Published online Aug 26, 2025. doi: 10.4330/wjc.v17.i8.107991
Revised: May 28, 2025
Accepted: July 14, 2025
Published online: August 26, 2025
Processing time: 141 Days and 12.1 Hours
The ground-breaking development of the incretin agonists by manipulation of the incretin system, including the gut hormones glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), as well as the pancreatic hor
Core Tip: Several incretin co-agonists have been developed in the recent years with outstanding metabolic and weight loss benefits revolutionising management of obesity and type 2 diabetes mellitus (T2DM). Management of obesity and T2DM with these glucagon-like peptide-1/glucose-dependent insulinotropic polypeptide/glucagon co-agonists/poly-agonists are associated with remarkable weight loss, improvement of glycated hemoglobin, albuminuria, lipid profile, liver fat and sleep apnea among patients. All these benefits are also associated with marked improvement in cardiovascular outcomes in patients treated with these medications. This clinical update review explores current evidence and emerging research questions regarding the cardiometabolic benefits associated with rational use of incretin co-agonists.
- Citation: Bhat S, Fernandez CJ, Lakshmi V, Pappachan JM. Efficacy and safety of incretin co-agonists: Transformative advances in cardiometabolic healthcare. World J Cardiol 2025; 17(8): 107991
- URL: https://www.wjgnet.com/1949-8462/full/v17/i8/107991.htm
- DOI: https://dx.doi.org/10.4330/wjc.v17.i8.107991
Incretin hormones are gastrointestinal peptides, secreted in response to nutrient intake, involved in regulating a variety of homeostatic human metabolic functions. Glucose-dependent insulinotropic polypeptide (GIP) produced from K cells of the proximal gut and glucagon-like peptide-1 (GLP-1) from the L cells of the lower intestine are the main incretin hor
As the endogenous incretin hormones have very short biological half-lives, pharmacological manipulation of the incretin system using various therapeutic agents has been developed to obtain the treatment-related clinical benefits over the past 2 decades. Some of these new molecules possess outstanding therapeutic advantages in the day-to-day manage
GLP-1 receptor agonist (GLP-1RA) are well known for their effects on glycaemic control in diabetes mellitus as well as obesity. Besides the effect on blood glucose levels, GLP-1RAs may have beneficial effects on body composition, bodyweight, body mass index (BMI), and waist circumference. They have been found to increase the high-density lipoprotein (HDL)-cholesterol level as well as reduce the total-cholesterol, low density lipoprotein (LDL)-cholesterol, and triglyceride levels[5]. Because of the favourable effects on adiposity and type 2 diabetes mellitus (T2DM) a paradigm shift in the management of diabesity (diabetes as a direct consequence of obesity) was adopted by most diabetologists with the judicious use of GLP-1RAs in the past 2 decades.
Once weekly subcutaneous (s/c) exenatide at a dose of 2 mg reduced mean glycated hemoglobin (HbA1c) by 1.9%, improved diastolic blood pressure, lipids and body weight (-3.0 kg)[6]. The newer GLP-1RAs possess better efficacy in managing diabesity as proven by inter-class comparator randomised controlled trials. For e.g., the SUSTAIN 3 trial, a head-to-head comparison between once weekly s/c semaglutide 1.0 mg and once weekly s/c exenatide 2.0 mg, demon
Liraglutide Effect and Action in Diabetes phase 3 studies investigated the efficacy and safety of liraglutide in comparison with other anti-diabetic agents[8]. These trials found that a greater proportion of participants achieved reduction of HbA1c levels below 6.5% with liraglutide compared to other anti-diabetic agents including glimepiride, rosiglitazone, metformin and insulin glargine. The efficacy of the dose of 1.8 mg was higher than that of 1.2 mg for HbA1c reduction[8].
Liraglutide use at a dose of 1.8 mg has been observed to achieve a mean HbA1c reduction of -1.22% and weight loss of -4.23 kg in patients after bariatric surgery, proving useful in those with persistent T2DM post metabolic surgery[9]. At 3.0 mg, the weight loss was greater (9.2 kg) in post-bariatric surgery patients[10].
A meta-analysis of 14 studies demonstrated that liraglutide 3.0 mg achieved a mean weight loss of 4.91 kg, and the weight loss was greater in patients without diabetes mellitus than those with diabetes mellitus[11]. In a meta-analysis comparing it with the older GLP-1RAs, liraglutide was found to be as effective as dulaglutide, albiglutide, and twice-daily exenatide in terms of HbA1c reduction while achieving greater weight loss than the latter, with the difference being nearly 1.0 kg[12].
In children and adolescents, liraglutide 1.8 mg, when added to metformin, has been found to reduce HbA1c by 0.64% at 28 weeks, but higher efficacy with a longer duration of treatment of 56 weeks was limited by gastrointestinal side effects[13]. Weight loss achieved in this age group has been observed to be up to 2.13 kg in a meta-analysis of seven randomized controlled trials. However, liraglutide did not improve blood pressure, lipid profile, or fasting insulin levels[14].
Liraglutide may have a role as an adjunct to insulin treatment in patients with type 1 diabetes especially in those with overweight/obesity[15]. However, hypoglycemia and ketoacidosis from insulin dose reduction are potential risks in such patients.
Once-weekly dulaglutide vs once-daily liraglutide in metformin-treated patients with T2DM, a randomised, open-label, phase 3, non-inferiority trial (AWARD-6), demonstrated that 1.5 mg of weekly dulaglutide was non-inferior to daily liraglutide of 1.8 mg in terms of HbA1c reduction (-1.42% vs -1.36%)[16]. Dulaglutide use was also associated with a significant reduction of HbA1c of -0.33% compared to insulin glargine[17]. Once weekly dosing advantage makes this GLP-1RA molecule an attractive choice for those with needle phobia. In patients with T2DM, it also caused weight loss (-0.86 kg) and reduction of waist circumference and BMI as seen in a meta-analysis of 18 studies[18].
Albiglutide is a once weekly GLP-1RA with a starting dose of 30 mg that can be up titrated to 50 mg weekly. The HARMONY phase 3 trials 1 to 5 showed that at the end of 3 years, albiglutide achieved an HbA1c reduction of -0.37% in rescue free population and -0.68% among all participants including those that required rescue therapy with other anti-diabetics (if they did not meet glycaemic goals)[19]. In the HARMONY 7 trial that compared once weekly albiglutide with once daily liraglutide, the HbA1c reduction, was higher with the latter as was weight reduction at 32 weeks (-0.64 kg vs -2.19 kg)[20]. This lower efficacy compared to liraglutide makes albiglutide less attractive in managing patients with diabesity.
Semaglutide as a subcutaneous injection in doses of 0.5 mg, 1.0 mg, and 2.0 mg once weekly is approved for management of T2DM and in a dose of 2.4 mg weekly for chronic weight management[21]. In the STEP 4 randomized controlled trial, subcutaneous semaglutide in a dose of 2.4 mg weekly was found to achieve a weight loss of up to 17.4%, which was sustained over 68 weeks in participants with obesity and without diabetes mellitus. This was associated with an improvement in waist circumference, BMI, lipid profile, fasting blood glucose, and HbA1c levels[22]. Semaglutide may achieve a significantly greater weight loss compared to liraglutide as per comparative studies (-13.8% to -15.8% vs -6.4% to -7.8%)[23-25]. In the phase 3b SUSTAIN 10 trial, subcutaneous semaglutide at 1.0 mg once weekly was found to be superior to once daily 1.2 mg liraglutide in HbA1c reduction (1.7% vs 1%)[26].
Oral semaglutide has been approved for T2DM. In the PIONEER 4 phase 3b trial, oral semaglutide was found to be non-inferior to once daily subcutaneous liraglutide for HbA1c reduction at 26 weeks and superior at 52 weeks. Sema
Orforglipron is a non-peptide, oral, small molecule GLP-1RA in phase 3 trials (ATTAIN-1) in the treatment of obesity and T2DM[30]. It can cause significant weight loss of up to 15%. The efficacy for causing weight loss of orforglipron among various GLP-1RAs is second only to Cagri-Sema[31].
Danuglipron is another non-peptide oral GLP-1RA in phase 3/2 of development. When administered orally at a dose of 120mg twice a day to participants with T2DM and overweight/obesity, the drug achieved a placebo-adjusted weight loss of 4.2 kg and an HbA1c reduction of 1.2%[32]. However, the drug caused nearly 50%discontinuation due to gastroin
Efpeglenatide is a long-acting GLP-1RA developed by a single amino acid modification in exendin providing a flexible dosing of once weekly and possibly once monthly[33]. This drug is undergoing phase 3 trials in the treatment of obesity and/or T2DM. The doses investigated are 2, 4, and 6 mg. Maximum weight loss was observed with efpeglenatide 4 mg (-2.3 kg at 30 weeks and -3.6 kg at 56 weeks)[34]. Maximum HbA1c reduction was observed with efpeglenatide 6 mg
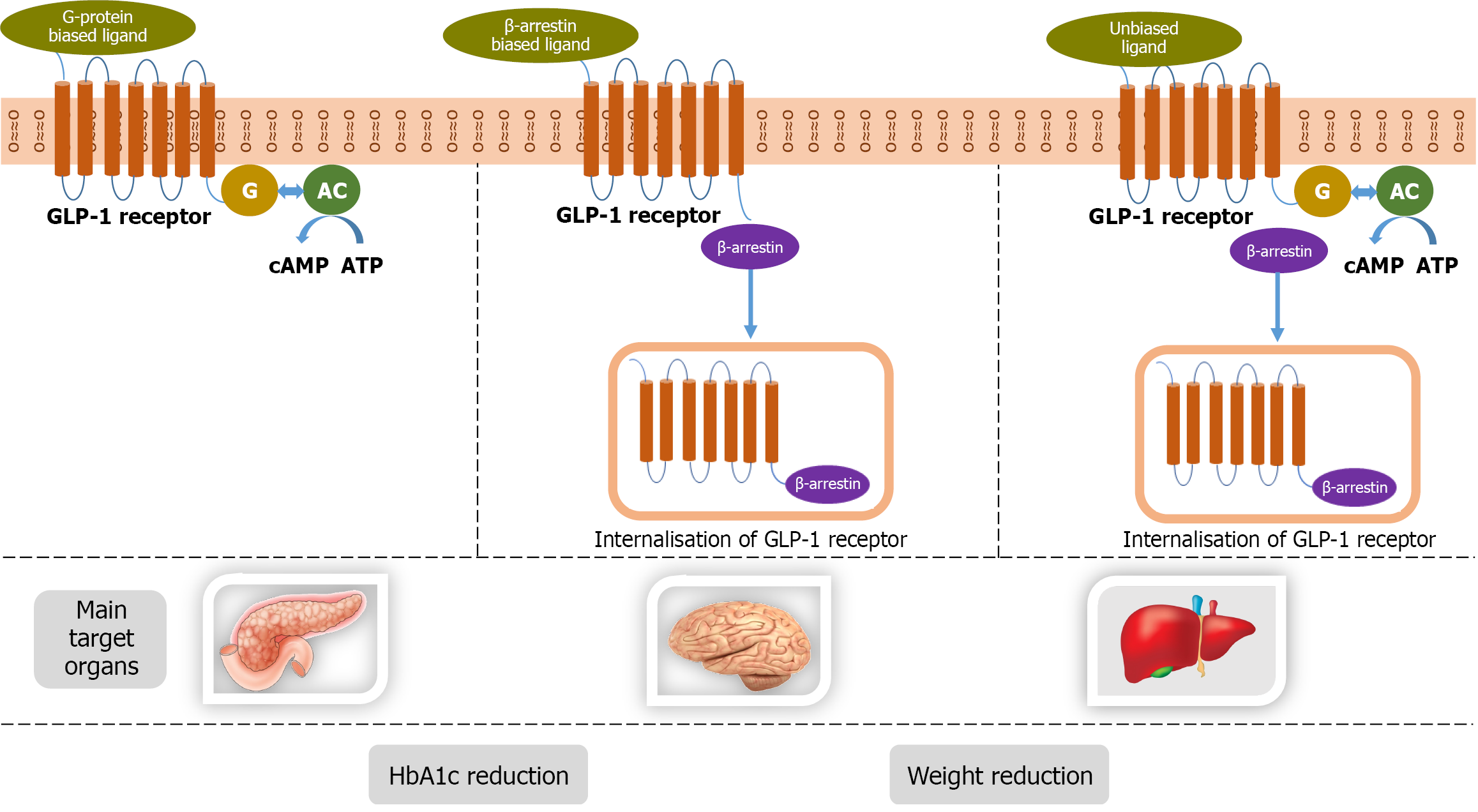
Ecnoglutide is a new long-acting “biased” GLP-1RA in phase 2/1 of development. The activation of the GLP-1 receptor (GLP-1R) triggers various intracellular signaling events, including cAMP generation, β-arrest in recruitment, and GLP-1R internalisation. This biased GLP-1RA favours cAMP production over β-arrest in recruitment, leading to reduced GLP-1R internalisation and enhanced insulin secretion. The biased GLP-1RAs like ecnoglutide have higher efficacy compared to unbiased GLP-1RAs in lowering glucose and weight[35,36]. In a phase 2 trial, the proportion of participants who achieved HbA1c below 7% with ecnoglutide was 68% to 84% compared to 21% in the placebo group. Other than improve
TG103 is a recombinant GLP-1/Fc fusion protein developed for once weekly injection. This drug is in phase 2/1 of development. The drug could cause a weight loss of greater than 6% in patients with overweight/obesity[39].
The PEGylated exenatide, PB-119, for once-weekly subcutaneous administration is in phase 2/3 of development[40]. A phase 3 trial from China showed a 1.49% HbA1c reduction and 13.94 kg of weight reduction at 52 weeks.
GLP-1RAs have been found to significantly reduce major adverse CV events (MACE), including CV and all-cause mortality, stroke, and myocardial infarction, with a trend to reduce heart failure[41]. Liraglutide was the first GLP-1RA to demonstrate CV benefit in terms of reduction in CV events and heart failure[42]. The risks of three-point MACEs (CV death, myocardial infarction, and stroke) are significantly reduced by albiglutide, dulaglutide, liraglutide, and subcuta
GLP-1RAs may be associated with an increase in heart rate. The risk for ventricular arrhythmias has been noted to be greater with higher doses and those with higher BMI, and it needs further studies for confirmation[45]. However, semaglutide may be associated with a reduction in the incidence of atrial fibrillation in individuals with high CV risk by up to 40%. This benefit, observed irrespective of the route of administration, has been suggested as a drug-specific effect of semaglutide rather than a class effect[46].
GLP-1RAs may confer benefits to patients with CKD. Liraglutide was the first of the GLP-1RAs to provide renal benefit to patients by reducing persistent macroalbuminuria[47]. In patients with diabetes mellitus and CKD with albuminuria, 1 mg weekly subcutaneous semaglutide reduced major kidney disease events which was a composite of the onset of kidney failure (dialysis, transplantation, or an estimated glomerular filtration rate (eGFR) of < 15 mL per minute per 1.73 m2), at least a 50% reduction in the eGFR from baseline, or death from kidney-related or CV causes[48]. Efpeglinatide is also associated with improved renal outcomes in patients with diabetes mellitus and high CV risk[43].
In patients with obstructive sleep apnoea, liraglutide 3.0 mg improved apnoea-hypopnea index and systolic blood pressure[49]. GLP-1RAs have been investigated in fatty liver disease in patients with and without diabetes mellitus as they reduce hepatocyte glucose and lipid accumulation and improve fibrosis. In a meta-analysis of 16 studies, Liraglutide was found to significantly reduce alanine transaminase (ALT) levels in patients with diabetes mellitus and metabolic dysfunction-associated fatty liver disease (MAFLD)[50]. Exenatide and Liraglutide have been shown to significantly reduce liver fat content in patients with diabetes mellitus, with the former being more efficacious[51]. Semaglutide achieved significant resolution of metabolic dysfunction-associated steatohepatitis (MASH) in a phase 2 trial but no improvement in liver fibrosis[52]. It is being evaluated for biopsy-proven MASH and fibrosis in phase 3 trials[53]. In a systematic review including 2178 patients, GLP-1RAs, namely exenatide, dulaglutide, liraglutide, or semaglutide, improved CRP levels and caused histological resolution of MASH with no worsening of fibrosis[54].
In addition to improving the metabolic parameters, GLP-1RAs reduce testosterone levels in women with polycystic ovary syndrome and obesity[55]. Interestingly, a meta-analysis of four randomized controlled trials showed that in patients with T2DM and psoriasis, liraglutide at a dose of 1.2 mg daily reduced the severity of psoriasis independently of weight loss or glycemic control, possibly due to its anti-inflammatory effect on invariant natural killer T cells, CD T cells, andinterleukin-17[56].
In patients with familial partial lipodystrophy, GLP-1RAs were found to reduce HbA1c, weight, fasting blood glucose levels, and triglyceride levels[57]. Exenatide long-acting release and liraglutide had beneficial effects on weight, BMI, waist circumference, and systolic as well as diastolic blood pressure in patients of schizophrenia on anti-psychotic therapy[58].
GIP is secreted by the enteroendocrine K cells in duodenum and jejunum, while GLP-1 is secreted by the enteroendocrine L cells in ileum and colon. In the physiological state, even in patients with diabetes, GLP-1 and GIP levels increase post prandially with the increase in molar concentration of GIP higher by 3 to 4 times compared to GLP-1. Both GIP receptor (GIPR) and GLP-1R belong to the class B family of 7-transmembrane G protein-coupled receptors (GPCRs) of the glu
In pancreatic beta cells, GLP-1 can activate both G proteins Gαs and Gαq, whereas GIP selectively activates Gαs[59]. GPCRs act downstream via predominantly two pathways, G proteins’ mediated cAMP and ERK1/2 activation and G protein-independent β-arrest in signalling. G protein mediated signalling is known to affect β cell signalling via alte
Agonist-dependent GPCR internalization is commonly mediated by β-arrest in recruitment of endosomal trafficking machinery[35]. Peptide ligands may vary in their degree of effect on GLP-1R internalization and recycling and inter
Certain co-agonists may have balanced action at two receptors such as GIPR and GLP-1R while others may have imbalanced action at either receptor leading variations in metabolic actions[36]. GIP and GLP-1 may have distinct effect on pancreatic islets depending on blood glucose levels. During hypoglycemia GIP is glucagonotropic via α cells while GLP-1A is insulinostatic via β cells. During hyperglycemia, although both peptides cause insulinotropic effect via β cells, this effect of GIP has been found to be significantly diminished in patients with diabetes. GIPRs are abundant in adipose tissue and agonism leads to lipoprotein lipase activation and uptake of triglycerides into adipose tissue. GIP also in
The GLP-1 and GIP potentiate insulin secretion in response to meals and exert a trophic action on pancreatic β-cells. The effect on insulin secretion is additive and much more than that of either molecule alone. While GLP-1 inhibits glucagon secretion from α-cells of the pancreas, GIP may stimulate glucagon secretion, especially during hypoglycemia, func
This was the first dual GIP/ GLP-1RA with a balanced action on both the GIPR and GLP-1R. Although phase 1 trials showed improvement in body weight and glucose levels, it did not fare better than the liraglutide in these terms in its phase 2b trial[64].
The second dual GIP/GLP-1 agonist that was developed, tirzepatide, has an unbalanced pharmacology, unlike RG7697, with a stronger agonism at the GIPR than at the GLP-1R. Also, tirzepatide has been shown to selectively cause G protein mediated cAMP signalling compared to β-arrest in recruitment at the GLP-1R, which may beneficially impactGLP-1R trafficking and augment its cellular response[36].
Tirzepatide became the first FDA-approved dual incretin agonist for T2DM and obesity in 2022 and 2023, respectively. It caused a superior reduction in HbA1c levels as well as body weight over semaglutide in a phase 3 trial[65]. In the SURMOUNT 2 trial on patients with T2DM and obesity, at the end of 72 weeks, tirzepatide 10 and 15mg once weekly subcutaneously achieved a reduction in body weight significantly greater than that by placebo (-12.8% and -14.7% vs
In a phase 2 trial involving 190 adults with MASH and moderate to severe liver fibrosis (stages 2 or 3), tirzepatide, at the end of 52 weeks, caused resolution of MASH without worsening of fibrosis compared to placebo[68]. In 731 patients with heart failure and preserved ejection fraction and a BMI of at least 30 kg/m2, tirzepatide reduced the risk of composite of death from CV causes or worsening heart failure, compared to placebo[69]. Unlike in the phase 2 trial with dulaglutide as a comparator, where the 15 mg dose of tirzepatide was used upfront, in the SURPASS 1 trial, a lower starting dose and slower monthly escalation was used to improve gastrointestinal tolerability. Nausea was reported in 12%, 13% and 16% participants on 5 mg, 10 mg and 15 mg of tirzepatide respectively[67].
The concept of co-agonism at the GLP-1R and glucagon receptor (GCGR) originated with the observed body weight-lowering effect of the endogenous gut peptide oxyntomodulin, which is released from posttranslational modifications of proglucagon having an agonistic action on both GLP-1R and GCGR[70,71]. GLP-1and glucagon are peptides that have similar N-terminal sequences and act on structurally related receptors. In animal models and humans, glucagon in fusion has been found to increase energy expenditure and cause thermogenesis through mechanisms mediated by brown adipose tissue (BAT) as well as those in dependent of BAT.
GCGR agonism has been shown to slightly raise fasting blood glucose levels and the HbA1c. The trophic action of GLP-1R agonism on the β-cells of the pancreas causing increased insulin secretory response is postulated to offset the hyperglycaemic effect of glucagon agonism[72]. Only those GLP-1R and GCGR dual-agonists with a balanced activity at these receptors, including mazdutide, cotadutide, survodutide, and SAR425899, exhibit glucose lowering, though all GLP-1R and GCGR dual agonists achieve significant weight lowering effects[72].
When combined with GLP-1RAs, the GCGR agonists have been found to act synergistically in causing remarkable weight loss, greater than that due to GLP-1R agonism alone. Glucagon agonism increases hepatic glucose production, clearing the liver of its glycogen stores and increasing the β-oxidation of fatty acids by the liver. GCGR agonism-mediated increased energy expenditure combined with the anorectic effect of GLP-1R agonism could achieve greater weight loss, reduction in hepatic fat content, and resolution of MASH. Dual GLP-1R/GCGR agonists have been developed as revolutionary anti-obesity medications[73,74].
Another unique feature of co-agonists is reduced metabolic adaptation, i.e., a smaller change in the sleeping metabolic rate with weight loss, leading to more successful weight reduction compared to previous anti-obesity medications[75]. However, unlike the GLP-1/GIP co-agonists, the GLP-1R/GCGR agonists are associated with gastrointestinal side effects of GLP-1RAs[76].
Mazdutide is a synthetic peptide analogue of oxyntomodulin with an addition of a fatty acyl moiety to increase half-life[77]. It is a once-weekly subcutaneous dual GLP-1R and GCGR agonist with a balanced activity at both receptors. It has been studied in phase 1 and phase 2 trials at doses of 3 mg, 4.5 mg, and 6 mg. It has been shown to reduce weight by 6.22% in both individuals with and without T2DM, the effective weight loss being greater in the latter group. It also reduces HbA1c levels, fasting blood glucose, total-cholesterol, LDL-cholesterol, HDL-cholesterol, and triglyceride levels and thereby positively affects cardiometabolic health. It was associated with gastrointestinal side effects, including nausea, vomiting, and reduced appetite[77,78].
Cotadutide is a dual GLP-1R/GCGR agonist with a balanced activity at both receptors. Once weekly subcutaneous injection reduces blood glucose levels as well as weight. It acts by delaying gastric emptying as well as by exerting an insulinotropic effect[79]. It has been shown to reduce liver glycogen and fat compared to liraglutide and placebo. Its development for metabolic-associated fatty liver disease (MAFLD) and CKD was discontinued at the phase 2 Level[80].
SAR425899 is a novel dual agonist at the GLP-1R and GCGR derived from exendin-4 with a balanced activity at both receptors. In a phase 2b trial in patients with T2DM, SAR425899 significantly reduced HbA1c and body weight. In this study, postprandial glycemic control achieved by SAR425899 was significantly greater than that achieved by liraglutide, probably due to the enhanced β-cell responsiveness caused by the former[81].
It is a dual GLP-1R/GCGR receptor agonist investigated in a dose of 10 mg once weekly subcutaneously. The efficacy of efinopegdutide for causing weight loss in patients with obesity is comparable to that of semaglutide[24]. However, it showed superior weight loss compared to liraglutide (-6.9% vs -5.8%). It had a favourable effect on blood pressure and lipid profile, with a slight increase in HDL-cholesterol (potential cardiometabolic benefits). However, the HbA1c remained unchanged, and fasting blood glucose increased while both were reduced in the liraglutide group[76]. In a phase 2a trial, efinopegdutide reduced liver fat content to a greater extent than semaglutide at 24 weeks in patients with MAFLD[82].
There are no published data available at present with this molecule, but the company OPKO Health reports that this dual GLP-1R/GCGR agonist caused weight loss in patients with T2DM and obesity. It is also being investigated for manage
It is a long-acting GLP-1R/GCGR dual agonist with a balanced activity at both receptors, developed for once-weekly subcutaneous administration for obesity with and without T2DM investigated in two phase 3 trials, SYNCHRONIZE-1 and SYNCHRONIZE-2, respectively[84]. It is currently in phase 3 of development. Its CV safety will be evaluated in the SYNCHRINIZE-CVOT[85]. In a meta-analysis of 29 randomized controlled trials of pharmacological interventions for biopsy-proven MASH, survodutide and tirzepatide ranked among the most effective therapies for MASH resolution without worsening of fibrosis[86].
It is a long-acting GLP-1/GCGR dual agonist administered once weekly being investigated (phase 2) for MAFLD. It has been found to reduce liver fat content, markers of hepatic inflammation, and body weight by 4.6%[87].
CagriSema (cagrilintide 2.4 mg/semaglutide 2.4 mg) is a combination of the dual amylin and calcitonin receptor agonist, namely cagrilintide, and the GLP-1RA, namely semaglutide, developed for once-weekly subcutaneous administration for weight management[88]. In a phase 2 trial in participants with T2DM, at the end of 32 weeks, the mean change in weight of CagriSema (-15.6%) was greater than that of semaglutide (-5.1%) or cagrilintide (-8.1%) but the change in HbA1c and fasting blood glucose by the combination did not surpass that of semaglutide[88]. In a meta-analysis of 29 studies on the efficacy and safety of GLP-1RAs, CagriSema was shown to have the best effect on weight loss, which was dose-dependent, while the gastrointestinal adverse effects did not increase with dose escalation[31]. It is currently in phase 3 trials (REDEFINE1 and 3)[89]. In a network meta-analysis of 29 studies comparing data from a total of 10333 participants on various medication including semaglutide, liraglutide, exenatide, cagrilintide, CagriSema, JNJ-64565111, orforglipron, and efpeglenatide, the maximum weight loss was achieved with CagriSema (mean weight change of -14.13 kg and a percentage weight loss of -15.44%) followed by orforglipron and semaglutide, with the weight change proportionate to the doses used. While the reduction in HbA1c with CagriSema and semaglutide were greater with higher doses, there was an increase in HbA1c with higher doses of liraglutide, thus potentially discouraging the use of higher doses of lira
Retatrutide is a novel long-acting triple agonist developed for once-weekly subcutaneous administration. It has a balanced GLP-1R and GCGR activity but greater GIPR activity. It is in phase 3 of its development. It has demonstrated the highest achievable weight loss with pharmacologic treatment, which is up to 24.2% at the end of 48 weeks with the 12 mg dose in the phase 2 clinical trials involving patients with obesity and no diabetes mellitus[90]. This weight loss did not appear to plateau at the end of the trial, thus potentially causing greater weight loss with further treatment duration[90]. Retatrutide has been associated with improvement in blood pressure as well as lipid profile. In the phase 2 trial in patients without T2DM, the drug demonstrated an improvement in fasting blood glucose levels and HbA1c, with 72%of participants who had prediabetes at baseline reverting to normoglycemia. In a phase 2 sub-study, retatrutide reduced liver fat and the fibrosis biomarkers K-18 and pro-C3 in MASH at the end of 24 and 48 weeks[91]. All these clinical and biochemical benefits are expected to improve the cardiometabolic outcomes to be proven in the near future once these trials are completed.
Efocipegtrutide, a triple GLP-1/GIP/GCGR agonist, has demonstrated significant liver fat reduction after 12 weeks in participants with MAFLD and is now in phase 2 development for MASH[92].
Dual and triple receptor agonists are likely more effective for weight loss than GLP-1R monoagonists. In a systematic review and network meta-analysis of 27 randomized controlled trials of seven GLP-1RAs and incretin polyagonists for weight loss in obesity, retatrutide in doses of 8 and 12 mg as well as tirzepatide 15 mg were relatively the most efficacious in causing weight loss and reducing waist circumference, compared to mazdutide, semaglutide, liraglutide, orforglipron and beinaglutide[93]. The weight lowering effect was greater with longer treatment duration and in those with higher BMI. In another systematic review and network meta-analysis comparing 16 incretin agonists from 76 randomized controlled trials involving 39246 adults, CagriSema was found to cause the greatest weight loss (-14.03 kg), followed by tirzepatide, retatrutide, orforglipron, semaglutide, and liraglutide[94]. Such a sustained weight loss may be a significant advantage over the GLP-1RAs, whose effects on body weight decline gradually with long-term use. In the same study, tirzepatide achieved the greatest HbA1c reduction (-2.1%), followed by mazdutide (-2.09%) and CagriSema (-1.8%)[94]. These outstanding metabolic benefits associated with poly-agonists are going to revolutionise the CV therapeutic land
The American Diabetic Association guidelines 2025 recommend use of GLP-1RAs such as semaglutide or dual agonists such as tirzepatide for their greater weight loss efficacy in patients with diabetes and overweight or obesity[95]. The guidelines also suggest consideration of these drugs with or without pioglitazone in patients with biopsy-proven MASH or those at high risk for liver fibrosis. Concurrent use of incretin agonists along with DPP4 inhibitors is not recommended due to absence of additional glucose lowering benefits with the combination and the potential for higher adverse effects. In insulin-sufficient individuals, GLP-1RAs or GLP-1R/GIPR dual agonists are preferred over insulin. In insulin-deficient individuals, combining the incretin agonists with insulin has been suggested to balance the weight gain effect of insulin with the weight lowering effects of the latter[96].
The dual GLP-1R and GCGR agonists have been associated with an increased heart rate, greater than that with GLP-1RAs due to the cardio-stimulant effect of glucagon.
Tirzepatide was found to cause an increase in mean pulse rate of 1-2 beats per min at the end of 40 weeks[67]. A similar increase in heart rate was observed with the GLP-1, GIP, GCG receptor triple agonist, retatrutide up to 24 weeks of duration but declined with further treatment duration. However, no serious arrhythmia was observed[90].
Some of the most reported adverse effects with incretin agonists are gastrointestinal events, including diarrhoea, nausea, and vomiting. These side effects are supposed to be lower with GLP-1/GIP co-agonists compared to GLP-1 RAs. As the gastrointestinal effect of GLP-1 agonism is neutralised by the anti-emetic effect of GIP agonism, tirzepatide has been found to have good gastrointestinal tolerance, unlike the GLP-1 mono-agonists[36]. These have been observed with increasing frequency at higher doses, especially with tirzepatide, semaglutide, dulaglutide, lixisenatide, and retatrutide[94].
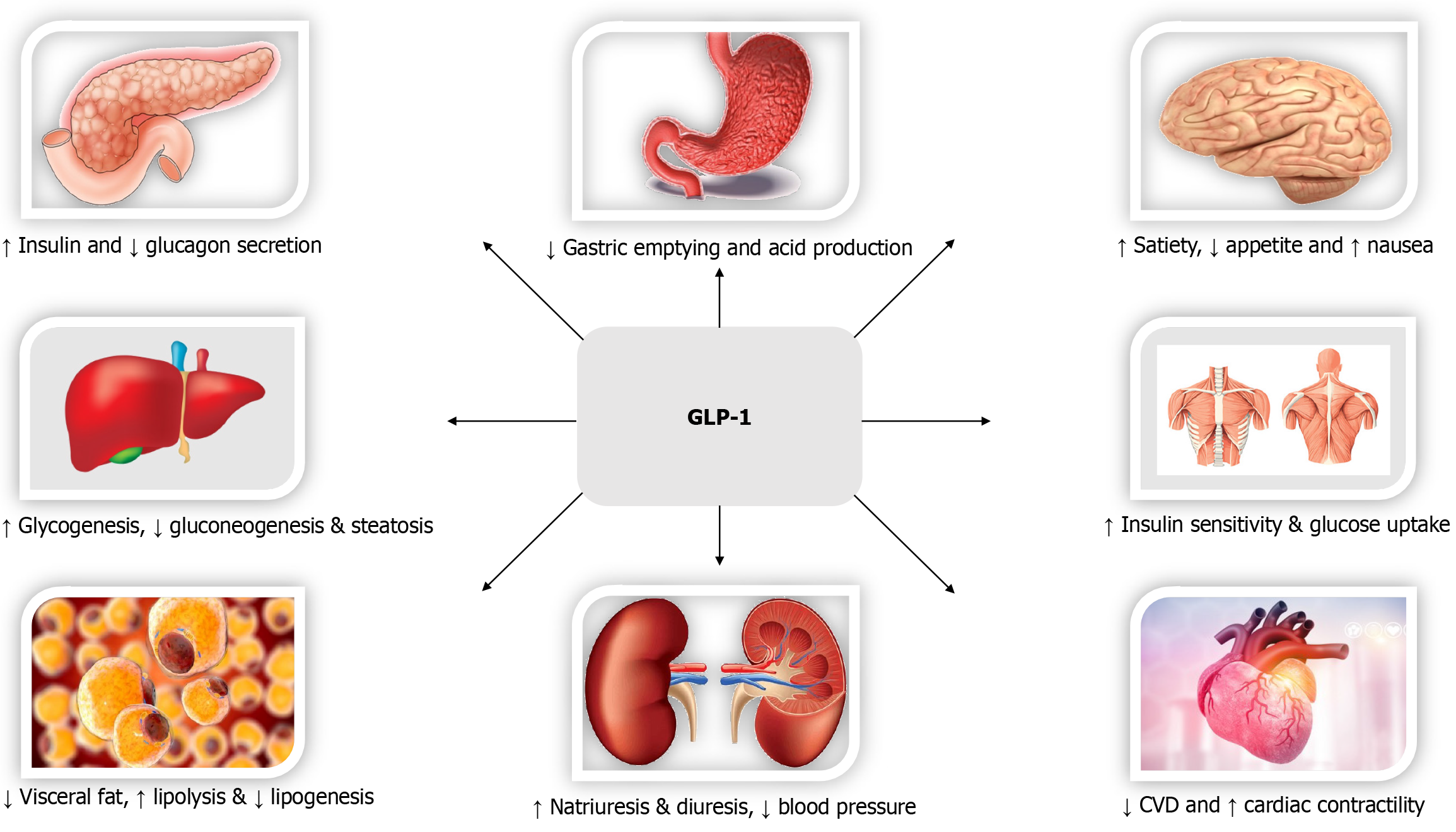
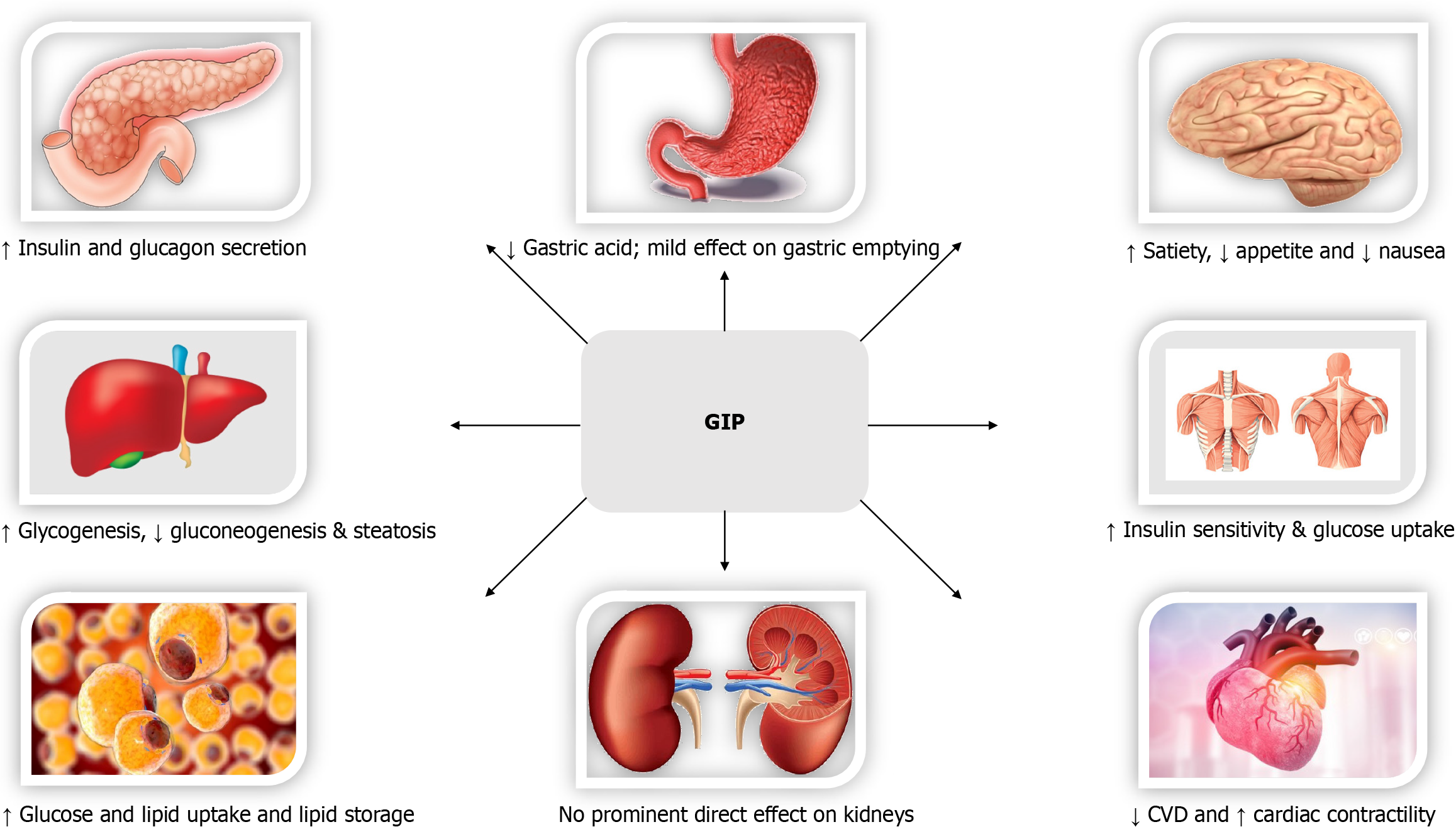
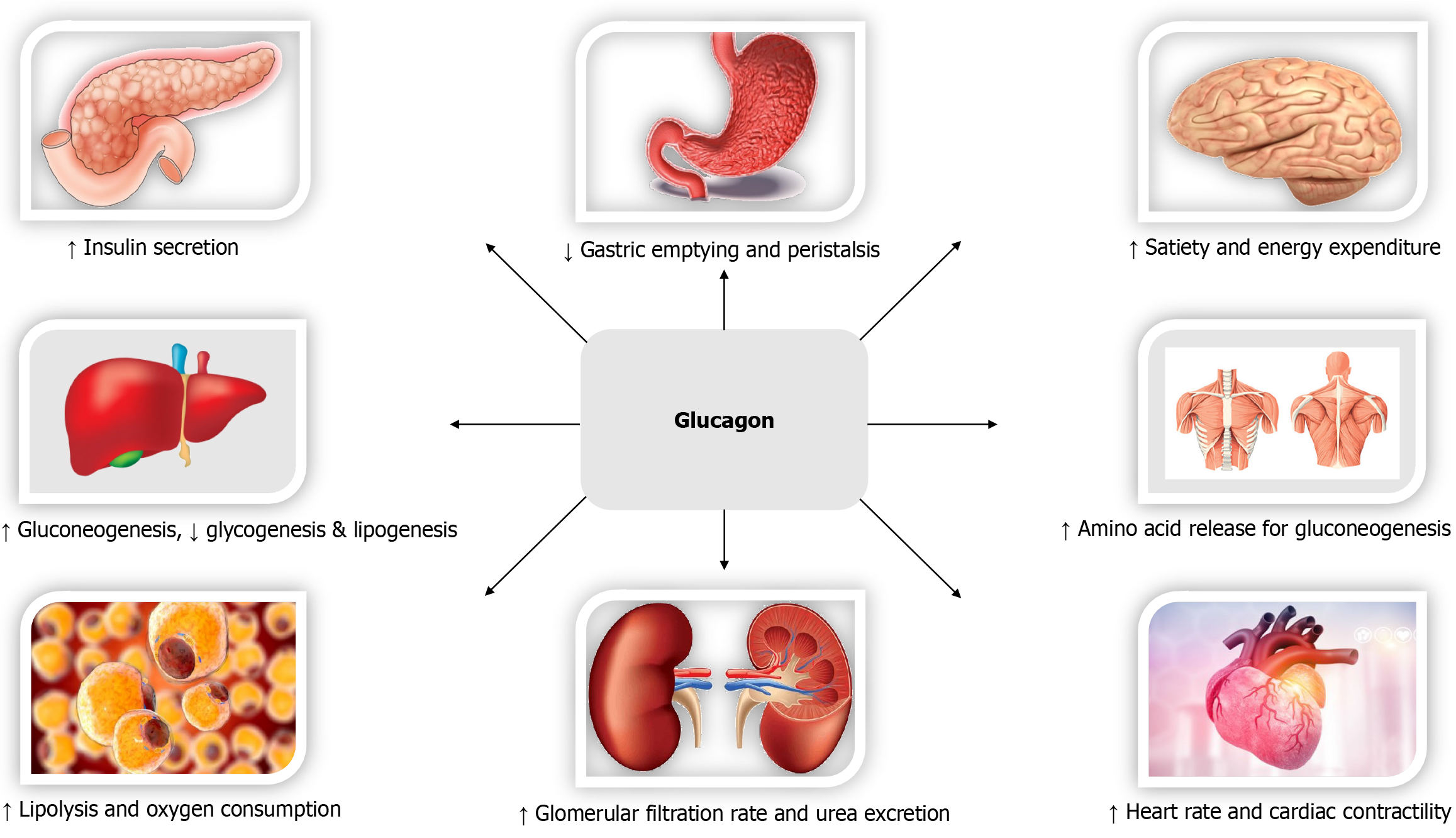
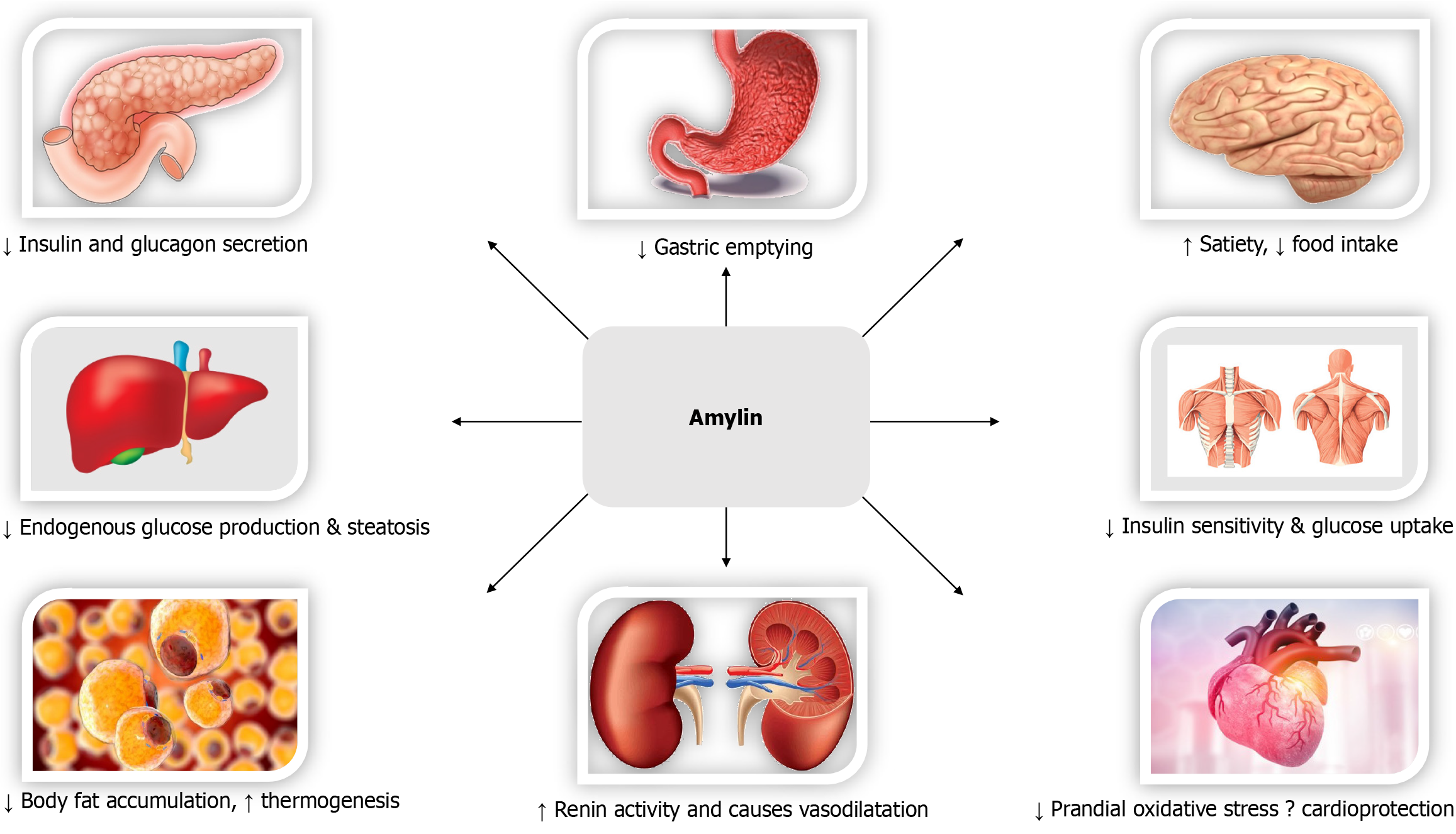
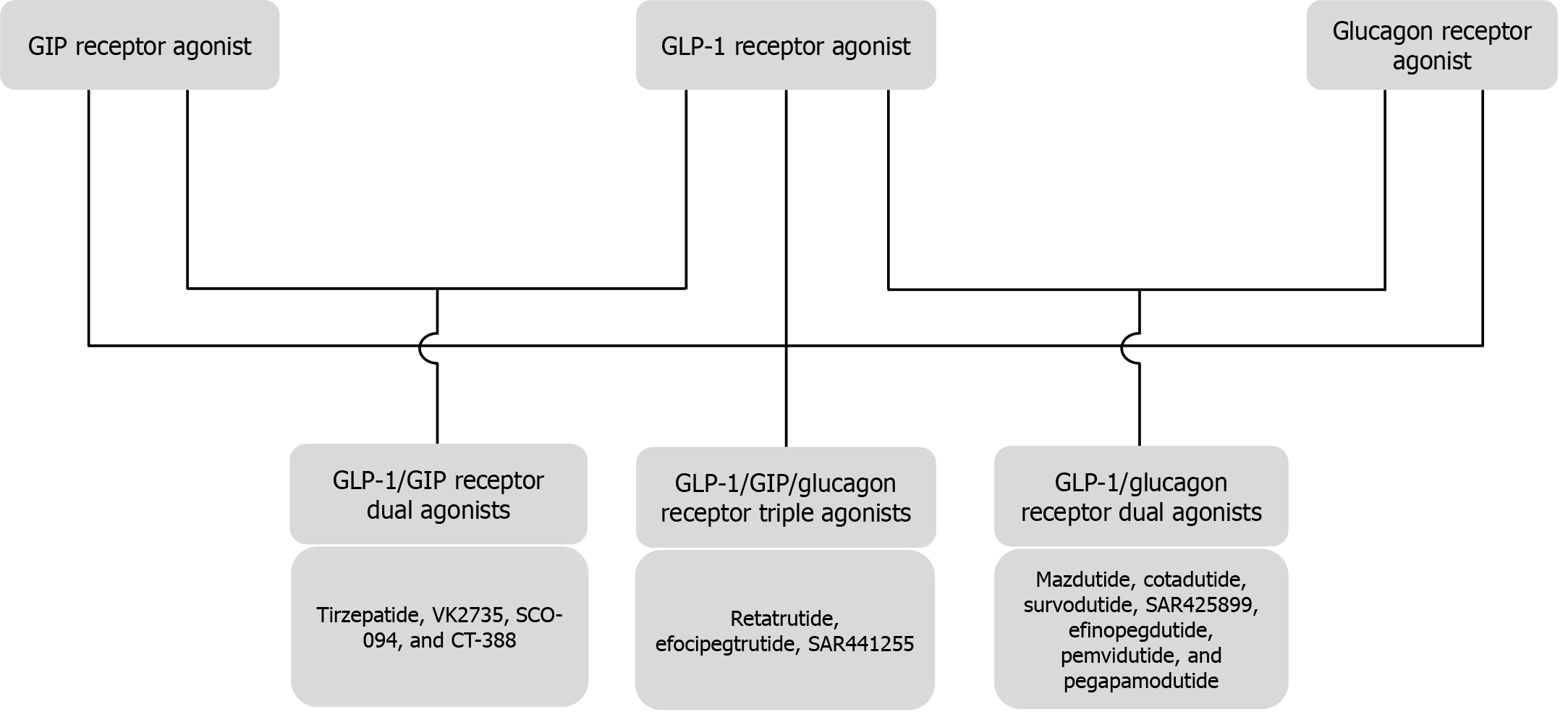
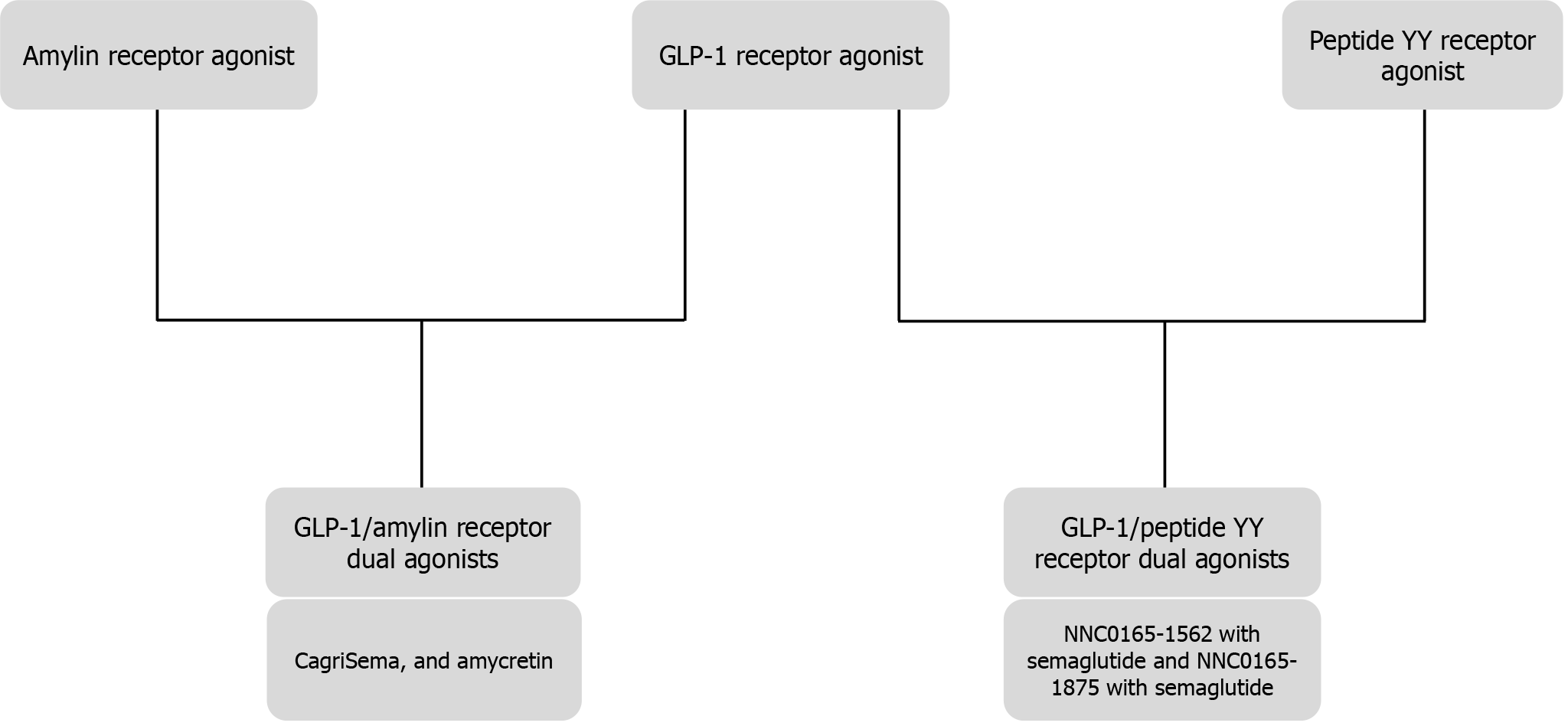
Biological effects of GLP-1 (Figure 2)[97], GIP (Figure 3)[98,99], Glucagon (Figure 4)[72], and Amylin (Figure 5)[100] at various organ/tissue levels are schematically depicted below. An overview of the various incretin co-agonists and their combinations is given in Figure 6, and that of GLP-1RAs with amylin and peptide YY agonists is shown in Figure 7.
Several new incretin co-agonists are currently under development and some of these molecules are expected to have outstanding cardiometabolic benefits. Amycretin (NNC0487-0111), a GLP-1/amylin receptor dual agonist by Novo Nordisk, has completed phase 1 development and is found to reduce body weight by 13%[101,102]. CT-996 (Carmot Therapeutics) is another oral GLP-1RA in phase 1 development for once-daily use[103]. VK2735 (Viking Therapeutics), SCO-094 (Scohia Pharma), and CT-388 (Carmot Therapeutics) are the GLP-1R and GIPR dualagonists in phase 1 development[103]. MariTide (AMG 133) by Amgen is an investigational bispecific molecule developed by conjugating a fully human monoclonal GIPR antagonist antibody to two GLP-1R agonist peptides[104]. Although seemingly contradictory, both GIP agonism, as seen with tirzepatide, and GIP antagonism, as observed with MariTide, have shown to synergise with GLP-1R agonism to promote remarkable weight loss[105]. Two peptide YY/GLP-1R dual agonists (NNC0165-1562 with semaglutide and NNC0165-1875 with semaglutide) from Novo Nordisk are undergoing phase 1 and phase 2 clinical trials, respectively[103].
Long-term CV safety of newer drugs such as retatrutide would be required for use in high-risk patients especially those with established atherosclerotic CV disease. The effect of incretin agonists especially with glucagon agonism, on heart rate and incidence of arrhythmias would need further research. Variable responses to incretin agonists remain a therapeutic challenge in management of metabolic diseases. Genetic missense variations in receptors including loss of function and gain of function mutations have been documented in GIPR, GLP1-R and GCCRs and may lead to reduced efficacy of drugs[106]. Head-to-head trials comparing various incretin agonists such as tirzepatide and retatrutide would help answer certain questions such as comparable efficacy of weight loss potential, efficacy regarding organ protection (liver, renal, CV etc.) and longer-term safety issues.
A major setback for most of the currently available incretin agonists is the high costs which make them less accessible to patients in resource poor countries. Because of the profound benefits and potential disease modifying properties of these agents, public health authorities and governments should consider provision of subsidised availability of these drugs to such patients. Another issue is the lack of adequate data on head-to-head comparison of oral vs injectable versions of different GLP-1RA molecules in terms of efficacy, safety and cost effectiveness. Oral agents may be more acceptable to some patients especially those with needle phobia and such comparative study data should provide adequate evidence for informed decision making in clinical practice.
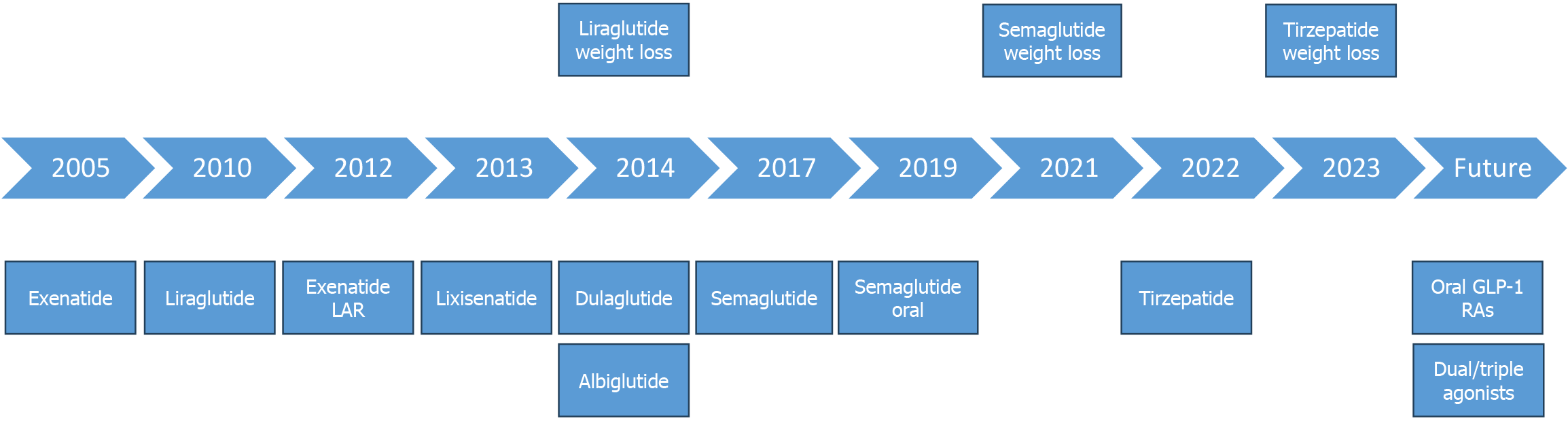
The stages of development of various incretin agonists are depicted in the Figure 8.
The incretin agonists are ground-breaking therapies for diabesity. The weight-reducing efficacy of dual agonists is probably the highest so far reported by any pharmacological therapy for obesity. The limiting gastrointestinal side effects of GLP-1 mono-agonists have been surpassed to some extent by the dual GIP/GLP-1 agonists. Future studies may throw light on the effect on CV health and the risk of tachyarrhythmias with these agents. With parallel effects on metabolic parameters other than blood glucose and weight, such as blood pressure, fatty liver, and lipid profile, the incretin co-agonists are a great leap forward in cardiometabolic healthcare.
We are thankful to Annlyn Vinu Thomas for providing us the audio clip for the core tip of this article.
| 1. | Nauck MA, Meier JJ. Incretin hormones: Their role in health and disease. Diabetes Obes Metab. 2018;20 Suppl 1:5-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 528] [Article Influence: 75.4] [Reference Citation Analysis (0)] |
| 2. | Meier JJ, Gallwitz B, Siepmann N, Holst JJ, Deacon CF, Schmidt WE, Nauck MA. Gastric inhibitory polypeptide (GIP) dose-dependently stimulates glucagon secretion in healthy human subjects at euglycaemia. Diabetologia. 2003;46:798-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 243] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 3. | Nauck MA, Heimesaat MM, Orskov C, Holst JJ, Ebert R, Creutzfeldt W. Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest. 1993;91:301-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1148] [Cited by in RCA: 1205] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 4. | Campbell JE, Drucker DJ. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013;17:819-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 918] [Cited by in RCA: 1062] [Article Influence: 88.5] [Reference Citation Analysis (0)] |
| 5. | Ansari HUH, Qazi SU, Sajid F, Altaf Z, Ghazanfar S, Naveed N, Ashfaq AS, Siddiqui AH, Iqbal H, Qazi S. Efficacy and Safety of Glucagon-Like Peptide-1 Receptor Agonists on Body Weight and Cardiometabolic Parameters in Individuals With Obesity and Without Diabetes: A Systematic Review and Meta-Analysis. Endocr Pract. 2024;30:160-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 39] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 6. | Wysham CH, MacConell LA, Maggs DG, Zhou M, Griffin PS, Trautmann ME. Five-year efficacy and safety data of exenatide once weekly: long-term results from the DURATION-1 randomized clinical trial. Mayo Clin Proc. 2015;90:356-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 7. | Ahmann AJ, Capehorn M, Charpentier G, Dotta F, Henkel E, Lingvay I, Holst AG, Annett MP, Aroda VR. Efficacy and Safety of Once-Weekly Semaglutide Versus Exenatide ER in Subjects With Type 2 Diabetes (SUSTAIN 3): A 56-Week, Open-Label, Randomized Clinical Trial. Diabetes Care. 2018;41:258-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 407] [Article Influence: 58.1] [Reference Citation Analysis (0)] |
| 8. | Blonde L, Russell-Jones D. The safety and efficacy of liraglutide with or without oral antidiabetic drug therapy in type 2 diabetes: an overview of the LEAD 1-5 studies. Diabetes Obes Metab. 2009;11 Suppl 3:26-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 173] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 9. | Miras AD, Pérez-Pevida B, Aldhwayan M, Kamocka A, McGlone ER, Al-Najim W, Chahal H, Batterham RL, McGowan B, Khan O, Greener V, Ahmed AR, Petrie A, Scholtz S, Bloom SR, Tan TM. Adjunctive liraglutide treatment in patients with persistent or recurrent type 2 diabetes after metabolic surgery (GRAVITAS): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2019;7:549-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 115] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 10. | Mok J, Adeleke MO, Brown A, Magee CG, Firman C, Makahamadze C, Jassil FC, Marvasti P, Carnemolla A, Devalia K, Fakih N, Elkalaawy M, Pucci A, Jenkinson A, Adamo M, Omar RZ, Batterham RL, Makaronidis J. Safety and Efficacy of Liraglutide, 3.0 mg, Once Daily vs Placebo in Patients With Poor Weight Loss Following Metabolic Surgery: The BARI-OPTIMISE Randomized Clinical Trial. JAMA Surg. 2023;158:1003-1011. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 72] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 11. | Konwar M, Bose D, Jaiswal SK, Maurya MK, Ravi R. Efficacy and Safety of Liraglutide 3.0 mg in Patients with Overweight and Obese with or without Diabetes: A Systematic Review and Meta-Analysis. Int J Clin Pract. 2022;2022:1201977. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 12. | Hu EH, Tsai ML, Lin Y, Chou TS, Chen TH. A Review and Meta-Analysis of the Safety and Efficacy of Using Glucagon-like Peptide-1 Receptor Agonists. Medicina (Kaunas). 2024;60:357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 13. | Tamborlane WV, Barrientos-Pérez M, Fainberg U, Frimer-Larsen H, Hafez M, Hale PM, Jalaludin MY, Kovarenko M, Libman I, Lynch JL, Rao P, Shehadeh N, Turan S, Weghuber D, Barrett T; Ellipse Trial Investigators. Liraglutide in Children and Adolescents with Type 2 Diabetes. N Engl J Med. 2019;381:637-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 231] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 14. | Gou H, Zhai Y, Guo J. Efficacy and safety of liraglutide for weight management in children and adolescents: a systematic review and meta-analysis of randomized controlled trials. Eur J Pediatr. 2023;182:5095-5108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Dejgaard TF, von Scholten BJ, Christiansen E, Kreiner FF, Bardtrum L, von Herrath M, Mathieu C, Madsbad S; ADJUNCT ONE and ADJUNCT TWO Investigators. Efficacy and safety of liraglutide in type 1 diabetes by baseline characteristics in the ADJUNCT ONE and ADJUNCT TWO randomized controlled trials. Diabetes Obes Metab. 2021;23:2752-2762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 16. | Dungan KM, Povedano ST, Forst T, González JG, Atisso C, Sealls W, Fahrbach JL. Once-weekly dulaglutide versus once-daily liraglutide in metformin-treated patients with type 2 diabetes (AWARD-6): a randomised, open-label, phase 3, non-inferiority trial. Lancet. 2014;384:1349-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 373] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 17. | Xu J, Yao D, Xia J. Efficacy and safety of dulaglutide compared with glargine in patients with type 2 diabetes: A systematic review and meta-analysis. J Clin Pharm Ther. 2021;46:1245-1253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Li Y, Gong X, Găman MA, Hernández-Wolters B, Velu P, Li Y. The effect of subcutaneous dulaglutide on weight loss in patients with Type 2 diabetes mellitus: Systematic review and meta-analysis of randomized controlled trials. Eur J Clin Invest. 2024;54:e14125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 19. | Home PD, Ahrén B, Reusch JEB, Rendell M, Weissman PN, Cirkel DT, Miller D, Ambery P, Carr MC, Nauck MA. Three-year data from 5 HARMONY phase 3 clinical trials of albiglutide in type 2 diabetes mellitus: Long-term efficacy with or without rescue therapy. Diabetes Res Clin Pract. 2017;131:49-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 20. | Pratley RE, Nauck MA, Barnett AH, Feinglos MN, Ovalle F, Harman-Boehm I, Ye J, Scott R, Johnson S, Stewart M, Rosenstock J; HARMONY 7 study group. Once-weekly albiglutide versus once-daily liraglutide in patients with type 2 diabetes inadequately controlled on oral drugs (HARMONY 7): a randomised, open-label, multicentre, non-inferiority phase 3 study. Lancet Diabetes Endocrinol. 2014;2:289-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 248] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 21. | Amaro A, Sugimoto D, Wharton S. Efficacy and safety of semaglutide for weight management: evidence from the STEP program. Postgrad Med. 2022;134:5-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 22. | Rubino D, Abrahamsson N, Davies M, Hesse D, Greenway FL, Jensen C, Lingvay I, Mosenzon O, Rosenstock J, Rubio MA, Rudofsky G, Tadayon S, Wadden TA, Dicker D; STEP 4 Investigators. Effect of Continued Weekly Subcutaneous Semaglutide vs Placebo on Weight Loss Maintenance in Adults With Overweight or Obesity: The STEP 4 Randomized Clinical Trial. JAMA. 2021;325:1414-1425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 584] [Cited by in RCA: 686] [Article Influence: 171.5] [Reference Citation Analysis (0)] |
| 23. | O'Neil PM, Birkenfeld AL, McGowan B, Mosenzon O, Pedersen SD, Wharton S, Carson CG, Jepsen CH, Kabisch M, Wilding JPH. Efficacy and safety of semaglutide compared with liraglutide and placebo for weight loss in patients with obesity: a randomised, double-blind, placebo and active controlled, dose-ranging, phase 2 trial. Lancet. 2018;392:637-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 510] [Article Influence: 72.9] [Reference Citation Analysis (0)] |
| 24. | Wen J, How-Volkman C, Truong A, Nadora D, Bernstein EM, Akhtar M, Puglisi J, Frezza E. Comparative Efficacy of Semaglutide Versus Liraglutide or Efinopegdutide on Weight Loss in Obese Patients: A Systematic Review and Meta-Analysis. Cureus. 2024;16:e75304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 25. | Rubino DM, Greenway FL, Khalid U, O'Neil PM, Rosenstock J, Sørrig R, Wadden TA, Wizert A, Garvey WT; STEP 8 Investigators. Effect of Weekly Subcutaneous Semaglutide vs Daily Liraglutide on Body Weight in Adults With Overweight or Obesity Without Diabetes: The STEP 8 Randomized Clinical Trial. JAMA. 2022;327:138-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 457] [Cited by in RCA: 515] [Article Influence: 171.7] [Reference Citation Analysis (0)] |
| 26. | Capehorn MS, Catarig AM, Furberg JK, Janez A, Price HC, Tadayon S, Vergès B, Marre M. Efficacy and safety of once-weekly semaglutide 1.0mg vs once-daily liraglutide 1.2mg as add-on to 1-3 oral antidiabetic drugs in subjects with type 2 diabetes (SUSTAIN 10). Diabetes Metab. 2020;46:100-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 263] [Article Influence: 52.6] [Reference Citation Analysis (0)] |
| 27. | Pratley R, Amod A, Hoff ST, Kadowaki T, Lingvay I, Nauck M, Pedersen KB, Saugstrup T, Meier JJ; PIONEER 4 investigators. Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): a randomised, double-blind, phase 3a trial. Lancet. 2019;394:39-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 369] [Article Influence: 61.5] [Reference Citation Analysis (0)] |
| 28. | Knop FK, Aroda VR, do Vale RD, Holst-Hansen T, Laursen PN, Rosenstock J, Rubino DM, Garvey WT; OASIS 1 Investigators. Oral semaglutide 50 mg taken once per day in adults with overweight or obesity (OASIS 1): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2023;402:705-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 170] [Article Influence: 85.0] [Reference Citation Analysis (0)] |
| 29. | Lütkemeyer C, Pasqualotto E, Ferreira ROM, Chavez MP, Petris I Jr, Dos Santos HV, Wille JM, Hohl A, Ronsoni MF, van de Sande-Lee S. Effects of once-daily oral orforglipron on weight and metabolic markers: a systematic review and meta-analysis of randomized controlled trials. Arch Endocrinol Metab. 2024;68:e230469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 30. | Li H, Yu G, Huang Q, Yang B, Nie J, Liu Y, Tu X. Efficacy and safety of GLP-1RAs for people with obesity: A systematic review based on RCT and Bayesian network meta-analysis. Biomed Pharmacother. 2024;171:116150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 31. | Saxena AR, Frias JP, Brown LS, Gorman DN, Vasas S, Tsamandouras N, Birnbaum MJ. Efficacy and Safety of Oral Small Molecule Glucagon-Like Peptide 1 Receptor Agonist Danuglipron for Glycemic Control Among Patients With Type 2 Diabetes: A Randomized Clinical Trial. JAMA Netw Open. 2023;6:e2314493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 81] [Reference Citation Analysis (0)] |
| 32. | Yoon KH, Kang J, Kwon SC, Trautmann ME, Hompesch M, Stewart J, Sorli CH. Pharmacokinetic and dose-finding studies on efpeglenatide in patients with type 2 diabetes. Diabetes Obes Metab. 2020;22:1292-1301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 33. | Frias JP, Choi J, Rosenstock J, Popescu L, Niemoeller E, Muehlen-Bartmer I, Baek S. Efficacy and Safety of Once-Weekly Efpeglenatide Monotherapy Versus Placebo in Type 2 Diabetes: The AMPLITUDE-M Randomized Controlled Trial. Diabetes Care. 2022;45:1592-1600. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 34. | Jones B, Buenaventura T, Kanda N, Chabosseau P, Owen BM, Scott R, Goldin R, Angkathunyakul N, Corrêa IR Jr, Bosco D, Johnson PR, Piemonti L, Marchetti P, Shapiro AMJ, Cochran BJ, Hanyaloglu AC, Inoue A, Tan T, Rutter GA, Tomas A, Bloom SR. Targeting GLP-1 receptor trafficking to improve agonist efficacy. Nat Commun. 2018;9:1602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 178] [Cited by in RCA: 163] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 35. | Gieroba B, Kryska A, Sroka-Bartnicka A. Type 2 diabetes mellitus - conventional therapies and future perspectives in innovative treatment. Biochem Biophys Rep. 2025;42:102037. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 36. | Willard FS, Douros JD, Gabe MB, Showalter AD, Wainscott DB, Suter TM, Capozzi ME, van der Velden WJ, Stutsman C, Cardona GR, Urva S, Emmerson PJ, Holst JJ, D'Alessio DA, Coghlan MP, Rosenkilde MM, Campbell JE, Sloop KW. Tirzepatide is an imbalanced and biased dual GIP and GLP-1 receptor agonist. JCI Insight. 2020;5:e140532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 221] [Cited by in RCA: 272] [Article Influence: 54.4] [Reference Citation Analysis (0)] |
| 37. | Guo W, Xu Z, Zou H, Li F, Li Y, Feng J, Zhu Z, Zheng Q, Zhu R, Wang B, Li Y, Hao S, Qin H, Jones CL, Adegbite E, Telusca L, Fenaux M, Zhong W, Junaidi MK, Xu S, Pan H. Discovery of ecnoglutide - A novel, long-acting, cAMP-biased glucagon-like peptide-1 (GLP-1) analog. Mol Metab. 2023;75:101762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 38. | Zhu D, Wang W, Tong G, Ma G, Ma J, Han J, Zhang X, Liu Y, Gan S, Qin H, Zheng Q, Ning J, Zhu Z, Guo M, Bu Y, Li Y, Jones CL, Fenaux M, Junaidi MK, Xu S, Pan H. Efficacy and safety of GLP-1 analog ecnoglutide in adults with type 2 diabetes: a randomized, double-blind, placebo-controlled phase 2 trial. Nat Commun. 2024;15:8408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 39. | Lin D, Xiao H, Yang K, Li J, Ye S, Liu Y, Jing S, Lin Y, Yang Y, Huang L, Yuan J, Li Z, Yang J, Gao H, Xie Y, Xu M, Yan L. Safety, tolerability, pharmacokinetics, and pharmacodynamics of TG103 injection in participants who are overweight or obese: a randomized, double-blind, placebo-controlled, multiple-dose phase 1b study. BMC Med. 2024;22:209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 40. | Yan X, Ma J, Liu Y, Wang X, Li S, Yan S, Mo Z, Zhu Y, Lin J, Liu J, Jia Y, Liu L, Ding K, Xu M, Zhou Z. Efficacy and safety of visepegenatide, a long-acting weekly GLP-1 receptor agonist as monotherapy in type 2 diabetes mellitus: a randomised, double-blind, parallel, placebo-controlled phase 3 trial. Lancet Reg Health West Pac. 2024;47:101101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 41. | Nreu B, Dicembrini I, Tinti F, Sesti G, Mannucci E, Monami M. Major cardiovascular events, heart failure, and atrial fibrillation in patients treated with glucagon-like peptide-1 receptor agonists: An updated meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis. 2020;30:1106-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 42. | Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, Steinberg WM, Stockner M, Zinman B, Bergenstal RM, Buse JB; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2016;375:311-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4164] [Cited by in RCA: 4909] [Article Influence: 545.4] [Reference Citation Analysis (0)] |
| 43. | Gerstein HC, Sattar N, Rosenstock J, Ramasundarahettige C, Pratley R, Lopes RD, Lam CSP, Khurmi NS, Heenan L, Del Prato S, Dyal L, Branch K; AMPLITUDE-O Trial Investigators. Cardiovascular and Renal Outcomes with Efpeglenatide in Type 2 Diabetes. N Engl J Med. 2021;385:896-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 477] [Article Influence: 119.3] [Reference Citation Analysis (0)] |
| 44. | McGuire DK, Marx N, Mulvagh SL, Deanfield JE, Inzucchi SE, Pop-Busui R, Mann JFE, Emerson SS, Poulter NR, Engelmann MDM, Ripa MS, Hovingh GK, Brown-Frandsen K, Bain SC, Cavender MA, Gislum M, David JP, Buse JB; SOUL Study Group. Oral Semaglutide and Cardiovascular Outcomes in High-Risk Type 2 Diabetes. N Engl J Med. 2025;392:2001-2012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 37] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 45. | Wu S, Lu W, Chen Z, Dai Y, Chen K, Zhang S. Association of glucagon-like peptide-1 receptor agonists with cardiac arrhythmias in patients with type 2 diabetes or obesity: a systematic review and meta-analysis of randomized controlled trials. Diabetol Metab Syndr. 2022;14:195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 46. | Saglietto A, Falasconi G, Penela D, Francia P, Sau A, Ng FS, Dusi V, Castagno D, Gaita F, Berruezo A, De Ferrari GM, Anselmino M. Glucagon-like peptide-1 receptor agonist semaglutide reduces atrial fibrillation incidence: A systematic review and meta-analysis. Eur J Clin Invest. 2024;54:e14292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 47. | Mann JFE, Ørsted DD, Brown-Frandsen K, Marso SP, Poulter NR, Rasmussen S, Tornøe K, Zinman B, Buse JB; LEADER Steering Committee and Investigators. Liraglutide and Renal Outcomes in Type 2 Diabetes. N Engl J Med. 2017;377:839-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 740] [Cited by in RCA: 869] [Article Influence: 108.6] [Reference Citation Analysis (0)] |
| 48. | Perkovic V, Tuttle KR, Rossing P, Mahaffey KW, Mann JFE, Bakris G, Baeres FMM, Idorn T, Bosch-Traberg H, Lausvig NL, Pratley R; FLOW Trial Committees and Investigators. Effects of Semaglutide on Chronic Kidney Disease in Patients with Type 2 Diabetes. N Engl J Med. 2024;391:109-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 560] [Article Influence: 560.0] [Reference Citation Analysis (1)] |
| 49. | Blackman A, Foster GD, Zammit G, Rosenberg R, Aronne L, Wadden T, Claudius B, Jensen CB, Mignot E. Effect of liraglutide 3.0 mg in individuals with obesity and moderate or severe obstructive sleep apnea: the SCALE Sleep Apnea randomized clinical trial. Int J Obes (Lond). 2016;40:1310-1319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 299] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 50. | Zhao Y, Zhao W, Bu H, Toshiyoshi M, Zhao Y. Liraglutide on type 2 diabetes mellitus with nonalcoholic fatty liver disease: A systematic review and meta-analysis of 16 RCTs. Medicine (Baltimore). 2023;102:e32892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 51. | Deng M, Wen Y, Yan J, Fan Y, Wang Z, Zhang R, Ren L, Ba Y, Wang H, Lu Q, Fan H. Comparative effectiveness of multiple different treatment regimens for nonalcoholic fatty liver disease with type 2 diabetes mellitus: a systematic review and Bayesian network meta-analysis of randomised controlled trials. BMC Med. 2023;21:447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 52. | Newsome PN, Buchholtz K, Cusi K, Linder M, Okanoue T, Ratziu V, Sanyal AJ, Sejling AS, Harrison SA; NN9931-4296 Investigators. A Placebo-Controlled Trial of Subcutaneous Semaglutide in Nonalcoholic Steatohepatitis. N Engl J Med. 2021;384:1113-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 513] [Cited by in RCA: 1212] [Article Influence: 303.0] [Reference Citation Analysis (0)] |
| 53. | Newsome PN, Sanyal AJ, Engebretsen KA, Kliers I, Østergaard L, Vanni D, Bugianesi E, Rinella ME, Roden M, Ratziu V. Semaglutide 2.4 mg in Participants With Metabolic Dysfunction-Associated Steatohepatitis: Baseline Characteristics and Design of the Phase 3 ESSENCE Trial. Aliment Pharmacol Ther. 2024;60:1525-1533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 45] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 54. | Fang L, Li J, Zeng H, Liu J. Effects of GLP-1 receptor agonists on the degree of liver fibrosis and CRP in non-alcoholic fatty liver disease and non-alcoholic steatohepatitis: A systematic review and meta-analysis. Prim Care Diabetes. 2024;18:268-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 55. | Austregésilo de Athayde De Hollanda Morais B, Martins Prizão V, de Moura de Souza M, Ximenes Mendes B, Rodrigues Defante ML, Cosendey Martins O, Rodrigues AM. The efficacy and safety of GLP-1 agonists in PCOS women living with obesity in promoting weight loss and hormonal regulation: A meta-analysis of randomized controlled trials. J Diabetes Complications. 2024;38:108834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 56. | Chang G, Chen B, Zhang L. Efficacy of GLP-1rA, liraglutide, in plaque psoriasis treatment with type 2 diabetes: a systematic review and meta-analysis of prospective cohort and before-after studies. J Dermatolog Treat. 2022;33:1299-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 57. | Foss-Freitas MC, Imam S, Neidert A, Gomes AD, Broome DT, Oral EA. Efficacy and Safety of Glucagon-Like Peptide 1 Agonists in a Retrospective Study of Patients With Familial Partial Lipodystrophy. Diabetes Care. 2024;47:653-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 58. | Khaity A, Mostafa Al-Dardery N, Albakri K, Abdelwahab OA, Hefnawy MT, Yousef YAS, Taha RE, Swed S, Hafez W, Hurlemann R, Elsayed MEG. Glucagon-like peptide-1 receptor-agonists treatment for cardio-metabolic parameters in schizophrenia patients: a systematic review and meta-analysis. Front Psychiatry. 2023;14:1153648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 59. | Novikoff A, O'Brien SL, Bernecker M, Grandl G, Kleinert M, Knerr PJ, Stemmer K, Klingenspor M, Zeigerer A, DiMarchi R, Tschöp MH, Finan B, Calebiro D, Müller TD. Spatiotemporal GLP-1 and GIP receptor signaling and trafficking/recycling dynamics induced by selected receptor mono- and dual-agonists. Mol Metab. 2021;49:101181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 56] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 60. | Oliveira de Souza C, Sun X, Oh D. Metabolic Functions of G Protein-Coupled Receptors and β-Arrestin-Mediated Signaling Pathways in the Pathophysiology of Type 2 Diabetes and Obesity. Front Endocrinol (Lausanne). 2021;12:715877. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 61. | Liu QK. Mechanisms of action and therapeutic applications of GLP-1 and dual GIP/GLP-1 receptor agonists. Front Endocrinol (Lausanne). 2024;15:1431292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 62. | Skow MA, Bergmann NC, Knop FK. Diabetes and obesity treatment based on dual incretin receptor activation: 'twincretins'. Diabetes Obes Metab. 2016;18:847-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 63. | Liskiewicz A, Khalil A, Liskiewicz D, Novikoff A, Grandl G, Maity-Kumar G, Gutgesell RM, Bakhti M, Bastidas-Ponce A, Czarnecki O, Makris K, Lickert H, Feuchtinger A, Tost M, Coupland C, Ständer L, Akindehin S, Prakash S, Abrar F, Castelino RL, He Y, Knerr PJ, Yang B, Hogendorf WFJ, Zhang S, Hofmann SM, Finan B, DiMarchi RD, Tschöp MH, Douros JD, Müller TD. Glucose-dependent insulinotropic polypeptide regulates body weight and food intake via GABAergic neurons in mice. Nat Metab. 2023;5:2075-2085. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 47] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 64. | Frias JP, Bastyr EJ 3rd, Vignati L, Tschöp MH, Schmitt C, Owen K, Christensen RH, DiMarchi RD. The Sustained Effects of a Dual GIP/GLP-1 Receptor Agonist, NNC0090-2746, in Patients with Type 2 Diabetes. Cell Metab. 2017;26:343-352.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 247] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 65. | Frías JP, Davies MJ, Rosenstock J, Pérez Manghi FC, Fernández Landó L, Bergman BK, Liu B, Cui X, Brown K; SURPASS-2 Investigators. Tirzepatide versus Semaglutide Once Weekly in Patients with Type 2 Diabetes. N Engl J Med. 2021;385:503-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 982] [Article Influence: 245.5] [Reference Citation Analysis (0)] |
| 66. | Garvey WT, Frias JP, Jastreboff AM, le Roux CW, Sattar N, Aizenberg D, Mao H, Zhang S, Ahmad NN, Bunck MC, Benabbad I, Zhang XM; SURMOUNT-2 investigators. Tirzepatide once weekly for the treatment of obesity in people with type 2 diabetes (SURMOUNT-2): a double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2023;402:613-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 292] [Article Influence: 146.0] [Reference Citation Analysis (0)] |
| 67. | Rosenstock J, Wysham C, Frías JP, Kaneko S, Lee CJ, Fernández Landó L, Mao H, Cui X, Karanikas CA, Thieu VT. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): a double-blind, randomised, phase 3 trial. Lancet. 2021;398:143-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 549] [Article Influence: 137.3] [Reference Citation Analysis (0)] |
| 68. | Loomba R, Hartman ML, Lawitz EJ, Vuppalanchi R, Boursier J, Bugianesi E, Yoneda M, Behling C, Cummings OW, Tang Y, Brouwers B, Robins DA, Nikooie A, Bunck MC, Haupt A, Sanyal AJ; SYNERGY-NASH Investigators. Tirzepatide for Metabolic Dysfunction-Associated Steatohepatitis with Liver Fibrosis. N Engl J Med. 2024;391:299-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 247] [Article Influence: 247.0] [Reference Citation Analysis (0)] |
| 69. | Packer M, Zile MR, Kramer CM, Baum SJ, Litwin SE, Menon V, Ge J, Weerakkody GJ, Ou Y, Bunck MC, Hurt KC, Murakami M, Borlaug BA; SUMMIT Trial Study Group. Tirzepatide for Heart Failure with Preserved Ejection Fraction and Obesity. N Engl J Med. 2025;392:427-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 177] [Article Influence: 177.0] [Reference Citation Analysis (0)] |
| 70. | McFarlin BE, Duffin KL, Konkar A. Incretin and glucagon receptor polypharmacology in chronic kidney disease. Am J Physiol Endocrinol Metab. 2024;326:E747-E766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 71. | Pocai A. Action and therapeutic potential of oxyntomodulin. Mol Metab. 2014;3:241-251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 137] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 72. | Winther JB, Holst JJ. Glucagon agonism in the treatment of metabolic diseases including type 2 diabetes mellitus and obesity. Diabetes Obes Metab. 2024;26:3501-3512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 73. | Sánchez-Garrido MA, Brandt SJ, Clemmensen C, Müller TD, DiMarchi RD, Tschöp MH. GLP-1/glucagon receptor co-agonism for treatment of obesity. Diabetologia. 2017;60:1851-1861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 131] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 74. | Elvert R, Bossart M, Herling AW, Weiss T, Zhang B, Kannt A, Wagner M, Haack T, Evers A, Dudda A, Keil S, Lorenz M, Lorenz K, Riz M, Hennerici W, Larsen PJ. Team Players or Opponents: Coadministration of Selective Glucagon and GLP-1 Receptor Agonists in Obese Diabetic Monkeys. Endocrinology. 2018;159:3105-3119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 75. | Corbin KD, Carnero EA, Allerton TD, Tillner J, Bock CP, Luyet PP, Göbel B, Hall KD, Parsons SA, Ravussin E, Smith SR. Glucagon-like peptide-1/glucagon receptor agonism associates with reduced metabolic adaptation and higher fat oxidation: A randomized trial. Obesity (Silver Spring). 2023;31:350-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 76. | Alba M, Yee J, Frustaci ME, Samtani MN, Fleck P. Efficacy and safety of glucagon-like peptide-1/glucagon receptor co-agonist JNJ-64565111 in individuals with obesity without type 2 diabetes mellitus: A randomized dose-ranging study. Clin Obes. 2021;11:e12432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 77. | Nalisa DL, Cuboia N, Dyab E, Jackson IL, Felix HJ, Shoki P, Mubiana M, Oyedeji-Amusa M, Azevedo L, Jiang H. Efficacy and safety of Mazdutide on weight loss among diabetic and non-diabetic patients: a systematic review and meta-analysis of randomized controlled trials. Front Endocrinol (Lausanne). 2024;15:1309118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 78. | Zhang B, Cheng Z, Chen J, Zhang X, Liu D, Jiang H, Ma G, Wang X, Gan S, Sun J, Jin P, Yi J, Shi B, Ma J, Ye S, Wang G, Ji L, Gu X, Yu T, An P, Deng H, Li H, Li L, Ma Q, Qian L, Yang W. Efficacy and Safety of Mazdutide in Chinese Patients With Type 2 Diabetes: A Randomized, Double-Blind, Placebo-Controlled Phase 2 Trial. Diabetes Care. 2024;47:160-168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 79. | Parker VER, Robertson D, Wang T, Hornigold DC, Petrone M, Cooper AT, Posch MG, Heise T, Plum-Moerschel L, Schlichthaar H, Klaus B, Ambery PD, Meier JJ, Hirshberg B. Efficacy, Safety, and Mechanistic Insights of Cotadutide, a Dual Receptor Glucagon-Like Peptide-1 and Glucagon Agonist. J Clin Endocrinol Metab. 2020;105:dgz047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 83] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 80. | Parker VER, Robertson D, Erazo-Tapia E, Havekes B, Phielix E, de Ligt M, Roumans KHM, Mevenkamp J, Sjoberg F, Schrauwen-Hinderling VB, Johansson E, Chang YT, Esterline R, Smith K, Wilkinson DJ, Hansen L, Johansson L, Ambery P, Jermutus L, Schrauwen P. Cotadutide promotes glycogenolysis in people with overweight or obesity diagnosed with type 2 diabetes. Nat Metab. 2023;5:2086-2093. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 31] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 81. | Schiavon M, Visentin R, Göbel B, Riz M, Cobelli C, Klabunde T, Dalla Man C. Improved postprandial glucose metabolism in type 2 diabetes by the dual glucagon-like peptide-1/glucagon receptor agonist SAR425899 in comparison with liraglutide. Diabetes Obes Metab. 2021;23:1795-1805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 82. | Romero-Gómez M, Lawitz E, Shankar RR, Chaudhri E, Liu J, Lam RLH, Kaufman KD, Engel SS; MK-6024 P001 Study Group. A phase IIa active-comparator-controlled study to evaluate the efficacy and safety of efinopegdutide in patients with non-alcoholic fatty liver disease. J Hepatol. 2023;79:888-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 96] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 83. | Jepsen MM, Christensen MB. Emerging glucagon-like peptide 1 receptor agonists for the treatment of obesity. Expert Opin Emerg Drugs. 2021;26:231-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 64] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 84. | Wharton S, le Roux CW, Kosiborod MN, Platz E, Brueckmann M, Jastreboff AM, Ajaz Hussain S, Pedersen SD, Borowska L, Unseld A, Kloer IM, Kaplan LM; SYNCHRONIZE‐1 and ‐2 trial committees and investigators. Survodutide for treatment of obesity: rationale and design of two randomized phase 3 clinical trials (SYNCHRONIZE™-1 and -2). Obesity (Silver Spring). 2025;33:67-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 85. | Kosiborod MN, Platz E, Wharton S, le Roux CW, Brueckmann M, Ajaz Hussain S, Unseld A, Startseva E, Kaplan LM; SYNCHRONIZE–CVOT Trial Committees and Investigators. Survodutide for the Treatment of Obesity: Rationale and Design of the SYNCHRONIZE Cardiovascular Outcomes Trial. JACC Heart Fail. 2024;12:2101-2109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 86. | Souza M, Al-Sharif L, Antunes VLJ, Huang DQ, Loomba R. Comparison of pharmacological therapies in metabolic dysfunction-associated steatohepatitis for fibrosis regression and MASH resolution: Systematic review and network meta-analysis. Hepatology. 2025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 87. | Harrison SA, Browne SK, Suschak JJ, Tomah S, Gutierrez JA, Yang J, Roberts MS, Harris MS. Effect of pemvidutide, a GLP-1/glucagon dual receptor agonist, on MASLD: A randomized, double-blind, placebo-controlled study. J Hepatol. 2025;82:7-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 32] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 88. | Frias JP, Deenadayalan S, Erichsen L, Knop FK, Lingvay I, Macura S, Mathieu C, Pedersen SD, Davies M. Efficacy and safety of co-administered once-weekly cagrilintide 2·4 mg with once-weekly semaglutide 2·4 mg in type 2 diabetes: a multicentre, randomised, double-blind, active-controlled, phase 2 trial. Lancet. 2023;402:720-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 153] [Article Influence: 76.5] [Reference Citation Analysis (0)] |
| 89. | Kokkorakis M, Chakhtoura M, Rhayem C, Al Rifai J, Ghezzawi M, Valenzuela-Vallejo L, Mantzoros CS. Emerging pharmacotherapies for obesity: A systematic review. Pharmacol Rev. 2025;77:100002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 90. | Jastreboff AM, Kaplan LM, Frías JP, Wu Q, Du Y, Gurbuz S, Coskun T, Haupt A, Milicevic Z, Hartman ML; Retatrutide Phase 2 Obesity Trial Investigators. Triple-Hormone-Receptor Agonist Retatrutide for Obesity - A Phase 2 Trial. N Engl J Med. 2023;389:514-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 551] [Cited by in RCA: 455] [Article Influence: 227.5] [Reference Citation Analysis (0)] |
| 91. | Sanyal AJ, Kaplan LM, Frias JP, Brouwers B, Wu Q, Thomas MK, Harris C, Schloot NC, Du Y, Mather KJ, Haupt A, Hartman ML. Triple hormone receptor agonist retatrutide for metabolic dysfunction-associated steatotic liver disease: a randomized phase 2a trial. Nat Med. 2024;30:2037-2048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 74] [Article Influence: 74.0] [Reference Citation Analysis (0)] |
| 92. | Abdelmalek MF, Suzuki A, Sanchez W, Lawitz E, Filozof C, Cho H, Baek E, Choi J, Baek S. A phase 2, adaptive randomized, double-blind, placebo-controlled, multicenter, 52-week study of HM15211 in patients with biopsy-confirmed non-alcoholic steatohepatitis - Study design and rationale of HM-TRIA-201 study. Contemp Clin Trials. 2023;130:107176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 93. | Xie Z, Zheng G, Liang Z, Li M, Deng W, Cao W. Seven glucagon-like peptide-1 receptor agonists and polyagonists for weight loss in patients with obesity or overweight: an updated systematic review and network meta-analysis of randomized controlled trials. Metabolism. 2024;161:156038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 94. | Yao H, Zhang A, Li D, Wu Y, Wang CZ, Wan JY, Yuan CS. Comparative effectiveness of GLP-1 receptor agonists on glycaemic control, body weight, and lipid profile for type 2 diabetes: systematic review and network meta-analysis. BMJ. 2024;384:e076410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 155] [Reference Citation Analysis (12)] |
| 95. | American Diabetes Association Professional Practice Committee. 8. Obesity and Weight Management for the Prevention and Treatment of Type 2 Diabetes: Standards of Care in Diabetes-2025. Diabetes Care. 2025;48:S167-S180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 96. | American Diabetes Association Professional Practice Committee. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Care in Diabetes-2025. Diabetes Care. 2025;48:S181-S206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 82] [Reference Citation Analysis (0)] |
| 97. | Ganakumar V, Fernandez CJ, Pappachan JM. Antidiabetic combination therapy and cardiovascular outcomes: An evidence-based approach. World J Diabetes. 2025;16:102390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 98. | Ciardullo S, Morieri ML, Daniele G, Fiorentino TV, Mezza T, Tricò D, Consoli A, Del Prato S, Giorgino F, Piro S, Solini A, Avogaro A. GLP1-GIP receptor co-agonists: a promising evolution in the treatment of type 2 diabetes. Acta Diabetol. 2024;61:941-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 99. | Samms RJ, Coghlan MP, Sloop KW. How May GIP Enhance the Therapeutic Efficacy of GLP-1? Trends Endocrinol Metab. 2020;31:410-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 260] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 100. | Nauck MA, Quast DR, Wefers J, Pfeiffer AFH. The evolving story of incretins (GIP and GLP-1) in metabolic and cardiovascular disease: A pathophysiological update. Diabetes Obes Metab. 2021;23 Suppl 3:5-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 206] [Article Influence: 51.5] [Reference Citation Analysis (0)] |
| 101. | Rudzki G, Knop-Chodyła K, Piasecka Z, Kochanowska-Mazurek A, Głaz A, Wesołek-Bielaska E, Woźniak M. Managing Post-Transplant Diabetes Mellitus after Kidney Transplantation: Challenges and Advances in Treatment. Pharmaceuticals (Basel). 2024;17:987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 102. | Bailey CJ, Flatt PR, Conlon JM. Multifunctional incretin peptides in therapies for type 2 diabetes, obesity and associated co-morbidities. Peptides. 2025;187:171380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 103. | Melson E, Ashraf U, Papamargaritis D, Davies MJ. What is the pipeline for future medications for obesity? Int J Obes (Lond). 2025;49:433-451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 95] [Article Influence: 95.0] [Reference Citation Analysis (0)] |
| 104. | Véniant MM, Lu SC, Atangan L, Komorowski R, Stanislaus S, Cheng Y, Wu B, Falsey JR, Hager T, Thomas VA, Ambhaikar M, Sharpsten L, Zhu Y, Kurra V, Jeswani R, Oberoi RK, Parnes JR, Honarpour N, Neutel J, Strande JL. A GIPR antagonist conjugated to GLP-1 analogues promotes weight loss with improved metabolic parameters in preclinical and phase 1 settings. Nat Metab. 2024;6:290-303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 86] [Article Influence: 86.0] [Reference Citation Analysis (0)] |
| 105. | Killion EA, Chen M, Falsey JR, Sivits G, Hager T, Atangan L, Helmering J, Lee J, Li H, Wu B, Cheng Y, Véniant MM, Lloyd DJ. Chronic glucose-dependent insulinotropic polypeptide receptor (GIPR) agonism desensitizes adipocyte GIPR activity mimicking functional GIPR antagonism. Nat Commun. 2020;11:4981. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 106] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 106. | Austin GO, Tomas A. Variation in responses to incretin therapy: Modifiable and non-modifiable factors. Front Mol Biosci. 2023;10:1170181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |