GENETIC BASIS
Molecular genetic studies have confirmed that 40%-60%[6] are mutations in genes encoding sarcomere structural proteins. Twenty-seven pathogenic genes have been found to be associated with HCM. These genes encode thick myofilament, thin myofilament, Z-disk structural proteins or calcium regulation related proteins. The remaining 50% of HCM do not find a clear pathogenic gene mutation, the cause of which is unknown. In some previous HCM diagnostic guidelines[6,7], it is believed that congenital metabolic diseases, neuromuscular diseases, mitochondrial diseases, malformation syndrome, systemic amyloidosis, etc.[7]. These rare clinical diseases are also classified as HCM, but the latest guidelines[1,2] exclude these diseases from HCM because of their clear etiology, prognosis and treatment are quite different from HCM.
Sarc
HCM, as a Mendelian autosomal dominant genetic disorder, has variable penetrance and is associated with genetic variations in the encoding of myocardial sarcomere proteins involved in contractile function[8]. The first gene related to HCM (MYH7) was identified in 1989[9], and its gene library expanded to include seven additional sarcomere genes (MYBPC3,TNNT2, TPM1, MYL2, MYL3, TNNI3, ACTC1) in the 1990s[10]. In patients with HCM and pathogenic sarcomere gene variants, the two most common genes are β - MYH7 and MYBPC3, which are found in 70% of variant positive patients, while other genes (TNNI3, TNNT2, TPM1, MYL2, MYL3, ACTC1) each account for a small proportion of patients (1% to 5%). Among these genes, over 2000 variants have been identified[11], most of which are “proprietary” (specific to individual families). Each descendant of affected family members has a 50% chance of inheriting this variant[12].
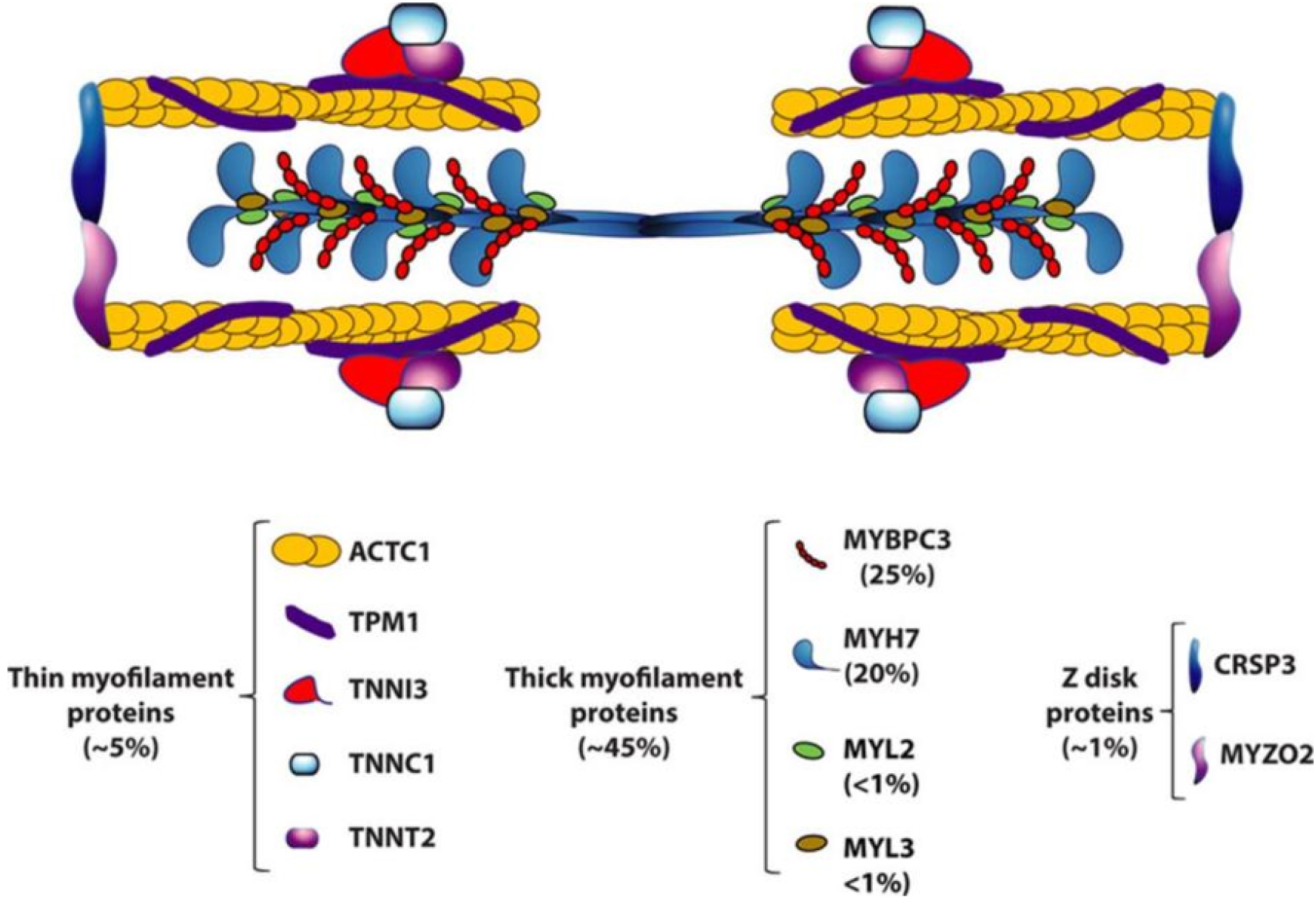
Established causal genes for HCM and their population frequencies are listed in Figure 1[12]. A schematic structure of a sarcomere composed of thick and thin filaments and Z discs is depicted along with its protein constituents involved in HCM. Lorenzini et al[13] conducted a retrospective analysis of carriers of sarcomere protein gene mutations identified during home screening. The estimated HCM penetrance rate at 15 years follow-up was 46%, and the frequency of the pathogenic gene was as follows: MYBPC3 (43.2%), MYH7 (24.2%), TNNI3 (13.7%), TNNT2 (11.9%), TPM1 (3.2%), MYL2 (2.1%), ACTC1 (0.4%), MYL3 (0%). In addition, 4 individuals (1.4%) carried multiple pathogenicity/Likely pathogenicity variants (3 MYBPC3 and TNNI3, and 1 MYH7 and TNNT2). According to the family line, the frequency of pathogenic genes is: MYBPC3 (45.5%), MYH7 (28.2%), TNNI3 (10.9%), TNNT2 (9.6%), TPM1 (1.9%), MYL2 (1.9%), ACTC1 (0.6%), MYL3 (0%); two families (1.3%) carry multiple pathogenicity/Likely pathogenicity variants. In a multivariate model adjusted for age and stratified by CMR, the independent predictors of HCM development were male gender and electrocardiographic abnormalities, with the TNNI3 variant having the lowest risk.
Figure 1 Hypertrophic cardiomyopathy as a disease of sarcomere proteins[12].
Citation: Marian AJ, Braunwald E. Hypertrophic Cardiomyopathy: Genetics, Pathogenesis, Clinical Manifestations, Diagnosis, and Therapy. Circ Res 2017; 121: 749-770. Copyright© 2017 American Heart Association, Inc.. Published by Wolters Kluwer Health, Inc. ( https://www.ahajournals.org/open-access-information).
Non-Sarc
In the Human Gene Mutation Database, professional (version 2015.3), there are 51 gene variants associated with HCM, including 8 fully validated sarcomere genes, 31 non-sarcomere genes, and 12 genes associated with complex phenotypes including left ventricular hypertrophy. The 8 core sarcomere genes (MYH7, MYBPC3, TNNT2, TPM1, MYL2, MYL3, TNNI3, and ACTC1) were validated completely because a detailed familial linkage study identified them as HCM genes. In addition, variants in 5 genes (ACTA1, COX15, MRPL3, MYO6 and SLC25A5) have been reported in other rare diseases, and most of these variants show a recessive mode of inheritance. There is evidence[14] to suggest that 11 genes play a major pathogenic role in HCM: Strong evidence (PLN, CSRP3[15], FHL1[16]), moderate evidence (CRYAB, ACTN2[17,18], FLNC[19], MYOZ2[20]), or weak evidence (TNNC1, MYH6, TRIM55[21], TRIM63[21]). In recent years, other non-sarcomere genes have also been found to be associated with the pathogenesis of HCM, such as the sodium channel gene SCN5A[22], which was confirmed to play an important role in the pathogenesis of HCM. In addition, our center found that the calcium channel gene CACNA1C has a gene accumulation effect with MYH7 gene in a large family with HCM[23,24].
Genetic screening
Echocardiography and CMR have a synergistic effect on the diagnosis of proband and family screening. Genetic testing can identify affected individuals without HCM phenotype, and its important role is to perform next-generation (cascade) testing on high-risk and usually asymptomatic family members. When the proband’s pathological sarcomere mutation test is positive, relatives who test negative for the same (or other) sarcomere mutation and are considered unaffected may be released from lifelong imaging examinations and clinical monitoring. In addition, the targeted gene testing team can identify non sarcomere cardiac, metabolic, or systemic diseases clinically similar to HCM that are associated with LV hypertrophy, such as lysosomal storage diseases like lysosome-associated membrane protein 2 (Danon) cardiomyopathy, (protein kinase, AMP-activated, non-catalytic, gamma-2) cardiac syndrome, Fabry disease, Noonan syndrome, and other RASopathy, as well as transthyretin cardiac amyloidosis. Due to the significant differences in natural course and treatment strategies between these phenotypic copies and sarcomere HCM, differential diagnosis defines the key role of genetic testing in such patients.
HCM is mainly a sarcomere disease, therefore, first-line gene testing mainly includes the detection of pathogenic HCM genes[25]: MYH7, MYBPC3, TNNI3, TNNT2, TPM1, MYL2, MYL3, and ACTC1. Pathogenic variants are typically identified in approximately 30% of sporadic cases and 60% of familial cases[26]. The identification of uncertain significant variations is not a clinically actionable outcome, but further research can be conducted at the clinical or research level to elucidate the pathogenicity of the variation (for example, through co segregation analysis of family members, DNA testing of parents to determine whether uncertain significant variations is a functional study from scratch). Genetic testing can be performed using various technical platforms, including gene panels, whole exome sequencing, or whole genome sequencing[27]. Generally, after the detection of eight first-line gene panels in the clinic, the whole exome gene detection or even the whole genome detection can be selected to find the possible new disease causing gene after the relevant disease causing gene is not found. However, the whole exome and whole genome detection have certain difficulties in the subsequent interpretation of gene data, and they can be applied to the interpretation of clinical data only after the large family and subsequent functional validation.
Recent studies have emphasized that specific genetic variations associated with the development of cardiomyopathy, particularly HCM, can guide rational drug design and the development of novel drug therapies[28]. For example, long-term administration of the small molecule MYK-461 has been shown to slow down the development of HCM and decrease pro-fibrotic gene expression in mice with myosin heavy chain heterozygous variants[29]. In addition, determining the diagnostic and prognostic impact of known pathological variations on sarcomere genes has laid the foundation for potential gene therapy. For example, clustered regularly interspaced short palindromic repeats - clustered regularly interspaced short palindromic repeats-associated protein 9 targets the GAGT deletion in MYBPC3 in human embryos and expresses functional cMYPPC in MYBPC3 gene knockout mice using adeno-associated virus therapy[30,31], to achieve the effect gene therapy. Overall, we found that specific genetic variations are associated with specific prognostic outcomes, which reinforces the role of genetic testing in personalized care for cardiomyopathy. In addition, to investigate the impact of common genetic variations on the risk of HCM, Harper et al[32] conducted two independent, unrelated, multi lineage case-control genome-wide association studies recruiting HCM patients from the HCM registry (2541 unselected cases compared to 40283 United Kingdom Biobank controls) and a comparative disease of rare biological resources (239 sarcomere negative cases compared to 7203 controls). Multi gene risk distribution, combined with pathogenic risk factors, drives individual susceptibility, and the management of sarcomere negativity is also an important aspect.
Genetic testing in HCM has several clinical benefits, including confirming diagnosis, preclinical diagnosis, cascading genetic testing within families, and guiding reproductive decision-making. The cascade gene testing in this family can identify individuals who carry pathogenic variants and require continuous monitoring, while those who do not carry the variant can be freed from lifelong clinical monitoring. After genetic testing, clinically actionable results (i.e., possible pathogenicity or pathogenicity) can provide diagnostic clarification for the proband and offer the possibility of cascading (predictive) testing for high-risk family members[12,33,34]. The most important is that the current assisted reproductive technology can screen embryos that do not carry disease causing genes in vitro and implant them into the mother, fundamentally reducing the number of patients with HCM and achieving true eugenics.
CLINICAL PHENOTYPE DIFFERENCES AMONG DIFFERENT GENOTYPES
The clinical first-line genes of HCM include mutations in genes encoding MYH7 and MYBPC3, accounting for about 80% of the positive cases of HCM by genetic testing; Next, TNNI3 and TNNT2 accounted for about 15% of HCM patients with positive genetic testing, and finally TPM1, ACTC1, MYL2 and MYL3 were relatively rare.
Morphological differences
The baseline data roughly divides patients into two categories. One group is sarcomere mutation positive, more likely to exhibit ventricular septal curvature with more fibrosis but less resting obstruction, while the other group is sarcomere mutation negative, more likely to exhibit isolated basal septal hypertrophy with obstruction but less fibrosis[35].
Compared with patients with sarcomere mutation negative HCM, those with sarcomere mutation positive HCM have a higher decrease in global longitudinal strain and a higher degree of LV myocardial fibrosis, indicating that sarcomere mutations may lead to LV diastolic dysfunction, thereby causing early progression of late stage heart failure[36]. Research has confirmed that pathogenic or potentially pathogenic sarcomere mutations are significantly associated with a large number of LV replacement fibrosis and extracellular volume fraction, especially in the interventricular septum. Similarly, Wang et al[37] found that late gadolinium enhancement ≥ 15% is three times associated with sudden cardiac death (SCD) risk, which supports the association between poor prognosis in HCM patients and sarcomere mutations[38]. More evidence also suggested that HCM patients carrying genetic mutations are more susceptible to increased interventricular septum and interventricular septal thickness compared to patients without genetic mutations[39]. It was found that mid septal hypertrophy is mainly associated with MYBPC3 variant, while a higher septum-to-posterior wall ratio correlated with MYH7 variant. Mutations in MYH7, MYBPC3, and other sarcomere or myofilament genes (TNNI3, TPM1, and TNNT2) have been shown to be associated with increased hypertrophy of the LV anterior wall, interventricular septum, and lateral wall. In contrast, ALPK3-associated hypertrophy chiefly presented in the apical region, while hypertrophy related to TTN and OBSCN mutations exhibited a uniform distribution across the myocardium. The pattern of hypertrophy varies with the type and category of gene mutation, which provides valuable diagnostic insights for early clinical identification of patients with sarcomere variation, so as to carry out genetic testing and family protection as soon as possible.
Pathological differences
Sarcomeric HCM is associated with worse outcomes than diseases caused by non sarcomeric mutations. Patients carrying sarcomere mutations, with strong evidence of pathogenicity, show clinical disease at an earlier age and have a greater burden, HCM related complications occurred earlier. After controlling for gender and ethnicity, the presence of sarcomere mutations increased the risk of all adverse outcomes by more than 2-fold, with the highest risk of ventricular arrhythmias[40]. Moreover, in recent years, our center’s research found that carrying pathogenic sarcomere mutations while carrying rare calcium gene variants (even without sufficient evidence to prove the pathogenicity of these variants) would have a more severe HCM phenotype and prognosis, suggesting that carrying pathogenicity is one of the important factors for the poor prognosis of HCM patients[24].
There is a direct link between Sarcs and poor microcirculation remodeling in HCM. HCM patients with sarcomere filament mutations are characterized by more severe microvascular dysfunction, which is the reason for the increased long-term prevalence of ventricular dysfunction and heart failure in genotype positive patients[41]. Patients with sarcomere mutation positive HCM have a higher degree of myocardial fibrosis and more severe ventricular diastolic dysfunction, which may lead to early onset of late stage heart failure[36]. Research has shown that they suffer from fatigue limitation and congestion symptoms, which are mainly the result of diastolic dysfunction and increased ventricular stiffness, leading to elevated left atrial and LV end diastolic pressure, resulting in pulmonary congestion and decreased exercise capacity[42,43]. Research has found that Sarc + patients with HCM have a higher lifetime risk than Sarc - patients and develop late diastolic heart failure (New York Heart Association III/IV symptoms) at an earlier age. CMR examination confirms that Sarc + patients have more severe LV diastolic dysfunction, providing a reasonable link between late LV diastolic dysfunction and Sarcs[36].
Overall, patients carrying sarcomere mutations have strong evidence of pathogenicity and exhibit clinical symptoms at an earlier age[40]. Several studies have reported that compared to HCM patients with negative sarcomere mutations, HCM patients carrying pathogenic/potentially pathogenic sarcomere mutations have a poorer prognosis. This includes early onset, high incidence rate of SCD, high incidence rate and overall mortality of atrial fibrillation, ventricular arrhythmia, heart failure[44-48], and they also tend to have more serious hypertrophy, microvascular dysfunction and myocardial fibrosis[7,49].
In addition, our center’s research has found that compared with patients with single Sarcs, patients with dual gene mutations are older, have a higher percentage of HCM family history, thicker walls, higher LVOT pressure gradients, more pathological Q waves, and more bundle branch block. During the follow-up period, the primary endpoint of patients with dual gene mutations was higher than the other three groups. Multivariate analysis showed that dual gene variation was an independent predictor of the primary endpoint event in patients, confirming the cumulative effect of dual gene variation, which resulted in more severe HCM phenotype and prognosis in patients[24].
Thin-filament mutations vs thick-filament mutations in Sarc + patients
Sarcomere is the basic unit of myocardial fiber, and its components include thick myofilament, thin myofilament, Z-line, M-line, etc. Gene variation can translate proteins with biological function defects by changing the amino acid sequence. It can also cause structural or functional abnormalities of sarcomere or sarcomere related proteins by reducing the expression level of the encoded protein, which will lead to abnormal myocardial contraction, impaired diastolic function, increased energy consumption, and ultimately cardiac hypertrophy. The thick myofilament are mainly composed of myosin, with the head and tail of myosin molecules arranged in a spiral shape to form the thick myofilament. The myosin head has ATPase activity and can bind to actin and generate force. Common HCM pathogenic thick myofilament genes include MYH7, MYL2, MYL3 and MYBPC3. The thin myofilament is mainly composed of actin, tropomyosin and troponin complex. Common HCM pathogenic thin myofilament genes include TNNT2, TNNI3, TPM1 and ACTC1.
Coppini et al[49] showed that patients with thin-filament mutations exhibited: (1) Milder and atypical distribution of LV hypertrophy and fewer occurrences of outflow tract obstruction compared to those with thick filamentous HCM; (2) The progression rate is higher in functional class III or IV of the New York Heart Association; (3) The incidence of systolic dysfunction or restrictive LV filling was higher during the last evaluation; (4) The prevalence of three-phase LV filling mode increased by 2.4 times; and (5) The incidence of malignant ventricular arrhythmia and SCD is similar[50].
In patients with HCM, thin filament mutations are associated with an increased likelihood of late stage LV dysfunction and heart failure compared to thick filament disease, but the risk of arrhythmia is comparable. Three phase LV filling is particularly common in filamentous HCM, reflecting severe diastolic dysfunction. The study supports the hypothesis that thin-filament HCM is phenotypically different from the more common thick-filament HCM[51-54]. Specific differences in LV morphology, function, and remodeling have been identified, indicating unique potential pathophysiological mechanisms. In the initial assessment, patients with thin-filaments showed smaller LV hypertrophy, typically developing in an apical or concentric pattern, while patients with thick-filaments almost universally exhibited classic asymmetric LV hypertrophy, including basal septum and anterior wall[55]. Therefore, dynamic LVOT obstruction is less common in the thin-filament cohort. The unique clinical and biophysical features of HCM associated with thin-filament mutations are different from the more common thick-filament mutations.
Thin-filament HCM is more likely to be associated with non significant and atypical distribution of LV hypertrophy, increased fibrosis, poor LV remodeling leading to functional deterioration, and more frequent occurrence of three-phase LV filling, reflecting severe diastolic dysfunction[51-54]. The management strategy should consider sufficient monitoring to detect LV dysfunction and symptom progression early, but the risk of arrhythmia does not seem to increase solely due to thin-filament mutation genotype.
MYH7 mutation vs MYBPC3 mutation in thick-filament
It is presumed that mutations in the mechanical enzyme β-cardiac myosin, which drives ventricular contraction, cause HCM by affecting the power output of myosin movement[56]. Β-myosin is a hexamer composed of two heavy chains, two essential light chains and two regulatory light chains. Heavy chains can be further divided into heavy meromyosin and light meromyosin. Heavy meromyosinconsists of sub-fragment 1 and sub-fragment 2, the former is the motor or head domain of myosin and the latter is the first about 40% of the α - helical coiled coil tail[57]. Light meromyosin is the distal tail of myosin, which contains the sequences responsible for thin filament formation. MYH7 181-937 is recognized as an important functional region of the head and neck, and the variation is likely to affect the protein function, and the clinical phenotype is more severe. There are relatively few pathogenic mutations in the rod, even if the disease occurs, the clinical phenotype caused is relatively mild.
Truncated mutations (encoding cMyPPC) or myosin missense mutations result in high contraction and low relaxation. Genetic and biochemical methods have shown that the gradual loss of cMyPPC leads to mutual enhancement of myosin contractility and changes in the dynamic conformation of myosin during relaxation. A treatment strategy that weakens cMyPPC activity may rescue the decrease in myocardial contractility in dilated cardiomyopathy patients, while MYK-461 inhibition of myosin benefits most mutated HCM patients[58].
Generally, there is no significant difference between the location of mutations in the encoded protein domain and the HCM phenotype. However, mutations in the MYH7 gene exhibit a strong preference for the globular head and hinge regions of myosin heavy chain protein, although there have also been descriptions of mutations leading to changes in the rod domain[59]. Phenotypically, mutations in the converter domain and flat surface area of the spherical head of MYH7 are associated with early disease onset. Overall, MYH7 and MYBPC3 are not only the two most common pathogenic genes involved in the basal septum in the typical form of HCM, but also the two most common genes in apical HCM[60].
Previous research data has shown that carriers of MYBPC3 mutations are older than carriers of MYH7 mutations (P = 0.01)[61]. Compared with MYBPC3 or other Sarcs, patients with pathogenic or potentially pathogenic MYH7 mutations tend to have higher late gadolinium enhancement and extracellular volume fractions%[38].
Troponin gene specific prognostic association in thin-filament mutations
The variation of troponin encoding genes is a clear cause of cardiomyopathy, but there is significant clinical heterogeneity. Early studies have clearly shown that compared to sarcomere negative patients, sarcomere positive patients have a poorer prognosis and higher endpoints for cardiovascular death, progression to end-stage heart failure, and non fatal stroke. At the individual gene level, some early correlations have been identified, such as the pathogenicity of residue 92 in TNNT2 and an increase in SCD, as well as an increase in the mutation rate of codon 145 in TNNI3 leading to restrictive cardiomyopathy (RCM) or HCM[60,62]. With the advancement of genetic testing and newly discovered variations, the Troponin gene specific prognostic association has been determined.
The data compiled by Coppini et al[49] shows that TNNC1 probands have the youngest age of diagnosis and the highest incidence of potentially fatal events. TNNT2 and TNNI3 probands have similar mortality, SCD, and ventricular fibrillation records, while TNNI3 probands tend to have RCM. Diagnostic hotspots were isolated in the N-terminus, tropomyosin binding domain, and alpha helic-1 of TNNT2, and probands carrying mutations at these locations showed significant differences in SCD/ventricular fibrillation; P = 0.004, SCD/death/transplantation (P = 0.053), and diagnostic age (P = 0.052); Most of the hotspots in TNNI3 probands are concentrated in the inhibitory region, switch peptide region, and C-terminus, with a higher incidence of heart failure (P = 0.030), as well as less common subtypes of cardiomyopathy such as RCM (P = 0.008). No diagnostic hotspots were found in the TNNC1 proband hotspots, possibly due to the small number of probands and/or the high pathogenicity of all hotspots.
PERSPECTIVE
Applying gene loading PheWAS to a large biobank based on healthcare may elucidate the medical impact of gene variations on human disease phenotypes[63]. The logical first step is to perform PheWAS only on pLOF variants, which has the advantage of being able to inquire about the maximum effect of gene burden on the relevant phenotype, but due to low frequency, it may lead to insufficient efficacy. Studies have shown that clustering pLOF alone for gene load association testing may be more suitable for certain genes (such as MYBPC3), while including other pDM variants selected based on computer prediction may be more advantageous in increasing the number of effect alleles for other genes (such as MYH7)[64].
A key goal of the precision medicine program is to promote a platform through which healthcare providers can make accurate diagnoses based on various personalized health data, including personal genetic information. Genetic testing for HCM patients can determine the exact genetic cause of the disease, improve diagnostic accuracy for patients with ambiguous diagnoses, and allow for cascading screening of family members[65]. However, pathogenic HCM variants discovered in more comprehensive sequencing may also be overlooked in clinical practice, and it is crucial to use the genome first approach of WES to review clinical charts of carriers of pathogenic and potentially harmful variants[66].
In addition, Green et al[29] inhibited the development of ventricular hypertrophy, myocardial cell disorder, and myocardial fibrosis through early and long-term administration of MYK-461, and reduced the expression of hypertrophy and pro fibrotic genes in mice carrying heterozygous human mutations in myosin heavy chain. Identified the small molecule MYK-461, which reduces contractility by decreasing the activity of adenosine triphosphatase in cardiac myosin heavy chain. High dynamic contraction is crucial for the pathology of HCM, and sarcomere contraction inhibitors may be a valuable treatment for HCM.
CONCLUSION
Multiple mechanisms reflecting the diversity of pathogenic genes and mutations are related to the pathogenesis of HCM. The mechanism events in HCM can be divided into four groups of interlocking mechanisms. The main flaw is mutation. The initial or proximal phenotype is defined as the phenotype resulting from the direct impact of mutations on the structure and function of sarcomere proteins. The intermediate (or secondary) phenotype includes molecular changes that occur in response to changes in the structure and function of sarcomere proteins. Examples of the latter include changes in gene expression and activation of signaling pathways, such as the mitogen-activated protein kinase and transforming growth factor beta 1 pathways. The tertiary effect is accompanied by histological and pathological phenotypes, which are the result of numerous secondary molecular events in the myocardium, such as activation of the hypertrophic signaling pathway. These molecular and histological changes lead to the clinical phenotype of HCM (grade four). It should be noted that there are mechanistic differences between HCM cases caused by sarcomere protein mutations and phenotypic conditions, as the latter’s ventricular hypertrophy may be at least partially due to the storage of substances (such as glycogen) and partially due to functional defects in muscle cells, such as impaired contraction.
Sarcomere mutations are associated with early onset age and high incidence rate, and the existence of sarcomere mutations makes the prognosis worse. Age and sarcomere mutation status at the time of diagnosis can predict prognosis, and patients with pathogenic/potentially pathogenic sarcomere mutations have a 2-fold higher risk of developing adverse outcomes compared to those without mutations.
In patients with sarcomere mutations, compared with patients with thick filament mutations, patients with thin filament mutations are associated with an increased likelihood of late stage LV dysfunction and heart failure, reflecting severe diastolic dysfunction. Comparing the two most common coarse muscle filament genes MYH7 and MYBPC3, MYBPC3 mutation carriers have a higher age, but the difference in myocardial fibrosis is not significant.
Among patients with thin myofilament gene mutations, TNNC1 positive probands had the worst prognosis. The mapping of troponin variants identified positions in TNNT2 and TNNI3 that are associated with enhanced pathogenicity, diagnosis of RCM, and increased risk of sudden death, that is, de novo variants, homozygotes, and compound heterozygotes have a poor prognosis. Of course, the research on the relationship between genotype and clinical phenotype is still controversial, and further large sample multi center long-term follow-up research is urgently needed. In this paper, the close relationship between HCM hypertrophy pattern and genetic variation was combed and insights were put forward, which provided a theoretical basis for genetic testing and personalized management of HCM patients.