Published online Mar 26, 2024. doi: 10.4330/wjc.v16.i3.161
Peer-review started: July 30, 2023
First decision: October 9, 2023
Revised: November 21, 2023
Accepted: January 12, 2024
Article in press: January 12, 2024
Published online: March 26, 2024
Processing time: 235 Days and 2.8 Hours
Patients with tetralogy of Fallot (TOF) often have arrhythmias, commonly being atrial fibrillation (AF). Radiofrequency ablation is an effective treatment for AF and does not usually cause severe postoperative hypoxemia, but the risk of complications may increase in patients with conditions such as TOF.
We report a young male patient with a history of TOF repair who developed severe hypoxemia after radiofrequency ablation for AF and was ultimately confirmed to have a new right-to-left shunt. The patient subsequently underwent atrial septal occlusion and eventually recovered.
Radiofrequency ablation may cause iatrogenic atrial septal injury; thus possible complications should be predicted in order to ensure successful treatment and patient safety.
Core Tip: More attention should be paid to patient hemodynamics before and after radiofrequency ablation in those with a potential risk of right-to-left shunt such as tetralogy of Fallot patients. These patients may need to be further evaluated before or during surgery to make safer treatment decisions. This case may provide an important reference for the proper preparation and perioperative management of atrial fibrillation under special circumstances.
- Citation: Li ZH, Lou L, Chen YX, Shi W, Zhang X, Yang J. Severe hypoxemia after radiofrequency ablation for atrial fibrillation in palliatively repaired tetralogy of Fallot: A case report. World J Cardiol 2024; 16(3): 161-167
- URL: https://www.wjgnet.com/1949-8462/full/v16/i3/161.htm
- DOI: https://dx.doi.org/10.4330/wjc.v16.i3.161
Tetralogy of Fallot (TOF) has been reported to be the most common cyanotic congenital heart disease with an incidence of 0.34 per 1000 Live births[1,2]. With the development of medical technology, the survival rate of patients with TOF has significantly increased; however, long-term complications can occur. Atrial arrhythmia is one of the most common late complications after the repair of TOF (and is generally related to myopathy caused by atrial surgical scarring, right atrial dilatation, and valve reflux[3,4]). However, rhythm control and heart rate control are not effective in preventing atrial fibrillation (AF) in patients with TOF[4], and radiofrequency ablation has become an important method of treating these patients[5]. Multiple studies have confirmed the safety and feasibility of this treatment[6,7], but few reports have focused on the risk of transatrial septal puncture in patients with TOF. Here, we report a case of severe hypoxemia in a patient with TOF who developed a right-to-left shunt after radiofrequency ablation for AF. This case provides an important reference for the surgical risk assessment and final decision in the treatment of similar patients, and may effectively prevent the occurrence of serious complications.
A 32-year-old man visited our center due to “heart palpitations for 2 mo”.
In the past 2 mo, the patient had persistent palpitations without obvious inducement, but there was no discomfort such as chest pain and dyspnea.
The patient had sustained palpitations recently and had a history of similar attacks in the past. He completed a 12-lead electrocardiogram (ECG) in the outpatient clinic and was diagnosed with AF. The patient still had palpitations and discomfort after taking metoprolol sustained-release tablets for heart rate control, and was admitted to the hospital for catheter ablation of AF. He had a history of TOF, and underwent palliative correction surgery 20 years ago, involving correction of complex congenital heart disease and pulmonary artery artificial vascular implantation.
The patient denied any family history of congenital heart disease and AF.
Surgical scars were seen on the chest, arrhythmia was present, a systolic murmur was heard in the auscultation area of the pulmonary valve, and the cardiac boundary was enlarged.
No obvious abnormalities were found during preoperative examinations such as routine blood and liver and kidney function tests.
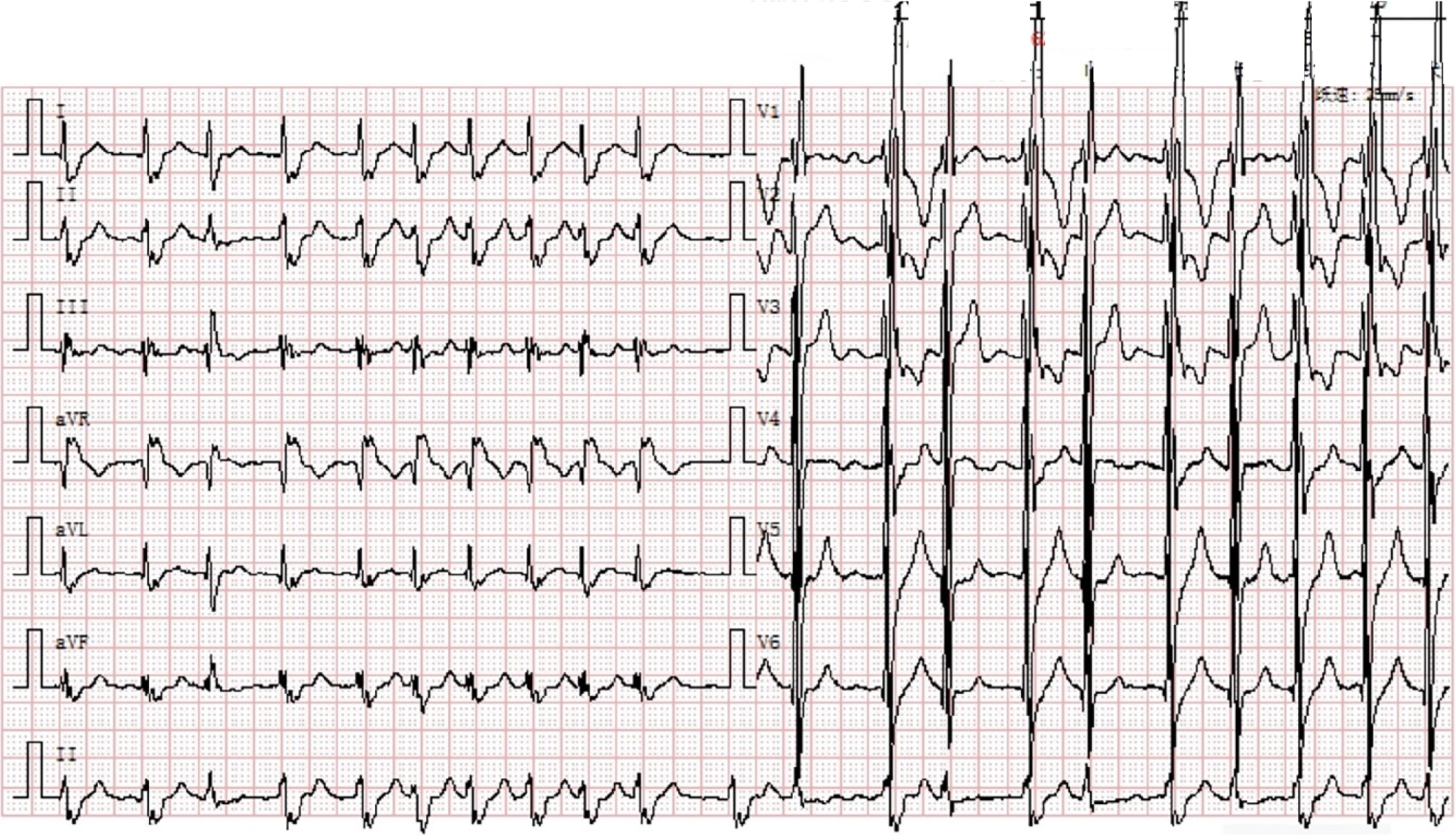
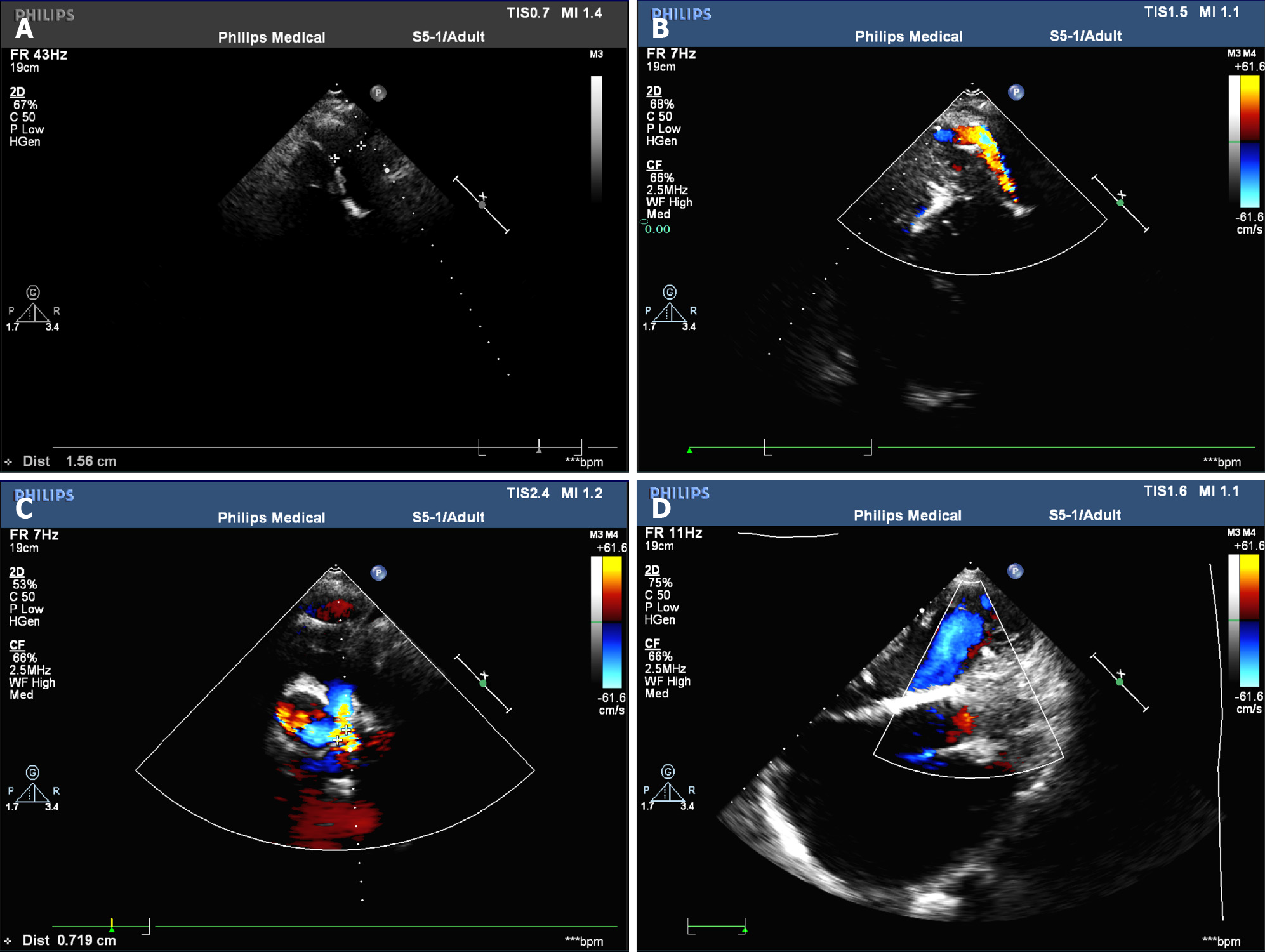
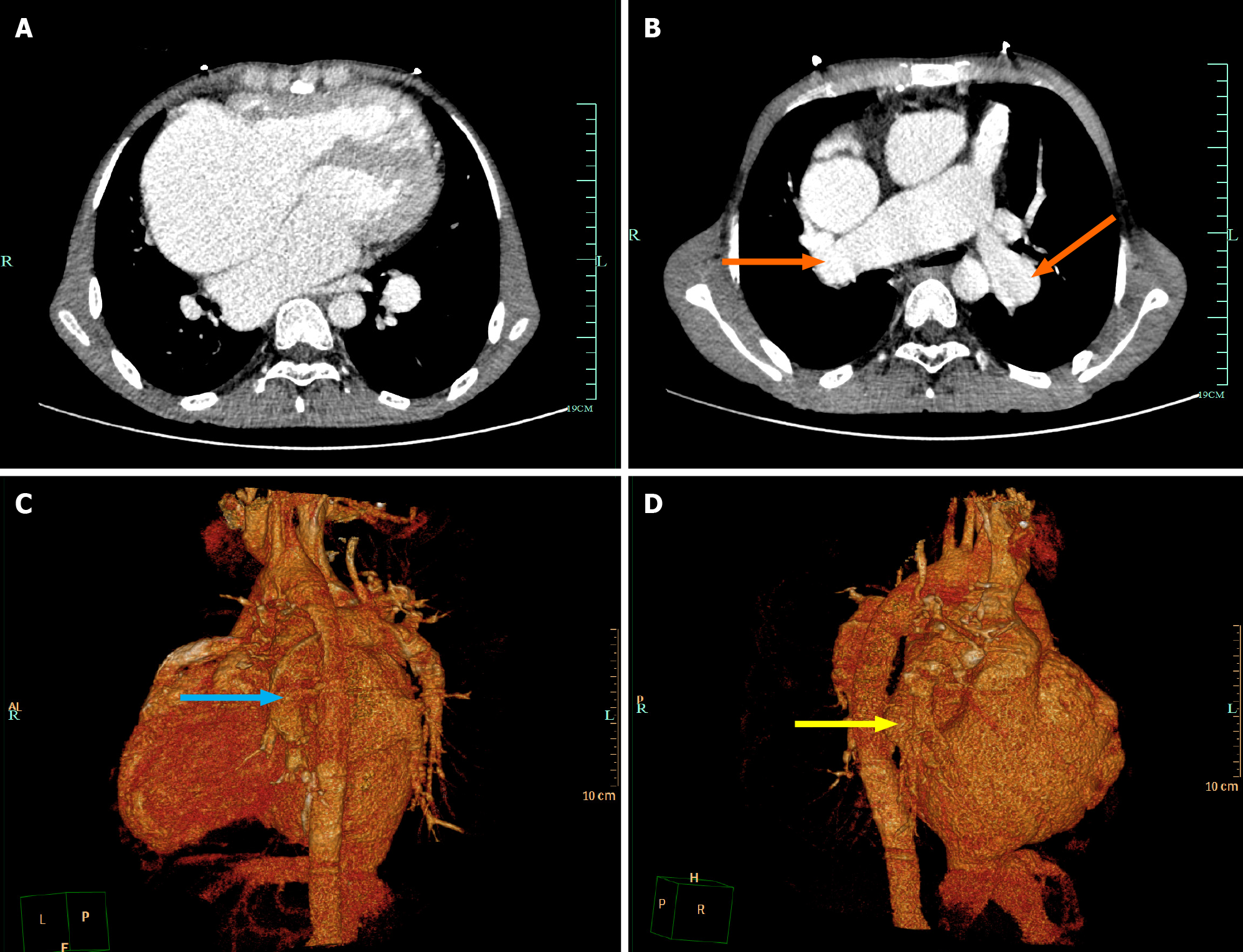
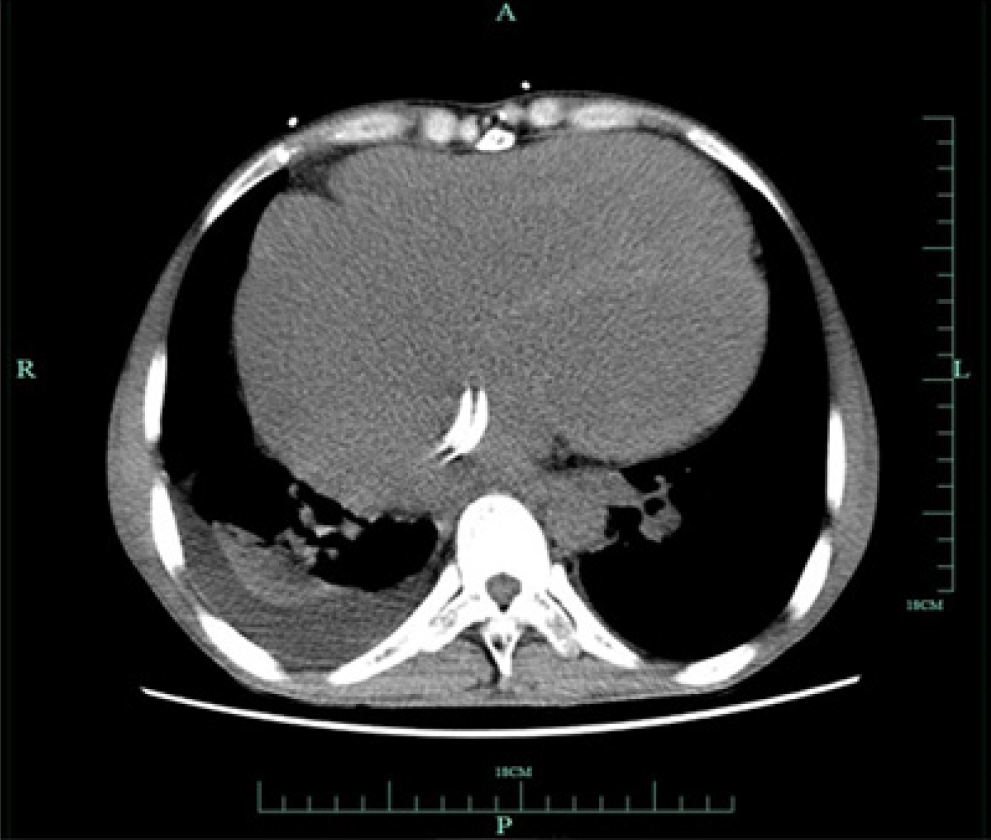
The 12-lead ECG showed AF (Figure 1). Transthoracic echocardiography confirmed the changes after TOF correction and pulmonary artery implantation, with the formation of collateral circulation between the descending aorta and the left pulmonary artery, no shunt at the ventricular septum level, enlargement of the right heart, and moderate to severe tricuspid regurgitation (Figure 2). Transesophageal echocardiography did not show the atrial septal shunt, which ruled out the possibility of “pentalogy of Fallot” in this patient. In addition, pulmonary venous computed tomography angiography (CTA) was performed preoperatively to evaluate pulmonary venous structure and function, showing that the right heart was significantly enlarged, and no obvious filling defect was found in the lumen. An artificial blood vessel shadow was seen between the right pulmonary artery and the right ventricle. The left side of the descending aorta was connected to the left pulmonary artery by another artificial blood vessel (Figure 3). These two artificial vessels may have reduced the symptoms of right ventricular outflow tract obstruction.
Based on the clinical manifestations and imaging findings, the patient was initially diagnosed with AF, and post-repair for TOF.
After completing the evaluation, pulmonary vein isolation and BOX isolation were performed under the guidance of the CARTO® system, and an ablation catheter was delivered to the bilateral pulmonary veins by an atrial septal puncture method under the guidance of ultrasound to complete electrical isolation during the operation. Intraoperative oxygen saturation was maintained at 90%-93%. No obvious complications were observed following catheter withdrawal. After ablation, the ECG showed sinus rhythm and dual-source atrial premature contractions. The outcome of the operation was satisfactory and met our expectations.
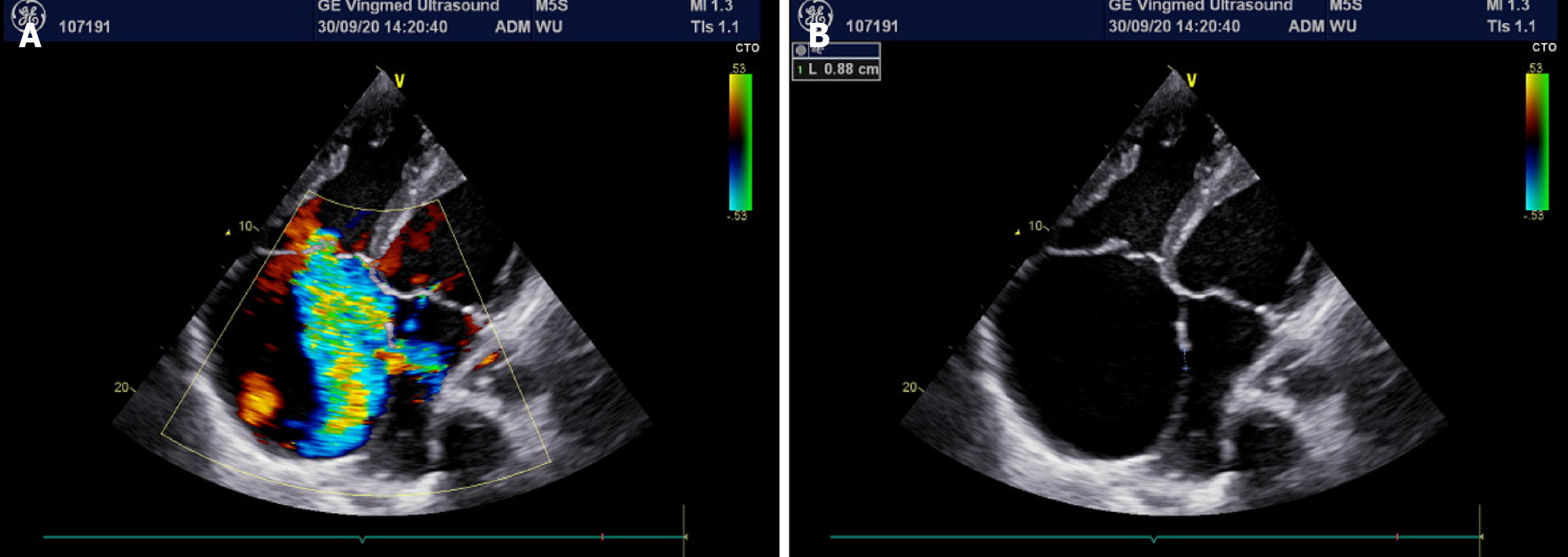
On the second day after ablation, the patient complained of chest tightness and shortness of breath without obvious inducement, and blood oxygen saturation decreased gradually, reaching a minimum of 50%. Oxygen saturation was only maintained at 70%-75% by oxygen inhalation. Physical examination revealed poor mental status, distention of the jugular vein, cyanosis of the mouth and lips, and decreased temperature in the extremities. Arterial blood gas analysis showed that the partial pressure of carbon dioxide was 25 mmHg, the partial pressure of oxygen was 42 mmHg, and the blood pH and bicarbonate concentration were within the normal range. Blood tests showed a brain natriuretic peptide level of 2716 ng/mL. In light of the patient’s history of congenital heart disease, acute heart failure was considered, an echocardiogram was performed, and a new right-to-left shunt approximately 9 mm wide was identified (Figure 4). After multidisciplinary consultation, hypoxemia due to circulatory hypoxia caused by an arteriovenous shunt, and atrial septal defect repair surgery were considered. However, blood oxygen saturation should be maintained before surgery to prevent damage to important organs, while endotracheal intubation was of little help to correct hypoxemia in this patient and may even have aggravated hypoxia. Therefore, the patient was transferred to the intensive care unit and a veno-venous extracorporeal membrane oxygenation intubation was performed. Postoperative oxygen saturation was maintained above 90%. Arterial blood gas analysis showed 29 mmHg of carbon dioxide and 85 mmHg of oxygen.
When the patient’s vital signs became stable, we communicated with his family and an atrial defect closure was subsequently performed with insertion of a 14 mm occluder (Figure 5). Oxygen saturation was maintained above 90% in the shutdown state of extracorporeal membrane oxygenation after occlusion. Following administration of anti-infection and anticoagulation therapy, the patient’s condition improved and he was discharged.
The patient’s oxygen saturation was normal and there were no episodes of AF after the operation.
AF has been reported with a higher probability in patients with TOF and occurs at a younger age than in the general population[8]. Ablative therapy has been proved to be an effective curative treatment modality for AF[5]. A previous study proved that AF progresses more rapidly in patients with TOF and conventional antiarrhythmic drugs are not effective[4]. With the development of electrophysiological techniques, the success rate of ablation at the lesion site in the presence of complex arrhythmogenic scarring and substrate in patients with TOF has improved. Current reports focus on the recurrence of postoperative arrhythmias and the quality of life of patients, and have confirmed the efficacy of radiofrequency ablation in the treatment of AF after repair of TOF[7,9]. However, there have been few reports on the risks of radiofrequency ablation in patients with TOF. Due to congenital abnormalities, patients with TOF may not be able to tolerate atrial septal puncture during radiofrequency ablation in terms of cardiac infrastructure and hemodynamics.
Typically, transseptal puncture for radiofrequency ablation of AF does not cause serious complications and does not require special treatment[9]. However, in this report, atrial septal puncture resulted in severe hypoxemia. It is known that patients with TOF usually develop right ventricular outflow tract obstruction, and this hemodynamic abnormality may still be present even after surgical treatment. In this case, the patient underwent TOF palliative surgery, in which the implantation of artificial blood vessels relieved some, but not all, of the pulmonary hypertension. In addition, during the preoperative ECG examination, the right heart was enlarged and the tricuspid valve showed medium-severe regurgitation, which suggested that during the systolic period, a large amount of blood flow regurgitated into the right atrium, resulting in high pressure in the right atrium. If an atrial septal defect is present at the same time, a right-to-left shunt is likely to form, resulting in hypoxemia. Transatrial septal puncture during radiofrequency ablation resulted in this condition followed by the subsequent development of hypoxemia. Unfortunately, we failed to accurately assess the right atrial pressure and predict the risk of atrial septal puncture leading to subsequent disease.
Usually, no serious pulmonary hypertension occurs after correction of TOF[10], but there are still a small number of patients with significantly increased pressure in the right ventricular outflow tract[11,12]. This long-term complication not only affects the quality of life of patients, but also increases the potential risk of atrial septal puncture therapy. Preoperative elevated pulmonary artery pressure has been shown to promote the risk of right-to-left shunt in iatrogenic atrial septal defects[13]. In addition, the persistence of an iatrogenic atrial septal defect leading to refractory hypoxemia has been reported in several cases[14,15], and studies have shown that patients with iatrogenic atrial septal defects are more likely to have hemodynamic abnormalities, heart failure, and other conditions[16,17]. These results suggest that patients with right ventricular outflow tract abnormalities and iatrogenic atrial septal defects are more likely to develop hypoxemia. Following the diagnosis and treatment of this critically ill patient, we believe that it may be possible to effectively reduce the occurrence of postoperative hypoxemia by accurately detecting the right heart pressure through the right cardiac catheter in advance for patients with right-to-left shunt risk. This method is not only suitable for TOF, but can also be used for preoperative evaluation of patients with pulmonary malformation, pulmonary embolism, chronic obstructive pulmonary disease, and other diseases to reduce the risk of surgery.
More attention should be paid to patient hemodynamics before and after radiofrequency ablation in those with a potential risk of right-to-left shunt such as TOF patients. These patients may need to be further evaluated before or during surgery to make safer treatment decisions. This case provides an important reference for appropriate treatment decisions and perioperative management of AF under special circumstances.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jain S, India; Ueda H, Japan S-Editor: Lin C L-Editor: Wang TQ P-Editor: Yuan YY
| 1. | van der Linde D, Konings EE, Slager MA, Witsenburg M, Helbing WA, Takkenberg JJ, Roos-Hesselink JW. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol. 2011;58:2241-2247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2512] [Cited by in RCA: 2198] [Article Influence: 157.0] [Reference Citation Analysis (0)] |
| 2. | Marelli AJ, Mackie AS, Ionescu-Ittu R, Rahme E, Pilote L. Congenital heart disease in the general population: changing prevalence and age distribution. Circulation. 2007;115:163-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1181] [Cited by in RCA: 1197] [Article Influence: 66.5] [Reference Citation Analysis (0)] |
| 3. | Khairy P, Aboulhosn J, Gurvitz MZ, Opotowsky AR, Mongeon FP, Kay J, Valente AM, Earing MG, Lui G, Gersony DR, Cook S, Ting JG, Nickolaus MJ, Webb G, Landzberg MJ, Broberg CS; Alliance for Adult Research in Congenital Cardiology (AARCC). Arrhythmia burden in adults with surgically repaired tetralogy of Fallot: a multi-institutional study. Circulation. 2010;122:868-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 385] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 4. | Ramdjan TTTK, Mouws EMJP, Teuwen CP, Sitorus GDS, Houck CA, Bogers AJJC, de Groot NMS. Progression of late postoperative atrial fibrillation in patients with tetralogy of Fallot. J Cardiovasc Electrophysiol. 2018;29:30-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | de Groot NM, Lukac P, Schalij MJ, Makowski K, Szili-Torok T, Jordaens L, Nielsen JC, Jensen HK, Gerdes JC, Delacretaz E. Long-term outcome of ablative therapy of post-operative atrial tachyarrhythmias in patients with tetralogy of Fallot: a European multi-centre study. Europace. 2012;14:522-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Griffiths JR, Nussinovitch U, Liang JJ, Sims R, Yoneda ZT, Bernstein HM, Viswanathan MN, Khairy P, Srivatsa UN, Frankel DS, Marchlinski FE, Sandhu A, Shoemaker MB, Mohanty S, Burkhardt JD, Natale A, Lakireddy D, De Groot NMS, Gerstenfeld EP, Moore JP, Ávila P, Ernst S, Nguyen DT. Catheter Ablation for Atrial Fibrillation in Adult Congenital Heart Disease: An International Multicenter Registry Study. Circ Arrhythm Electrophysiol. 2022;15:e010954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 7. | Orczykowski M, Borowiec K, Biernacka E, Bodalski R, Urbanek P, Derejko P, Kodziszewska K, Woźniak O, Fronczak A, Marcinkiewicz K, Guzek K, Fil A, Warmiński G, Hoffman P, Bilińska M, Szumowski Ł. Ablation of atrial tachyarrhythmias late after surgical correction of tetralogy of Fallot: long-term follow-up. Kardiol Pol. 2018;76:1097-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Gazzaz TF, Elders B, Steve Fan CP, Manlhiot C, Seed M, Yoo SJ, van Arsdell G, Grosse-Wortmann L. The Modified History of Tetralogy of Fallot During Childhood and Adolescence. JACC Cardiovasc Imaging. 2021;14:1478-1480. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Raine D, O'Sullivan J, Chaudhari M, Hamilton L, Hasan A, Bourke JP. Ablation of atrial tachyarrhythmias late after surgical repair of tetralogy of Fallot. Cardiol Young. 2011;21:31-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 10. | Hu JJ, Bonnichsen CR, Dearani JA, Miranda WR, Johnson JN, Cetta F, Stephens EH, Aganga DO, Van Dorn CS. Adults With Tetralogy of Fallot: Early Postoperative Outcomes and Risk Factors for Complications. Mayo Clin Proc. 2021;96:2398-2406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 11. | Roisman ML, Beller BM, O'Keefe JD. Irreversible pulmonary hypertension after correction of tetralogy of Fallot. Chest. 1972;62:34-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 12. | Yasuhara J, Yamagishi H. Pulmonary arterial hypertension associated with tetralogy of Fallot. Int Heart J. 2015;56 Suppl:S17-S21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Hammerstingl C, Lickfett L, Jeong KM, Troatz C, Wedekind JA, Tiemann K, Lüderitz B, Lewalter T. Persistence of iatrogenic atrial septal defect after pulmonary vein isolation--an underestimated risk? Am Heart J. 2006;152:362.e1-362.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 66] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 14. | Kawaji T, Kaneda K, Kato M, Yokomatsu T. Iatrogenic Atrial Septal Defect Causing Position-Dependent Hypoxemia. JACC Cardiovasc Interv. 2020;13:2081-2082. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 15. | Sirker A, Hyde J, Hildick-Smith D. Refractory hypoxemia after mitral valve surgery: an unusual cause and its successful percutaneous treatment. J Invasive Cardiol. 2006;18:E86-E88. [PubMed] |
| 16. | Takaya Y, Akagi T, Hara H, Kanazawa H, Ikari Y, Isotani A, Shirai S, Kubo S, Morikawa T, Naganuma T, Saji M, Kuwata S, Hiasa G, Watanabe Y, Yamawaki M, Imai M, Matsumoto T, Yamamoto M, Murakami T, Asami M, Mizote I, Okai T, Bota H, Ito H. Iatrogenic Atrial Septal Defect Requiring Transcatheter Closure Following Transcatheter Mitral Valve Repair. Circ J. 2022;86:1740-1744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Lurz P, Unterhuber M, Rommel KP, Kresoja KP, Kister T, Besler C, Fengler K, Sandri M, Daehnert I, Thiele H, Blazek S, von Roeder M. Iatrogenic Atrial Septal Defects Following Transcatheter Mitral Valve Repair and Implications of Interventional Closure. JACC Cardiovasc Interv. 2021;14:2685-2694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |