a possible cause for myocardial ischemia. J Am Coll Cardiol. 1998;31:1089-1096. [PubMed] |
| 86. | Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med. 2007;356:830-840. [PubMed] |
| 87. | Maron MS, Olivotto I, Maron BJ, Prasad SK, Cecchi F, Udelson JE, Camici PG. The case for myocardial ischemia in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2009;54:866-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 203] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 88. | Petersen SE, Jerosch-Herold M, Hudsmith LE, Robson MD, Francis JM, Doll HA, Selvanayagam JB, Neubauer S, Watkins H. Evidence for microvascular dysfunction in hypertrophic cardiomyopathy: new insights from multiparametric magnetic resonance imaging. Circulation. 2007;115:2418-2425. [PubMed] |
| 89. | Bravo PE, Zimmerman SL, Luo HC, Pozios I, Rajaram M, Pinheiro A, Steenbergen C, Kamel IR, Wahl RL, Bluemke DA. Relationship of delayed enhancement by magnetic resonance to myocardial perfusion by positron emission tomography in hypertrophic cardiomyopathy. Circ Cardiovasc Imaging. 2013;6:210-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 90. | Soliman OI, Geleijnse ML, Michels M, Dijkmans PA, Nemes A, van Dalen BM, Vletter WB, Serruys PW, ten Cate FJ. Effect of successful alcohol septal ablation on microvascular function in patients with obstructive hypertrophic cardiomyopathy. Am J Cardiol. 2008;101:1321-1327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 91. | Olivotto I, Cecchi F, Gistri R, Lorenzoni R, Chiriatti G, Girolami F, Torricelli F, Camici PG. Relevance of coronary microvascular flow impairment to long-term remodeling and systolic dysfunction in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2006;47:1043-1048. [PubMed] |
| 92. | Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH, Hindricks G, Kirchhof P; ESC Committee for Practice Guidelines-CPG; Document Reviewers. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation--developed with the special contribution of the European Heart Rhythm Association. Europace. 2012;14:1385-1413. [PubMed] |
| 93. | Gaita F, Di Donna P, Olivotto I, Scaglione M, Ferrero I, Montefusco A, Caponi D, Conte MR, Nistri S, Cecchi F. Usefulness and safety of transcatheter ablation of atrial fibrillation in patients with hypertrophic cardiomyopathy. Am J Cardiol. 2007;99:1575-1581. [PubMed] |
| 94. | Spoladore R, Maron MS, D’Amato R, Camici PG, Olivotto I. Pharmacological treatment options for hypertrophic cardiomyopathy: high time for evidence. Eur Heart J. 2012;33:1724-1733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 130] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 95. | Ashrafian H, McKenna WJ, Watkins H. Disease pathways and novel therapeutic targets in hypertrophic cardiomyopathy. Circ Res. 2011;109:86-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 131] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 96. | Semsarian C, Ahmad I, Giewat M, Georgakopoulos D, Schmitt JP, McConnell BK, Reiken S, Mende U, Marks AR, Kass DA. The L-type calcium channel inhibitor diltiazem prevents cardiomyopathy in a mouse model. J Clin Invest. 2002;109:1013-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 107] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 97. | McTaggart DR. Diltiazem reverses tissue Doppler velocity abnormalities in pre-clinical hypertrophic cardiomyopathy. Heart Lung Circ. 2004;13:39-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 98. | Treatment of Preclinical Hypertrophic Cardiomyopathy With Diltiazem. Accessed December 5, 2012. Available from: http: //clinicaltrials.gov/ct2/show/NCT00319982. |
| 99. | Lee L, Horowitz J, Frenneaux M. Metabolic manipulation in ischaemic heart disease, a novel approach to treatment. Eur Heart J. 2004;25:634-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 121] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 100. | Tomberli B, Girolami F, Coppini R, Ferrantini C, Rossi A, Cecchi F, Olivotto I. [Management of refractory symptoms in hypertrophic cardiomyopathy with restrictive pathophysiology: novel perspectives for ranolazine]. G Ital Cardiol (Rome). 2012;13:297-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 101. | Oi S, Haneda T, Osaki J, Kashiwagi Y, Nakamura Y, Kawabe J, Kikuchi K. Lovastatin prevents angiotensin II-induced cardiac hypertrophy in cultured neonatal rat heart cells. Eur J Pharmacol. 1999;376:139-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 83] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 102. | Luo JD, Zhang WW, Zhang GP, Guan JX, Chen X. Simvastatin inhibits cardiac hypertrophy and angiotensin-converting enzyme activity in rats with aortic stenosis. Clin Exp Pharmacol Physiol. 1999;26:903-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 89] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 103. | Senthil V, Chen SN, Tsybouleva N, Halder T, Nagueh SF, Willerson JT, Roberts R, Marian AJ. Prevention of cardiac hypertrophy by atorvastatin in a transgenic rabbit model of human hypertrophic cardiomyopathy. Circ Res. 2005;97:285-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 124] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 104. | Teekakirikul P, Eminaga S, Toka O, Alcalai R, Wang L, Wakimoto H, Nayor M, Konno T, Gorham JM, Wolf CM. Cardiac fibrosis in mice with hypertrophic cardiomyopathy is mediated by non-myocyte proliferation and requires Tgf-β. J Clin Invest. 2010;120:3520-3529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 369] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 105. | Bauersachs J, Störk S, Kung M, Waller C, Fidler F, Hoyer C, Frantz S, Weidemann F, Ertl G, Angermann CE. HMG CoA reductase inhibition and left ventricular mass in hypertrophic cardiomyopathy: a randomized placebo-controlled pilot study. Eur J Clin Invest. 2007;37:852-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 106. | Nagueh SF, Lombardi R, Tan Y, Wang J, Willerson JT, Marian AJ. Atorvastatin and cardiac hypertrophy and function in hypertrophic cardiomyopathy: a pilot study. Eur J Clin Invest. 2010;40:976-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 107. | Tsybouleva N, Zhang L, Chen S, Patel R, Lutucuta S, Nemoto S, DeFreitas G, Entman M, Carabello BA, Roberts R. Aldosterone, through novel signaling proteins, is a fundamental molecular bridge between the genetic defect and the cardiac phenotype of hypertrophic cardiomyopathy. Circulation. 2004;109:1284-1291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 178] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 108. | de Resende MM, Kriegel AJ, Greene AS. Combined effects of low-dose spironolactone and captopril therapy in a rat model of genetic hypertrophic cardiomyopathy. J Cardiovasc Pharmacol. 2006;48:265-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 109. | MacDonald KA, Kittleson MD, Kass PH, White SD. Effect of spironolactone on diastolic function and left ventricular mass in Maine Coon cats with familial hypertrophic cardiomyopathy. J Vet Intern Med. 2008;22:335-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 110. | Zafarullah M, Li WQ, Sylvester J, Ahmad M. Molecular mechanisms of N-acetylcysteine actions. Cell Mol Life Sci. 2003;60:6-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 992] [Cited by in RCA: 996] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 111. | Poli G, Parola M. Oxidative damage and fibrogenesis. Free Radic Biol Med. 1997;22:287-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 347] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 112. | Marian AJ, Senthil V, Chen SN, Lombardi R. Antifibrotic effects of antioxidant N-acetylcysteine in a mouse model of human hypertrophic cardiomyopathy mutation. J Am Coll Cardiol. 2006;47:827-834. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 96] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 113. | Lombardi R, Rodriguez G, Chen SN, Ripplinger CM, Li W, Chen J, Willerson JT, Betocchi S, Wickline SA, Efimov IR. Resolution of established cardiac hypertrophy and fibrosis and prevention of systolic dysfunction in a transgenic rabbit model of human cardiomyopathy through thiol-sensitive mechanisms. Circulation. 2009;119:1398-1407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 100] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 114. | Elliott PM, Mohiddin SA. Almanac 2011: cardiomyopathies. The national society journals present selected research that has driven recent advances in clinical cardiology. Heart. 2011;97:1914-1919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |