Published online Jan 26, 2023. doi: 10.4330/wjc.v15.i1.33
Peer-review started: September 11, 2022
First decision: November 2, 2022
Revised: November 15, 2022
Accepted: November 15, 2022
Article in press: November 15, 2022
Published online: January 26, 2023
Processing time: 122 Days and 4.7 Hours
Takotsubo cardiomyopathy (TTC) can be diagnosed in patients presenting with clinical features of acute coronary syndrome (ACS) by using Mayo clinic criteria. Multiple precipitators have been attributed to causing TTC. Rarely it has been reported to occur following an acute envenomation.
This review describes the various patterns, mechanisms, and outcomes of envenomation induced TTC.
In this review, we included all studies on “TTC” and “envenomation “published in the various databases before June 2022. To be included in the review articles had to have a distinct diagnosis of TTC and an envenomation
A total of 20 patients with envenomation induced TTC were identified. Most episodes of envenomation induced TTC were reported following a bee sting, scorpion sting, and snake envenomation. Fear and anxiety related to the sting, direct catecholamine toxicity and administration of exogenous beta-adrenergic agents have been commonly postulated to precipitate TTC in these patients. 95% of these patients presented with a clinical picture of ACS. Most of these patients also fulfill at least 3 out of 4 criteria of Mayo clinic criteria for TTC. Echocardiographic evidence of Apical TTC was noted in 72% of patients. 94% of these patients had clinical improvement following optimal management and 35% of these patients were treated with guideline directed medications for heart failure.
Envenomation following multiple insect stings and reptile bites can precipitate TTC. Most reported envenomation related TTC has been due to bee stings and scorpion bites. Common mechanisms causing TTC were fear, anxiety, and stress of envenomation. Most of these patients present with clinical presentation of ACS, ST elevation, and elevated troponin. The most common type of TTC in these patients is Apical, which improved following medical management.
Core Tip: Multiple envenomations following insect stings and reptile bites can cause Takotsubo cardiomyopathy. Bee stings, wasp stings, scorpion stings, snake bites, spider bites, and jellyfish stings are the commonly reported precipitators of this cardiomyopathy. Multiple mechanisms have been postulated to cause this of which fear, anxiety, and stress of envenomation are the predominant ones. Patients usually present with clinical presentation of acute coronary syndrome, ST elevation, and elevated troponin. Echocardiography commonly shows an apical pattern of cardiomyopathy. This cardiomyopathy improves with medical management.
- Citation: Mishra AK, George AA, John KJ, Arun Kumar P, Dasari M, Afraz Pasha M, Hadley M. Takotsubo cardiomyopathy following envenomation: An updated review. World J Cardiol 2023; 15(1): 33-44
- URL: https://www.wjgnet.com/1949-8462/full/v15/i1/33.htm
- DOI: https://dx.doi.org/10.4330/wjc.v15.i1.33
Takotsubo cardiomyopathy (TTC), aka stress cardiomyopathy, is a reversible left ventricular dysfunction syndrome precipitated by multiple emotional and environmental triggers[1]. Patient with TTC presents with the clinical picture of an acute coronary syndrome (ACS) including chest pain, abnormal cardiac enzymes, and electrocardiography (EKG) following a distinct trigger or acute stress[2]. It is most commonly reported in postmenopausal women worldwide. TTC has been reported in around 2% of patients presenting with an initial diagnosis of ACS[3].
As per the Revised Mayo Clinic Criteria, TTC can be diagnosed in the presence of the following four criteria: (1) Transient left ventricular (LV) dyskinesis extending beyond a single coronary distribution, (2) Absence of acute plaque rupture or obstructive coronary artery disease, (3) New EKG abnormalities or significant troponin elevation, and (4) absence of myocarditis or pheochromocytoma[4]. Most of the time with conservative medical management recovery of LV function occurs within 21 d in up to 95% of patients[3].
While the exact mechanisms resulting in the precipitation of TTC are not fully understood, most often it has been reported following an episode of sudden physical and emotional stressors resulting in high-catecholamine states. Over the last two decades, multiple different physical stressors have been reported to precipitate TTC[3,5]. Although uncommon TTC has been reported in patients following an episode of acute envenomation[6,7]. In this narrative review, we aimed to describe the current literature on the pattern, mechanism, and outcomes of envenomation induced TTC.
We searched the PubMed and Medline databases for the terms “TTC” and “envenomation”. We expanded our search using the names of various agents of envenomation including “snake”, and “scorpion” and “wasp” and “bee” and “spider” and “jellyfish”. We also searched in the google scholar and Reference Citation Analysis database with similar terms. We screened the references of the initially identified manuscripts for additional manuscripts. To be included in the review articles had to have a distinct diagnosis of TTC and envenomation. Revised Mayo Clinical Criteria was used to screen for diagnosis of TTC. Envenomation had to be confirmed based on the toxidrome specific and unique to the agent. Although evidence of the absence of coronary artery disease based on cardiac catheterization was preferred, we did include reports using an alternating modality of imaging as well. All the studies published in English, including adults with an episode of recent envenomation and subsequent diagnosis of TTC were eligible to be included in this review. In this review, we included all studies published before June 2022. All the articles were independently screened by 2 different physicians. Articles lacking clinical details, opinions, comments, and letters were excluded from this review.
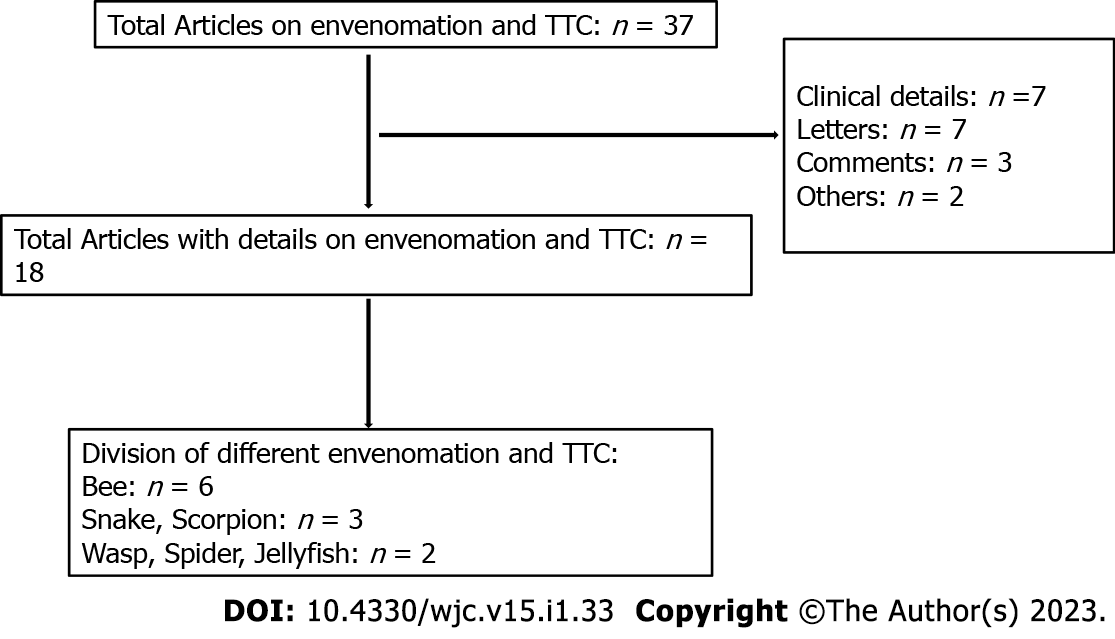
As shown in the strobe diagram a total of 37 articles were identified (Figure 1). Among these 18 articles were eligible to be included in the review. Six of these articles were on bee sting precipitating TTC, 3 articles each on snake envenomation and scorpion sting, and 2 articles each on wasp, spider, and jellyfish envenomation induced TTC. A total of 20 patients with envenomation induced TTC were reported from thirteen different countries around the world. Tables 1 and 2 describe the details of the presentation, pattern, management, and outcome in each of these envenomations. We have described the characteristics of each of these individual envenomation related TTC below.
| Ref. | Year of publication | Country | Age | Gender | Comorbidity | Clinical presentation |
| Bee | ||||||
| Mishra et al[8] | 2016 | USA | 63 | F | Unknown | Rash, diaphoresis, chest pain, dyspnea |
| Aono et al[9] | 2019 | Japan | 87 | F | Unknown | Multiple bee stings with multi-organ failure |
| Seecheran et al[10] | 2021 | Caribbean | 48 | F | None | Obtunded, somnolent |
| Winogradow et al[12] | 2011 | Germany | 37 | F | Unknown | Local itching, spreading hives |
| Winogradow et al[12] | 2011 | Germany | 70 | F | Unknown | Swelling of throat, chest pain |
| Ghanim et al[11] | 2015 | Israel | 37 | F | None | Rash, dyspnea, impaired consciousness |
| Wasp | ||||||
| Scheiba et al[13] | 2011 | Germany | 81 | M | Unknown | Absent pulses, gasping, unconsciousness |
| Geppert et al[14] | 2010 | Germany | 70 | F | Unknown | Chest pain and dyspnoea |
| Spider | ||||||
| Alexakis et al[16] | 2015 | Greece | 64 | F | Hyperthyroidism | Nausea, tremor, infraorbital edema, diffuse abdominal pain, lower extremity muscle cramps |
| Isbister et al[17] | 2015 | Australia | 33 | M | Unknown | Perioral paresthesia, widespread fasciculation, diaphoresis, hypersalivation, dyspnea |
| Isbister et al[17] | 2015 | Australia | 13 | F | Unknown | Localized intense pain, urinary incontinence, profuse sweating, and vomiting |
| Snake | ||||||
| Van Rensburg et al[18] | 2015 | South Africa | 58 | M | None | Bilateral ptosis |
| Murase et al[19] | 2012 | Japan | 56 | F | Unknown | Swelling of left foot |
| Delumgahawaththa et al[40] | 2021 | Sri Lanka | 66 | F | Hypertension, hyperlipidemia | Central chest pain |
| Scorpion | ||||||
| Abroug et al[29] | 2018 | Tunisia | 36 | F | Unknown | Pulmonary edema, shock |
| Abroug et al[29] | 2017 | Tunisia | 45 | F | Unknown | Altered level of consciousness, respiratory failure, shock |
| Miranda et al[23] | 2015 | Brazil | 7 | M | Unknown | Localized pain, vomiting, profuse sweating, dyspnea |
| Jain et al[27] | 2006 | India | 35 | M | Unknown | Mild local pain, irritation |
| Jellyfish | ||||||
| Bianchi et al[24] | 2011 | Italy | 53 | F | Unknown | Chest pain, fatigue, localized intense itch |
| Tiong et al[25] | 2009 | Australia | 26 | M | Unknown | Localized pain, restlessness, agitation, palpitations |
| Ref. | Agent | Mayo criteria | Type | Mechanism | Rx | Outcome |
| Mishra et al[8] | Bee | Apical hypokinesia, LVEF – 40 to 45%; Absence of CAD; ST elevation, T wave inversion Troponin elevation | Apical | -Cytotoxic storm; -Direct cardiotoxic effects of bee venom; -Anaphylactic reaction | -ACEI; -BB | Improved |
| Aono et al[9] | Bees (Multiple) | Apical hypokinesia; Absence of CAD; ST elevation in I, II, aVl, aVf, V2 to V6 and raised Troponin | Apical | -Multi organ failure; -Death by cardiac rupture | ||
| Seecheran et al[10] | African Bee | Apical hypokinesia; Absence of CAD; ST elevation in V1, V2, V3, raised Troponin | Apical | -Cytokine storm; -Anaphylaxis | -ARNI; -Eplerenone; -Bisoprolol; -Dapagliflozin | Improved |
| Winogradow et al[12] | Bee | Anterior, lateral, and Posterior hypokinesia; Absence of CAD; ST elevation in VI and raised Troponin | Reverse | - | -BB; -ACEI; -Aldosterone agonist | - |
| Winogradow et al[12] | Bee | Apical wall hypokinesia; Absence CAD; VFIB | Apical | -Hypersensitivity; -Epinephrine Injection | - | - |
| Ghanim et al[11] | Bees | Basal and Mid hypokinesia; Absence of CAD; ST elevation in lateral leads and depression in anterior and inferior leads and raised Troponin | Reverse | -Catecholamine storm; -Epinephrine injection | -IABP; -VAECMO; -Adrenaline; -Norepinephrine and Dopamine | Improved |
| Scheiba et al[13] | Wasp | Apical and anteroseptal hypokinesia, LVEF – 45%; Absence of CAD; ST elevation in I, aVL, V2-V4 and Troponin elevation | Apical | -Hypersensitivity; -Epinephrine Injection | -BB; -ACEI; -Aspirin | Improved |
| Geppert et al[14] | Wasp | Apical (anterior and inferior) and anteroseptal hypokinesia (46%); Absence of CAD; ST segment elevations in the leads, V1–V3, T-negatives in leads I, aVL and V2 –V4 and raised Troponin | Apical | -Epinephrine injection | -Bisoprolol; -Ramipril; -Aspirin | Improved |
| Alexakis et al[16] | Black widow spider | Hypokinesis of the basal and middle segments of the LV wall with apical sparing, LVEF – 36%; CAG not done; T wave flattening in aVL, and Troponin elevation | Reverse | -Alpha-ladrotoxin mediated catecholamine storm; -Cytokine storm | - BB; -ACEI; -Aspirin; -Clopidogrel | Improved |
| Isbister et al[17] | Funnel web spider | Diffuse global hypokinesia with LVEF of 20%; CAG not done; Diffuse ST changes and troponin elevation | - | -Toxin; -Antivenom; -Catecholamine storm | Improved | |
| Isbister et al[17] | Funnel web spider | Reduced EF- 45%; CAG not done; ST depression in the inferior and anterior leads and elevated troponin | - | -Toxin; -Antivenom; -Adrenaline | - | Improved |
| Van Rensburg et al[18] | Cape Cobra | Mid and apical LV segment hypo or akinetic, LVEF: 27%, Absent CAD; New ST elevation across anterolateral leads, subsequent EKG showing T wave inversions in anterolateral leads, raised troponin | Mid ventricular and apical; (Apical ballooning) | -Catecholamine storm; -Cytokine storm; -Catecholamine; release associated with Intensive Care Unit medication | Supportive care; Anti-snake venom administered for snake bite | Recovery of RWMA within a week (ECHO) |
| Murase et al[19] | Gloydius blomhoffii Snake (Mamushi) | Abnormal LV systolic function, apical akinesis, hyperdynamic basal segment of LV; No stenotic lesions noted on the CT scan; EKG changes T inversions in 1, a VL, V1-6, raised troponin | Apical | -Unknown | Supportive management; No Anti snake venom | Complete recovery (ECHO); (4 d) |
| Delumgahawaththa et al[40] | Unidentified (Possibly Russel Viper) | Hypokinetic mid and apex of LV segments, with ballooning, LVEF: 35%; Absence of CAD; EKG changes with ST elevation in 1 and a VL, T wave inversion II, III, a VF, V 2-6, Troponin elevation | Mid LV and Apical LV | -Catecholamine storm; -Direct myocardial toxicity; -Microvascular spasm | ACEI, BB, Diuretic, single antiplatelet, statin; Anti-snake venom | Partial recovery in 1 mo and complete in 5 mo (ECHO) |
| Abroug et al[29] | Androctonus australis | Patient 1: Basal ballooning of left and right ventricles; CAG not done; Troponin elevation; ST depression in four precordial leads; No other causes to explain the cardiac dysfunction. Patient 2: Impaired systolic function on echocardiogram. Basal ballooning on cardiac MRI in 24 hours; CAG not done; Troponin and ProBNP elevation; No other causes to explain the cardiac dysfunction | Basal ballooning in both patients | -Catecholamine storm; -Microvascular dysfunction | -CPAP, Dobutamine infusion; -No management mentioned | Patient 1: Discharged in 5 d, MRI in one month was normal. Patient 2: Discharged in 4 d with normal LV function on repeat echocardiography examination before discharge |
| Miranda et al[23] | Tityus serrulatus | Apical ballooning with hypokinesia; CAG not done; ST segment elevation in V1-V3, DI and aVL; No other causes to explain the cardiac dysfunction | Apical | -Alpha toxins act on voltage-gated sodium channels causing catecholamine storm; -Cytokine storm | -IV furosemide | Day 6 - asymptomatic, discharged; Cardiac MRI in 7 mo showed complete recovery of wall motion in apical region |
| Jain et al[27] | Mesobuthus tamulus | Apical, apicolateral hypokinesia; CAG not done; ST segment elevationin V1-V3 and T wave inversion; Absence of other causes of cardiac dysfunction | Apical | -Catecholamine storm; -Microvascular dysfunction | -Systemic steroids, inotropic drugs; | -Improved LVEF in 7 d; -Cerebellar infarction with clinical signs |
| Bianchi et al[24] | Pelagia noctiluca - A common Mediterranean jellyfish | Apical akinesis with severe left ventricular dysfunction; Absence of CAG; Acute myocardial infarction including persistent ST elevation despite thrombolysis performed for symptom relief and raised Troponin | Apical and mid-ventricular | -Direct toxicity; -Catecholamine storm; -Cytokine storm | -Symptomatic management | Improved. Completely symptom free, improved LVEF in 7 d |
| Tiong et al[25] | Carukia barnesi - A cubozoan/box jellyfish found in Australia | Mid-ventricular stress cardiomyopathy with apical sparing; No CAG; Persistent hyperacute T waves, rise in troponin | Mid-ventricular ballooning with apical sparing | -Sympathetic overdrive; -Catecholamine storm | -Supportive with high flow oxygen, morphine; -Magnesium sulfate IV | - Improved, discharged in 3 d |
On bee sting precipitating TTC the total number of articles identified from various databases was 10, among which 6 articles with complete patient details were eligible to be included in the study. A total of 6 patients were reported to have TTC following a bee sting[8-12] . All these patients fulfilled at least 3 of the 4 mayo criteria for TTC as shown in Table 2. The mean age of these patients was 57, and all of them were females. The most common pattern of TTC noted was an apical type (67%). The various mechanisms that were postulated to precipitate TTC episodes were an anaphylactic reaction, epinephrine injection, catecholamine storm, cytokine storm, and direct cardiotoxic effect of the venom. Treatment details were available in 4 out of the 6 patients. Beta-blockers (BB) were used in 3 patients; Angiotensin converting enzyme inhibitors (ACEI) were used in 2 patients. Good outcome was reported in 4 patients, but multiorgan failure and death by cardiac rupture was reported in 1 patient. Interestingly improvement in a patient with cardiogenic shock was reported following mechanical circulatory support and extracorporeal membrane oxygenation.
The total number of articles on wasp envenomation and TTC identified from various databases was 5, among which 2 articles were eligible to be included in the study. A total of 2 patients were reported to have TTC following wasp envenomation[12-15]. Both the patient’s fulfilled at least 3 of the 4 mayo criteria for TTC as shown in Table 2. The mean age of the patients was 75 and one of them was male. An apical pattern of TTC was noted in both patients. The mechanisms postulated to precipitate TTC episodes were an anaphylactic reaction and epinephrine injection secondary to the effect of the venom. Treatment details were available for both patients. BB and ACEI were used in both patients. No mortality was reported, and both patients had clinical improvement.
The total number of articles identified from various databases describing spider bite and TTC was 4, among which 2 articles had patient details and were eligible to be included in the study. A total of 3 patients were reported to have TTC following spider envenomation[16,17]. All these patients fulfilled at least 2 of the 4 mayo criteria for TTC as shown in Table 2. The mean age of the patients was 37 and two of them were female. Details of the pattern of TTC were not available in 2 of the 3 patients. The various mechanisms that were postulated to precipitate TTC episodes were a direct cardiotoxic effect of the venom, catecholamine storm, epinephrine, and anti-venom injection. Treatment details were not available for most of the patients. BB and ACEI were used in one patient. All three patients had improvement following treatment.
Snake envenomation precipitating TTC related articles identified from databases were 4, among which 3 articles were eligible to be included in the study. A total of 3 patients were reported to have TTC following snake bite and subsequent envenomation[18-20]. All patients fulfilled at least 3 of the 4 mayo clinic criteria for TTC as shown in Table 2. The mean age of the patients was 60 and two of them were female. An apical type of TTC was noted in all patients with additional involvement of the mid left ventricle in two. Catecholamine surge secondary to emotional stress because of snake bite was thought to be the underlying pathology of TTC. Only one patient was treated with guideline directed therapy for LV dysfunction with BB, ACEI, and a diuretic, while others received supportive care. All the patients recovered completely.
Scorpion sting related TTC articles that were identified from databases were 6. Three articles were eligible to be included in our study. A total of four patients were reported to have TTC following scorpion bite related envenomation[6,7,21-23]. All patients fulfilled at least 3 of the 4 mayo clinic criteria for TTC as shown in Table 2. The mean age of the patients was 31 and the gender distribution was equal. Apical type of TTC was noted in 2 patients and basal type of TTC in the other two patients. Abnormal response to catecholamines mediated through microvascular dysfunction, alpha toxins acting on sodium channels in the autonomic center leading to sympathetic excitation and release of catecholamines as well as a massive outpouring of catecholamines were suggested to be the precipitators of TTC. Among the four patients, the first patient was treated with continuous positive airway pressure and dobutamine infusion, the second patient with intravenous furosemide, and the third patient with systemic steroids along with inotropic drugs. Except for the utilization of diuretics, these 3 patients were not treated with guideline directed therapy for LV dysfunction. However, complete recovery was reported in all patients within seven days.
Regarding Jelly Fish envenomation precipitating TTC, three articles were identified from databases. Two articles with one patient each had TTC following a jellyfish envenomation[24,25]. These patients fulfilled at least 3 of the 4 mayo clinic criteria for TTC as shown in Table 2. The mean age of the patients was 40 and one of them was male. Mid ventricular ballooning was noticed in both patients, with one patient showing apical involvement as well. Precipitators of TTC in these patients were thought to be secondary to stress, venom, and excess endogenous catecholamine release. These 2 patients were not treated with any medications for guideline directed therapy for LV dysfunction. Both patients were treated symptomatically and completely recovered within a week.
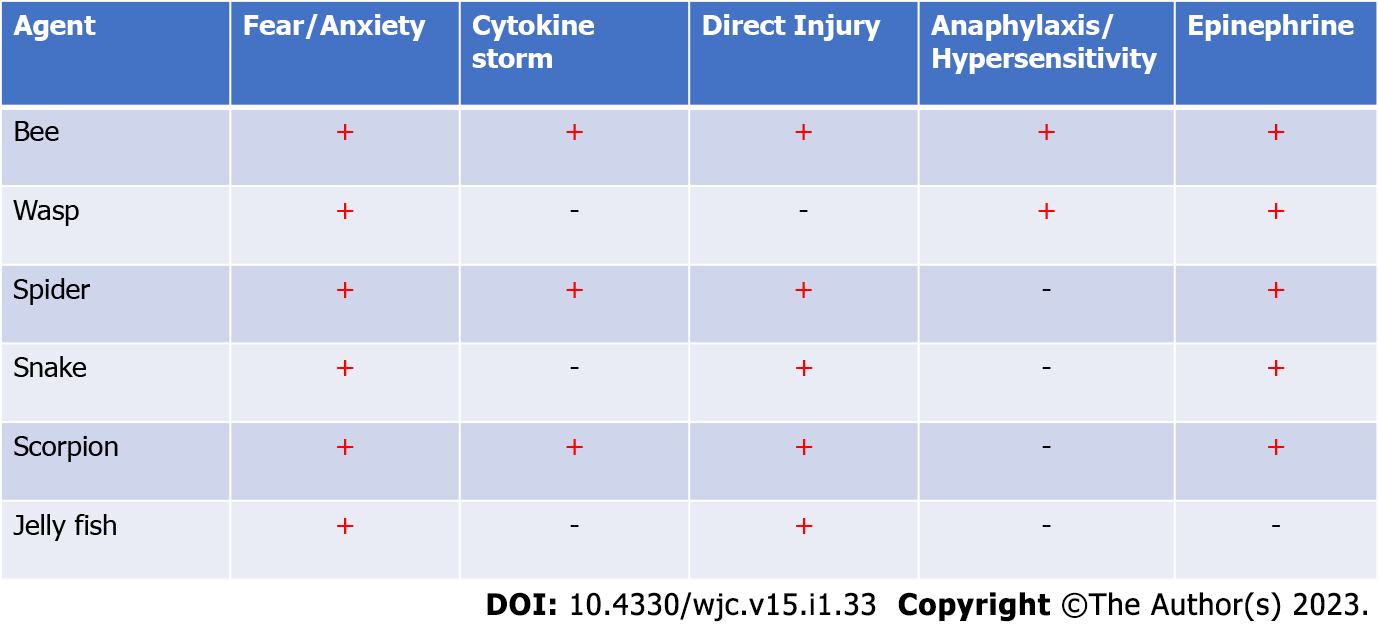
TTC, aka stress induced cardiomyopathy, transient apical ballooning syndrome, and broken heart syndrome is a reversible cardiomyopathy that is increasingly being diagnosed in patients presenting with ACS following a triggering event, precipitator. Multiple emotional, environmental, physical, and iatrogenic triggers have consistently been reported to precipitate such events[1-3,5,26]. While multiple stressors have been known to predispose TTC, in this review article, we highlight the multiple reported cases of TTC following envenomation which has not been reviewed before. In this descriptive review, we also discuss the various mechanisms of envenomation that have been postulated to precipitate TTC in the medical literature. Envenomation and stings have various cardiac complications including vasovagal episodes, myocarditis, ACS, and TTC. In this review, we have specifically focused on their implications in the presence of TTC. As mentioned in Figure 2 multiple mechanisms have been postulated to result in sympathetic activation which ultimately precipitates the transient LV dysfunction. Excessive sympathetic stimulation in these patients could be initiated by stress and subsequently worsen in the presence of another mechanism. Five distinct mechanisms have been consistently reported across the literature.
The sudden and accidental exposure to a sting or a bite that is perceived as threatening can result in peak stress. Acute emotional stress is known to increase regional cerebral blood flow in the hippocampus, brainstem, and basal ganglia and increase brain activation along with an increasing concentration of epinephrine and norepinephrine in the brain simultaneously. After an episode of acute stress, the interaction of the neocortex, limbic system, reticular activating system, brain stem, and spinal cord results in the interpretation of the stimulus as a threat. Subsequently, activation of the brainstem results in a neural stress response which results in the secretion of norepinephrine from locus coeruleus in the brain which then stimulates the hypothalamic-pituitary-adrenal axis. The sympathetic pathway through the posterior hypothalamus descends to the spinal cord and facilitates the release of norepinephrine. This increased catecholamine secretion from sympathetic nerve endings and secretion of epinephrine and norepinephrine from chromaffin cells in the adrenal medulla can subsequently affect the cardiac adrenergic receptors. Which subsequently contributes to the classical presentation of TTC. As shown in Figure 2, emotional stress was the precipitator of TTC across all the bites[18,27-29].
During an animal bite or an envenomation, venom is injected with the help of stinging apparatus into the dermal-epidermal junction, subcutaneous tissues, vessels, or muscle fibers. Venoms are chemically a diverse mixture of proteins, amines, and salts produced for predation or protection and can result in significant injury on injection to humans as well. Following the bite metalloproteinase, and L-Amino acid oxidase present in these venoms result in local dermal proteolysis, tissue destruction, and apoptosis, subsequently providing access to toxins to circulation, and target organ. Once within circulation, these toxins can alter the structure and function of endothelial cells. These agents are not only highly proinflammatory resulting in the secretion of excessive inflammatory markers they also precipitate dysregulation of neutrophils, mononuclear phagocytic system, and granulocytes thus accelerating inflammation and cell damage. Dysregulation and excessive stimulation of these cytokines, and pro inflammatory mediators result in cytokine storm causing multiorgan dysfunction. Multiple infections [including Novel coronavirus disease 19 (COVID-19)], toxins, cancers, and diseases are known to cause TTC due to cytokine storms precipitated by endothelial dysfunction[18,30,31].
Emotional stress like pheochromocytoma is associated with an excessive outburst of catecholamine. In patients with TTC, catecholamine levels can be extremely high as compared to other cardiac diseases. Similarly, patients with TTC have been shown to have higher ventricular catecholamine sensitivity. Alpha ladrotoxin in spider venom and alpha toxin in scorpion venom has been reported to precipitate catecholamine storm. Subsequently, excessive norepinephrine released from cardiac sympathetic nerve terminals can result in a reduction of cardiac myocyte viability. Transient elevations of catecholamines in TTC result in localized focal mononuclear inflammatory cells, fibrosis, and contractions bands in cardiac myocytes. This is evident with elevated cardiac enzymes and localized areas of edema, without late enhancement in magnetic resonance imaging. Direct catecholamine injury was also commonly reported to be the precipitate TTC following all the envenomations[28,31-33].
Anaphylaxis secondary to bites and envenomation is immunoglobulin E (IgE) mediated. In patients with prior sensitization, systemic reactions can occur in up to 9% of patients. Major allergens like Antigen 5, phospholipase A1, phospholipase A2, hyaluronidase, and acid phosphatase can precipitate classic Ig E mediated anaphylactic response. Subsequently, mast cells and basophils degranulate releasing proinflammatory markers including histamine and proteases. Which results in the activation, influx, and migration of neutrophils and lymphocytes and the secretion of chemokines and cytokines. Anaphylaxis induced myocardial injury can be diverse. In a study including 300 patients with anaphylaxis myocardial injury was reported in 7.3% of patients. The etiologies of myocardial injury were anaphylactic cardiomyopathy, TTC, and Kounis syndrome. The causes of excessive catecholamine release precipitating TTC in anaphylaxis are multifactorial. Histamine can stimulate the adrenal medulla directly to produce catecholamine, compensatory catecholamine release due to activation of the renin-angiotensin-aldosterone axis, and external administration of adrenaline for anaphylaxis and norepinephrine for persistent shock. This form of TTC was only reported following bee and wasp stings[3,28,31,33].
In normal patients beta1-adrenergic receptor (B1AR) and B2AR receptors density are highest in the apex, thus making it susceptible to exposure to higher levels of catecholamine. Following exposure to supratherapeutic doses of catecholamine signal trafficking of catecholamine receptors occurs, which is a form of protective response of cardiac myocytes to stress. This switching of Gs to Gi coupling of B2AR receptors results in reduced inotropy and apoptosis of cardiac myocytes. This supratherapeutic level of catecholamine surge is classical of pheochromocytoma thus requiring evaluation of the same and has been incorporated into the Mayo clinic criteria for diagnosis of TTC. Exogenous catecholamines have been consistently reported to precipitate TTC. TTC has been reported following the administration of epinephrine to patients subcutaneously, intravenously, and through inhalation. Iatrogenic large volume adrenaline injection has also been reported to precipitate TTC. As shown in Image 2 exogenous catecholamine injection was the second most reported mechanism of TTC after emotional stress in these patients[11-13,28,31,34-37].
Multiple other mechanisms including microvascular dysfunction and spasm, abnormal myocardial metabolism and intracellular lipid accumulation, myocardial inflammation, estrogen excess, and endothelial dysfunction have been postulated[28,31,38]. In patients with envenomation induced TTC, multiple mechanisms can initiate and precipitate cardiac dysfunction.
In this narrative review, we summarized the clinical features following envenomation induced TTC. Most insect stings and reptile bites are accidental. Following these bites and stings, clinical manifestations can vary from an asymptomatic state and mild local reactions to severe multiorgan dysfunction based on the dose and the characteristics of the venom. TTC can occur irrespective of prior cardiac comorbidities and in absence of traditional cardiac risk factors. To summarize the clinical features of these envenomations with TTC we combined the episodes of TTC following all the envenomation. To date envenomation resulting in TTC has been described in the above-mentioned animals. Almost all these patients presented with a clinical picture of ACS (95%) and had EKG changes and elevated troponin (100% each). Sixty percent of these patients had ST segment elevation (n = 12), and 15% of them had ST depression and T wave inversion simultaneously (n = 3). Among patients with echocardiographic evidence of TTC (n = 18), Apical TTC was found in 72% of patients (n = 13), reverse TTC in 28% (n = 5), and midventricular TTC was reported in 17% (n = 3) of patients. Details of Coronary angiogram (CAG) were mentioned in 85% of patients (n = 17) and all the CAG were negative for any acute obstructive disease.
Conservative medical management was initially done on all the patients. Management was mostly physician directed and clinical feature driven. Medications for heart failure management were given to 35% (n = 7) of patients. These 7 patients were treated with ACEI, and BB together. Ionotropic agents were used in 15% (n = 3) and aldosterone agonist was used in 10% (n = 2) of patients. Details of clinical outcomes were available for 18 patients among whom 17 (94%) had clinical improvement. Improvement in LV ejection fraction (LVEF) and regional wall motion abnormality was reported in all patients with clinical improvement. Death occurred in one patient following multi organ dysfunction and cardiac rupture after multiple bee stings. As per the author’s knowledge, this is the first review summarizing the various mechanisms, clinical features, management, and outcome following envenomation’s precipitating TTC[34,35,39].
This review has a few limitations. All patients with TTC secondary to envenomation were included from case studies spanning more than a decade. Most studies did not rule out pheochromocytoma. Most of these patients did not have baseline echocardiography before admission. Studies also lacked details of CAG at presentation and echocardiographic details during follow up. Even though multiple mechanisms of TTC were considered, case studies did not report levels of inflammatory markers, catecholamine levels, IgE levels, Eosinophil counts, or advanced cardiac imaging. Most of these reports did not mention the different components of the venom which were instrumental in the precipitation of TTC[39,40]. Classification of the severity of these toxidromes, varied treatment protocols and non-availability of recurrence, functional status at discharge, and long term follow up data continue to be a challenge[3,4,26,33,33,39-41]. However, this review has the strength of describing the patterns and mechanisms of envenomation related TTC and their outcome. We also recommend that future case studies should focus on using a uniform diagnostic criterion for diagnosing and reporting TTC from episodes of stings or envenomation. Future studies should identify specific components in the venom precipitating catecholamine storm, microvascular injury, and myocardial damage[42-44]. Uniform echocardiography at diagnosis and follow up should be done consistently. All available details including imaging modalities and pattern of cardiac involvement should be consistently reported[45]. Given the rarity of these events, randomized trials on these specific etiologies might be difficult, interim optimal medical management during the envenomation and goal directed therapy for heart failure should be considered in patients who have evidence of LV dysfunction with low ejection fraction, unless complete recovery of clinical symptoms, signs, and LVEF is documented at the time of discharge from hospital. Guideline directed heart failure medications including BB and ACEI can be administered and consistent reporting of these subgroups of patients should be encouraged.
In conclusion, multiple insect stings and reptile bites related envenomation can precipitate TTC. Most envenomation related TTC has been due to bee stings and scorpion bites. Fear and anxiety related to the sting, direct catecholamine toxicity, and administration of exogenous beta-adrenergic agents have been commonly postulated to precipitate TTC in these patients. Most of these patients present with clinical presentation of ACS, ST elevation, and elevated troponin. Most of these patients also fulfill at least 3 out of 4 criteria of Mayo clinic criteria for TTC. The most common type of TTC in these patients is Apical, which improves following medical management. One third of these patients were treated with ACEI and BB with reported improvement in outcome.
Multiple different physical, emotional and iatrogenic stressors have been known to precipitate stress induced cardiomyopathy also called takotsubo cardiomyopathy (TTC).
Rarely TTC has been reported in patients following an episode of acute envenomation secondary to insect stings and reptile bites.
This review aimed to narrate the current literature on the patterns, mechanisms, and outcomes of envenomation induced TTC.
This review included all studies on “TTC” and “envenomation “published in PubMed, Google Scholar, and Reference Citation Analysis databases before June 2022. Articles had to have a distinct diagnosis of TTC with Revised Mayo Clinical Criteria and documented envenomation.
Envenomation related TTC was reported from all over the world. The reports of envenomation induced TTC were reported mostly following a bee sting, scorpion sting, and snake envenomation. Fear and anxiety related to the sting, direct catecholamine toxicity, and administration of exogenous beta-adrenergic agents were postulated to precipitate TTC in these patients. Most patients presented with a clinical picture of acute coronary syndrome (ACS), ST elevation, and elevated troponin. Echocardiographic evidence of Apical TTC was noted in most of the patients. Almost all of these patients had clinical improvement following optimal management.
Envenomation following multiple insect stings and reptile bites can precipitate TTC. Most reported envenomation related TTC has been due to bee and scorpion stings. Common mechanisms causing TTC following envenomation are fear, anxiety, and stress. Most of these patients present with clinical presentation of ACS. The most common type of TTC in these patients is Apical, which improved following medical management.
Multiple envenomations following insect stings and reptile bites can cause TTC. Worldwide bee stings, wasp stings, scorpion stings, snake bites, spider bites, and jellyfish stings are the commonly reported precipitators of this cardiomyopathy. Multiple mechanisms have been postulated to cause this of which fear, anxiety, and stress of envenomation are the predominant ones. Patients usually present with a clinical presentation of ACS. Echocardiography commonly shows an apical pattern of cardiomyopathy. This cardiomyopathy improves with medical management.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Teragawa H, Japan; Wang Y, China S-Editor: Liu GL L-Editor: A P-Editor: Liu GL
| 1. | Prasad A, Lerman A, Rihal CS. Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): a mimic of acute myocardial infarction. Am Heart J. 2008;155:408-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1193] [Cited by in RCA: 1297] [Article Influence: 76.3] [Reference Citation Analysis (0)] |
| 2. | John K, Lal A, Mishra A. A review of the presentation and outcome of takotsubo cardiomyopathy in COVID-19. Monaldi Arch Chest Dis. 2021;91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | Murakami T, Komiyama T, Kobayashi H, Ikari Y. Gender Differences in Takotsubo Syndrome. Biology (Basel). 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 4. | Sahu KK, Mishra AK, Lal A. Newer Insights Into Takotsubo Cardiomyopathy. Am J Med. 2020;133:e318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | George AA, John KJ, Selvaraj V, Mishra AK. Endocrinological abnormalities and Takotsubo cardiomyopathy. Monaldi Arch Chest Dis. 2021;91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Akashi YJ, Goldstein DS, Barbaro G, Ueyama T. Takotsubo cardiomyopathy: a new form of acute, reversible heart failure. Circulation. 2008;118:2754-2762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 662] [Cited by in RCA: 590] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 7. | Ben Jemaa A, Bahloul M, Kallel H, Turki O, Dlela M, Bouaziz M. [Inverted Takotsubo Syndrome due to Severe Scorpion Envenomation: Report of one Case]. Med Trop Sante Int. 2021;1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 8. | Mishra S, Mishra A, Mishra JP. Bee sting: A rare cause of Takotsubo Cardiomyopathy. Int J Cardiol. 2016;223:787-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Aono J, Saito M, Inaba S, Kurata A, Uetani T, Annen S, Higashi H, Higaki J, Ikeda S. Multiple Bee Sting-Induced Life-Threatening Takotsubo Cardiomyopathy. Circ J. 2019;83:489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Seecheran RV, Ramdin R, Singh S, Seecheran V, Persad S, Peram L, Raza SS, Seecheran NA. Africanized Honey Bee Sting-Induced Stress-Related Cardiomyopathy: A Bee or Octopus Trap. Cureus. 2021;13:e16681. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 11. | Ghanim D, Adler Z, Qarawani D, Kusniec F, Amir O, Carasso S. Takotsubo cardiomyopathy caused by epinephrine-treated bee sting anaphylaxis: a case report. J Med Case Rep. 2015;9:247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Winogradow J, Geppert G, Reinhard W, Resch M, Radke PW, Hengstenberg C. Tako-tsubo cardiomyopathy after administration of intravenous epinephrine during an anaphylactic reaction. Int J Cardiol. 2011;147:309-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Scheiba N, Viedt-Suelmann C, Schäkel K. Tako-tsubo cardiomyopathy after a hymenoptera sting and treatment with catecholamines. Acta Derm Venereol. 2011;91:593-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Geppert G, Radke PW, Kurowski V, Hunold P, Schunkert H. [Wasp sting, adrenaline injection and acute thoracic pain: an un usual case of stress-induced (tako-tsubo) cardiomyopathy]. Med Klin (Munich). 2010;105:246-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Hausen S, Treusch A, Hermes C, Boekstegers P. [Wasp-sting-induced pheochromozytoma crisis with stress-related cardiomyopathy (Takotsubo)]. Med Klin Intensivmed Notfmed. 2014;109:621-624. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 16. | Alexakis LC, Arapi S, Stefanou I, Gargalianos P, Astriti M. Transient reverse takotsubo cardiomyopathy following a spider bite in Greece: a case report. Medicine (Baltimore). 2015;94:e457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Isbister GK, Sellors KV, Beckmann U, Chiew AL, Downes MA, Berling I. Catecholamine-induced cardiomyopathy resulting from life-threatening funnel-web spider envenoming. Med J Aust. 2015;203:302-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Van Rensburg A, Kyriakakis C. A tale of a cobra and an octopus: Takotsubo cardiomyopathy following a snake bite. Am J Med. 2015;128:e5-e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Murase K, Takagi K. Takotsubo cardiomyopathy in a snake bite victim: a case report. Pan Afr Med J. 2012;13:51. [PubMed] |
| 20. | Mishra AK, Dai Q, Sahu KK, ElMeligy A. Atypical Takotsubo Cardiomyopathy in COVID-19. Am J Med Sci. 2021;362:e41-e42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Miranda CH, Braggion-Santos MF, Schmidt A, Pazin Filho A, Cupo P. Evolution of the electrocardiogram QRS complexes voltage in scorpion envenomation-induced Takotsubo syndrome. Am J Emerg Med. 2015;33:837-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Madias JE. Voltage attenuation of the electrocardiogram QRS complexes in a patient with "scorpion envenomation"-induced Takotsubo syndrome. Am J Emerg Med. 2015;33:838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 23. | Miranda CH, Braggion-Santos MF, Schmidt A, Pazin-Filho A, Cupo P. The first description of cardiac magnetic resonance findings in a severe scorpion envenomation: Is it a stress-induced (Takotsubo) cardiomyopathy like? Am J Emerg Med. 2015;33:862.e5-862.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Bianchi R, Torella D, Spaccarotella C, Mongiardo A, Indolfi C. Mediterranean jellyfish sting-induced Tako-Tsubo cardiomyopathy. Eur Heart J. 2011;32:18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Tiong K. Irukandji syndrome, catecholamines, and mid-ventricular stress cardiomyopathy. Eur J Echocardiogr. 2009;10:334-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | John Binu A, Kumar Mishra A, Gunasekaran K, Iyadurai R. Cardiovascular manifestations and patient outcomes following snake envenomation: a pilot study. Trop Doct. 2019;49:10-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Jain MK, Indurkar M, Kastwar V, Malviya S. Myocarditis and multiple cerebral and cerebellar infarction following scorpion sting. J Assoc Physicians India. 2006;54:491-492. [PubMed] |
| 28. | Pelliccia F, Kaski JC, Crea F, Camici PG. Pathophysiology of Takotsubo Syndrome. Circulation. 2017;135:2426-2441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 471] [Article Influence: 58.9] [Reference Citation Analysis (0)] |
| 29. | Abroug F, Ouanes I, Maatouk M, Golli M, Ouanes-Besbes L. Inverted Takotsubo syndrome in Androctonus australis scorpion envenomation. Clin Toxicol (Phila). 2018;56:381-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 30. | George AA, Mishra AK, Sargent J. Letter to the Editor Regarding "Pipeline Embolization in Patients with Posterior Circulation Subarachnoid Hemorrhages: Is Takotsubo Cardiomyopathy a Limiting Factor? World Neurosurg. 2020;144:303-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 31. | Lyon AR, Citro R, Schneider B, Morel O, Ghadri JR, Templin C, Omerovic E. Pathophysiology of Takotsubo Syndrome: JACC State-of-the-Art Review. J Am Coll Cardiol. 2021;77:902-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 190] [Article Influence: 47.5] [Reference Citation Analysis (0)] |
| 32. | de Pender AM, Winkel KD, Ligthelm RJ. A probable case of Irukandji syndrome in Thailand. J Travel Med. 2006;13:240-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 33. | Ryan RYM, Seymour J, Loukas A, Lopez JA, Ikonomopoulou MP, Miles JJ. Immunological Responses to Envenomation. Front Immunol. 2021;12:661082. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 34. | Surineni K, Afzal MR, Barua R, Parashara D. Epinephrine-Induced Takotsubo Cardiomyopathy. Fed Pract. 2016;33:28-30. [PubMed] |
| 35. | Cha YS, Kim H, Bang MH, Kim OH, Kim HI, Cha K, Lee KH, Hwang SO. Evaluation of myocardial injury through serum troponin I and echocardiography in anaphylaxis. Am J Emerg Med. 2016;34:140-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 36. | Spina R, Song N, Kathir K, Muller DWM, Baron D. Takotsubo cardiomyopathy following unintentionally large subcutaneous adrenaline injection: a case report. Eur Heart J Case Rep. 2018;2:yty043. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 37. | Mendoza I, Novaro GM. Repeat recurrence of takotsubo cardiomyopathy related to inhaled beta-2-adrenoceptor agonists. World J Cardiol. 2012;4:211-213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 38. | Li P, Li C, Mishra AK, Cai P, Lu X, Sherif AA, Jin L, Wang B. Impact of malnutrition on in-hospital outcomes in takotsubo cardiomyopathy. Nutrition. 2022;93:111495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 39. | Mishra A, Binu A, Abraham G, Vanjare H, George T, Iyadurai R. Cerebrovascular Injury Following Scorpion Sting and Snake Envenomation: A Case Series. Can J Neurol Sci. 2018;45:669-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 40. | Delumgahawaththa WMS, Roshanthan S, Sathananthan PP, Seneviratne NHG. Chest Pain Following an Unidentified Snake Bite. Sri Lankan Journal of Cardiology. 2021;4:52-57. |
| 41. | Sahu KK, Mishra AK, Doshi A, Martin KB. Heartbroken twice: a case of recurrent Takatsubo cardiomyopathy. BMJ Case Rep. 2020;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 42. | Boyd B, Solh T. Takotsubo cardiomyopathy: Review of broken heart syndrome. JAAPA. 2020;33:24-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 43. | George AA, John KJ, Jha A, Mishra AK. Infections precipitating Takotsubo cardiomyopathy, an uncommon complication of a common infection. Monaldi Arch Chest Dis. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 44. | Sunil KK, Joseph JK, Joseph S, Varghese AM, Jose MP. Cardiac Involvement in Vasculotoxic and Neurotoxic Snakebite - A not so Uncommon Complication. J Assoc Physicians India. 2020;68:39-41. [PubMed] |
| 45. | Nayar J, John K, Philip A, George L, George A, Lal A, Mishra A. A Review of Nuclear Imaging in Takotsubo Cardiomyopathy. Life (Basel). 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |