Published online May 26, 2025. doi: 10.4330/wjc.v17.i5.105919
Revised: March 26, 2025
Accepted: May 7, 2025
Published online: May 26, 2025
Processing time: 102 Days and 2.4 Hours
Atrial fibrillation (AF) associated with chronic kidney disease (CKD) is a prevalent condition in the United States, significantly impacting global morbidity and mortality. Understanding temporal patterns in AF-related mortality among CKD patients is crucial for effective clinical and public health strategies.
To investigate AF-CKD comorbidity and mortality on the national level.
Death certificates from the Centers for Disease Control Wide-Ranging Online Data for Epidemiologic Research database spanning 2011-2020 were analyzed to investigate AF-related CKD mortality in adults aged 35 to 85 or more years. Age-adjusted mortality rates (AAMRs) per 100000 persons and annual percent change (APC) were calculated, stratified by year, sex, race/ethnicity, and geographic region.
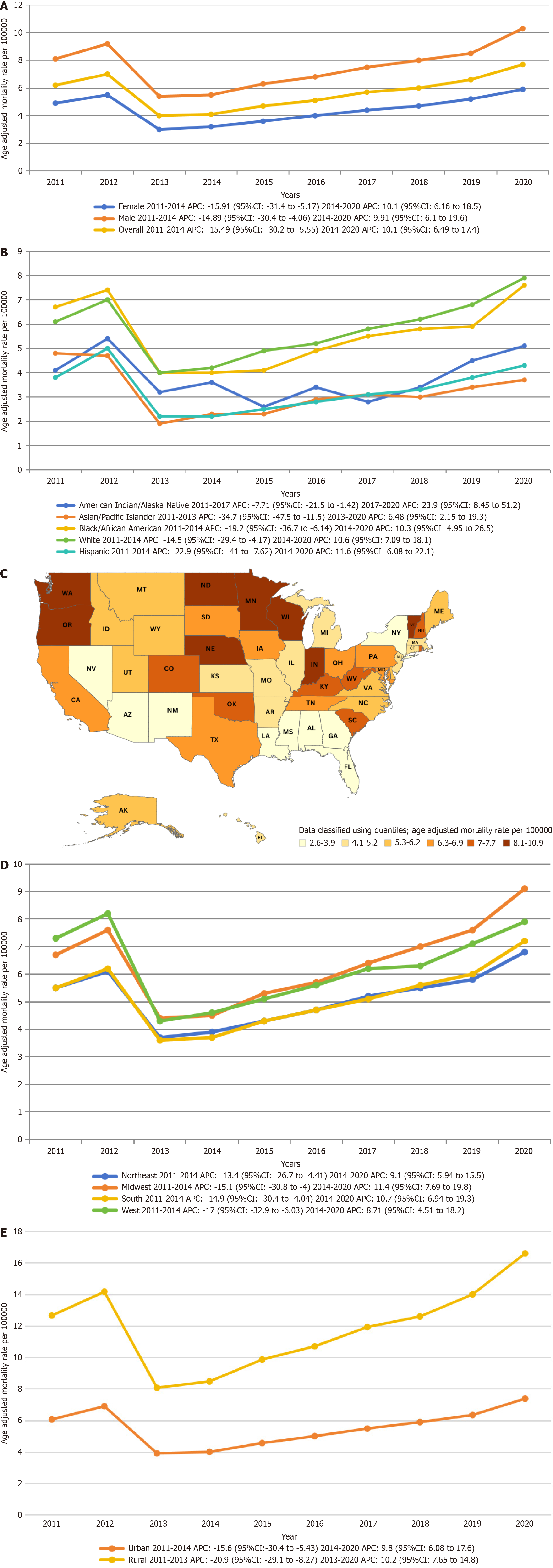
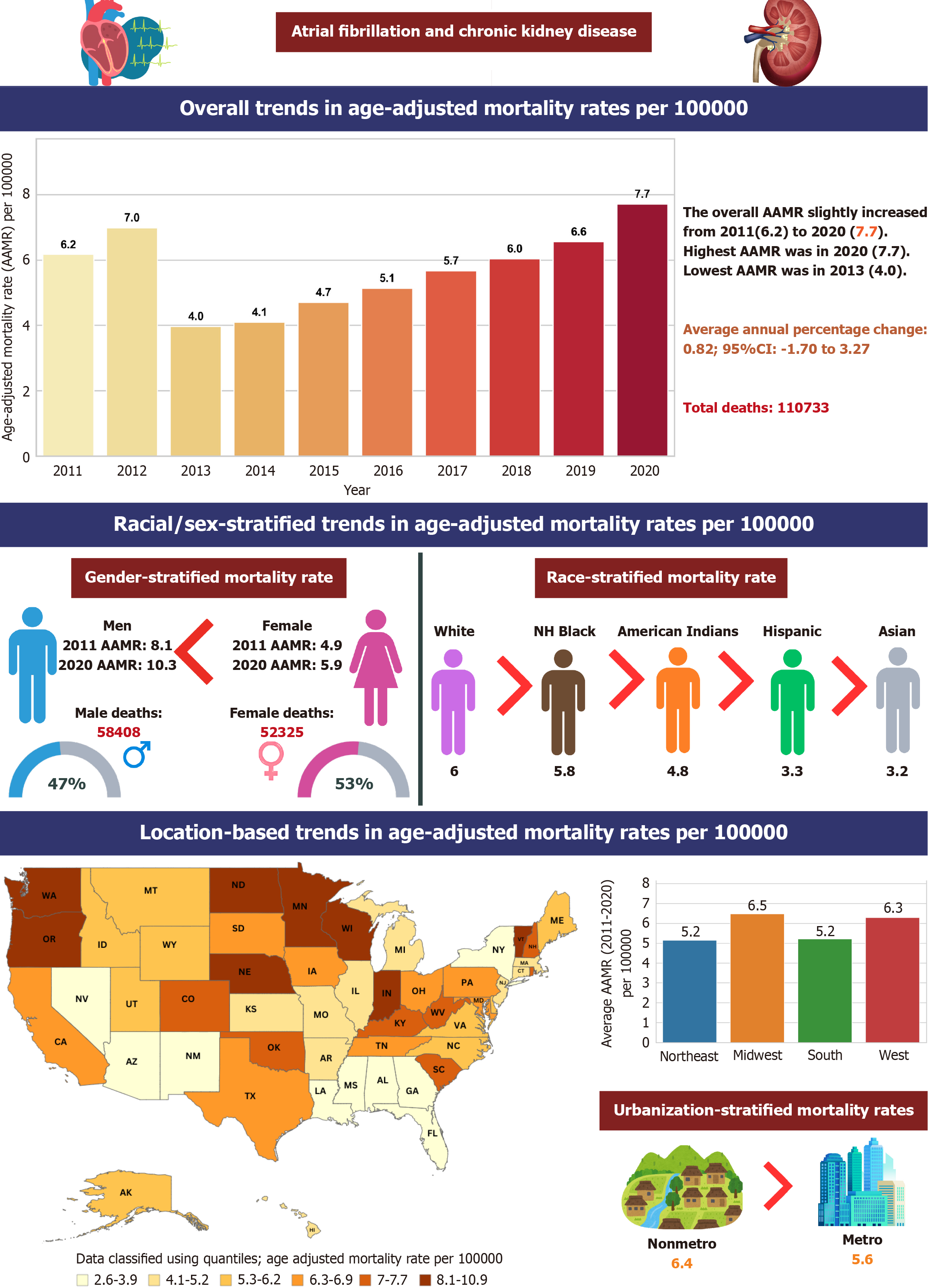
A total of 110733 deaths occurred among adults (aged 35-85 or more years) related to AF associated with CKD in the United States. Overall AAMR declined from 8.1 in 2011 to 5.5 in 2014 (APC: -14.89; 95% confidence interval (CI): -30.44 to -4.06), followed by an increase to 10.3 in 2020 (APC: 9.91; 95%CI: 6.1-19.62). Men had higher AAMRs than women (men: 7.6, 95%CI: 7.6-7.7). Non-Hispanic White adults had the highest AAMR (7.8), followed by non-Hispanic Black (5). States in the top 90th percentile had approximately four times higher AAMRs than those in the lower 10th percentile. AAMR also varied by region (Midwest: 7.6, West: 6.7, Northeast: 6.3, South: 5.6), with nonmetropolitan areas exhibiting higher AF-associated CKD mortality.
Temporal trends in AF-related mortality among CKD patients showed fluctuations over the study period, with notable disparities across demographic and geographic factors. Targeted interventions are warranted to mitigate the burden of AF associated with CKD and reduce mortality rates in the United States.
Core Tip: Chronic kidney disease (CKD) could contribute to atrial fibrillation with increased mortality; our study investigates this correlation using the Centers for Disease Control’s national database over the last 2 decades. The age-adjusted mortality rate for CKD-related atrial fibrillation deaths in adults decreased from 2011 to 2014, followed by an increase from 2014 to 2020. Study noticed a variation in mortality based on gender (higher in men), race (higher in non-Hispanic White patients), Midwest states (Indiana, and Minnesota), and Nonmetropolitan areas. That result illustrates different disparities in two correlated common chronic morbidity that would guide healthcare providers and policymakers work on achieving more efficient and equitable healthcare system.
- Citation: Abdullah Naveed M, Ahmed F, Ali A, Eltawansy S, Afzaal Z, Azeem B, Kashan M, Kamel O, Jain H, Ahmed M, Aman K, Zahid H, Iqbal R, Ullah A, Naveed Zafar M, Lajczak P, Obi O, Ahmed R. National adult mortality trends due to chronic kidney disease-related atrial fibrillation in the United States from year 2011-2020. World J Cardiol 2025; 17(5): 105919
- URL: https://www.wjgnet.com/1949-8462/full/v17/i5/105919.htm
- DOI: https://dx.doi.org/10.4330/wjc.v17.i5.105919
Chronic kidney disease (CKD) and atrial fibrillation (AF) represent two significant health burdens within the United States, each associated with increased morbidity and mortality rates[1]. CKD’s prevalence has surged over decades in the US, with estimates reaching 26 million adults, up from approximately 10% to > 13% within a decade[2]. Simultaneously, AF, the prevalent cardiac arrhythmia globally, affects millions. Projections suggest 6-12 million in the United States and 17.9 million in Europe by 2050 and 2060, respectively[3]. The coexistence of AF and CKD significantly heightens the risk of adverse cardiovascular events and mortality[4]. Both conditions are prevalent, with CKD patients at heightened risk of developing AF[5].
The intersection of CKD and AF presents a formidable challenge to healthcare providers. Individuals with CKD are more prone to developing AF due to various factors, including fluid overload, electrolyte imbalances, and structural cardiac changes[6,7]. AF complicates CKD management by increasing thromboembolic events and worsening cardiova
The comprehensive investigation uses the Centers for Disease Control and Prevention’s Wide-Ranging Online Data for Epidemiologic Research database to collect information from death certificates[10]. The study focused on instances of persons who died from CKD related to AF between 2011 and 2020. The study used the diagnostic code “I 48” “N18.0 to N18.9” from the 10th version of the International Classification of Diseases and Related Health Problems[11]. The dataset comprises information regarding the causes of death from death certificates from all 50 states and the district of Colum
Our study information was obtained utilizing death certificates that included population size, demographics, location by state, urban status[13], facility type, and Census Bureau categories[14]. The differences in the coding practices over the years or the potential incorrect data found in the database could possibly skew results and have been acknowledged in the limitations of our manuscript.
We assessed mortality rates per 100000 individuals and computed both age-unadjusted and age-adjusted data during the study period. We classified these rates by year, gender, race/ethnicity, state, and urban/ rural status, with 95% confi
CKD-related AF affected 110733 adults (aged 35-85 or more years) between 2011 and 2020 (Table 1). Of these, 42.5% occurred within medical facilities, 24.2% occurred in nursing homes/Long-term care facilities, 6.6% occurred in hospices, 23% occurred at home, and 3.7% at other places (Table 2).
| Year | Overall | Women | Men | NH White | NH Black or African American | NH Asian or Pacific Islander | NH American Indian or Alaska Native | Hispanic or Latino | Population |
| 2011 | 10682 | 5237 | 5445 | 8897 | 1001 | 286 | 40 | 439 | 164802438 |
| 2012 | 12411 | 6048 | 6363 | 10273 | 1145 | 301 | 52 | 611 | 166516716 |
| 2013 | 7227 | 3407 | 3820 | 6136 | 637 | 130 | 34 | 282 | 168240727 |
| 2014 | 7694 | 3674 | 4020 | 6488 | 676 | 172 | 38 | 305 | 170292776 |
| 2015 | 9022 | 4247 | 4775 | 7682 | 721 | 189 | 34 | 367 | 172416615 |
| 2016 | 9990 | 4698 | 5292 | 8379 | 876 | 242 | 49 | 423 | 173964174 |
| 2017 | 11329 | 5309 | 6020 | 9447 | 1041 | 275 | 35 | 511 | 176104659 |
| 2018 | 12366 | 5764 | 6602 | 10314 | 1114 | 282 | 52 | 581 | 177613416 |
| 2019 | 13650 | 6475 | 7175 | 11364 | 1181 | 339 | 66 | 675 | 179040846 |
| 2020 | 16362 | 7466 | 8896 | 13532 | 1534 | 385 | 81 | 816 | 180565367 |
| Total | 110733 | 52325 | 58408 | 92512 | 9926 | 2601 | 481 | 5010 | 1729557734 |
| Year | Medical facility | Nursing home/long-term care facility | Hospices | Home |
| 2011 | 4691 | 2843 | 469 | 2241 |
| 2012 | 5181 | 3301 | 698 | 2731 |
| 2013 | 3222 | 1813 | 401 | 1472 |
| 2014 | 3464 | 1922 | 405 | 1633 |
| 2015 | 3832 | 2300 | 627 | 1953 |
| 2016 | 4347 | 2417 | 697 | 2197 |
| 2017 | 4797 | 2721 | 845 | 2549 |
| 2018 | 5184 | 2983 | 911 | 2896 |
| 2019 | 5542 | 3144 | 1121 | 3332 |
| 2020 | 6811 | 3340 | 1094 | 4495 |
| Total | 47071 | 26784 | 7268 | 25499 |
The overall AAMR for CKD-related AF deaths in adults was 6.2 in 2011 and increased to 7.7 in 2020. (APC: 0.82; 95%CI:
| Year interval | APC (95%CI) |
| Overall | |
| 2011 to 2014 | -15.49 (-30.23 to -5.55) |
| 2014 to 2020 | 10.12 (6.49-17.41) |
| Men | |
| 2011 to 2014 | -14.89 (-30.44 to -4.06) |
| 2014 to 2020 | 9.91 (6.10-19.62) |
| Women | |
| 2011 to 2014 | -15.91 (-31.40 to -5.17) |
| 2014 to 2020 | 10.09 (6.16-18.51) |
| NH White | |
| 2011 to 2014 | -14.49 (-29.44 to -4.17) |
| 2014 to 2020 | 10.63 (7.09-18.11) |
| NH Black or African American | |
| 2011 to 2014 | -19.23 (-36.71 to -6.14) |
| 2014 to 2020 | 10.28 (4.94-26.47) |
| NH American Indian or Alaska native | |
| 2011 to 2017 | -7.17 (-21.47 to -1.42) |
| 2017 to 2020 | 23.97 (8.45-51.18) |
| Hispanic or Latino | |
| 2011 to 2014 | -22.94 (-41.00 to -7.62) |
| 2014 to 2020 | 11.59 (6.08-22.08) |
| NH Asian or Pacific Islander | |
| 2011 to 2013 | -34.76 (-47.48 to -11.50) |
| 2013 to 2020 | 6.48 (2.15-19.29) |
| Nonmetropolitan areas | |
| 2011 to 2013 | -20.90 (-29.08 to -8.27) |
| 2013 to 2020 | 10.17 (7.65-14.83) |
| Metropolitan area | |
| 2011 to 2014 | -15.59 (-30.45 to -5.43) |
| 2014 to 2020 | 9.80 (6.08-17.61) |
| Northeast region | |
| 2011 to 2014 | -13.42 (-26.72 to -4.41) |
| 2014 to 2020 | 9.12 (5.94-15.48) |
| Midwest region | |
| 2011 to 2014 | -15.05 (-30.78 to -4.00) |
| 2014 to 2020 | 11.41 (7.69-19.80) |
| South region | |
| 2011 to 2014 | -14.92 (-30.38 to -4.04) |
| 2014 to 2020 | 10.67 (6.94-19.25) |
| West region | |
| 2011 to 2014 | -16.99 (-32.90 to -6.03) |
| 2014 to 2020 | 8.71 (4.51-18.15) |
| Year | Men | Women | Overall |
| 2011 | 8.1 (7.9-8.3) | 4.9 (4.8-5.1) | 6.2 (6.1-6.3) |
| 2012 | 9.2 (9-9.4) | 5.5 (5.4-5.7) | 7 (6.9-7.1) |
| 2013 | 5.4 (5.2-5.6) | 3 (2.9-3.1) | 4 (3.9-4.1) |
| 2014 | 5.5 (5.3-5.7) | 3.2 (3.1-3.3) | 4.1 (4-4.2) |
| 2015 | 6.3 (6.1-6.5) | 3.6 (3.5-3.8) | 4.7 (4.6-4.8) |
| 2016 | 6.8 (6.6-7) | 4 (3.8-4.1) | 5.1 (5-5.2) |
| 2017 | 7.5 (7.4-7.7) | 4.4 (4.3-4.5) | 5.7 (5.6-5.8) |
| 2018 | 8 (7.8-8.2) | 4.7 (4.6-4.8) | 6 (5.9-6.1) |
| 2019 | 8.5 (8.3-8.7) | 5.2 (5.1-5.3) | 6.6 (6.4-6.7) |
| 2020 | 10.3 (10.1-10.5) | 5.9 (5.7-6) | 7.7 (7.6-7.8) |
| Total | 7.6 (7.6-7.7) | 4.5 (4.4-4.5) | 5.8 (5.7-5.8) |
Adult men had consistently higher AAMRs than adult women throughout the study period (overall AAMR men: 7.6, 95%CI: 7.6-7.7; women: 4.5, 95%CI: 4.4-4.5). In 2011, the AAMR for adult men was 8.1 (95%CI: 7.9-8.3), which decreased to 5.5 in 2014 (APC: -14.89; 95%CI: -30.44 to -4.06), followed by an increase to 10.3 in 2020 (APC: 9.91; 95%CI: 6.1-19.62). Similarly, the AAMR for adult women in 2011 was 4.9 (95%CI: 4.8-5.1), which decreased to 3.2 in 2014 (APC: -15.91; 95%CI: -31.4 to -5.17), followed by an increase to 5.9 in 2020 (APC: 10.09; 95%CI: 6.16-18.51). The AAMR for adult men and women at the end of the study period was 10.3 and 5.9, respectively (men: 95% Cl: 10.1-10.5; women: 95%CI: 5.7-6) (Figure 1A, Tables 3 and 4).
Per race/ethnicity, AAMRs demonstrated highest values in non-Hispanic (NH) White patients, NH Black or African American, NH American Indian or Alaska native, NH Asian or Pacific Islander, and lastly Hispanic or Latino populations (overall AAMR NH White: 7.8, 95%CI: 7.7-7.8; NH Black or African American: 5, 95%CI: 4.9-5.1; NH American Indian or Alaska native: 3.8, 95%CI: 3.5-4.2; NH Asian or Pacific Islander: 2.6, 95%CI: 2.5-2.7; Hispanic or Latino: 2.2, 95%CI: 2.1-2.2).
The AAMRs for NH White and NH Black or African American exhibited similar trends over the study period. Both groups saw a decrease from 2011 to 2014 (NH White: APC: -14.49, 95%CI -29.44 to -4.17; NH Black or African American: APC: -19.23, 95%CI: -36.71 to -6.14), followed by an increase from 2014 to 2020 (NH White: APC: 10.63, 95%CI: 7.09-18.11; NH Black or African American: APC: 10.28, 95%CI: 4.94-26.47). The AAMRs for NH American Indian or Alaska Native decreased from 2011 to 2017 (APC: -7.71; 95%CI: -21.47 to -1.42), and then significantly increased from 2017 to 2020 (APC: 23.97; 95%CI: 8.45-51.18). The AAMRs for NH Asian or Pacific Islanders significantly decreased from 2011 to 2013 (APC:
| Year | NH White | NH Black or African American | NH American Indian or Alaska native | Hispanic or Latino | NH Asian or Pacific Islander |
| 2011 | 6.3 (6.1-6.4) | 6.9 (6.5-7.4) | 5 (3.5-6.9) | 3.8 (3.4-4.1) | 4.8 (4.2-5.4) |
| 2012 | 7.1 (6.9-7.2) | 7.6 (7.1-8) | 6.3 (4.6-8.4) | 5 (4.6-5.4) | 4.7 (4.2-5.2) |
| 2013 | 4.1 (4-4.2) | 4.2 (3.8-4.5) | 4.1 (2.8-5.8) | 2.2 (1.9-2.4) | 1.9 (1.6-2.2) |
| 2014 | 4.3 (4.2-4.4) | 4.1 (3.8-4.4) | 4.4 (3.1-6) | 2.2 (2-2.5) | 2.3 (2-2.7) |
| 2015 | 5 (4.9-5.1) | 4.3 (3.9-4.6) | 3.2 (2.2-4.5) | 2.5 (2.2-2.7) | 2.4 (2-2.7) |
| 2016 | 5.4 (5.3-5.5) | 5 (4.7-5.4) | 4.4 (3.2-5.9) | 2.8 (2.5-3) | 2.9 (2.5-3.3) |
| 2017 | 6 (5.9-6.1) | 5.7 (5.3-6.1) | 3.4 (2.3-4.7) | 3.1 (2.8-3.4) | 3.1 (2.7-3.4) |
| 2018 | 6.4 (6.3-6.6) | 6 (5.6-6.3) | 4.4 (3.3-5.9) | 3.3 (3-3.6) | 3 (2.6-3.3) |
| 2019 | 7 (6.9-7.1) | 6.1 (5.8-6.5) | 5.6 (4.3-7.2) | 3.8 (3.5-4.1) | 3.4 (3-3.8) |
| 2020 | 8.3 (8.1-8.4) | 7.8 (7.4-8.2) | 6.6 (5.2-8.2) | 4.3 (4-4.6) | 3.7 (3.3-4) |
| Total | 7.8 (0-6) | 5.1 (0.1-5.8) | 4.2 (0.2-4.8) | 2.2 (0-3.3) | 2.7 (0.1-3.2) |
A significant difference in AAMR was observed in different states, with the AAMRs ranging from 2.6 (95%CI: 2.3-2.9) in Nevada to 10.9 (95%CI: 10.6-11.3) in Minnesota. States belonging to the top 90th percentile were Indiana, Minnesota, Nebraska, North Dakota, Oregon, Vermont, Washington, and Wisconsin which had approximately four times the AAMRs compared with states that fell into the lower 10th percentile, namely, Alabama, Arizona, Florida, Georgia, Loui
| State | Age-adjusted rate (95%CI) |
| Alabama | 3.8 (3.6-4.1) |
| Alaska | 5.3 (4.4-6.3) |
| Arizona | 3.9 (3.7-4) |
| Arkansas | 4.1 (3.8-4.4) |
| California | 6.3 (6.2-6.5) |
| Colorado | 7.6 (7.3-8) |
| Connecticut | 4.7 (4.4-5) |
| Delaware | 5.4 (4.8-6) |
| District of Columbia | 4.5 (3.8-5.3) |
| Florida | 3.3 (3.2-3.4) |
| Georgia | 3.6 (3.4-3.7) |
| Hawaii | 4.3 (3.9-4.7) |
| Idaho | 5.8 (5.3-6.3) |
| Illinois | 4.2 (4.1-4.4) |
| Indiana | 8.9 (8.6-9.2) |
| Iowa | 6.5 (6.2-6.9) |
| Kansas | 4.2 (3.9-4.5) |
| Kentucky | 7.7 (7.4-8.1) |
| Louisiana | 3.4 (3.2-3.7) |
| Maine | 5.9 (5.4-6.4) |
| Maryland | 6.4 (6.1-6.7) |
| Massachusetts | 5.2 (5-5.4) |
| Michigan | 5.1 (4.9-5.3) |
| Minnesota | 10.9 (10.6-11.3) |
| Mississippi | 3.8 (3.5-4.1) |
| Missouri | 4.7 (4.5-4.9) |
| Montana | 5.6 (5.1-6.2) |
| Nebraska | 8.7 (8.1-9.2) |
| Nevada | 2.6 (2.3-2.9) |
| New Hampshire | 7.3 (6.8-7.9) |
| New Jersey | 5.2 (5-5.4) |
| New Mexico | 3.3 (3-3.6) |
| New York | 3.5 (3.4-3.6) |
| North Carolina | 6.2 (6-6.4) |
| North Dakota | 8.9 (8.1-9.8) |
| Ohio | 6.5 (6.3-6.7) |
| Oklahoma | 7.5 (7.1-7.8) |
| Oregon | 9.8 (9.4-10.2) |
| Pennsylvania | 6.9 (6.7-7) |
| Rhode Island | 7.3 (6.7-7.9) |
| South Carolina | 7 (6.7-7.3) |
| South Dakota | 6.6 (6-7.3) |
| Tennessee | 6.9 (6.7-7.2) |
| Texas | 6.5 (6.3-6.6) |
| Utah | 5.3 (4.9-5.7) |
| Vermont | 8.1 (7.3-9) |
| Virginia | 5.3 (5.1-5.5) |
| Washington | 9 (8.7-9.3) |
| West Virginia | 7.7 (7.2-8.2) |
| Wisconsin | 8.4 (8.1-8.6) |
| Wyoming | 6.2 (5.3-7) |
On average, throughout the study period the highest mortality was observed in the Midwestern region (AAMR: 7.6; 95%CI: 7.5-7.7), followed by the Western (AAMR: 6.7; 95%CI: 6.6-6.8), Northeastern (AAMR: 6.3; 95%CI: 6.2-6.4), and Southern (AAMR: 5.6; 95%CI: 5.6-5.7) regions (Figure 1D, Tables 3 and 7). Nonmetropolitan areas had consistently higher AAMRs than metropolitan areas throughout the study period, with overall AAMRs of 8 (95%CI: 7.9-8.1) and 6.1 (95%CI: 6.1-6.2), respectively. AAMRs of metropolitan areas decreased from 2011 to 2014 (APC: -15.59; 95%CI: -30.45 to -5.43), followed by an increase till the end of the study in 2020 (APC: 9.80; 95%CI: 6.08-17.61). While AAMRs of Nonmetropolitan significantly decreased from 2011 to 2013 (APC: -20.90; 95%CI: -29.08 to -8.27), followed by an increase till 2020 (APC: 10.17; 95%CI: 7.65 to 14.83) (Figure 1E, Tables 3 and 8).
| Year | Northeast | Midwest | South | West |
| 2011 | 5.5 (5.2-5.7) | 6.7 (6.4-6.9) | 5.5 (5.3-5.7) | 7.3 (7-7.6) |
| 2012 | 6.1 (5.9-6.4) | 7.6 (7.3-7.8) | 6.2 (6-6.4) | 8.2 (7.9-8.5) |
| 2013 | 3.7 (3.5-3.9) | 4.4 (4.2-4.6) | 3.6 (3.4-3.7) | 4.3 (4.1-4.5) |
| 2014 | 3.9 (3.7-4.1) | 4.5 (4.3-4.7) | 3.7 (3.6-3.9) | 4.6 (4.4-4.8) |
| 2015 | 4.3 (4.1-4.5) | 5.3 (5.1-5.6) | 4.3 (4.1-4.4) | 5.1 (4.9-5.3) |
| 2016 | 4.7 (4.5-5) | 5.7 (5.5-5.9) | 4.7 (4.5-4.8) | 5.6 (5.4-5.8) |
| 2017 | 5.2 (4.9-5.4) | 6.4 (6.2-6.7) | 5.1 (5-5.3) | 6.2 (5.9-6.4) |
| 2018 | 5.5 (5.2-5.7) | 7 (6.7-7.2) | 5.6 (5.4-5.8) | 6.3 (6-6.5) |
| 2019 | 5.8 (5.5-6) | 7.6 (7.4-7.9) | 6 (5.8-6.1) | 7.1 (6.9-7.4) |
| 2020 | 6.8 (6.6-7.1) | 9.1 (8.9-9.4) | 7.2 (7-7.4) | 7.9 (7.6-8.1) |
| Total | 5.2 (5.1-5.2) | 6.5 (6.4-6.6) | 5.2 (5.2-5.3) | 6.3 (6.2-6.4) |
| Year | Metropolitan | Nonmetropolitan |
| 2011 | 6.1 (6-6.2) | 6.6 (6.3-6.9) |
| 2012 | 6.9 (6.8-7.1) | 7.3 (7-7.6) |
| 2013 | 3.9 (3.8-4) | 4.2 (3.9-4.4) |
| 2014 | 4 (3.9-4.1) | 4.5 (4.2-4.7) |
| 2015 | 4.6 (4.5-4.7) | 5.3 (5-5.5) |
| 2016 | 5 (4.9-5.1) | 5.7 (5.4-6) |
| 2017 | 5.5 (5.4-5.6) | 6.4 (6.2-6.7) |
| 2018 | 5.9 (5.8-6) | 6.7 (6.4-7) |
| 2019 | 6.4 (6.2-6.5) | 7.7 (7.4-7.9) |
| 2020 | 7.4 (7.3-7.5) | 9.2 (8.9-9.5) |
| Total | 5.6 (5.6-5.7) | 6.4 (6.3-6.5) |
Our results show that the overall mortality rates for AF and CKD increased from 2011 to 2020. AMR was consistently greater for men than for women and for non-metropolitan areas than for metropolitan areas. The highest overall AAMR was reported in the Midwest region and states like Indiana and Minnesota. The lowest overall AAMR was reported in the Southern region and states like Alabama and Arizona. The values were the highest for NH white populations and lowest for Hispanic/Latino populations.
The study provides valuable insights into the temporal trends in mortality associated with AF among adults diagnosed with CKD in the United States over a decade, from 2011 to 2020. Previous investigations into CKD-related mortality have shown notable declines, indicating improvements in healthcare strategies (Figure 2)[18]. Our findings reveal a notewor
When categorized by sex, male patients consistently showed higher AAMRs as compared to female counterparts throughout the study period. These differences can be attributed to several factors. This observation aligns with broader trends in cardiovascular and renal health, where males often face a higher burden of disease and mortality compared to females. Firstly, males tend to have a higher prevalence of risk factors for AF, such as hypertension, obesity, and coronary artery disease, which are also common comorbidities in CKD[25-27]. Additionally, biological differences between sexes, including hormonal influences and genetic predispositions, may contribute to varying susceptibilities to AF-related complications. Furthermore, disparities in healthcare access and utilization, as well as differences in adherence to treatment regimens, may result in suboptimal management of AF and CKD among males.
When analyzed according to race/ethnicity, the analysis revealed that NH White patients exhibited notably elevated AAMRs compared to their counterparts. These differences may arise from variations in socioeconomic status, healthcare access, prevalence of comorbidities, and genetic predispositions. NH Additionally, recent research also found that white males have the highest burden of AF[28]. Possible contributors include differences in comorbidity prevalence, delays in diagnosis and treatment, variations in care quality, and genetic and biological factors. Moreover, some groups may have higher rates of obesity, hypertension, and other cardiovascular risk factors known to increase the likelihood of developing AF[29].
Another significant finding of our study underscores geographic disparities, with marked variations in AAMRs observed across states and regions. With the AAMRs ranging from 2.6 to 10.9 in Nevada and Minnesota respectfully. The analysis also disclosed that the Midwestern region consistently demonstrated the highest AAMRs. These regional differences may stem from various factors distinctive to the CKD population. For example, variances in healthcare infrastructure and accessibility to specialized care might affect the proper timely diagnosis and management of AF complications. Furthermore, socioeconomic elements like income inequality and educational attainment could impact the access to healthcare services and compliance with treatment plans among CKD patients with AF[30,31]. Additionally, contextual poverty and its impact on nutrition can further exacerbate these disparities[32]. A meta-analysis further underscores the significance of socioeconomic elements, revealing their strong association with CKD[33]. Moreover, disparities in the prevalence of risk factors such as high blood pressure, diabetes, and obesity among regions might contribute to variations in mortality rates.
Persistent disparities exist between metropolitan and nonmetropolitan areas, with the latter consistently displaying higher AAMRs associated with AF among CKD patients. Contributing to this discrepancy are health behaviors, such as hypertension, diabetes, and obesity, which are not well managed and treated in rural areas compared to urban areas in the United States. These challenges are often due to barriers such as limited access to healthcare, financial constraints, and cultural differences[34]. Patients with CKD in rural areas face significant challenges in accessing kidney replacement therapy, leading to worse outcomes compared to their urban counterparts[35]. These challenges are particularly pronounced in vulnerable populations, such as the urban poor, who have a higher risk of progression to end-stage renal disease[36]. Addressing these determinants has the potential to narrow the gap in mortality rates between metropolitan and nonmetropolitan areas.
Irrespective of the stratification by sex, race/ethnicity, urbanization or United States census regions, the overall AAMRs had a concerning upward trend in the years 2014-2020. This increase in the last years of the study could be due to the COVID-19 pandemic. Its impact was wide ranging from affecting the physical health of the general population and complicating existing chronic conditions to the overuse of medical facilities for COVID-19 which could have potentially disrupted the healthcare access for general population that did not present with acute symptoms. The pandemic could be a comprehensive explanation for the elevated mortality in the final years of the study period.
Our findings have shown a significant association between AF and CKD that adds greatly to the morbidity and mortality in the United States as well as globally. This prevalence of the disease poses an inevitable burden that threatens overall national health. There is a need of development of targeted interventions and policies specifically targeting the identified high-risk demographics. This will help mitigate the burden of the disease and improve the morbidity and mortality linked with CKD and AF in the United States.
This database might result in errors or omissions. The outcomes for patients aged 35 and older might not be generalizable to younger ages. Analyzing this data does not involve detailed clinical data, such as biomarkers and treatment modalities, and it impedes a comprehensive understanding of mortality drivers. The absence of scrutiny for the definite classification of both studied diseases impedes interpretation that would guide towards proper interventions. Furthermore, this study did not account for socioeconomic factors, potential confounders such as comorbid conditions, and medical management modalities.
In conclusion, our study underscores a notable increase in mortality linked to AF associated with CKD among adults aged 35 to 85 or more years from 2011 to 2020. Exceptionally high mortality rates were observed among NH White populations, males, residents in the Midwest United States regions, and those in non-metropolitan areas. Addressing mortality associated with AF and CKD complications demands enhanced preventive and management strategies, es
| 1. | Matsushita K, Ballew SH, Wang AY, Kalyesubula R, Schaeffner E, Agarwal R. Epidemiology and risk of cardiovascular disease in populations with chronic kidney disease. Nat Rev Nephrol. 2022;18:696-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 290] [Reference Citation Analysis (0)] |
| 2. | Weiner DE. Public health consequences of chronic kidney disease. Clin Pharmacol Ther. 2009;86:566-569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 3. | Lippi G, Sanchis-Gomar F, Cervellin G. Global epidemiology of atrial fibrillation: An increasing epidemic and public health challenge. Int J Stroke. 2021;16:217-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 220] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 4. | Zoccali C, Mark PB, Sarafidis P, Agarwal R, Adamczak M, Bueno de Oliveira R, Massy ZA, Kotanko P, Ferro CJ, Wanner C, Burnier M, Vanholder R, Mallamaci F, Wiecek A. Diagnosis of cardiovascular disease in patients with chronic kidney disease. Nat Rev Nephrol. 2023;19:733-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 36] [Reference Citation Analysis (0)] |
| 5. | McManus DD, Saczynski JS, Ward JA, Jaggi K, Bourrell P, Darling C, Goldberg RJ. The Relationship Between Atrial Fibrillation and Chronic Kidney Disease : Epidemiologic and Pathophysiologic Considerations for a Dual Epidemic. J Atr Fibrillation. 2012;5:442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 6. | Song J, Navarro-Garcia JA, Wu J, Saljic A, Abu-Taha I, Li L, Lahiri SK, Keefe JA, Aguilar-Sanchez Y, Moore OM, Yuan Y, Wang X, Kamler M, Mitch WE, Ruiz-Hurtado G, Hu Z, Thomas SS, Dobrev D, Wehrens XH, Li N. Chronic kidney disease promotes atrial fibrillation via inflammasome pathway activation. J Clin Invest. 2023;133:e167517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 7. | Heo NJ, Rhee SY, Waalen J, Steinhubl S. Chronic kidney disease and undiagnosed atrial fibrillation in individuals with diabetes. Cardiovasc Diabetol. 2020;19:157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Airy M, Schold JD, Jolly SE, Arrigain S, Bansal N, Winkelmayer WC, Nally JV Jr, Navaneethan SD. Cause-Specific Mortality in Patients with Chronic Kidney Disease and Atrial Fibrillation. Am J Nephrol. 2018;48:36-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Tapoi L, Ureche C, Sascau R, Badarau S, Covic A. Atrial fibrillation and chronic kidney disease conundrum: an update. J Nephrol. 2019;32:909-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Centers for Disease Control and Prevention. CDC WONDER. [cited 10 February 2025]. Available from: https://wonder.cdc.gov/. |
| 11. | World Health Organization. ICD-10 version: 2019. [cited 10 February 2025]. Available from: https://icd.who.int/browse10/2019/en. |
| 12. | Cuschieri S. The STROBE guidelines. Saudi J Anaesth. 2019;13:S31-S34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 387] [Cited by in RCA: 2050] [Article Influence: 341.7] [Reference Citation Analysis (1)] |
| 13. | Centers for Disease Control and Prevention. NCHS Urban-Rural Classification Scheme for Counties. [cited 10 February 2025]. Available from: https://www.cdc.gov/nchs/data-analysis-tools/urban-rural.html. |
| 14. | United States Census Bureau. Census bureau economic briefing room. [cited 10 February2025]. Available from: https://www.census.gov/economic-indicators/. |
| 15. | Centers for Disease Control and Prevention. WISQARS Frequently Asked Questions. [cited 10 February 2025]. Available from: https://www.cdc.gov/injury/wisqars/mapping_help/crude_rate.htm. |
| 16. | National Library of Medicine. Finding and Using Health Statistics. [cited 10 February 2025]. Available from: https://www.nlm.nih.gov/oet/ed/stats/02-600.html. |
| 17. | National Cancer Institute. JoinPoint Regression Program. [cited 10 February 2025]. Available from: https://surveillance.cancer.gov/joinpoint/. |
| 18. | Silver SA, Bell CM, Chertow GM, Shah PS, Shojania K, Wald R, Harel Z. Effectiveness of Quality Improvement Strategies for the Management of CKD: A Meta-Analysis. Clin J Am Soc Nephrol. 2017;12:1601-1614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Shi Y, Xiong J, Chen Y, Deng J, Peng H, Zhao J, He J. The effectiveness of multidisciplinary care models for patients with chronic kidney disease: a systematic review and meta-analysis. Int Urol Nephrol. 2018;50:301-312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 20. | Hsu HT, Chiang YC, Lai YH, Lin LY, Hsieh HF, Chen JL. Effectiveness of Multidisciplinary Care for Chronic Kidney Disease: A Systematic Review. Worldviews Evid Based Nurs. 2021;18:33-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 21. | Llewellyn S. The Chronic Care Model, Kidney Disease, and Primary Care: A Scoping Review. Nephrol Nurs J. 2019;46:301-328. [PubMed] |
| 22. | Chadban SJ, Ahn C, Axelrod DA, Foster BJ, Kasiske BL, Kher V, Kumar D, Oberbauer R, Pascual J, Pilmore HL, Rodrigue JR, Segev DL, Sheerin NS, Tinckam KJ, Wong G, Knoll GA. KDIGO Clinical Practice Guideline on the Evaluation and Management of Candidates for Kidney Transplantation. Transplantation. 2020;104:S11-S103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 365] [Article Influence: 73.0] [Reference Citation Analysis (0)] |
| 23. | Sidhu B, Mavilakandy A, Hull KL, Koev I, Vali Z, Burton JO, Ng GA. Atrial Fibrillation and Chronic Kidney Disease: Aetiology and Management. Rev Cardiovasc Med. 2024;25:143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 24. | Gansevoort RT, Hilbrands LB. CKD is a key risk factor for COVID-19 mortality. Nat Rev Nephrol. 2020;16:705-706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 135] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 25. | Yoon YH, Park GM, Lee JY, Lee JH, Roh JH, Kim TO, Lee PH, Choe J, Kim YH, Lee SW. Relationship between sexual differences and cardiovascular risk factors in the prevalence of asymptomatic coronary disease. Int J Cardiol. 2023;370:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 26. | Davis E, Higgins MK, Wood KA, Cimiotti J, Gary RA, Dunbar SB. Sex Differences in Cardiac Risk Factors in Young Adults: A Secondary Analysis and Surveillance Study. J Cardiovasc Nurs. 2023;38:168-178. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 27. | Sah M, Saa L, P V. Ethnicity and risk factors among Indian coronary artery disease patients. Bioinformation. 2023;19:19-23. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 28. | Borzecki AM, Bridgers DK, Liebschutz JM, Kader B, Kazis LE, Berlowitz DR. Racial differences in the prevalence of atrial fibrillation among males. J Natl Med Assoc. 2008;100:237-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 90] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 29. | Centers for Disease Control and Prevention (CDC). Differences in prevalence of obesity among black, white, and Hispanic adults - United States, 2006-2008. MMWR Morb Mortal Wkly Rep. 2009;58:740-744. [PubMed] |
| 30. | Nicholas SB, Kalantar-Zadeh K, Norris KC. Socioeconomic disparities in chronic kidney disease. Adv Chronic Kidney Dis. 2015;22:6-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 173] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 31. | Morton RL, Schlackow I, Mihaylova B, Staplin ND, Gray A, Cass A. The impact of social disadvantage in moderate-to-severe chronic kidney disease: an equity-focused systematic review. Nephrol Dial Transplant. 2016;31:46-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 82] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 32. | Gutiérrez OM. Contextual poverty, nutrition, and chronic kidney disease. Adv Chronic Kidney Dis. 2015;22:31-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 33. | Vart P, Gansevoort RT, Joosten MM, Bültmann U, Reijneveld SA. Socioeconomic disparities in chronic kidney disease: a systematic review and meta-analysis. Am J Prev Med. 2015;48:580-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 81] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 34. | Ricci-Cabello I, Ruiz-Perez I, Rojas-García A, Pastor G, Gonçalves DC. Improving diabetes care in rural areas: a systematic review and meta-analysis of quality improvement interventions in OECD countries. PLoS One. 2013;8:e84464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 35. | Scholes-Robertson NJ, Howell M, Gutman T, Baumgart A, SInka V, Tunnicliffe DJ, May S, Chalmers R, Craig J, Tong A. Patients' and caregivers' perspectives on access to kidney replacement therapy in rural communities: systematic review of qualitative studies. BMJ Open. 2020;10:e037529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 36. | Hall YN, Choi AI, Chertow GM, Bindman AB. Chronic kidney disease in the urban poor. Clin J Am Soc Nephrol. 2010;5:828-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |