Published online May 26, 2013. doi: 10.4330/wjc.v5.i5.141
Revised: April 24, 2013
Accepted: May 8, 2013
Published online: May 26, 2013
Processing time: 103 Days and 7.3 Hours
AIM: To assess role of combined modality of mechanical fragmentation and intralesional thrombolysis in patients with massive pulmonary embolism presenting subacutely.
METHODS: Eight of 70 patients presenting in tertiary care centre of North India with massive pulmonary embolism within 4 years had subacute presentation (symptom onset more than 2 wk). These patients were subjected to pulmonary angiography with intention to treat basis via mechanical breakdown and intra lesional thrombolysis. Mechanical breakdown of embolus was accomplished with 5-F multipurpose catheter to re-establish flow, followed by intralesional infusion of urokinase (4400 IU/kg over 10 min followed by 4400 IU/kg per hour over 24 h).
RESULTS: Eight patients, mean age 47.77 ± 12.20 years presented with subacute pulmonary embolism (mean duration of symptoms 2.4 wk). At presentation, mean heart rate, shock index, miller score and mean pulmonary pressures were 101.5 ± 15.2/min, 0.995 ± 0.156, 23.87 ± 3.76 and 37.62 ± 6.67 mmHg which reduced to 91.5 ± 12.2/min (P = 0.0325), 0.789 ± 0.139 (P = 0.0019), 5.87 ± 1.73 (P = 0.0000004) and 27.75 ± 8.66 mmHg (P = 0.0003) post procedurally. Mean BP improved from 80.00 ± 3.09 mmHg to 90.58 ± 9.13 mmHg (P = 0.0100) post procedurally. Minor complications in the form of local hematoma-minor hematoma in 1 (12.5%), and pseudoaneurysm (due to femoral artery puncture) in 1 (12.5 %) patient were seen. At 30 d and 6 mo follow up survival rate was 100% and all the patients were asymptomatic and in New York Heart Association class 1.
CONCLUSION: Combined modality of mechanical fragmentation and intralesional thrombolysis appears to be a promising alternative to high risk surgical procedures in patients with subacute massive pulmonary embolism.
Core tip: Patients with massive pulmonary embolism presenting subacutely (> 2 wk) have high mortality and older clots in these patients may be less amenable to thrombolysis with increased likelihood of recurrence and thromboembolic pulmonary hypertension. Eight of 70 patients with massive pulmonary embolism presenting subacutely were subjected to mechanical breakdown and intra lesional thrombolysis with urokinase (4400 IU/kg over 10 min followed by 4400 IU/kg per hour over 24 h). Post procedurally, patients documented significant improvement in hemodynamic parameters with 100% survival at 30 d and 6 mo followup. This modality appears to be a promising alternative to high risk surgical procedures in such patients.
- Citation: Mohan B, Chhabra ST, Aslam N, Wander GS, Sood NK, Verma S, Mehra AK, Sharma S. Mechanical breakdown and thrombolysis in subacute massive pulmonary embolism: A prospective trial. World J Cardiol 2013; 5(5): 141-147
- URL: https://www.wjgnet.com/1949-8462/full/v5/i5/141.htm
- DOI: https://dx.doi.org/10.4330/wjc.v5.i5.141
Massive pulmonary embolism (PE) is a life-threatening condition with a high early mortality rate due to acute right ventricular failure and cardiogenic shock[1-3]. In addition to the rapid initiation of anticoagulation therapy with intravenous (IV) unfractionated heparin, potentially life-saving therapy includes thrombolysis, surgical embolectomy, or catheter thrombectomy. The traditional window period for thrombolysis in patients presenting with acute massive pulmonary embolism is two weeks[4]. In the present review we propose another subset of patients with massive pulmonary embolism presenting subacutely (> 2 wk) who appear to benefit maximally with mechanical breakdown and thrombolysis. These patients presenting subacutely have high mortality and may not respond to standard anticoagulant or thrombolytic therapy with high likelihood of recurrence and development of thromboembolic pulmonary hypertension[5].
The present study has been conducted as an open non comparative prospective trial in the department of cardiology of our institution, a tertiary care centre in North India over a time span of four years (2007-2011). Approval for the same was obtained from ethical committee of the institute.
Eight of the 70 patients presenting with massive pulmonary embolism had subacute presentation with presenting symptoms of two to four weeks duration. Massive pulmonary embolism was defined as pulmonary arterial occlusion of more than 50% as confirmed by pulmonary angiographic score (Miller Index) and/or presence of hemodynamic impairment i.e., mean pulmonary artery pressure > 25 mmHg and/or shock index > 1. Shock index equals heart rate divided by systolic systemic blood pressure.
After obtaining bed side transthoracic echocardiography to confirm the suspicion of pulmonary embolism, to estimate pulmonary arterial pressure and to exclude right atrial or ventricular thrombi, patients underwent emergent right heart catheterization and pulmonary angiography. Patients who showed a rapid deterioration of their cardiopulmonary condition were put on oxygen supplementation with noninvasive pressure support or intubation. Positive inotropic and vasoactive support with catecholamines was supplemented according to the patient’s hemodynamic condition prior to right heart catheterization and pulmonary angiography.
The criteria for inclusion were patients who received emergency catheter directed intervention due to angiographically confirmed subacute massive PE (miller index > 0.6) with involvement of central pulmonary artery and hemodynamic shock defined as shock index (i.e., heart rate/systolic blood pressure) score of > 0.8. Patients with acute presentation (< 2 wk) and those who were hemodynamically stable (shock index < 0.8) and sub massive PE (Miller index of < 0.6 and central pulmonary artery not involved) were excluded. Echocardiographic criteria for diagnosis of subacute PE were right ventricular (RV) wall thickness > 5 mm; tricuspid regurgitant jet velocity > 3.7 m/s; the occurrence of both a dilated RV cavity with normal interventricular septal motion; an inspiratory collapse of the inferior vena cava[6].
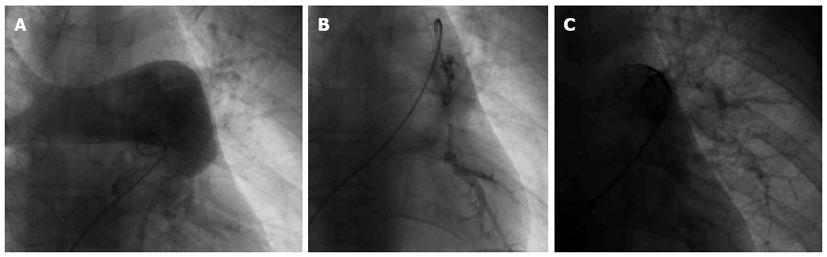
Informed, written consent was obtained. Under local anaesthesia, 5F femoral sheath was introduced in femoral vein for procedure. Initially with 5F multipurpose catheter, right heart study was performed and pulmonary artery pressure recorded. Subsequently, 5F multipurpose (cordis) catheter was used to obtain pulmonary angiogram after injecting 10-15 mL non ionic contrast dye with hand injection (Figure 1A). After confirming the diagnostic criteria, mechanical fragmentation was initiated; 0.35” guide wire was passed; multiple rotatory movements were given in embolus. Further mechanical breakdown was done with 5F multipurpose catheter and pig tail catheter (Figure 1B). The pig tail was kept inside the large significant embolus for urokinase therapy.
After ensuring flow across pulmonary artery; urokinase in dosage of 4400 IU/Kg body weight was given intralesional over 10 min and 4400 IU/kg per hour for 24 h through pig tail catheter kept in pulmonary artery. Follow up angiogram was done 24 h post procedure (Figure 1C). Patient’s blood pressure and heart rate were monitored every hour. Clinical follow up and simultaneous hemodynamic data was obtained. Shock index was calculated on hourly basis. Technical success was defined as reduction in baseline miller index following treatment. Clinical success was defined as stabilization of hemodynamic parameters, resolution of shock, complete weaning off of inotropic support and survival until discharge from the hospital.
Miller index was used to calculate the angiographic scores for the degree of pulmonary embolism[7]. The Miller score is composed of an objective score for arterial obstruction and a subjectively determined score for reduction of peripheral perfusion. The right pulmonary artery is assigned 9 and the left is assigned 7 segmental arteries. Partial or complete occlusion of a segmental artery receives a point score of 1. Proximal pulmonary embolism is scored equal to the number of segmental arteries arising distally according to the anatomic subdivisions. The maximal score for obstruction is 16. Reduction of peripheral perfusion is scored by dividing each lung into upper, middle and lower zones and using a four point scale. Maximal score of reduced perfusion in both lungs is 18. The maximal Miller score for both lungs is 34. A Miller score of 17 or more indicates a greater than 50% obstruction of pulmonary vascular bed and forms an angiographic definition of a massive PE. The Miller index is Miller score divided by 34 (range 0.0 to 1.0)[8].
The Miller index was recorded in our study at the time of initial pulmonary angiogram and after 24 h of urokinase infusion. Major procedural complications were defined as: hemorrhage requiring transfusion, perforation of cardiopulmonary structures, anaphylaxis from contrast injection, arrhythmias with hemodynamic decompensation (blocks), worsening pulmonary artery hypertension, hypoxia or shock and/or death during the procedure.
Minor complications were defined as transient catheter-induced arrhythmia, mild contrast reactions, catheter-related infection and small hematomas not requiring transfusion. Major hematoma was defined as hematoma requiring one or more blood transfusion. Minor hematoma was defined as spontaneously resolving hematoma not requiring blood transfusion. The data was analyzed using students t test for comparison of paired samples. A P value of < 0.05 was considered to be statistically significant.
Over the span of four years, 70 patients presented with massive pulmonary embolism of whom 8 (11.43%) presented subacutely (2-4 wk). There were 6 males and 2 females and the average age of patients was 47.77 ± 12.20 years. The average duration of symptoms prior to presentation in emergency was 2.4 wk (range: 2-4 wk). All the patients had tachycardia (heart rate > 100/min) and tachypnoea at the time of presentation (Table 1).
| No. | Duration | PA involved | PAP | Mean PAP | Heart rate | BP | Mean BP | SI | Miller score | |||||||
| Before | After | Before | After | Before | After | Before | After | Before | After | Before | After | Before | After | |||
| 1 | 2 wk | RPA | 88/26/48 | 58/18 | 46 | 30.66 | 84 | 72 | 100/70 | 130/70 | 80 | 90 | 0.84 | 0.55 | 22 | 3 |
| 2 | 15 d | Both PA | 46/24/34 | 28/12 | 31.33 | 17.33 | 102 | 90 | 106/72 | 110/80 | 83.33 | 90 | 0.96 | 0.81 | 26 | 8 |
| 3 | 15 d | RPA occlusion | 70/30 | 60/30 | 43.33 | 40 | 102 | 110 | 110/70 | 120/80 | 83.33 | 93.33 | 0.92 | 0.91 | 22 | 5 |
| 4 | 15 d | B/L PE (TB) | 50/24 | 40/14 | 32.66 | 22.66 | 98 | 94 | 100/70 | 130/90 | 80 | 103.33 | 0.98 | 0.72 | 25 | 5 |
| 5 | 4 wk | B/L PTE | 89/20 | 68/20 | 43 | 36 | 122 | 96 | 100/70 | 100/70 | 80 | 80 | 1.22 | 0.96 | 26 | 8 |
| 6 | 3 wk | Clot at MPA | 50/70 | 28/10 | 30 | 16 | 102 | 90 | 100/70 | 130/90 | 80 | 103.33 | 1.02 | 0.69 | 25 | 7 |
| 7 | 2 wk | B/L main segment | 78/25 | 50/24 | 42.66 | 32.66 | 122 | 102 | 100/70 | 110/70 | 80 | 83.33 | 1.22 | 0.92 | 28 | 6 |
| 8 | 2 wk | B/L | 56/20 | 50/15 | 32 | 26.66 | 80 | 78 | 100/60 | 104/70 | 73.33 | 81.33 | 0.8 | 0.75 | 25 | 5 |
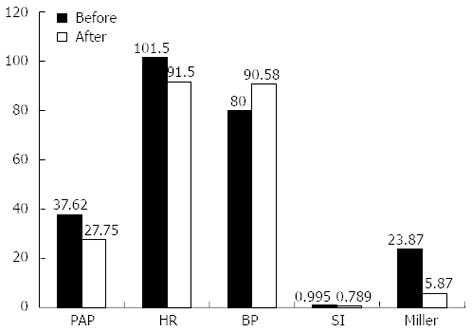
Catheter directed mechanical breakdown combined with intraembolus thrombolysis with urokinase was performed in all cases with subacute massive PE. There was a statistically highly significant fall in mean pulmonary artery pressure from 37.62 ± 6.67 to 27.75 ± 8.66 mmHg (P = 0.0003) 24 h post procedure, Mean systemic blood pressure rose significantly from 80.00 ± 3.09 mmHg to 90.58 ± 9.13 mmHg post procedure (P = 0.0100), The arterial oxygen saturation showed significant rise from base line levels 88.5% ± 2.8% to 98.6% ± 2.07% (P < 0.001). The mean heart rate prior to procedure was 101.5 ± 15.2 beats per minute. Twenty-four hours post procedure it showed a significant decrease to 91.5 ± 12.2 bpm (P = 0.0325). There was a highly significant fall in mean shock index from 0.995 ± 0.156 prior to procedure to 0.789 ± 0.139 (P = 0.0019) post procedurally. The 2 hourly change in shock index was also recorded. It was found that shock index continued to decrease up to 24 h with significant abrupt fall within first 8 h. The decrease in Miller score was highly significant when check pulmonary angiography was performed after 24 h [23.87 ± 3.76 to 5.87 ± 1.73 pre procedure (P = 0.0000004)] after 24 h (Table 2 and Figure 2).
| PAP | HR | BP | SI | Miller score | ||||||
| Before | After | Before | After | Before | After | Before | After | Before | After | |
| mean ± SD | 37.62 ± 6.67 | 27.75 ± 8.66 | 101.5 ± 15.2 | 91.5 ± 12.2 | 80.00 ± 3.09 | 90.58 ± 9.13 | 0.995 ± 0.156 | 0.789 ± 0.139 | 23.87 ± 3.76 | 5.87 ± 10.73 |
| mean ± SE | 37.62 ± 2.4 | 27.75 ± 3.1 | 101.5 ± 5.4 | 91.5 ± 4.3 | 80.00 ± 1.1 | 90.58 ± 3.2 | 0.995 ± 0.055 | 0.789 ± 0.049 | 23.87 ± 1.3 | 5.87 ± 0.61 |
| t | 6.346 | 2.659 | 3.499 | 4.809 | 17.686 | |||||
| P | 0.0003 | 0.0325 | 0.0100 | 0.0019 | 0.000000 | |||||
| df | 7 (highly significant) | 7 (significant) | 7 (significant) | 7 (highly significant) | 7 (highly significant) | |||||
Minor complications in the form of local hematoma-minor hematoma in 1 (12.5%), and pseudoaneurysm (due to femoral artery puncture) in 1 (12.5%) patient were seen.
All the patients were discharged on oral anticoagulants. At 30 d and 6 mo of follow up survival rate was 100% and all the patients were asymptomatic and in New York Heart Association class 1.
Pulmonary embolic disease can present in many ways ranging from mild pleuritic pain to sudden fatal collapse. Since the presentation is so varied, classification of these patients into different clinical subgroups using the history, echocardiography and pulmonary angiographic findings is needed before possible differences in clinical course, response to treatment, and late prognosis can be considered[9].
Major pulmonary embolism occurring insidiously over several weeks (subacute massive pulmonary embolism) has a high mortality and may not respond well to standard anticoagulant or thrombolytic treatment[5]. Treated acute massive pulmonary embolism has a good long-term prognosis[3,10-12], and recurrent pulmonary embolism, poor resolution of pulmonary artery obstruction, and the development of pulmonary hypertension are extremely rare[13,14]. This is not surprising since most of these patients have welldefined and often temporary factors predisposing to embolism, the embolus is of recent formation, and it is susceptible to both therapeutic and natural lysis. In contrast, in subacute massive pulmonary embolism the predisposing factor is often unknown and potentially might continue to operate after initial treatment, causing recurrence of emboli. Furthermore, older clot, accumulated in the pulmonary circulation over a period of weeks, might be expected to lyse less easily. If so, thromboembolic pulmonary hypertension ought to be more likely to develop in subacute rather than in acute massive pulmonary embolism. Henceforth in this subset of patients presenting subacutely, mechanical breakdown and intrapulmonary thrombolysis might be more effective than usual intravenous thrombolysis or anticoagulation. In patients with subacute PE, with hypotension and borderline hemodynamics, systemic thrombolysis might not be possible making local thrombolysis with mechanical breakdown an attractive possibility. Moreover, mechanical breakdown might be less invasive and score over the traditional surgical approach (thromboendarterectomy) for these patients.
The thrombolytic employed in our study, urokinase (UK), specifically catalyzes the cleavage of the Arg-Val bond in plasminogen to form plasmin which breaks down the fibrin polymers of blood clots. Among the plasminogen activators, UK provides a superior alternative for the simple reasons of it being more potent as compared to tissue-plasminogen activator and non-antigenic by virtue of its human origin unlike streptokinase[15]. Weitz et al[16] in a study found that UK has direct catalytic activity against fibrinogen and renders it less clottable by thrombin by releasing fibrinopeptide B, a potent chemoattractant. Henceforth they concluded that urokinase may participate in processes extending beyond fibrinolysis, a property which might especially be relevant in our patients with subacute PE and relatively older thrombus in process of organization. Moreover in a randomized controlled multicenter trial of recombinant tissue plasminogen activator[17] (rt-PA) versus urokinase in the treatment of acute pulmonary embolism, Goldhaber et al[3] found that despite rapid clot lysis at 2 h by rt-PA; at 24 h both drug regimens had produced equally good reperfusion. Also, in terms of cost and availability in developing nations UK might be a preferred option.
In our study significant reduction in shock index, Miller index and mean pulmonary artery pressure was recorded in 8 patients 24 h post procedure. The hemodynamic improvement recorded was maximum in first 8 h after procedure, though the improvement continued to occur over period of 24 h. At 6 mo of follow up survival rate was 100% and all the 8 patients were asymptomatic. The proposed mechanisms of early rapid hemodynamic improvements in our patients could be increased exposure of fibrin on clot surfaces caused by fragmentation accelerating the thrombolytic action. Also when there is total occlusion of pulmonary artery occlusion by an embolus, any fluid infused will theoretically make only evanescent contact with thrombus and be washed into the non occluded ipsilateral and contralateral pulmonary artery. After fragmentation, infused thrombolytics will have greater contact with the distal thrombus throughout the pulmonary arterial tree. This especially could be helpful in patients with subacute PE in whom older clot, accumulated in the pulmonary circulation over a period of weeks, might be expected to lyse less easily. Moreover 5F multipurpose and then pigtail catheter was employed in our study for mechanical fragmentation of this organizing old clot, which could be an added advantage[18-21].
The consensus statement recommends IV fibrinolytic therapy for patients with massive PE with low risk of bleeding complications (class IIa; level of evidence B) and for patients with submassive PE judged to have clinical evidence of adverse prognosis (new hemodynamic instability, worsening respiratory insufficiency, severe RV dysfunction, or major myocardial necrosis) and low risk of bleeding complications (class IIb; level of evidence C). Fragmentation of clot in the main or lobar pulmonary arteries to restore pulmonary perfusion alone or followed by local thrombolysis is an alternative for patients with massive PE and contraindications to fibrinolysis or who remain unstable after receiving fibrinolysis (class IIa; level of evidence C) and emergency surgical thrombectomy is unavailable or not preferred[22].
Patients presenting with systolic pulmonary artery pressures ≥ 50 mmHg at the time of acute pulmonary embolism are very likely to suffer from chronic thromboembolic pulmonary hypertension (CTEPH) even if the diagnosis has not been established earlier. Beyond 2 wk, patients with subacute massive pulmonary embolism are no longer candidates for traditional thrombolytic therapy and in presence of massive PE the modality of treatment for is pulmonary endarterectomy (PEA). When performed in experienced centers and in carefully selected patients, PEA in patients with CTEPH provides remarkable results with a periprocedural mortality rate of < 5% to 11%, nearly normalized hemodynamics, and substantial improvement in clinical symptoms[23-25]. In a comprehensive review of 1500 PEA procedures performed at a center in California, there was an almost linear relationship between preoperative pulmonary vascular resistance and perioperative mortality. In a series from France[25], the mortality rate was 4% when the preoperative pulmonary vascular resistance was < 900 dyne.s/cm5 but increased to 10% in patients with resistances between 900 and 1200 dyne.s/cm5and to 20% for higher resistances[25]. Postoperative residual pulmonary hypertension has been identified as the most important predictor of death. In the largest series published thus far, patients with a postoperative pulmonary vascular resistance > 500 dyne.s/cm5 had a mortality rate of 30.6% (15 of 49 patients), whereas those with a postoperative resistance < 500 dyne.s/cm5 had a mortality rate of 0.9% (4 of 434 patients)[24]. Taken together, these data suggest that technical operability must not necessarily confer a benefit to every patient with CTEPH. Dartevelle et al[25] have suggested that patients should be selected for PEA only if a reduction in pulmonary vascular resistance by > 50% can be predicted.
The 8 patients with massive PE presenting sub acutely who underwent mechanical breakdown and thrombolysis had significant immediate, 30 d and 6 mo improvement in hemodynamics and clinical profile. This technique is less invasive, inexpensive with probably similar if not more mortality benefits than surgical procedure (100% survival at 6 mo in this study). Moreover, it may prevent development of CTEPH in patients presenting with subacute massive pulmonary embolism in whom window period for traditional systemic thrombolysis is over and thrombus is in process of organizing. Larger, multicenter and randomised trials should be performed to further study the role of mechanical breakdown and intrapulmonary thrombolysis in this subset of patients[26,27].
In conclusion, subacute massive pulmonary embolism has a high mortality and may not respond well to standard anticoagulant or thrombolytic treatment, as older clot accumulated in the pulmonary circulation over a period of weeks might be expected to lyse less easily. With survival rate of 100%, improved hemodynamics and clinical profile at 6 mo, in this subset of patients, mechanical breakdown followed by intrapulmonary thrombolysis appears to be an attractive option. Larger, multicenter and randomised trials with longer follow up are required to study the role of this less invasive and inexpensive technique in terms of immediate mortality benefits and prevention of recurrent PE/progression to CTEPH.
In subacute massive pulmonary embolism older clots accumulated over period of weeks may be less amenable to thrombolysis with increased likelihood of recurrence and development of thromboembolic pulmonary hypertension. In these patients mechanical breakdown of thrombus followed by urokinase infusion may be cost-effective, minimally invasive, and potentially life-saving procedure by accelerating velocity of thrombolysis and increasing surface area of clot being lysed. Moreover, combined modality of mechanical fragmentation and intralesional thrombolysis appears to be a promising alternative to high risk surgical procedures in patients with subacute massive pulmonary embolism.
Though not many areas are involved in studying the combined modality of mechanical breakdown and intralesional thrombolysis in patients with subacute massive pulmonary embolism; Kuo et al, based on a recent meta-analysis of 594 patients from 35 nonrandomized studies (six prospective with 94 patients, 29 retrospective with 500 patients) reported pooled clinical success rate from catheter based therapy to be 86.5% (95%CI: 82.2-90.2) in patients with massive pulmonary embolism. Moreover, pulmonary endarteractomy is being performed in patients with chronic thromboembolic pulmonary hypertension at specialized centers in California and France and have reported an almost linear relationship between preoperative pulmonary vascular resistance and perioperative mortality.
This is the first study conducted in the patients of subacute massive pulmonary embolism with mechanical breakdown and thrombolysis as a method of treatment. Prior studies on combined modality of mechanical breakdown and intralesional thrombolysis have involved patients with acute massive pulmonary embolism. Moreover at our tertiary care centre in North India, authors have reported excellent outcomes when this modality was employed in patients with failed thrombolysis as published in JOIC.
The study highlights the role of combined modality of mechanical breakdown and intralesional thrombolysis in patients with subacute massive pulmonary embolism. This modality is an attractive, less invasive alternative and might help to avoid surgical management (pulmonary endarterectomy) with excellent long term results in these patients. Moreover, in developing nations where cost is an important issue, this technique appears to be cost effective.
Subacute pulmonary embolism: Patients with massive pulmonary embolism presenting subacutely i.e., more than 2 wk from symptom onset. Massive pulmonary embolism: Defined as pulmonary arterial occlusion of more than 50% as confirmed by pulmonary angiographic score (miller index) and/or presence of hemodynamic impairment i.e., mean pulmonary artery pressure > 25 mmHg and/or shock index > 1. Shock index equals heart rate divided by systolic systemic blood pressure.
The paper is interesting and well written, my major comment refers to overall presentation of data: this paper is a report of 8 cases and should be presented as is.
P- Reviewer Leroyer C S- Editor Gou SX L- Editor A E- Editor Zhang DN
| 1. | Heit JA, Silverstein MD, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ. Predictors of survival after deep vein thrombosis and pulmonary embolism: a population-based, cohort study. Arch Intern Med. 1999;159:445-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 489] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 2. | Wood KE. Major pulmonary embolism: review of a pathophysiologic approach to the golden hour of hemodynamically significant pulmonary embolism. Chest. 2002;121:877-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 542] [Cited by in RCA: 516] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 3. | Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER). Lancet. 1999;353:1386-1389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1917] [Cited by in RCA: 1830] [Article Influence: 70.4] [Reference Citation Analysis (0)] |
| 4. | Daniels LB, Parker JA, Patel SR, Grodstein F, Goldhaber SZ. Relation of duration of symptoms with response to thrombolytic therapy in pulmonary embolism. Am J Cardiol. 1997;80:184-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 119] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 5. | Ellis DA, Neville E, Hall RJ. Subacute massive pulmonary embolism treated with plasminogen and streptokinase. Thorax. 1983;38:903-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Kasper W, Geibel A, Tiede N, Bassenge D, Kauder E, Konstantinides S, Meinertz T, Just H. Distinguishing between acute and subacute massive pulmonary embolism by conventional and Doppler echocardiography. Br Heart J. 1993;70:352-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 69] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | Miller GA, Sutton GC, Kerr IH, Gibson RV, Honey M. Comparison of streptokinase and heparin in treatment of isolated acute massive pulmonary embolism. Br Med J. 1971;2:681-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 265] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 8. | Schmitz-Rode T, Janssens U, Duda SH, Erley CM, Günther RW. Massive pulmonary embolism: percutaneous emergency treatment by pigtail rotation catheter. J Am Coll Cardiol. 2000;36:375-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 109] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 9. | Horan LG, Flowers NC, Havelda CJ. Relation between right ventricular mass and cavity size: an analysis of 1500 human hearts. Circulation. 1981;64:135-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Kasper W, Konstantinides S, Geibel A, Olschewski M, Heinrich F, Grosser KD, Rauber K, Iversen S, Redecker M, Kienast J. Management strategies and determinants of outcome in acute major pulmonary embolism: results of a multicenter registry. J Am Coll Cardiol. 1997;30:1165-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 714] [Cited by in RCA: 633] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 11. | Lobo JL, Zorrilla V, Aizpuru F, Uresandi F, Garcia-Bragado F, Conget F, Monreal M. Clinical syndromes and clinical outcome in patients with pulmonary embolism: findings from the RIETE registry. Chest. 2006;130:1817-1822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 12. | Lin BW, Schreiber DH, Liu G, Briese B, Hiestand B, Slattery D, Kline JA, Goldhaber SZ, Pollack CV. Therapy and outcomes in massive pulmonary embolism from the Emergency Medicine Pulmonary Embolism in the Real World Registry. Am J Emerg Med. 2012;30:1774-1781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 13. | Hall RJ, Sutton GC, Kerr IH. Long-term prognosis of treated acute massive pulmonary embolism. Br Heart J. 1977;39:1128-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 69] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Paraskos JA, Adelstein SJ, Smith RE, Rickman FD, Grossman W, Dexter L, Dalen JE. Late prognosis of acute pulmonary embolism. N Engl J Med. 1973;289:55-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 106] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Kunamneni A, Ravuri BD, Ellaiah P, Prabhakhar T, Saisha V. Urokinase-A strong plasminogen activator–a review. Biotechnol Mol Biol Rev. 2008;3:58-70. |
| 16. | Weitz JI, Leslie B. Urokinase has direct catalytic activity against fibrinogen and renders it less clottable by thrombin. J Clin Invest. 1990;86:203-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Urokinase versus tissue plasminogen activator in pulmonary embolism. Lancet. 1988;2:691-692. [PubMed] |
| 18. | Brady AJ, Crake T, Oakley CM. Percutaneous catheter fragmentation and distal dispersion of proximal pulmonary embolus. Lancet. 1991;338:1186-1189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 74] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Schmitz-Rode T, Günther RW, Pfeffer JG, Neuerburg JM, Geuting B, Biesterfeld S. Acute massive pulmonary embolism: use of a rotatable pigtail catheter for diagnosis and fragmentation therapy. Radiology. 1995;197:157-162. [PubMed] |
| 20. | Schmitz-Rode T, Janssens U, Schild HH, Basche S, Hanrath P, Günther RW. Fragmentation of massive pulmonary embolism using a pigtail rotation catheter. Chest. 1998;114:1427-1436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 67] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Tajima H, Murata S, Kumazaki T, Nakazawa K, Abe Y, Komada Y, Niggemann P, Takayama M, Tanaka K, Takano T. Hybrid treatment of acute massive pulmonary thromboembolism: mechanical fragmentation with a modified rotating pigtail catheter, local fibrinolytic therapy, and clot aspiration followed by systemic fibrinolytic therapy. AJR Am J Roentgenol. 2004;183:589-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 42] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Jaff MR, McMurtry MS, Archer SL, Cushman M, Goldenberg N, Goldhaber SZ, Jenkins JS, Kline JA, Michaels AD, Thistlethwaite P. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation. 2011;123:1788-1830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1460] [Cited by in RCA: 1566] [Article Influence: 111.9] [Reference Citation Analysis (0)] |
| 23. | Klepetko W, Mayer E, Sandoval J, Trulock EP, Vachiery JL, Dartevelle P, Pepke-Zaba J, Jamieson SW, Lang I, Corris P. Interventional and surgical modalities of treatment for pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43:73S-80S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 121] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 24. | Jamieson SW, Kapelanski DP, Sakakibara N, Manecke GR, Thistlethwaite PA, Kerr KM, Channick RN, Fedullo PF, Auger WR. Pulmonary endarterectomy: experience and lessons learned in 1,500 cases. Ann Thorac Surg. 2003;76:1457-162; discussion 1457-162;. [PubMed] |
| 25. | Dartevelle P, Fadel E, Mussot S, Chapelier A, Hervé P, de Perrot M, Cerrina J, Ladurie FL, Lehouerou D, Humbert M. Chronic thromboembolic pulmonary hypertension. Eur Respir J. 2004;23:637-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 305] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 26. | Kuo WT, Gould MK, Louie JD, Rosenberg JK, Sze DY, Hofmann LV. Catheter-directed therapy for the treatment of massive pulmonary embolism: systematic review and meta-analysis of modern techniques. J Vasc Interv Radiol. 2009;20:1431-1440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 333] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 27. | Mohan B, Mahajan V, Chhabra ST. Combined modality of mechanical breakdown and intraembolus thrombolysis in failed systemic thrombolysis of subacute pulmonary embolism patients. J Interv Cardiol. 2010;23:479-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |