Published online Aug 26, 2025. doi: 10.4330/wjc.v17.i8.111159
Revised: July 2, 2025
Accepted: August 5, 2025
Published online: August 26, 2025
Processing time: 58 Days and 7.7 Hours
Cardiac myxoma, a benign intracardiac tumor, is traditionally excised via conventional sternotomy, which is invasive and associated with longer recovery times. Minimally invasive robotic surgery has emerged as a potential alternative, offe
To assess robotic surgery vs sternotomy for cardiac myxoma regarding operative times, hospital stay, transfusions, and complications.
A systematic review was performed using EMBASE, OVID, Scopus, PubMed, Cochrane, and ScienceDirect databases to identify studies comparing robotic surgery and sternotomy for cardiac myxoma excision. Continuous outcomes were analyzed using mean differences (MDs), and categorical outcomes with odds ratios (ORs) and 95% confidence intervals (95%CIs). A random-effects model was used to pool data, accounting for study heterogeneity.
Six studies involving 425 patients (180 robotic, 245 conventional) were included. Robotic surgery significantly increased cross-clamp time (MD = 12.03 minutes, 95%CI: 2.14-21.92, P = 0.02) and cardiopulmonary bypass time (MD = 28.37 minutes, 95%CI: 11.85-44.89, P = 0.001). It reduced hospital stay (MD = -1.86 days, 95%CI: -2.45 to
Robotic surgery for cardiac myxoma excision prolongs operative times but shortens hospital stays and reduces transfusion needs, suggesting enhanced recovery without compromising safety.
Core Tip: This meta-analysis compares robotic surgery to conventional sternotomy for cardiac myxoma excision, revealing that robotic surgery, using systems like the da Vinci surgical system, significantly reduces hospital stay by 1.86 days and blood transfusion needs (odds ratio = 0.30). Despite longer cardiopulmonary bypass and cross-clamp times, robotic surgery enhances recovery without increasing complications like atrial arrhythmia. These findings highlight robotic surgery’s potential as a minimally invasive alternative, offering improved outcomes for suitable patients.
- Citation: Khawar MMH, Ejaz H, Jaffar MS, Kashif M, Habib M, Mukhtar A, Riaz H, Shah SA, Muhammad A, Ali U, Saeed H, Buhadur Ali MK, Chhetri R. Comparative outcomes of robotic surgery vs conventional sternotomy for cardiac myxoma excision: A meta-analysis. World J Cardiol 2025; 17(8): 111159
- URL: https://www.wjgnet.com/1949-8462/full/v17/i8/111159.htm
- DOI: https://dx.doi.org/10.4330/wjc.v17.i8.111159
Cardiac myxoma is the most common primary tumor of the heart, with an incidence of 0.5 to 1 case per million individuals[1] or a global prevalence rate of 0.03%[2]. Although benign, it can prove life-threatening due to the high risk of embolism, obstruction, and sudden cardiac death associated with it[3]. Therefore, the presence of cardiac myxoma, most commonly found in the left atrium, often warrants surgical excision. Conventionally, cardiac myxomas have been managed via a median approach in which an incision is made through the sternum to achieve direct exposure of the heart[4]. Even though it remained the only treatment modality for a long time, the procedure had its shortcomings, including a risk of sternomediastinitis, a rare but life-threatening complication[5], and severe chronic pain and discomfort due to the incision made through the sternum, impacting the quality of life in the long run[6]. However, more recently, the advan
This new approach, utilizing a robotic surgical system, typically the da Vinci surgical system[8], promises increased precision in the surgical procedure due to enhanced 3-D visualization, along with a greater range of motion and dexterity provided by the robotic arms[9]. Moreover, due to its inherent minimally invasive nature, it allows for much smaller and cosmetically acceptable incisions[10] with lower post-operative pain[9]. However, despite all these facts, questions regarding the clinical efficacy and safety of robotic excision in comparison to the conventional approach still fog the discernment of surgeons[11]. This is primarily due to a lack of supporting evidence and conflicting results reported in individual-based studies[12]. All this accentuates the need for a robust comparative analysis.
This meta-analysis aims to bridge the knowledge gap by quantitatively synthesizing the available evidence comparing postoperative clinical and surgical outcomes in cardiac myxoma patients undergoing excision via conventional midline sternotomy and robotic-assisted surgery. Hence, this study aims to facilitate the surgeons in making a more patient-centric decision, allowing them to gauge the relative advantages and limitations of the mentioned approaches in line with the specifics of cardiac myxoma patients.
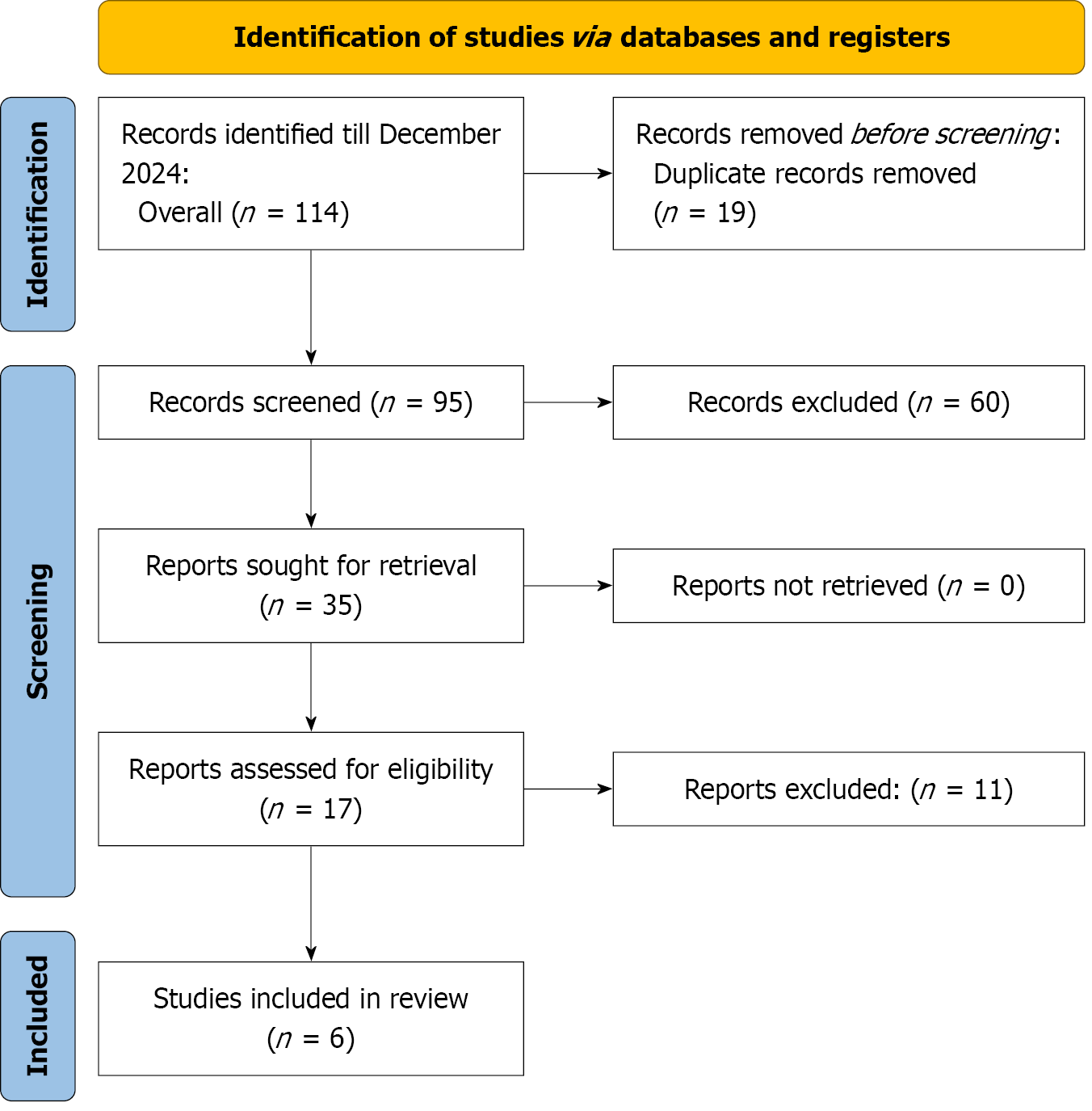
A comprehensive literature search was conducted in accordance with the PRISMA guidelines and recommended methodologies[13]. The search was conducted across various databases, including PubMed, EMBASE, OVID, and Scopus, from their inception to December 2024. To maximize the retrieval of relevant studies, a combination of keywords and MeSH terms was employed, including “Cardiac Myxoma” AND “Robotic Surgery” OR “Robotic Myxoma Excision” OR “Minimally Invasive Surgery” AND “Conventional Sternotomy” OR “Open Sternotomy” AND “Cardiac Surgery.” Detailed search strategy for different databases in the given Supplementary Table 1. Additional references were identified through manual searches of the reference lists of included articles. All identified articles underwent full-text screening for eligibility, with systematic application of inclusion and exclusion criteria.
Observational studies comparing robotic cardiac myxoma excision to conventional sternotomy were included in this meta-analysis. Studies were eligible if they reported quantitative data on at least one of the following outcomes: Cross-clamp time, cardiopulmonary bypass (CPB) time, length of hospital stay, blood transfusion rates, incidence of atrial arrhythmias, or ventilation duration.
Studies were excluded if they (1) Did not provide comparative data between robotic and conventional approaches; (2) Focused on conditions other than cardiac myxoma; (3) Were duplicate studies with overlapping patient data (only the most comprehensive or recent study was retained); or (4) Were case reports, conference abstracts, editorials, or expert opinions.
Two independent reviewers performed the search, screened titles and abstracts, and assessed full-text articles for eligibility. Disagreements were resolved through discussion or consultation with a third reviewer. Data extraction was conducted independently by the same reviewers and included details on study design, population characteristics, surgical approach, and reported outcomes.
A Newcastle-Ottawa Scale (NOS) was used to evaluate the quality of papers. Quality evaluation was conducted by two independent authors[14]. NOS consisted of three factors: Patient selection (4 points), the comparability of the groups (2 points), and the ascertainment of the exposure (3 points). The total score ranged from 0 (the worst) to 9 (the best). The quality was interpreted as good (score ≥ 7), moderate (score ≥ 5 to < 7), and poor (score ≤ 5). The discrepancy between the two authors was resolved by consensus.
Statistical analyses were conducted using Review Manager (version 5.3, Cochrane Collaboration). Dichotomous outcomes were analyzed using the odds ratio (OR) with 95% confidence intervals (95%CIs). In contrast, continuous outcomes were assessed by pooling mean differences (MDs) using the generic inverse variance method under a random-effects model. According to the Cochrane Handbook (chapter 9), heterogeneity was considered significant if the alpha value of the χ2 test was below 0.1. The interpretation of the I² test for heterogeneity was as follows: 0% to 40% was considered not significant, 30% to 60% indicated moderate heterogeneity, 50% to 90% represented substantial heterogeneity, and 75% to 100% signified considerable heterogeneity[15]. For each outcome, a forest plot was constructed to analyze the data visually. Additionally, a subgroup analysis by geographical location was conducted to investigate potential variations in outcomes across different regions. Following the statistical analysis, the quality of evidence for each outcome was evaluated using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach, which assesses domains such as risk of bias, inconsistency, imprecision, indirectness, and effect size to determine the certainty of the evidence.
Ethical approval and informed consent were not required for this study as it is a meta-analysis of previously published data. All included studies had obtained ethical approval from their respective institutions, as stated in their original publications
The characteristics of the included studies are summarized as follows (Figure 1): All six studies were observational and published between 2012 and 2024, representing data from Turkey, China, the United States, and the United Kingdom. The total number of patients across all studies was 425, with sample sizes for robotic surgery ranging from 9 to 60 and for sternotomy from 18 to 64. The mean ages of patients ranged between 47.7 ± 13.0 years and 59.4 ± 11.3 years, with no significant differences between the robotic and sternotomy groups. The proportion of male patients varied, with robotic cohorts ranging from 31% to 68.7% and sternotomy cohorts from 22% to 43.3%. Hypertension was reported in 10% to 37.5% of robotic patients and 11% to 50% of sternotomy patients. The prevalence of diabetes mellitus was generally low, with rates ranging from 6% to 12.5% in the robotic group and 0% to 28% in the sternotomy group (Table 1).
| Ref. | Country | Study design | Patients (n) | Age (mean ± SD) | Male (%) | Hypertension (%) | Diabetes mellitus (%) |
| Kadiroğulları et al[10], 2020 | Turkey | Observational Study | Robotic: 16; Sternotomy: 30 | Robotic: 53.2 ± 11.9; Sternotomy: 54.7 ± 13.2 | Robotic: 68.7; Sternotomy: 43.3 | Robotic: 37.5; Sternotomy: 50 | Robotic: 12.5; Sternotomy: 23.3 |
| Kesävuori et al[16],2015 | United Kingdom (London) | Observational Study | Robotic: 9; Sternotomy: 18 | Robotic: 58.8 ± 12.9; Sternotomy: 59.4 ± 11.3 | Robotic: 44; Sternotomy: 22 | Robotic: 11; Sternotomy: 22 | Robotic: 11; Sternotomy: 0 |
| Li and Gao[18], 2017 | China | Observational Study | Robotic: 60; Sternotomy: 60 | Robotic: 51.9 ± 12.3; Sternotomy: 50.7 ± 13.5 | Robotic: 41.7; Sternotomy: 39.2 | Robotic: 18.3; Sternotomy: 11.2 | Robotic: 8.3; Sternotomy: 4.8 |
| Liu et al[17], 2024 | China | Observational Study | Robotic: 30; Sternotomy: 64 | Robotic: 52.3 ± 15.3; Sternotomy: 54.5 ± 13.2 | Robotic: 43; Sternotomy: 31 | Robotic: 10; Sternotomy: 15.6 | Robotic: 6.7; Sternotomy: 10.9 |
| Schilling et al[20], 2012 | United States (Ohio) | Observational Study | Robotic: 16; Sternotomy: 29 | Robotic: 53.1 ± 15.2; Sternotomy: 58.8 ± 11.4 | Robotic: 31; Sternotomy: 28 | Not reported | Robotic: 6; Sternotomy: 28 |
| Yang et al[19], 2015 | China | Observational Study | Robotic: 49; Sternotomy: 44 | Robotic: 47.7 ± 13.0; Sternotomy: 51.2 ± 12.1 | Robotic: 48.9; Sternotomy: 40.9 | Robotic: 16.3; Sternotomy: 25 | Robotic: 10.2; Sternotomy: 15.9 |
Of the six studies included in the analysis, five were determined to be of good quality: Kadiroğulları et al[10] (2020) and Kesävuori et al[16] (2015) both scored 7/9, while Liu et al[17] (2024), Li and Gao[18] (2017), and Yang et al[19] (2015) each scored 8/9. One study, Schilling et al[20] (2012), was categorized as moderate quality with a score of 5/9. The quality assessment was independently performed by two authors, with any differences resolved through consensus. Overall, the studies included in this meta-analysis were assessed as moderate to high quality, supporting the reliability of the results (Supplementary Table 2).
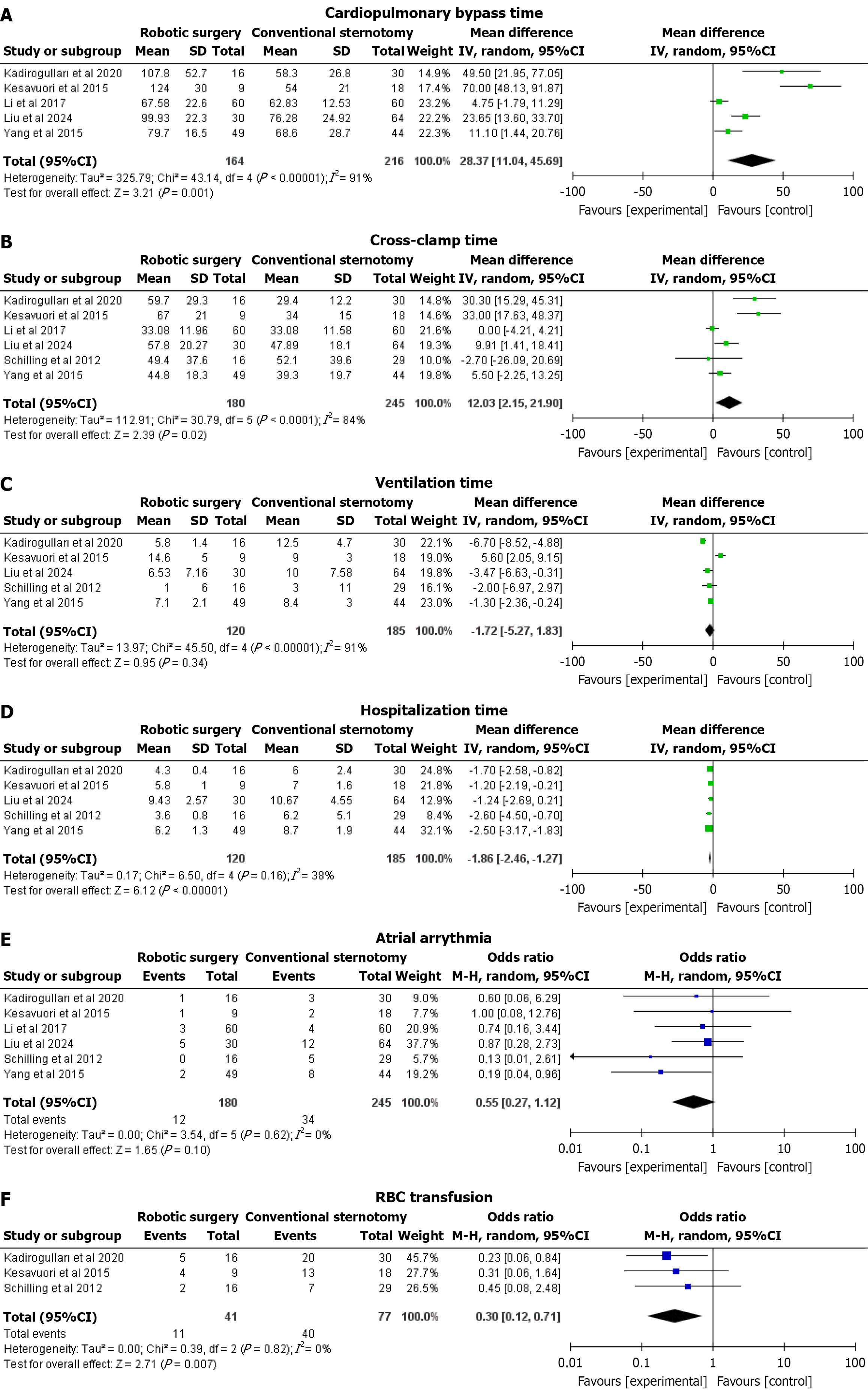
CPB time: Five studies reported this outcome. Robotic surgery was associated with a significantly longer CPB time compared to conventional sternotomy. The pooled MD was 28.37 minutes (95%CI: 11.04-45.69; P = 0.001), with considerable heterogeneity observed (I² = 91%) that dropped significantly (I² = 75%) after excluding Kesävuori et al[16] (2015) and Li and Gao[18] (2017; Figure 2A).
Cross-clamp time: Six studies reported this outcome. Robotic surgery also demonstrated a significantly greater cross-clamp time. The pooled MD was 12.03 minutes (95%CI: 2.15-21.90; P = 0.02), with substantial heterogeneity (I² = 84%) that dropped significantly (I² = 68%) after excluding Kesävuori et al[16] (2015) and Li and Gao[18] (2017; Figure 2B).
Ventilation time: Five studies reported this outcome. There was no statistically significant difference in ventilation time between robotic surgery and conventional sternotomy. The pooled MD was -1.72 minutes (95%CI: -5.27 to 1.83; P = 0.34), with considerable heterogeneity (I² = 91%) that dropped significantly (I² = 0%) after excluding Kesävuori et al[16] (2015) and Kadiroğulları et al[10] (2020; Figure 2C).
Hospitalization time: Five studies reported this outcome. Robotic surgery was associated with a significantly shorter hospitalization duration compared to conventional sternotomy. The pooled MD was -1.86 days (95%CI: -2.46 to -1.27; P < 0.00001), with low heterogeneity (I² = 38%; Figure 2D).
Atrial arrhythmia: Six studies reported this outcome. No significant difference was observed in the occurrence of atrial arrhythmia between the two surgical approaches. The pooled OR was 0.55 (95%CI: 0.27-1.12; P = 0.10), with no heterogeneity (I² = 0%; Figure 2E).
Red blood cell transfusion: Three studies reported this outcome. Robotic surgery significantly reduced the need for red blood cell (RBC) transfusions compared to conventional sternotomy. The pooled OR was 0.30 (95%CI: 0.12-0.71; P = 0.007), with no heterogeneity observed (I² = 0%; Figure 2F).
The studies were divided into two groups based on location, specifically Chinese studies and other studies. The subgroup analysis for outcomes with higher heterogeneity—ventilation time, CPB time, and cross-clamp time—in the context of cardiac myxoma excision reveals varied impacts of robotic surgery compared to conventional sternotomy. Ventilation time shows no significant overall difference (MD = -1.72 days, 95%CI: -5.27 to 1.83, P = 0.34), with high heterogeneity (I² = 91%), indicating inconsistent effects across subgroups. CPB time is significantly longer with robotic surgery (MD = 28.37 minutes, 95%CI: 11.85-44.89, P = 0.001), with notable subgroup variation (I² = 94.4%), particularly a greater increase in non-Chinese studies (MD = 61.53). Cross-clamp time shows no significant difference overall (MD = -0.70 minutes, 95%CI: -1.97 to 0.58, P = 0.29), with moderate heterogeneity (I² = 59%) and no significant subgroup differences (P = 0.63), indicating minimal variability between Chinese and other studies. These results highlight that while robotic surgery extends CPB time, the lack of significant differences in cross-clamp and ventilation times suggests influences from regional practices or study-specific factors may play a role in the observed heterogeneity (Supplementary Figures 1, 2 and 3).
The GRADE assessment of six outcomes comparing robotic surgery vs conventional sternotomy for cardiac myxoma excision indicates varying levels of evidence certainty. CPB time (MD = 28.37 minutes, 95%CI: 11.04–45.69) and cross-clamp time (MD = 12.03 min, 95%CI: 2.15-21.90) are rated as low certainty due to serious inconsistency (I² = 91% and 84%, respectively), though no serious concerns were noted for risk of bias, imprecision, or indirectness. Ventilation time (MD = -1.72 minutes, 95%CI: -5.27 to 1.83) is classified as very low certainty, reflecting serious inconsistency (I² = 91%) and imprecision, as the confidence interval includes no effect. Hospitalization time (MD = -1.86 days, 95%CI: -2.46 to -1.27) and RBC transfusion (OR = 0.30, 95%CI: 0.12-0.71) are rated as low certainty, with no serious inconsistency (I² = 38% and 0%, respectively) and no issues with risk of bias, imprecision, or indirectness. Atrial arrhythmia (OR = 0.55, 95%CI: 0.27-1.12) also has low certainty due to serious imprecision, with no inconsistency (I² = 0%) and no concerns for other domains. The absence of downgrading for risk of bias, indirectness, or publication bias, supported by moderate to high-quality studies and lack of funnel plot asymmetry, underscores the reliability of the study designs. These findings suggest that robotic surgery reduces hospitalization time and transfusion needs, while operative times and ventilation outcomes warrant cautious interpretation (Supplementary Table 3).
The use of robotics in various cardiac procedures has seen a surge in recent years, driven by improved outcomes and reduced adverse effects. To our knowledge, this is the first meta-analysis to explore the effectiveness and safety profile of robotic surgery compared to conventional sternotomy for the excision of atrial myxoma. The results of this meta-analysis suggest that robotic surgery effectively reduces hospitalization time, along with a lower rate of blood transfusion, compared to sternotomy. The significance of these outcomes is clinically relevant for the older population and patients with comorbidities who were previously considered high risk for sternotomy. There was no significant difference in ventilation time or rates of atrial arrhythmias. However, robotic surgery was associated with greater cross-clamp time and CPB time. Heterogeneity was observed in some outcomes, which reflects the variable surgical expertise and population in these studies.
The cross-clamp time and CPB time were significantly increased in the robotic surgery group, with MDs of 12.03 (95%CI: 2.15-21.90) and 28.37 (95%CI: 11.04-45.69), respectively. The pooled MD in our meta-analysis is in line with other studies evaluating the same intervention. The longer cross-clamp and CPB times are likely attributable to both the operative complexity of robotic surgery (including docking/undocking and changing instrument arms) and the steep learning curve associated with these procedures. However, it has been observed that this time difference will significantly reduce with an increasing number of cases, providing relative expertise and experience, which will help surgeons overcome the learning curve[21]. There was also heterogeneity present in the outcomes mentioned above, which differences in study population, operative methodology, and study conditions can explain.
The incidence of RBC infusion is significantly reduced in the robotic surgery group, with a pooled OR of 0.30 (95%CI: 0.12-0.71). Our findings are strongly coherent with other studies exploring the use of RS compared to sternotomy. This finding can be attributed to the fact that robotic surgery is less invasive, more precise, and less likely to damage critical blood vessels, resulting in reduced blood loss during surgery[22]. This finding has a strong clinical benefit in reducing surgery-induced hemodynamic complications, which can also be fatal, and severe adverse effects associated with blood transfusion. Additionally, no heterogeneity was observed in our findings.
The hospitalization rate also decreased in the robotic surgery group, with a pooled MD of -1.86 (95%CI: -2.46 to 1.27). Again, our finding aligns with previous literature examining the use of robotic surgery compared to sternotomy[23]. The decreased hospitalization rate is due to the less invasive nature of robotic surgery, resulting in fewer complications and post-operative trauma or pain. This demonstrates the clear benefits of robotic surgery in reducing hospitalization associated with infection and complications in post-operative patients. It also helps decrease hospitalization costs and improve patient satisfaction.
Our meta-analysis is limited by its reliance on retrospective observational studies, as no randomized controlled trials were identified, which increases the risk of bias that can only be mitigated through blinded randomized controlled trials (RCTs). The relatively small sample sizes, resulting from the limited number of published studies, further limit the robustness of our findings. Additionally, unmeasured confounding factors such as tumor size and surgeon volume may influence outcomes and were not accounted for in the analysis. Potential overlap between study centers could also introduce bias, though this was not specifically assessed. Furthermore, the study did not evaluate critical aspects such as cost or long-term quality of life outcomes, which are essential for a comprehensive comparison of robotic surgery vs sternotomy. Publication bias assessment was not conducted due to the inclusion of fewer than 10 studies, limiting our ability to detect potential reporting biases. Future RCTs with larger sample sizes are necessary to address these limi
Robotic surgery compared to conventional sternotomy has better post-operative outcomes and reduced complication rate. However, it lags behind sternotomy in a few intra-operative parameters, which will surely be improved with increased surgical expertise.
| 1. | Islam AKMM. Cardiac myxomas: A narrative review. World J Cardiol. 2022;14:206-219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 57] [Article Influence: 19.0] [Reference Citation Analysis (1)] |
| 2. | Grebenc ML, Rosado de Christenson ML, Burke AP, Green CE, Galvin JR. Primary cardiac and pericardial neoplasms: radiologic-pathologic correlation. Radiographics. 2000;20:1073-103; quiz 1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 354] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 3. | Saad EA, Mukherjee T, Gandour G, Fatayerji N, Rammal A, Samuel P, Abdallah N, Ashok T. Cardiac myxomas: causes, presentations, diagnosis, and management. Ir J Med Sci. 2024;193:677-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Ashinze P, Banerjee S, Egbunu E, Salawu W, Idris-agbabiaka A, Obafemi E, Olajuwon TJ, Chukwu B, Aremu SA, David OO, Alausa HM, Iwaloye FA. Cardiac myxomas: a review of current treatment approaches and emerging molecular therapies. Cardiothorac Surg. 2024;32:22. [DOI] [Full Text] |
| 5. | Robicsek F, Madjarov J. Complications of Midline Sternotomy [Internet]. In: Meyerson S, Baumgartner WA, Jacobs JP, editors. Pearson's General Thoracic Surgery. STS Cardiothoracic Surgery E-Book. Chicago: Society of Thoracic Surgeons; 2019. |
| 6. | Veal FC, Bereznicki LR, Thompson AJ, Peterson GM, Orlikowski CE. Pain and Functionality Following Sternotomy: A Prospective 12-Month Observational Study. Pain Med. 2016;17:1155-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Bussani R, Castrichini M, Restivo L, Fabris E, Porcari A, Ferro F, Pivetta A, Korcova R, Cappelletto C, Manca P, Nuzzi V, Bessi R, Pagura L, Massa L, Sinagra G. Cardiac Tumors: Diagnosis, Prognosis, and Treatment. Curr Cardiol Rep. 2020;22:169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 104] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 8. | Yale Medicine. Robot-Assisted Heart Surgery. [cited 27 January 2025]. Available from: https://www.yalemedicine.org/conditions/robotic-heart-surgery?form=MG0AV3. |
| 9. | Cleveland Clinic. Robotic heart surgery. [cited 27 January 2025]. Available from: https://my.clevelandclinic.org/health/treatments/17438-robotically-assisted-heart-surgery?form=MG0AV3. |
| 10. | Kadiroğulları E, Onan B, Aydın Ü, Başgöze S, Şen O. A comparison of robotically-assisted endoscopic versus sternotomy approach for myxoma excision: A single-center experience. Turk Gogus Kalp Damar Cerrahisi Derg. 2020;28:450-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 11. | Luo C, Zhu J, Bao C, Ding F, Mei J. Minimally invasive and conventional surgical treatment of primary benign cardiac tumors. J Cardiothorac Surg. 2019;14:76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 12. | Mendyka D, Płonek T, Jędrasek T, Korman A, Złotowska A, Jędrasek A, Skalik R, Kustrzycki W. The Therapeutic Potential of Different Surgical Approaches in the Management of Cardiac Myxoma: A Systematic Review. J Clin Med. 2024;14:121. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 13. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 40456] [Article Influence: 10114.0] [Reference Citation Analysis (2)] |
| 14. | Lo CK, Mertz D, Loeb M. Newcastle-Ottawa Scale: comparing reviewers' to authors' assessments. BMC Med Res Methodol. 2014;14:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 770] [Cited by in RCA: 1635] [Article Influence: 148.6] [Reference Citation Analysis (0)] |
| 15. | Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, Thomas J. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10:ED000142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1361] [Cited by in RCA: 2921] [Article Influence: 486.8] [Reference Citation Analysis (0)] |
| 16. | Kesävuori R, Raivio P, Jokinen JJ, Sahlman A, Vento A. Quality of life after robotically assisted atrial myxoma excision. J Robot Surg. 2015;9:235-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Liu Y, Ge S, Li X, Lu C, Zhang C, Liu Z. Comparative analysis of robotically-assisted versus conventional sternotomy approach in left atrial-myxoma resection: A single-center retrospective observational study. Asian J Surg. 2024;47:3877-3882. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 18. | Li SL, Gao CQ. [Robotic surgery versus conventional open chest surgery for heart tumor: a propensity score matching analysis]. Nanfang Yike Daxue Xuebao. 2017;37:1296-1300. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 19. | Yang M, Yao M, Wang G, Xiao C, Wu Y, Zhang H, Gao C. Comparison of postoperative quality of life for patients who undergo atrial myxoma excision with robotically assisted versus conventional surgery. J Thorac Cardiovasc Surg. 2015;150:152-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Schilling J, Engel AM, Hassan M, Smith JM. Robotic excision of atrial myxoma. J Card Surg. 2012;27:423-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Williams ML, Hwang B, Huang L, Wilson-Smith A, Brookes J, Eranki A, Yan TD, Guy TS, Bonatti J. Robotic versus conventional sternotomy mitral valve surgery: a systematic review and meta-analysis. Ann Cardiothorac Surg. 2022;11:490-503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 22. | Tan A, Ashrafian H, Scott AJ, Mason SE, Harling L, Athanasiou T, Darzi A. Robotic surgery: disruptive innovation or unfulfilled promise? A systematic review and meta-analysis of the first 30 years. Surg Endosc. 2016;30:4330-4352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 23. | Chuchulo A, Ali A. Is Robotic-Assisted Surgery Better? AMA J Ethics. 2023;25:E598-E604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |