Published online May 26, 2010. doi: 10.4330/wjc.v2.i5.125
Revised: April 23, 2010
Accepted: April 30, 2010
Published online: May 26, 2010
AIM: To evaluate efficacy and tolerability of the combination valsartan plus hydrochlorothiazide (160 mg and 25 mg daily, respectively) in young-middle aged males with high-normal blood pressure (BP) or first-degree arterial hypertension with evidence of target organ damage.
METHODS: Twenty males with high-normal BP or first-degree hypertension associated with left ventricular concentric remodeling and/or increased aortic stiffness were enrolled. BP at rest and during exercise, and echocardiographic parameters of the left ventricle (LV), were evaluated at baseline and after 3 mo of treatment. The effects of treatment on aortic stiffness, metabolic parameters, renal and erectile function were also assessed.
RESULTS: BP was significantly reduced by treatment both at rest (P < 0.001) and during exercise (P < 0.001), and 85% of patients achieved BP normalization (< 130/85 mmHg). Doppler echocardiography showed a significant reduction of LV mass (P < 0.005). LV hypertrophy was identified in 70% of subjects at baseline and in 5% after 3 mo of treatment. The ratio of early (E) to late (A) trans-mitral diastolic flow velocity increased, (P < 0.05), the relative wall thickness decreased (P < 0.05) and the left ventricular relaxation time shortened (P < 0.005). The left atrial diameter (P < 0.05) and the aortic diameter (P < 0.05) and stiffness (P < 0.005) also decreased.
CONCLUSION: The full-dose combination of valsartan plus hydrochlorothiazide produced optimal BP control with regression of target organ damage, already after 3 mo, without relevant side effects.
- Citation: Ruvolo A, Mercurio V, Fazio V, Carlomagno G, Russo T, Affuso F, Fazio S. Efficacy and safety of valsartan plus hydroclorothiazide for high blood pressure. World J Cardiol 2010; 2(5): 125-130
- URL: https://www.wjgnet.com/1949-8462/full/v2/i5/125.htm
- DOI: https://dx.doi.org/10.4330/wjc.v2.i5.125
Hypertension is one of the most prevalent cardiovascular risk factors in the adult population and is therefore considered an important medical and public health issue[1,2]. Both blood pressure (BP) values and duration of hypertension influence the level of risk for stroke, heart failure, atherosclerosis, heart attack and kidney disease[3,4]. In the new European Guidelines, hypertension is defined by a systolic BP (SBP) of > 139 mmHg and/or diastolic BP (DBP) of > 89 mmHg. To reduce cardiovascular risk, the European Guidelines recommend prompt treatment of patients affected by hypertension[5]. Moreover, longitudinal data from the Framingham Heart Study showed that subjects with a BP value of 130-139/85-89 mmHg have a more than twofold increase in the relative risk for cardiovascular diseases vs patients with BP ≤ 120/80 mmHg[6-8]. Furthermore, it has been reported that about 50% of pre-hypertensive individuals (SBP 120-139 mmHg, DBP 80-89 mmHg) have an excessive BP increase during exercise compared with normotensive subjects and echocardiographic evidence of structural and functional abnormalities of the left ventricle (LV)[9]. It is essential to identify these patients because, as with patients affected by mild hypertension, they are often under-treated despite their high cardiovascular risk and because they easily develop target organ damage and cardiovascular events in the mid to long term[10]. According to the most recent European Guidelines, the treatment of elevated BP in the pre-hypertensive-first degree range depends not only on the values of BP but also on the presence of other risk factors such as age, smoking, diabetes, sedentary lifestyle and, particularly, the presence of target organ damage. In addition to lifestyle recommendations (i.e. low sodium diet, weight loss, limited alcohol intake, smoking cessation and aerobic exercise)[11], there are now a wide range of drugs (i.e. diuretics, β-blockers, calcium-antagonists, ACE-inhibitors, and especially sartans) that may be used to treat arterial hypertension[1]. However, despite the more stringent guidelines and the rich therapeutic arsenal, it is estimated that only 58% of hypertensive individuals receive treatment and among these only 31% maintain good BP control[12]. This limited success is due to several factors, among which are the lack of diagnosis in many candidates in whom a pharmacologic treatment should be advised, inadequate treatment and poor compliance to pharmacologic therapies. Consequently, there is high incidence of cardiovascular events, morbidity, mortality and disability within the population, with significant increases in public spending.
Hypertension may be underrated also because it is not always possible to identify hypertensive subjects from the measurement of BP at rest alone. Indeed, many subjects may have normal BP at rest, but their pressure increases excessively during the psycho-physical stresses of everyday life[13,14]. Furthermore, poor compliance of some patients may be due to unpleasant side effects of prescribed drugs. Among these, erectile dysfunction is one of the most frequent causes of therapy discontinuity in male subjects[15]. On these premises, the aim of this study was to demonstrate the efficacy and safety of a prompt pharmacologic treatment with the combination of valsartan plus hydroclorothiazide in young-middle aged male subjects with slight hypertension and the presence of target organ damage.
Twenty young or middle-aged males with first-degree hypertension or high-normal BP, and with echocardiographic evidence of LV concentric remodeling and/or high vascular stiffness, naïve for antihypertensive treatment, were selected from our outpatient department and enrolled in this prospective, not controlled, 12-wk study. Each patient provided written informed consent to the study. The protocol was approved by the Ethics Committee of our Medical School, and the study was carried out according to the principles outlined in the Declaration of Helsinki.
Inclusion criteria for recruitment were age between 18 and 60 years, high-normal BP (130-139/85-89 mmHg), essential first-degree hypertension (BP 140-159/90-99 mmHg), and echocardiographic evidence of left ventricular concentric remodeling and/or increased aortic stiffness. Exclusion criteria were documented presence of ischemic heart disease, kidney or endocrine failure, inability to perform the bicycle-ergometer test, diabetes mellitus and valvular heart disease or arrhythmias. The characteristics of the study population are reported in Table 1.
| Patients | 20 |
| Age (yr) | 51 ± 9 |
| Weight (kg) | 77 ± 10.9 |
| Body surface area (m2) | 1.8 ± 0.15 |
| Body mass index (kg/m2) | 26.47 ± 2.9 |
| Systolic blood pressure (mmHg) | 141 ± 7 |
| Diastolic blood pressure (mmHg) | 89 ± 5.3 |
| Left ventricular hypertrophy (%) | 70 |
Patients consumed one tablet of the combination valsartan 160 mg plus hydroclorothiazide 25 mg every morning for 3 mo.
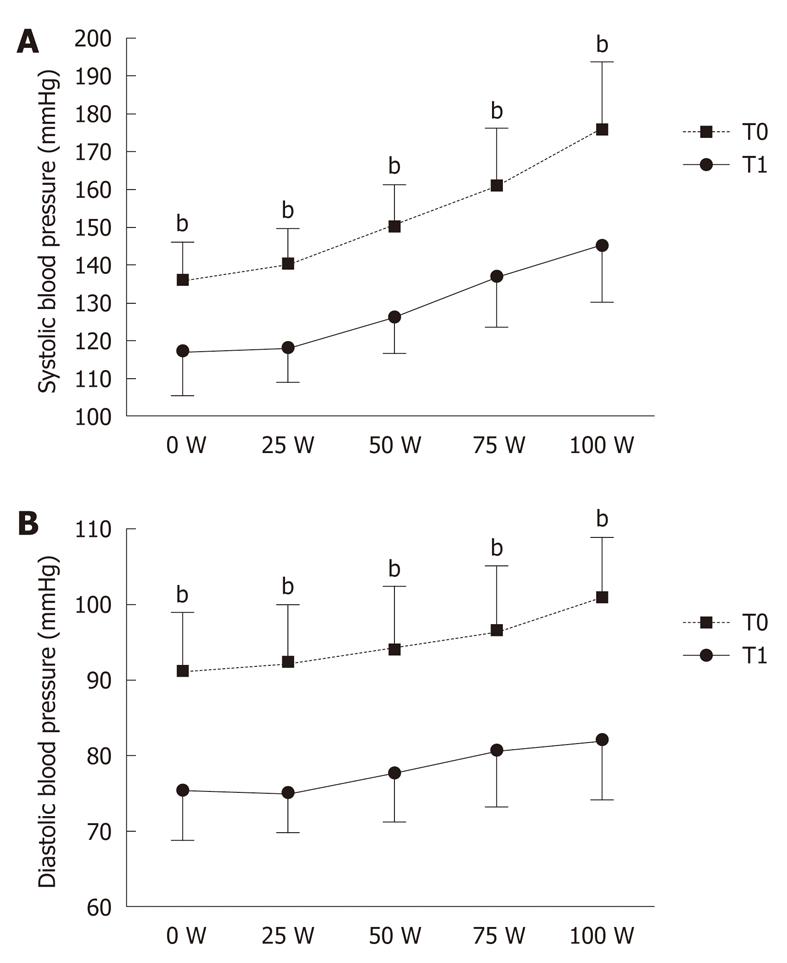
At baseline (T0) and after 3 mo of treatment (T1) we measured patients’ BP at rest in a sitting position and after a bicycle-ergometer test. This was performed by means of the version 5 CardioSoft software (General Electric, Freiburg, Germany), according to the Bruce Protocol. Each stage lasted 3 min, with progressive workload increments of 25 W[16]. All patients performed exercise until reaching a workload of 100 W. This level of exercise was selected because it is considered comparable to that carried out during everyday life. BP was measured at the end of each stage[9,17,18].
At T0 and T1, patients also underwent routine laboratory blood tests and transthoracic mono-two dimensional and Doppler echocardiography (i.e. pulsed wave, continuous wave and color Doppler) for the evaluation of morphological and functional cardiac parameters, according to the Guidelines of the American Society of Echocardiography[19]. Echocardiographic examinations were carried out with a commercially available ultrasonograph (Aplio CV, Toshiba Co., Otawara, Japan) equipped with a 3-MHz linear transducer (PST-30BT). In particular, we measured left ventricular mass (g/m²), relative wall thickness and the ratio of early (E) to late (A) trans-mitral diastolic flow velocity (E/A) and left ventricular relaxation time (msec) to evaluate left ventricular diastolic function. We also measured left atrial and aortic diameters (mm) and aortic stiffness (mmHg/mL), based on the pulse pressure/left ventricular stroke volume ratio[20].
All patients were asked to complete the International Index of Erectile Function (IIEF-5) questionnaire, which assesses erectile function, at T0 and T1[15]. The questionnaire consisted of five questions in the sexual sphere and a score was assigned to each item. The highest score possible was 25 and a score below 21 indicated erectile dysfunction.
Numeric variables are expressed as mean ± SD. Data were analyzed with standard statistical software (SPSS 14.0 program). We used the two-tailed unpaired Student’s t-test and the χ2 test for comparisons between mean values before and after treatment. A P value < 0.05 was considered statistically significant.
BP became normal (BP < 130/85 mmHg) in 85% of patients after 3 mo of treatment. In particular, rest BP values, both SBP and DBP, were significantly reduced after 3 mo of treatment: 141 ± 7 mmHg vs 116 ± 13 mmHg (P < 0.001) and 89 ± 5 mmHg vs 76 ± 7 mmHg (P < 0.001), respectively. In addition, treatment significantly reduced post-exercise SBP and DBP: 175 ± 16 mmHg vs 145 ± 15 mmHg (P < 0.001) and 101 ± 8 mmHg vs 82 ± 9 mmHg (P < 0.001), respectively (Figure 1).
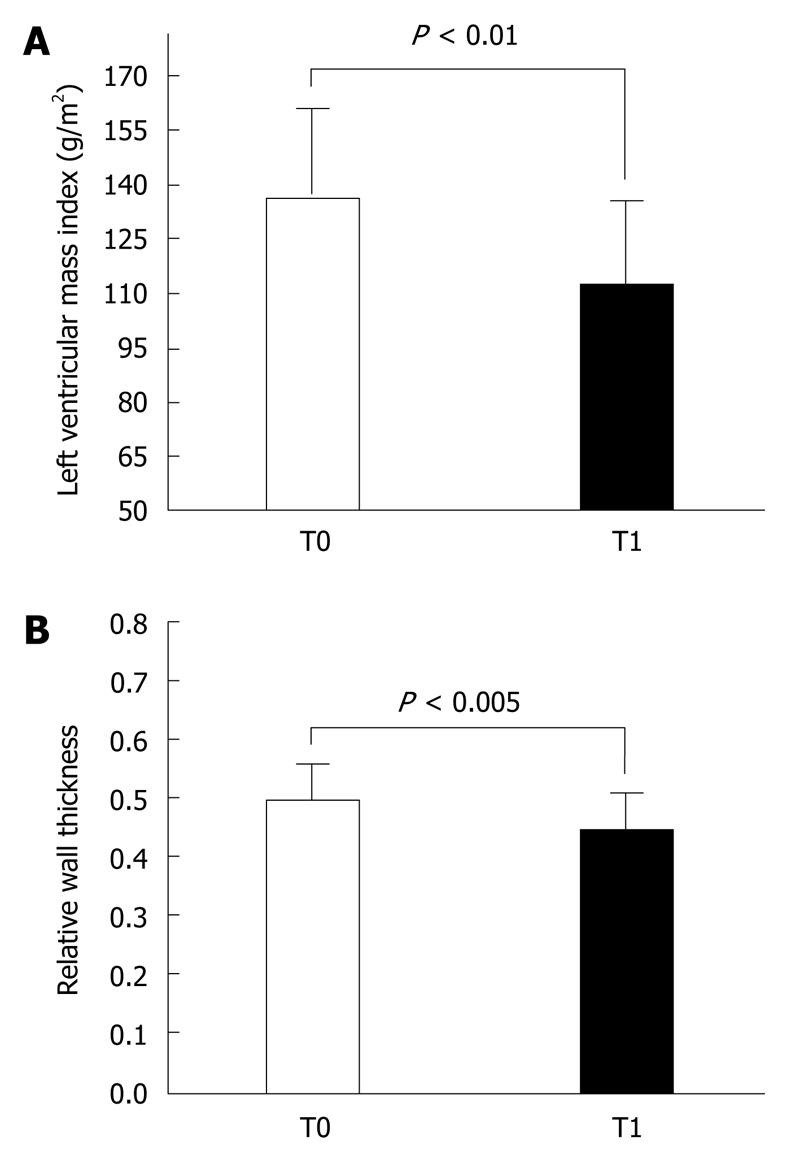
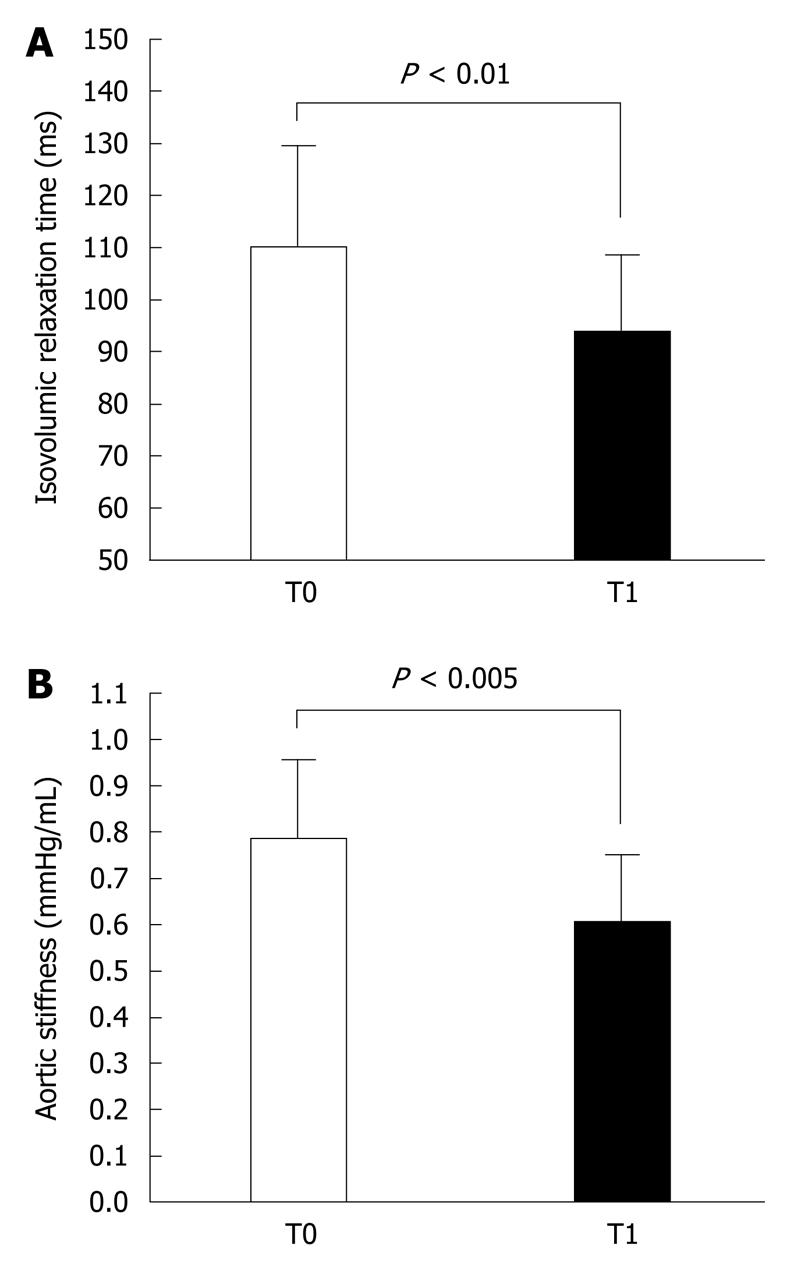
The Doppler-echocardiographic results were equally impressive. Regarding morphological features, there was a significant reduction of left ventricular mass (136 ± 27 g/m2vs 112 ± 25 g/m2, P < 0.005) (Figure 2A) and relative wall thickness (0.49 ± 0.06 vs 0.44 ± 0.07, P < 0.05) (Figure 2B). Furthermore, while concentric left ventricular hypertrophy was present in 70% of subjects at study entry, it was present in only 5% after 3 mo. Also, two diastolic functional parameters changed significantly: the E/A was increased (1.06 ± 0.32 vs 1.26 ± 0.36, P < 0.05), while the left ventricular relaxation time was shorter (111 ± 20 ms vs 94 ± 15 ms, P < 0.005) (Figure 3A). Finally, we observed a reduction in the left atrial diameter (36 ± 4 mm vs 33 ± 4 mm, P < 0.05) and a reduction in the aortic diameter (36 ± 4 mm vs 34 ± 3.4 mm, P < 0.05) and stiffness (0.78 ± 0.17 mmHg/mL vs 0.60 ± 0.15 mmHg/mL, P < 0.005) (Figure 3B).
No adverse effect resulted from treatment. In particular, metabolic parameters remained within normal values: low-density lipoprotein levels were 133 ± 28 mg/dL and 132 ± 25 mg/dL at T0 and T1, respectively, and fasting blood glucose levels were 90 ± 17 mg/dL and 90 ± 18 mg/dL at T0 and T1, respectively. Similarly, there were no significant changes in renal function or in the hydro-electrolytic balance.
Lastly, the IIEF-5 score showed that erectile function did not worsen after the combination treatment, the scores being 20.5 ± 3 at T0 and 21.4 ± 2 at T1.
The results of this study show that an early pharmacologic treatment with the combination valsartan plus hydrochlorothiazide (160 and 25 mg daily, respectively) in 20 young or middle-aged males with high-normal BP or first degree hypertension with evidence of target organ damage is very effective and well tolerated. Indeed, after 3 mo of treatment, BP was significantly reduced both at rest and during exercise and the proportion of patients reaching BP normalization (BP < 130/85 mmHg) was very high (85%).
Similarly, the treatment was very effective in counteracting target organ damage, thus highly reducing cardiovascular risk. In particular, left ventricular mass and relative wall thickness were significantly reduced; concentric left ventricular hypertrophy was present in 70% of subjects at study entry and was present in only 5% after 3 mo of treatment. This was associated with a significant improvement in relaxation, as shown by the significant increase in trans-mitral E/A and the shortening of left ventricular relaxation time. In addition, there was a significant decrease in left atrial diameter and a reduction in aortic diameter and stiffness, as estimated by the pulse pressure/left ventricular stroke volume ratio. These impressive results obtained after only 3 mo of treatment could have important implications. In fact, as shown in this and previous studies, many subjects with only high-normal BP or first-degree hypertension may have increased left ventricular mass and concentric hypertrophy associated with diastolic dysfunction mainly due to impaired myocardial relaxation[21,22]. Increased left ventricular mass is a recognized significant adverse prognostic factor[23]. Furthermore, many of the patients included in this study also have increased aortic stiffness, which is another adverse prognostic factor[24-26]. Consequently, a prompt pharmacologic intervention, which is able to normalize BP and correct these cardiovascular abnormalities, should reduce cardiovascular risk and improve the long-term prognosis even in the patients with high BP who are often untreated by the physician.
The striking results of this study were obtained without any metabolic side effects. In addition, the antihypertensive therapy used did not affect erectile function in our patients, with good compliance to treatment as derived by the tablet count and the results obtained. It is conceivable that the adverse effects that usually result from the administration of hydroclorothiazide on metabolism and erectile function have been counteracted by the favorable effects of valsartan[27,28]. After 12 mo of the same treatment, our 20 patients are stable and have not reported any adverse effects.
This study has some limitations. Optimal noninvasive assessment of diastolic ventricular function should be obtained with Tissue Doppler Imaging, because classic echocardiographic parameters may be influenced by several factors, such as afterload, ventricular filling and heart rate. Unfortunately, our echocardiographic equipment lacks the specific software to apply this technique. In the future, it would be useful to evaluate diastolic function in patients more accurately. Another limitation may be the small sample size and the open design; therefore a larger controlled study should be preformed to confirm our results.
Although a large, long-term, controlled study is needed to demonstrate whether this kind of therapeutic intervention could have a favorable prognostic impact, in our group of male patients with high BP, the early administration of full dose combination valsartan plus hydroclorothiazide produced good control of BP and, after only 3 mo, a regression of cardiac and aortic abnormalities with improved left ventricular relaxation and aortic stiffness without any relevant side effect.
Notwithstanding the stringent guidelines and the rich therapeutic arsenal, it is estimated that only 58% of hypertensive individuals receive treatment and, among these, only 31% maintain good blood pressure (BP) control. This limited success is due to several reasons among which the lack of diagnosis in many candidates in whom a pharmacologic treatment should be advised, inadequate treatment and poor compliance to pharmacologic therapies are certainly important. Consequently, there is high incidence of cardiovascular events, morbidity, mortality and disability of the population, with significant increase in public spending. Furthermore, the poor compliance of some patients with the prescribed drugs may be due to unpleasant side effects. Among these, erectile dysfunction is one of the most frequent causes of therapy discontinuity in the male subjects.
The aim of this study was to demonstrate the efficacy, safety and tolerability of a prompt pharmacologic treatment with the combination valsartan plus hydroclorothiazide in young-middle aged male subjects with slight hypertension and presence of target organ damage.
The results of this study show that an early pharmacologic treatment with the combination valsartan plus hydrochlorothiazide (160 and 25 mg daily, respectively) in 20 young or middle-aged men with high-normal BP or first degree hypertension with evidence of target organ damage, is very effective and well tolerated. Indeed, already after 3 mo of treatment, BP was significantly reduced both at rest and during exercise, the proportion of patients reaching BP normalization (BP < 130/85 mmHg) being very high (85%). Similarly, the treatment was very effective in counteracting target organ damage, thus highly reducing the cardiovascular risk. In particular, left ventricular mass and relative wall thickness were significantly reduced; concentric left ventricular hypertrophy was present in 70% of subjects at the study entry and only in 5% after 3 mo of treatment. In addition, the antihypertensive therapy used did not affect the erectile function in our patients, with good compliance to treatment
A prompt pharmacologic treatment should be applied in subjects with high-normal BP, first degree hypertension with evidence of target organ damage, in order to normalize BP and revert vascular and cardiac remodeling. The combination valsartan plus hydroclorothiazide could be a good choice.
The paper is well written and the conclusions are clear. Only a limited number of subjects were studied but they served as their own controls. References are appropriate.
Peer reviewer: T Fikret Ilgenli, MD, Associate Professor of Cardiology, Department of Cardiology, Gölcük Military Hospital, 41650-Gölcük, Kocaeli, Turkey
S- Editor Cheng JX L- Editor Lutze M E- Editor Zheng XM
| 1. | Lenfant C, Chobanian AV, Jones DW, Roccella EJ. Seventh report of the Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7): resetting the hypertension sails. Hypertension. 2003;41:1178-1179. |
| 2. | Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217-223. |
| 3. | Kannel WB. Blood pressure as a cardiovascular risk factor: prevention and treatment. JAMA. 1996;275:1571-1576. |
| 4. | Launer LJ, Masaki K, Petrovitch H, Foley D, Havlik RJ. The association between midlife blood pressure levels and late-life cognitive function. The Honolulu-Asia Aging Study. JAMA. 1995;274:1846-1851. |
| 5. | Summary of the 2007 European Society of Hypertension (ESH) and European Society of Cardiology (ESC) guidelines for the management of arterial hypertension. Vasc Health Risk Manag. 2007;3:783-795. |
| 6. | Qureshi AI, Suri MF, Kirmani JF, Divani AA, Mohammad Y. Is prehypertension a risk factor for cardiovascular diseases? Stroke. 2005;36:1859-1863. |
| 7. | Affuso F, Ruvolo A, Fazio S. Prehypertension and elevated risk of cardiovascular disease: physiopathologic mechanisms. Am J Med. 2007;120:e11. |
| 8. | Vasan RS, Larson MG, Leip EP, Evans JC, O'Donnell CJ, Kannel WB, Levy D. Impact of high-normal blood pressure on the risk of cardiovascular disease. N Engl J Med. 2001;345:1291-1297. |
| 9. | Fazio S, Palmieri EA, Izzo R, Affuso F, Romano M, Riccio G, Pilato G, Trimarco B, De Luca N. An exaggerated systolic blood pressure response to exercise is associated with cardiovascular remodeling in subjects with prehypertension. Ital Heart J. 2005;6:886-892. |
| 10. | Cuspidi C, Ambrosioni E, Mancia G, Pessina AC, Trimarco B, Zanchetti A. Role of echocardiography and carotid ultrasonography in stratifying risk in patients with essential hypertension: the Assessment of Prognostic Risk Observational Survey. J Hypertens. 2002;20:1307-1314. |
| 11. | Effects of weight loss and sodium reduction intervention on blood pressure and hypertension incidence in overweight people with high-normal blood pressure. The Trials of Hypertension Prevention, phase II. The Trials of Hypertension Prevention Collaborative Research Group. Arch Intern Med. 1997;157:657-667. |
| 12. | Hajjar I, Kotchen TA. Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988-2000. JAMA. 2003;290:199-206. |
| 13. | Millar-Craig MW, Bishop CN, Raftery EB. Circadian variation of blood-pressure. Lancet. 1978;1:795-797. |
| 14. | Miyai N, Arita M, Morioka I, Miyashita K, Nishio I, Takeda S. Exercise BP response in subjects with high-normal BP: exaggerated blood pressure response to exercise and risk of future hypertension in subjects with high-normal blood pressure. J Am Coll Cardiol. 2000;36:1626-1631. |
| 15. | Fogari R, Zoppi A. Effects of antihypertensive therapy on sexual activity in hypertensive men. Curr Hypertens Rep. 2002;4:202-210. |
| 16. | Bruce RA, McDonough JR. Stress testing in screening for cardiovascular disease. Bull N Y Acad Med. 1969;45:1288-1305. |
| 18. | Franz IW. Blood pressure measurement during ergometric stress testing. Z Kardiol. 1996;85 Suppl 3:71-75. |
| 19. | Gottdiener JS, Bednarz J, Devereux R, Gardin J, Klein A, Manning WJ, Morehead A, Kitzman D, Oh J, Quinones M. American Society of Echocardiography recommendations for use of echocardiography in clinical trials. J Am Soc Echocardiogr. 2004;17:1086-1119. |
| 20. | Palmieri V, Bella JN, Roman MJ, Gerdts E, Papademetriou V, Wachtell K, Nieminen MS, Dahlöf B, Devereux RB. Pulse pressure/stroke index and left ventricular geometry and function: the LIFE Study. J Hypertens. 2003;21:781-787. |
| 21. | Ganau A, Devereux RB, Roman MJ, de Simone G, Pickering TG, Saba PS, Vargiu P, Simongini I, Laragh JH. Patterns of left ventricular hypertrophy and geometric remodeling in essential hypertension. J Am Coll Cardiol. 1992;19:1550-1558. |
| 22. | Ren JF, Pancholy SB, Iskandrian AS, Lighty GW Jr, Mallavarapu C, Segal BL. Doppler echocardiographic evaluation of the spectrum of left ventricular diastolic dysfunction in essential hypertension. Am Heart J. 1994;127:906-913. |
| 23. | Verdecchia P, Schillaci G, Borgioni C, Ciucci A, Gattobigio R, Zampi I, Santucci A, Santucci C, Reboldi G, Porcellati C. Prognostic value of left ventricular mass and geometry in systemic hypertension with left ventricular hypertrophy. Am J Cardiol. 1996;78:197-202. |
| 24. | Blacher J, Guerin AP, Pannier B, Marchais SJ, Safar ME, London GM. Impact of aortic stiffness on survival in end-stage renal disease. Circulation. 1999;99:2434-2439. |
| 25. | Stefanadis C, Dernellis J, Tsiamis E, Stratos C, Diamantopoulos L, Michaelides A, Toutouzas P. Aortic stiffness as a risk factor for recurrent acute coronary events in patients with ischaemic heart disease. Eur Heart J. 2000;21:390-396. |
| 26. | Sugioka K, Hozumi T, Sciacca RR, Miyake Y, Titova I, Gaspard G, Sacco RL, Homma S, Di Tullio MR. Impact of aortic stiffness on ischemic stroke in elderly patients. Stroke. 2002;33:2077-2081. |
| 27. | Düsing R. Effect of the angiotensin II antagonist valsartan on sexual function in hypertensive men. Blood Press Suppl. 2003;2:29-34. |
| 28. | Fogari R, Zoppi A, Poletti L, Marasi G, Mugellini A, Corradi L. Sexual activity in hypertensive men treated with valsartan or carvedilol: a crossover study. Am J Hypertens. 2001;14:27-31. |