Published online Oct 26, 2013. doi: 10.4330/wjc.v5.i10.394
Revised: September 20, 2013
Accepted: October 11, 2013
Published online: October 26, 2013
Processing time: 129 Days and 10.6 Hours
The important role of atherosclerosis in pathophysiology of Alzheimer’s Disease has become evident. Mechanisms such as hyperlipidemia, inflammation, abdominal obesity and insulin resistance are important yet they may not fully explain the specific involvement of the Circle of Willis in these pathologies. The Circle of Wills is a complex geometrical structure which has several areas with different curvature as well as various branching angles of vessels composing the circle. The hemodynamics in this region should take into account the Dean number which indicates the influence of curvature on the resistance to blood flow. Thus, areas with various curvature and angles may have different hemodynamics and there are certain areas in the Circle of Willis that are more likely to develop atherosclerotic changes. Therefore, this could suggest the novel pathophysiological pathway resulting from the geometric peculiarities of the Circle of Willis. One of the directions of future research is to examine whether specific areas of the Circle of Willis are more likely to develop atherosclerotic changes compared to other ones. Selective areas of the Circle of Willis affected by atherosclerotic changes could indicate the primary role of atherosclerosis promoting Alzheimer’s disease although other pathophysiological mechanisms suggesting the opposite direction should be also examined in prospective studies.
Core tip: The Dean number can become an important local pathophysiological mechanism that can help to explain the specific involvement of the Circle of Willis in atherosclerosis and Alzheimer’s Disease as anatomically different parts of the Circle of Willis would exhibit various degree of the curvature which would predispose to Alzheimer’s disease. This could possibly explain some sporadic cases of Alzheimer’s disease in the presence of minimal damage from atherosclerosis as well as open up new avenues for prevention of sporadic Alzheimer’s disease.
- Citation: Ismailov RM. Circle of Willis atherosclerosis, Alzheimer’s disease and the Dean number. World J Cardiol 2013; 5(10): 394-396
- URL: https://www.wjgnet.com/1949-8462/full/v5/i10/394.htm
- DOI: https://dx.doi.org/10.4330/wjc.v5.i10.394
The important role of atherosclerosis in pathophysiology of Alzheimer’s disease has become evident. Studies that examined an association between the Circle of Willis atherosclerosis, Alzheimer’s disease and some other neurodegenerative conditions are examples of important research directions focused on probable influence of various vascular factors on Alzheimer’s disease[1,2]. On the other hand, those studies suggest that these pathologies could share some common pathophysiological mechanisms that yet need to be investigated. Some of such mechanisms such as hyperlipidemia, inflammation, abdominal obesity and insulin resistance were described by authors as probable candidates[1,2]. However, although all these factors are very important, they may not fully explain the specific involvement of the Circle of Willis in these pathologies.
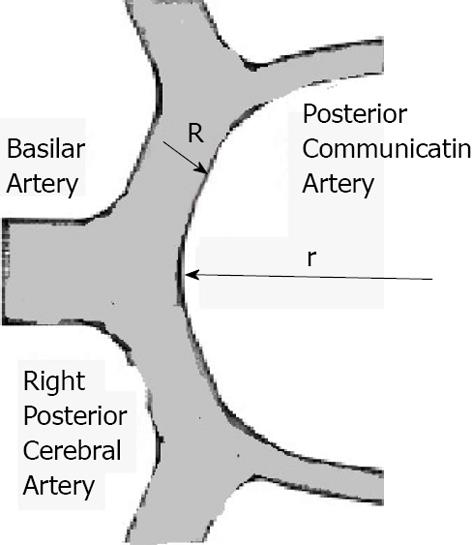
The Circle of Wills is a complex geometrical structure which has several areas with different curvature as well as various branching angles of vessels composing the circle. On the other hand, there are multiple anatomical variations of the Circle of Willis[3]. When a fluid runs through branching pipes a change of the its direction happens and similarly, when blood flows through the branching area in the Circle of Willis it changes direction. In general, taking into account that blood flow in the cardiovascular system is mostly laminar and the fact that branching areas of many arterial bifurcations have various angles, several hemodynamic factors (i.e., radius of curvature of internal wall at branching area, Reynolds number, diameters of bifurcating vessels, etc.) should be taken into account[4]. One of them is the degree of curvature or the Dean number (Di). The Dean number indicates the influence of curvature on the resistance to blood flow[4,5]. If flow is laminar, then the Dean number is determined as: D = 0.5 Re{[R(R/r;)]1/2} Where Re indicates Reynolds number, R is a radius of the vessel, r is a radius of the curvature[4] (Figure 1).
Thus, areas with various curvature and angles may have different hemodynamics. For example, hemodynamics in the area where the degree of curvature is substantial could be described by the so called “hemodynamic shade” zone[6]. This zone can be characterized by a secondary flow and a boundary, therefore, there is a significant deterioration of mass exchange due to the attachment of stacks of erythrocytes (rouleaux) to the vascular wall[6]. This could deteriorate the permeability of the endothelium and decrease the rate of removal of various particles such as lipids and lipoproteins, which in turn can lead to the formation of lipid stripes directed along the blood flow and located in the “hemodynamic shade” of the original attached rouleaux. This could also explain why hyperlipidemia could be one of the non specific yet contributing pathophysiological mechanisms in the development of the Circle of Willis atherosclerosis. Therefore, there are certain areas in the Circle of Willis that are more likely to develop atherosclerotic changes. As mentioned earlier, other factors such as hyperlipidemia or abdominal obesity should be taken into account as well.
Subsequently, with the development of atherosclerosis, vascular wall in the certain areas of the Circle of Willis (i.e., with substantial curvature) becomes less elastic and more rigid. This could result in the deterioration in the cyclic changes in the vascular wall deformation produced by cardiac contractions, and, therefore, in the performance of a “deformation pump”[7]. The operating principle of this pump is in the cyclic creation of the boundary layer and its separation[7]. This deformation pump is important to consider as it could influence the dynamics of the regional brain extravascular extracellular fluid which was previously studied with regard to amyloid beta-protein, amyloid-beta building blocks for plaques and subsequent involvement in neurodegeneration[8]. Such consideration of regional brain extravascular extracellular fluid dynamics is also particularly important in light of the fact that certain waste products such as glutamate or calcium can accumulate there causing degradation of certain cellular components thus playing an important role in the pathogenesis of Alzheimer’s disease[9,10]. A consideration of both the deformation pump and extravascular extracellular fluid could become an important link between Alzheimer’s disease and atherosclerosis.
All this could suggest the novel pathophysiological pathway resulting from the geometric peculiarities of the Circle of Willis. One of the directions of future research is to examine whether specific areas of the Circle of Willis are more likely to develop atherosclerotic changes compared to other ones. Selective areas of the Circle of Willis affected by atherosclerotic changes could indicate the primary role of atherosclerosis promoting Alzheimer’s disease. On the other hand, other pathophysiological mechanisms that could explore local factors (i.e., the Dean number) and suggesting the opposite direction should be also examined in prospective studies. For example, anatomically “different” parts of the Circle of Willis (i.e., narrowed branching areas) would exhibit various degree of the Dean number and this would predispose to Alzheimer’s disease. This could possibly explain some sporadic cases of Alzheimer’s disease in the presence of minimal damage from atherosclerosis in this area. More importantly, this would open up new avenues for prevention of sporadic Alzheimer’s disease in the light of the fact that this is an emerging health concern in the elderly. In addition, certain rheological factors such as blood viscosity should be taken into account as a contributing pathophysiological mechanism as well. In conclusion, more studies are needed to examine the common pathophysiological mechanisms related to both Alzheimer’s disease and various vascular pathologies. Such common pathophysiological pathways should take into account multiple factors such as hyperlipidemia, insulin resistance, certain local rheological and hemodynamic factors as well as potentially new contributing factors established in future research.
P- Reviewers Goldhammer E, Moriarty P S- Editor Wen LL L- Editor A E- Editor Yan JL
| 1. | Roher AE, Esh C, Kokjohn TA, Kalback W, Luehrs DC, Seward JD, Sue LI, Beach TG. Circle of willis atherosclerosis is a risk factor for sporadic Alzheimer’s disease. Arterioscler Thromb Vasc Biol. 2003;23:2055-2062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 215] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 2. | Yarchoan M, Xie SX, Kling MA, Toledo JB, Wolk DA, Lee EB, Van Deerlin V, Lee VM, Trojanowski JQ, Arnold SE. Cerebrovascular atherosclerosis correlates with Alzheimer pathology in neurodegenerative dementias. Brain. 2012;135:3749-3756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 204] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 3. | Kapoor K, Singh B, Dewan LI. Variations in the configuration of the circle of Willis. Anat Sci Int. 2008;83:96-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 132] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 4. | Caro CG, Pedley TJ. The Mechanics of the Circulation. Oxford, New York: Oxford University Press 1978; . |
| 5. | Ismailov RM. Arch vessel injury: geometrical considerations. Implications for the mechanism of traumatic myocardial infarction II. World J Emerg Surg. 2006;1:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Ismailov RM. Mathematical model of blunt injury to the vascular wall via formation of rouleaux and changes in local hemodynamic and rheological factors. Implications for the mechanism of traumatic myocardial infarction. Theor Biol Med Model. 2005;2:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Ismailov RM. New insights into the mechanism of Alzheimer’s disease: A multidisciplinary approach. In: Amazon Kindle 2009; . |
| 8. | Meyer-Luehmann M, Stalder M, Herzig MC, Kaeser SA, Kohler E, Pfeifer M, Boncristiano S, Mathews PM, Mercken M, Abramowski D. Extracellular amyloid formation and associated pathology in neural grafts. Nat Neurosci. 2003;6:370-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 104] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 9. | Cirrito JR, May PC, O’Dell MA, Taylor JW, Parsadanian M, Cramer JW, Audia JE, Nissen JS, Bales KR, Paul SM. In vivo assessment of brain interstitial fluid with microdialysis reveals plaque-associated changes in amyloid-beta metabolism and half-life. J Neurosci. 2003;23:8844-8853. [PubMed] |
| 10. | Khachaturian ZS. The role of calcium regulation in brain aging: reexamination of a hypothesis. Aging (Milano). 1989;1:17-34. [PubMed] |