Revised: December 21, 2011
Accepted: December 28, 2011
Published online: March 26, 2012
AIM: To test the efficacy of a proprietary nutraceutical combination in reducing insulin resistance associated with the metabolic syndrome (MetS).
METHODS: Sixty-four patients with MetS followed at a tertiary outpatient clinic were randomly assigned to receive either placebo or a proprietary nutraceutical combination (AP) consisting of berberine, policosanol and red yeast rice, in a prospective, double-blind, placebo-controlled study. Evaluations were performed at baseline and after 18 wk of treatment. The homeostasis model assessment of insulin resistance (HOMA-IR) index was the primary outcome measure. Secondary endpoints included lipid panel, blood glucose and insulin fasting, after a standard mixed meal and after an oral glucose tolerance test (OGTT), flow-mediated dilation (FMD), and waist circumference.
RESULTS: Fifty nine patients completed the study, 2 withdrew because of adverse effects. After 18 wk there was a significant reduction in the HOMA-IR index in the AP group compared with placebo (ΔHOMA respectively -0.6 ± 1.2 vs 0.4 ± 1.9; P < 0.05). Total and low density lipoprotein cholesterol also significantly decreased in the treatment arm compared with placebo (Δlow density lipoprotein cholesterol -0.82 ± 0.68 vs -0.13 ± 0.55 mmol/L; P < 0.001), while triglycerides, high density lipoprotein cholesterol, and the OGTT were not affected. In addition, there were significant reductions in blood glucose and insulin after the standard mixed meal, as well as an increase in FMD (ΔFMD 1.9 ± 4.2 vs 0 ± 1.9 %; P < 0.05) and a significant reduction in arterial systolic blood pressure in the AP arm.
CONCLUSION: This short-term study shows that AP has relevant beneficial effects on insulin resistance and many other components of MetS.
- Citation: Affuso F, Mercurio V, Ruvolo A, Pirozzi C, Micillo F, Carlomagno G, Grieco F, Fazio S. A nutraceutical combination improves insulin sensitivity in patients with metabolic syndrome. World J Cardiol 2012; 4(3): 77-83
- URL: https://www.wjgnet.com/1949-8462/full/v4/i3/77.htm
- DOI: https://dx.doi.org/10.4330/wjc.v4.i3.77
Metabolic syndrome (MetS) is a clustering of components associated with type 2 diabetes mellitus (T2D) and increased risk of cardiovascular (CV) events[1]. Although there are different criteria for the identification of MetS, scientific societies agree that abdominal obesity, impaired glucose metabolism, dyslipidemia and arterial hypertension represent the key components[2]. Estimates of its prevalence may vary depending on the definition, but the resulting increase in morbidity and mortality is a matter of great concern[3]. Even though MetS is characterized by wide phenotypic and biologic heterogeneity, insulin resistance and visceral obesity are the key features of the syndrome[1,4].
Since no single pathogenetic pathway has to date been identified as a valuable therapeutic target in the syndrome, current management still addresses the various components of MetS individually, by means of both lifestyle modifications and pharmacological therapy. This kind of approach is frequently burdened by therapeutic failure and patient frustration, and most often requires a multi-drug regime; therefore, the need of a process-oriented, disease-modifying treatment aimed at attenuating disease progression and reducing the risk of CV events is increasingly recognized.
A recent study by our group has investigated the effects of a proprietary nutraceutical combination (AP) on lipids and endothelial function, demonstrating how a treatment with a single tablet containing berberine (BRB), policosanol, and red yeast rice (RYR) significantly lowered total and low density lipoprotein cholesterol (LDL), simultaneously improving endothelial function, in a population with mild to moderate hypercholesterolemia. In addition, a subgroup of insulin-resistant patients showed significant improvements in the homeostasis model assessment of insulin resistance (HOMA-IR), QUICKI, and McAuley indices[5]. Since BRB has already shown effects on lipid metabolism, T2D, insulin resistance, and nitric oxide production[6-8], we hypothesized that its synergistic action with policosanols and RYR might be useful in the management of the different components of the MetS. Therefore, the aim of this study was to investigate the effects of AP in patients with MetS.
The study was a prospective, single-center, randomized, double-blind, placebo-controlled trial consisting of a screening visit and an 18 wk treatment period.
The study protocol was approved by the Ethics Committee of the University of Naples “Federico II”, and written informed consent was obtained from each patient. The study was conducted in accordance with the principles of the Declaration of Helsinki. The trial was registered at clinicaltrials.gov with ID NCT01087632.
Study participants were recruited between September 2009 and February 2010 at the outpatient clinic of our department.
Eligibility criteria were: (1) age between 18 and 65 years; (2) diagnosis of metabolic syndrome, defined as the presence of a waist circumference > 102 cm (male), > 88 cm (female), associated with at least two of the following: triglycerides ≥ 1.7 mmol/L; high density lipoprotein (HDL) < 1.03 mmol/L (male), < 1.29 mmol/L (female); systolic blood pressure ≥ 130 mmHg or diastolic blood pressure ≥ 85 mmHg or need for antihypertensive therapy; fasting glucose ≥ 5.6 mmol/L; and (3) ability to understand and give informed consent to clinical experimentation.
Exclusion criteria were: (1) proven intolerance to any component of AP; (2) pregnancy or breastfeeding; (3) treatment with hypoglycemic agents; (4) moderate to severe liver dysfunction (Child B-C); (5) abnormal renal function (serum creatinine greater than 2 mg/dL); (6) serum triglycerides > 5.6 mmol/L; and (7) a history or current symptoms of heart failure.
The nutraceutical combination used in this study consisted of a single tablet containing BRB 500 mg, RYR (monacolin K 3 mg), and policosanol 10 mg (AP, Armolipid Plus®, Rottapharm Madaus, Italy).
Patients with concomitant diseases were included provided their clinical conditions and treatment had been stable during the previous 6 mo. Sixty four patients meeting the eligibility criteria were enrolled in the study. Patients were randomized to receive either one tablet of AP or placebo once daily after supper. The placebo tablet, identical in taste and appearance to the AP tablet, consisted of inactive compound and did not contain any carbohydrates. Randomization and blinding were provided by Rottapharm Madaus SpA (Monza, Italy), which also funded the study.
The primary endpoint was the absolute change from baseline of the HOMA-IR index. Secondary endpoints were the reductions in fasting and post-prandial glucose and insulin levels, waist circumference, total cholesterol, LDL and triglycerides (TG), and the improvement in endothelial-dependent flow-mediated dilation (FMD) in relation to AP treatment.
Initial screening included medical history, physical examination, evaluation of anthropometric parameters, routine blood tests, serum renal and hepatic markers, measurements of serum insulin, glucose and lipids concentrations.
Study assessments were performed at baseline and after 18 wk of treatment. Patients were evaluated after an overnight fast of 14 h, with a physical examination and medical history; arterial blood pressure, anthropometric and impedentiometric parameters were measured. Endothelial-dependent dilation was assessed with the FMD of the brachial artery using Doppler ultrasonography, according to the Guidelines of the International Brachial Artery Reactivity Task Force[9]. The forearm was occluded by cuff inflation to at least 50 mmHg above systolic pressure for 5 min, resulting in a reactive hyperemia after the release of the cuff, and the increased shear stress led to endothelial-mediated vasodilatation. FMD was measured with an ultrasound scanner with a 7.5 MHz linear transducer (Toshiba PLT-704AT); five consecutive discrete measurements were obtained and averaged into the final value at each time point. Later, a blood sample was taken for biochemical measurements of glucose, insulin (radioimmunoassay method), lipids (total, LDL, HDL cholesterol and TG), serum renal and hepatic markers (creatinine, aspartate aminotransferase; alanine aminotransferase). Then, all the patients received a standard mixed meal, and blood samples were collected at 30, 60 and 120 min after meal consumption for the evaluation of serum glucose and insulin levels. The next day a standard oral glucose tolerance test (OGTT) was performed. The HOMA-IR index was calculated as follows: HOMA-IR = [fasting glucose (in mg/dL)/18] × [fasting insulin (in μUI/mL)/22.5].
All patients received dietary counseling from a specialist. At baseline, patients were asked to maintain their usual diet. Then, after 6 wk of treatment, they received an individualized isocaloric diet based on estimated ideal weight. Concomitant medications and adverse events were monitored throughout the study.
The study was powered in accordance with a predetermined statistical analysis plan. A sample size of 30 patients in each of the 2 study arms was calculated based on a predicted dropout rate of 6%, a power of 0.8, and an α-error probability of 0.05, to detect an absolute between-group difference of 0.8 in the change of HOMA-IR index given an expected standard deviation (SD) of variations of 1 between changes in the primary endpoint variable after treatment.
The paired-samples t test was used for within-group comparisons. The unpaired, two-tailed t test was used for between-group comparisons of baseline characteristics and changes after treatment. The data are presented as mean ± SD.
Sixty four patients meeting the eligibility criteria were randomized to receive a tablet of AP or placebo once a day after supper. Fifty nine patients (29 in the AP arm and 30 in the placebo arm) completed the study, whereas 3 were lost to follow-up and 2 withdrew from the study (one in the AP group and one in the P group) because of non-serious adverse events (constipation).
Patients’ clinical characteristics was shown in Table 1. At baseline, the 2 groups were comparable in age, sex, smoking status, concomitant medications, anthropometric parameters, lipid levels. In the placebo group, 8 patients were being treated with statins, and 25 patients with antihypertensive drugs (40% with β-blockers, 92% with angiotensin receptor blockers (ARB) or angiotensin converting enzyme inhibitors (ACE-I)). Similarly, in the AP group, 8 patients were being treated with statins, and 27 patients with antihypertensive drugs (41% with β-blockers, 96% with ARB or ACE-I). Baseline fasting glucose levels were significantly higher in the AP arm compared with the placebo group. However, the HOMA-IR index was comparable in the 2 groups.
| AP | Placebo | |
| Number (M/F) | 29 (20/9) | 30 (18/12) |
| Age | 53 ± 7 | 50 ± 12 |
| Weight (kg) | 90 ± 13 | 96 ± 18 |
| BMI | 32.2 ± 4.6 | 34.7 ± 5.1 |
| Waist circumference (cm) | 110 ± 9 | 115 ± 13 |
| Systolic blood pressure (mmHg) | 125 ± 13 | 125 ± 14 |
| Diastolic blood pressure (mmHg) | 78 ± 8 | 81 ± 8 |
| Fasting glucose (mmol/L) | 5.72 ± 1.22 | 4.72 ± 0.67a |
| Fasting insulin (pmol/L) | 90 ± 42 | 90 ± 69 |
| HOMA-IR | 3.2 ± 1. 5 | 2.7 ± 2.2 |
| Triglycerides (mmol/L) | 1.76 ± 0.86 | 1.92 ± 0. 83 |
| Total cholesterol (mmol/L) | 5.40 ± 1.00 | 5.09 ± 1.03 |
| HDL cholesterol (mmol/L) | 1.08 ± 0.26 | 1.18 ± 0.35 |
| LDL cholesterol (mmol/L) | 3.49 ± 0.19 | 3.05 ± 1. 00 |
| Flow mediated dilation (%) | 6.8 ± 3.1 | 6.8 ± 1.9 |
| Concomitant medications (n) | ||
| ACE-I/ARB | 26/29 | 24/30 |
| Statins | 8/29 | 8/30 |
| Beta-blockers | 10/29 | 10/30 |
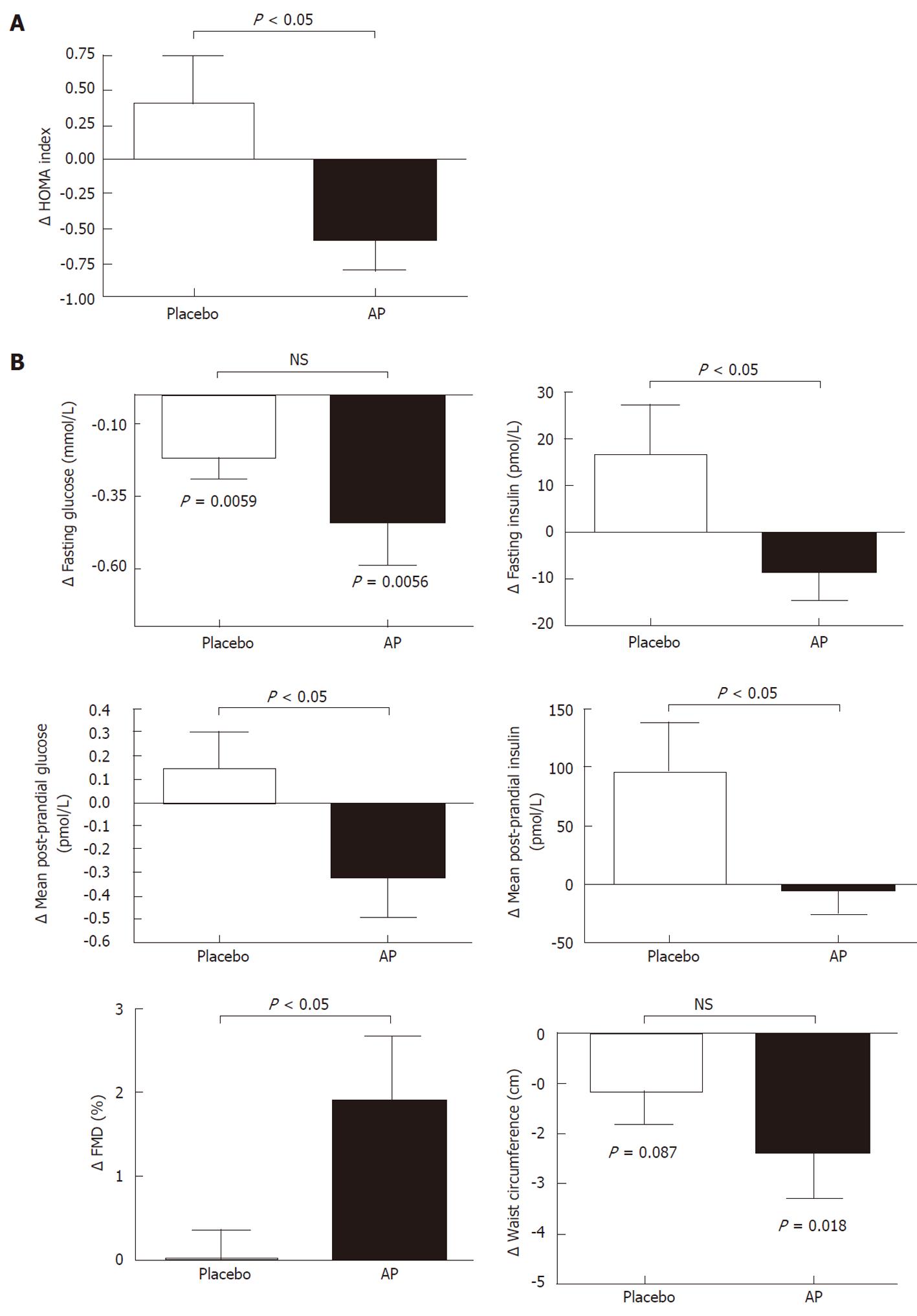
After 18 wk of treatment, the comparison of absolute changes from baseline between the 2 groups showed a significant decrease in the HOMA-IR index in the AP arm (from 3.2 ± 1.5 to 2.6 ± 1.3 vs from 2.7 ± 2.2 to 3.2 ± 2.6, P < 0.05) (Table 2, Figure 1A). Among secondary endpoints, although no significant difference was observed between the 2 arms in the reduction of fasting glucose (from 5.72 ± 1.22 to 5.28 ± 1.11 mmol/L vs from 4.72 ± 0.67 to 4.55 ± 0.67 mmol/L, P > 0.05), fasting insulin decreased significantly in the AP group as compared with placebo (from 90 ± 42 to 76 ± 42 pmol/L vs from 90 ± 69 to 111 ± 90 pmol/L, P < 0.05) (Figure 1B). In addition, there was a significant improvement in the post-prandial mean blood glucose and insulin levels in the AP arm compared with placebo (respectively from 7.00 ± 1.89 to 6.67 ± 1.61 mmol/L vs from 5.89 ± 1.33 to 6.05 ± 0.89 mmol/L, and from 306 ± 125 to 298 ± 159 pmol/L vs from 409 ± 194 to 507 ± 347 pmol/L, P < 0.05 for both) ((Figure 1B). No significant variations were observed for OGTT parameters both within-group and between-group.
| AP | Placebo | ||||||||
| Baseline | 18-wk | Within-group difference vs baseline (P) | Baseline | 18-wk | Within-group difference vs baseline (P) | Δ from baseline AP | Δ from baseline placebo | Between-group comparison of Δ (P) | |
| Number (M/F) | 29 (20/9) | 30 (18/12) | |||||||
| Age | 53 ± 7 | 50 ± 11.9 | |||||||
| Smoker | 3/29 | 3/30 | |||||||
| Weight (kg) | 90 ± 13 | 88 ± 12 | 0.008 | 96 ± 18 | 95 ± 18 | 0.011 | -1.6 ± 3.1 | -1.3 ± 2.6 | 0.657 |
| BMI (kg/m2) | 32.2 ± 4.6 | 31.7 ± 4.4 | 0.013 | 34.7 ± 5.1 | 34.2 ± 5.1 | 0.008 | -0.5 ± 1.1 | -0.5 ± 0.9 | 0.805 |
| Waist circumference (cm) | 110 ± 9 | 107 ± 9 | 0.018 | 115 ± 13 | 114 ± 13 | 0.087 | -2.4 ± 5.1 | -1.2 ± 3.6 | 0.301 |
| Systolic blood pressure (mmHg) | 125 ± 13 | 120 ± 9 | 0.037 | 125 ± 14 | 126 ± 12 | 0.585 | -5 ± 14 | 1 ± 12 | 0.047 |
| Diastolic blood pressure (mmHg) | 78 ± 8 | 75 ± 8 | 0.087 | 81 ± 8 | 78 ± 7 | 0.084 | -3 ± 8 | -3 ± 10 | 0.753 |
| Fasting glucose (mmol/L) | 5.72 ± 1.22 | 5.28 ± 1.11 | 0.006 | 4.72 ± 0.67 | 4.55 ± 0.67 | 0.006 | -0.44 ± 0.78 | -0.22 ± 0.39 | 0.186 |
| Fasting Insulin (pmol/L) | 90 ± 42 | 76 ± 42 | 0.165 | 90 ± 69 | 111 ± 90 | 0.119 | -14 ± 35 | 21 ± 57 | 0.042 |
| HOMA-IR | 3.2 ± 1.5 | 2.6 ± 1.3 | 0.019 | 2.8 ± 2.2 | 3.2 ± 2.6 | 0.259 | -0.6 ± 1.2 | 0.4 ± 1.9 | 0.023 |
| Mean postprandial glucose (mmol/L) | 7.00 ± 1.89 | 6.67 ± 1.61 | 0.085 | 5.89 ± 1.33 | 6.05 ± 0.89 | 0.309 | -0.33 ± 1.00 | 0.16 ± 0.78 | 0.046 |
| Mean postprandial Insulin (pmol/L) | 306 ± 125 | 298 ± 159 | 0.793 | 409 ± 194 | 507 ± 347 | 0.017 | -7 ± 115 | 100 ± 215 | 0.023 |
| OGTT Glucose at 2 h (mmol/L) | 8.89 ± 3.44 | 8.33 ± 3.00 | 0.216 | 6.94 ± 1.94 | 7.17 ± 1.89 | 0.478 | -10 ± 47 | 4 ± 25 | 0.153 |
| Triglycerides (mmol/L) | 1.76 ± 0.86 | 1.73 ± 0.85 | 0.933 | 1.92 ± 0.83 | 2.07 ± 1.28 | 0.375 | -0.01 ± 0.89 | 0.17 ± 1.03 | 0.467 |
| Total cholesterol (mmol/L) | 5.40 ± 1.00 | 4.60 ± 0.59 | < 0.001 | 5.09 ± 1.03 | 5.08 ± 0.82 | 0.253 | -0.82 ± 0.76 | -0.13 ± 0.60 | < 0.001 |
| HDL cholesterol (mmol/L) | 1.08 ± 0.26 | 1.08 ± 0.23 | 0.757 | 1.18 ± 0.35 | 1.1 ± 0.28 | 0.054 | 0.00 ± 0.13 | -0.08 ± 0.21 | 0.074 |
| LDL cholesterol (mmol/L) | 3.49 ± 0.19 | 2.69 ± 0.54 | < 0.001 | 3.05 ± 1 | 2.93 ± 0.99 | 0.200 | -0.82 ± 0.68 | -0.13 ± 0.55 | < 0.001 |
| Flow mediated dilation (%) | 6.8 ± 3.1 | 8.7 ± 3.3 | 0.021 | 6.8 ± 1.9 | 6.8 ± 2.1 | 0.982 | 1.9 ± 4.2 | 0 ± 1.9 | 0.032 |
Total cholesterol and LDL significantly decreased compared with placebo (respectively from 209 ± 38 to 178 ± 23 mg/dL vs from 198 ± 40 to 193 ± 31 mg/dL, and from 136 ± 8 to 105 ± 21 mg/dL vs from 119 ± 38 to 114 ± 38 mg/dL, P < 0.001), while no significant change was observed in HDL and TG levels (Figure 1B). Subjects who received AP had a significant improvement in FMD, whereas no change was observed in the placebo group (Table 2, Figure 1B); the mean values of the baseline brachial artery diameter were comparable between groups.
A slight reduction in body weight was observed in both groups. Waist circumference was significantly reduced in the AP group compared with baseline (from 110.3 ± 9.5 cm to 107.9 ± 8.6 cm, P < 0.001), but no significance was found compared with placebo.
After 18 wk of treatment, systolic blood pressure significantly decreased in the AP group, compared with placebo (from 125 ± 13 to 120 ± 9 mmHg vs from 125 ± 14 to 126 ± 12 mmHg, P < 0.05).
No changes in renal and hepatic parameters were observed throughout the study period. AP was generally well tolerated and no serious adverse event occurred.
This study shows that AP is effective and safe in reducing insulin resistance in a group of patients with MetS. We observed a significant reduction in the HOMA-IR index after 18 wk of treatment; this finding was accompanied by an improvement of postprandial glucose handling, as well as by beneficial effects on multiple clinical and biochemical parameters of MetS.
The HOMA index is a well validated surrogate measure of insulin resistance, and a strong predictor of CV risk in several classes of patients[10,11]. Mounting evidence from the last years supports the importance of the HOMA-IR index as a prognosticator even at values very close to the normal range, i.e., in patients not fitting a diagnosis of T2D. Recent studies demonstrated its independent value in the prediction of CV events in the general adult non-diabetic population[12,13]. In light of these epidemiological findings, our results appear strengthened in their clinical significance, strongly advocating further research in the field. The effect of AP on insulin resistance may be chiefly explained by the action of BRB on glucose metabolism. BRB has been used for more than 2000 years in traditional Asian medicine for the treatment of many unrelated disorders including diabetes mellitus. Two randomized controlled trials have investigated, to date, the metabolic effects of BRB on glucose metabolism in T2D patients: in both studies BRB was effective in improving glucose control and indices of insulin resistance, to an extent similar to commonly employed hypoglycemic agents, which were used as control[6,14]. Our results concerning insulin resistance substantially confirm that found in these reports in a different, “less advanced” population of patients with MetS. Of note, the dose of BRB employed in the aforementioned clinical experiences is much higher than the one used in our study (approximately twice as high).
Several experimental studies recently provided insights into the pharmacodynamic basis of such therapeutic effects of BRB, in which it differs substantially from all the most prescribed molecules in the field. In fact, BRB activates AMP-activated protein kinase leading to metabolic gene regulation, with beneficial effects on adipose tissue and muscle[7]; moreover, the AMP-mediated activation reduces insulin secretion by pancreatic β-cells[15]. Another recognized mechanism is the upregulation of insulin-receptor expression through protein kinase C activation[16]. On the other hand, evidence from both in vivo and in vitro studies suggest that part of the antihyperglycemic activity of BRB is due to a decrease in the availability of glucose after a meal. In particular, BRB suppresses intestinal disaccharidases, reducing the intestinal absorption of glucose[17]. This latter effect is very interesting and may explain the slight but significant reduction in postprandial glycemia observed in the treated group.
AP treatment led to a significant reduction in total and LDL cholesterol, confirming previous reports[5,18]. Interestingly, the therapeutic effect seemed evident also in those patients who were already under treatment with statins. These results are extremely meaningful in light of the recommendation to reduce LDL-cholesterol below 100 mg/dL to reduce the risk of CV events in patients with MetS. Both BRB and RYR monotherapy have been proven to reduce blood lipid levels at higher doses, even being advocated by many as a first-line therapy for statin-intolerant subjects; once again, the doses used in the present trial were much lower than the ones commonly used in the past monotherapy studies[19,20].
The positive effects on both glucose and lipid metabolism were accompanied by a marked improvement in endothelial function. In fact, this study shows a significant increase in FMD values in the AP group compared with the placebo group. Such a finding may arise not only from the improvement of metabolic alterations, but also from the demonstrated antiproliferative and vasodilatatory effects of BRB[21,22]. Moreover, BRB has shown beneficial effects on endothelial function also by inducing upregulation of the endothelial progenitor cells related to nitric oxide production. The importance of this improvement of vascular reactivity seems also reinforced by the unexpected reduction of blood pressure values in the treated arm.
In both groups, a slight reduction in body weight was observed and the magnitude of this reduction was comparable. This effect may be related to the diet. Nevertheless this slight weight loss in the placebo arm was not associated with an improvement in metabolic parameters, suggesting a beneficial effect of AP independent of weight loss. In addition, patients in the active arm also showed a trend to reduced waist circumference, which was not observed in the placebo arm. We can postulate that this reflects a better disposal of fat, with a relative reduction of visceral fat in the AP arm. The effect on waist circumference confirms previous results in animal models, in which treatment with BRB led to a significant reduction in abdominal fat[23]. However, we also acknowledge that study design and duration did not aim at demonstrating effects on anthropometric parameters.
In conclusion, we demonstrated that a combination of BRB, RYR and policosanol exerts beneficial effects on all components of MetS, despite the short duration of the study and the low doses of the individual components; the clinical benefit seems pleiotropic, involving both markers of insulin-resistance, dyslipidemia, and endothelial function. The treatment was well tolerated with negligible side effects.
Management of MetS is nowadays based on lifestyle intervention and treatment of its individual components. Effective prevention is based on strategizing health policies and mass intervention programs; anyway, given its high prevalence and significance, an effective therapy to contrast the cluster of components of MetS and reduce risk at the patient level is increasingly felt as an urgent need; if our results are confirmed by larger studies with harder outcome measures, we believe that nutraceuticals may play an important role in such a scenario, given their strong rationale, pleiotropic action, efficacy, and tolerability. Upcoming research shall also focus on dose ranging, patient selection and association studies with other “pharmaceutical” molecules, i.e., statins, polyunsaturated fatty acids, and metformin, possibly in the context of large multicenter trials.
The results of this study should be interpreted in light of some limitations. This was a short-term study, while metabolic interventions may require longer periods to assess stable beneficial effects. On one hand, this could have led to an underestimation of the actual effects of chronic treatment; on the other hand, some long-term safety and tolerability questions, as well as efficacy on hard clinical endpoints, remain unanswered and need further investigation.
The improvement in the HOMA-IR index could have been influenced, at least in part, by the dietary intervention; however, patients in the placebo arm, who followed the same diet, experienced a slight worsening of the index. Another point is that, although this was a randomized study, fasting glucose levels were not well balanced between the 2 groups; since the groups appeared comparable regarding all other measures of insulin resistance, including the predefined primary endpoint, this inequality seems to be acceptable and justified by the wide phenotypic variability of MetS.
The present study shows that the administration of AP in a group of MetS patients is safe and effective in reducing more than one feature of MetS. Further studies are needed to investigate whether long-term treatment with this kind of nutraceutical combination may prevent CV and T2D complications.
Part of the results of the present study were first presented at the American College of Cardiology 2011 Scientific Sessions (New Orleans, LA).
Metabolic syndrome is responsible for a large part of the increase in the incidence of cardiovascular events developed countries have been witnessing in the last decades. Patients are mostly treated by means of lifestyle modification, counseling and multiple drug therapies, with frequent nonadherence and treatment failure. In this paper, the authors assessed the clinical benefits of a nutraceutical containing berberine, policosanol, and red yeast rice, on insulin resistance and several other parameters of metabolic syndrome, on top of current guideline-oriented therapy.
Research has long been focusing on integrated pleiotropic medications and drug combinations in metabolic syndrome. However, most pharmacological approaches to date have been addressing the consequences of the disease (i.e., lipid levels and platelet hyperaggregability) more than its underlying mechanisms (i.e., insulin resistance, visceral obesity). On the other hand, nutritional or nutraceutical supplements have to date proven of little or no benefit in this patient population.
The findings can be of great interest to the readers, since authors demonstrate that a low dose of a nutraceutical combination effectively and safely reduces insulin resistance in patients with metabolic syndrome, with a general effect on clinical and biochemical parameters; these changes were accompanied by a significant improvement in vascular reactivity and systolic blood pressure. The addition of nutraceutics to lifestyle modification and pharmacological therapy might offer an innovation in this field, given the strong rationale, pleiotropic action, efficacy and good tolerability of these compounds.
The applicability of the results could be surprisingly wide, because of the availability of this specific nutraceutical combination in several countries, and its good safety and tolerability profiles. Single, isolated experiences on selected patients seem to be warranted, especially in the setting of primary prevention and in individuals refusing “traditional” drug therapy. Nonetheless, authors must acknowledge that much larger and long-term studies will be needed to expand indications to the whole metabolic syndrome-population in a more systematic fashion. The current scenario of clinical research in primary prevention would probably require years and thousands of patients to test such treatments on hard event-based endpoints; however, large trials based on well-validated surrogate endpoints will certainly follow in the near future.
The term “nutraceutical” derives form the words “nutrition” and “pharmaceutical”, and it can be defined as a product isolated or purified from foods, generally sold in medicinal forms, and demonstrated to have a medical benefit or to provide protection against chronic diseases. Many studies have investigated the beneficial properties of some of these substances on metabolic alterations, and the assessment of the most recent scientific evidence in the field led us to explore the potential efficacy on insulin resistance of a nutraceutical combination of berberine, red yeast rice, and policosanols.
This paper is well-written and interesting.
Peer reviewer: Hiroki Teragawa, MD, PhD, Department of Cardiovascular Medicine, Hiroshima University Graduate School of Biomedical Sciences, 1-2-3 Kasumi, Minami-ku, Hiroshima 734-8551, Japan
S- Editor Cheng JX L- Editor Cant MR E- Editor Li JY
| 1. | Cornier MA, Dabelea D, Hernandez TL, Lindstrom RC, Steig AJ, Stob NR, Van Pelt RE, Wang H, Eckel RH. The metabolic syndrome. Endocr Rev. 2008;29:777-822. [PubMed] |
| 2. | Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001;285:2486-2497. [PubMed] |
| 3. | Malik S, Wong ND, Franklin SS, Kamath TV, L'Italien GJ, Pio JR, Williams GR. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation. 2004;110:1245-1250. [PubMed] |
| 5. | Affuso F, Ruvolo A, Micillo F, Saccà L, Fazio S. Effects of a nutraceutical combination (berberine, red yeast rice and policosanols) on lipid levels and endothelial function randomized, double-blind, placebo-controlled study. Nutr Metab Cardiovasc Dis. 2010;20:656-661. [PubMed] |
| 6. | Zhang Y, Li X, Zou D, Liu W, Yang J, Zhu N, Huo L, Wang M, Hong J, Wu P. Treatment of type 2 diabetes and dyslipidemia with the natural plant alkaloid berberine. J Clin Endocrinol Metab. 2008;93:2559-2565. [PubMed] |
| 7. | Lee YS, Kim WS, Kim KH, Yoon MJ, Cho HJ, Shen Y, Ye JM, Lee CH, Oh WK, Kim CT. Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes. 2006;55:2256-2264. [PubMed] |
| 8. | Xu MG, Wang JM, Chen L, Wang Y, Yang Z, Tao J. Berberine-induced upregulation of circulating endothelial progenitor cells is related to nitric oxide production in healthy subjects. Cardiology. 2009;112:279-286. [PubMed] |
| 9. | Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257-265. [PubMed] |
| 10. | Hanley AJ, Williams K, Stern MP, Haffner SM. Homeostasis model assessment of insulin resistance in relation to the incidence of cardiovascular disease: the San Antonio Heart Study. Diabetes Care. 2002;25:1177-1184. [PubMed] |
| 11. | Bonora E, Formentini G, Calcaterra F, Lombardi S, Marini F, Zenari L, Saggiani F, Poli M, Perbellini S, Raffaelli A. HOMA-estimated insulin resistance is an independent predictor of cardiovascular disease in type 2 diabetic subjects: prospective data from the Verona Diabetes Complications Study. Diabetes Care. 2002;25:1135-1141. [PubMed] |
| 12. | Nakamura K, Sakurai M, Miura K, Morikawa Y, Ishizaki M, Yoshita K, Kido T, Naruse Y, Nakagawa H. Homeostasis model assessment of insulin resistance and the risk of cardiovascular events in middle-aged non-diabetic Japanese men. Diabetologia. 2010;53:1894-1902. [PubMed] |
| 13. | Bonora E, Kiechl S, Willeit J, Oberhollenzer F, Egger G, Meigs JB, Bonadonna RC, Muggeo M. Insulin resistance as estimated by homeostasis model assessment predicts incident symptomatic cardiovascular disease in caucasian subjects from the general population: the Bruneck study. Diabetes Care. 2007;30:318-324. [PubMed] |
| 14. | Zhang H, Wei J, Xue R, Wu JD, Zhao W, Wang ZZ, Wang SK, Zhou ZX, Song DQ, Wang YM. Berberine lowers blood glucose in type 2 diabetes mellitus patients through increasing insulin receptor expression. Metabolism. 2010;59:285-292. [PubMed] |
| 15. | Zhou L, Wang X, Shao L, Yang Y, Shang W, Yuan G, Jiang B, Li F, Tang J, Jing H. Berberine acutely inhibits insulin secretion from beta-cells through 3',5'-cyclic adenosine 5'-monophosphate signaling pathway. Endocrinology. 2008;149:4510-4518. [PubMed] |
| 16. | Kong WJ, Zhang H, Song DQ, Xue R, Zhao W, Wei J, Wang YM, Shan N, Zhou ZX, Yang P. Berberine reduces insulin resistance through protein kinase C-dependent up-regulation of insulin receptor expression. Metabolism. 2009;58:109-119. [PubMed] |
| 17. | Pan GY, Huang ZJ, Wang GJ, Fawcett JP, Liu XD, Zhao XC, Sun JG, Xie YY. The antihyperglycaemic activity of berberine arises from a decrease of glucose absorption. Planta Med. 2003;69:632-636. [PubMed] |
| 18. | Cicero AF, Rovati LC, Setnikar I. Eulipidemic effects of berberine administered alone or in combination with other natural cholesterol-lowering agents. A single-blind clinical investigation. Arzneimittelforschung. 2007;57:26-30. [PubMed] |
| 19. | Becker DJ, Gordon RY, Halbert SC, French B, Morris PB, Rader DJ. Red yeast rice for dyslipidemia in statin-intolerant patients: a randomized trial. Ann Intern Med. 2009;150:830-839. [PubMed] |
| 20. | Liu J, Zhang J, Shi Y, Grimsgaard S, Alraek T, Fønnebø V. Chinese red yeast rice (Monascus purpureus) for primary hyperlipidemia: a meta-analysis of randomized controlled trials. Chin Med. 2006;1:4. [PubMed] |
| 21. | Ko WH, Yao XQ, Lau CW, Law WI, Chen ZY, Kwok W, Ho K, Huang Y. Vasorelaxant and antiproliferative effects of berberine. Eur J Pharmacol. 2000;399:187-196. [PubMed] |
| 22. | Wang Y, Huang Y, Lam KS, Li Y, Wong WT, Ye H, Lau CW, Vanhoutte PM, Xu A. Berberine prevents hyperglycemia-induced endothelial injury and enhances vasodilatation via adenosine monophosphate-activated protein kinase and endothelial nitric oxide synthase. Cardiovasc Res. 2009;82:484-492. [PubMed] |