Published online Sep 26, 2022. doi: 10.4330/wjc.v14.i9.496
Peer-review started: May 2, 2022
First decision: May 31, 2022
Revised: July 6, 2022
Accepted: August 26, 2022
Article in press: Auguest 26, 2022
Published online: September 26, 2022
Processing time: 140 Days and 16.4 Hours
Coronary calcium poses a challenge for the interventional cardiologist often leading to stent under-expansion and subsequent ischemic events. Aggressive balloon post-dilatation though helpful is usually inadequate. Multiple plaque ablation techniques are in vogue, but they are technically demanding and are not without complications. Shockwave intravascular lithotripsy (S-IVL) has emerged as a user-friendly and effective mechanism for calcium management with a high safety margin. A series of trials (DISRUPT CAD I-IV) have demonstrated both short-term and long-term safety and efficacy of the technique. As experience with the technique grows more and more, therapy areas like stent restenosis are being covered by the S-IVL.
We report a series of 2 cases successfully managed with S-IVL therapy at our center. The first case is of a 57-year-old smoker who presented with acute coro
S-IVL is an emerging, efficient, user-friendly and safe therapy for managing intracoronary calcium in routine interventional practice.
Core Tip: The presence of severe calcification in coronary arteries poses a challenge for the interventional cardiologist. If inadequately addressed, it leads to inadequate stent expansion, difficulty in delivery of stent/balloon, balloon rupture, stent dislodgement, stent thrombosis and even perforation. Intravascular lithotripsy is a novel technique utilizing ultrasonic waves to disrupt the calcium in the vessel wall while retaining them in situ. With the accumulation of data and growing expertise of operators with intravascular lithotripsy, it is turning out to be an indispensable tool in catheterization laboratory. We present 2 cases in which intravascular lithotripsy was successfully utilized.
- Citation: Pradhan A, Vishwakarma P, Bhandari M, Sethi R. Intravascular lithotripsy for coronary calcium: A case report and review of the literature. World J Cardiol 2022; 14(9): 496-507
- URL: https://www.wjgnet.com/1949-8462/full/v14/i9/496.htm
- DOI: https://dx.doi.org/10.4330/wjc.v14.i9.496
Calcified coronary artery lesions continue to be one of the pivotal challenges faced by interventional cardiologists. Stent under-expansion due to calcified lesions is a strong predictor of procedural failure and may lead to various acute complications, such as stent thrombosis and long-term stent restenosis[1]. Although aggressive balloon dilatation can sometimes achieve adequate room for stent deployment, the degree of final luminal gain is often limited. Furthermore, in eccentric lesions, high-pressure balloon dilatation can lead to overstretching of the noncalcified wall with minimal impact on the calcified lesion, which exacerbates the risk for coronary dissection and perforation[2].
Shockwave intravascular lithotripsy (S-IVL) or intravascular lithotripsy (IVL; Shockwave Medical Inc., Santa Clara, CA, United States) has recently been approved by the United States Food and Drug Administration for plaque modification in calcified coronary lesions. Contrary to rotational athe
Case 1: Our patient was a 57-year-old man who smoked and was admitted with a diagnosis of acute coronary syndrome.
Case 2: A 58-year-old woman with chronic stable angina (CCS Class III) presented to a peripheral hospital where her coronary angiogram revealed triple vessel disease.
Case 1: He presented with intermittent retrosternal chest pain at rest with radiation to arm for the past 7 d.
Case 2: She had effort angina despite medical therapy for past 4 mo.
Cases 1 and 2: There was no past history of any specific illness.
Case 1: He was a chronic smoker, and there was no family history of coronary artery disease (CAD).
Case 2: She was a nonsmoker, non-diabetic and had no family history of CAD.
Case 1: He was hemodynamically stable at admission with blood pressure of 134/70 mmHg and pulse rate of 80/min.
Case 2: She was hemodynamically stable at admission with blood pressure of 124/78 mmHg and pulse rate of 68/min.
Case 1: A 12 lead electrocardiogram was normal except for a q wave and t wave inversion in lead III. His routine biochemistry and hemogram were within normal limits. His cardiac troponin value was 0.016 ng/mL (Normal < 0.014 ng/mL).
Case 2: A 12 lead electrocardiogram was unremarkable as was her routine biochemical and hematological profile.
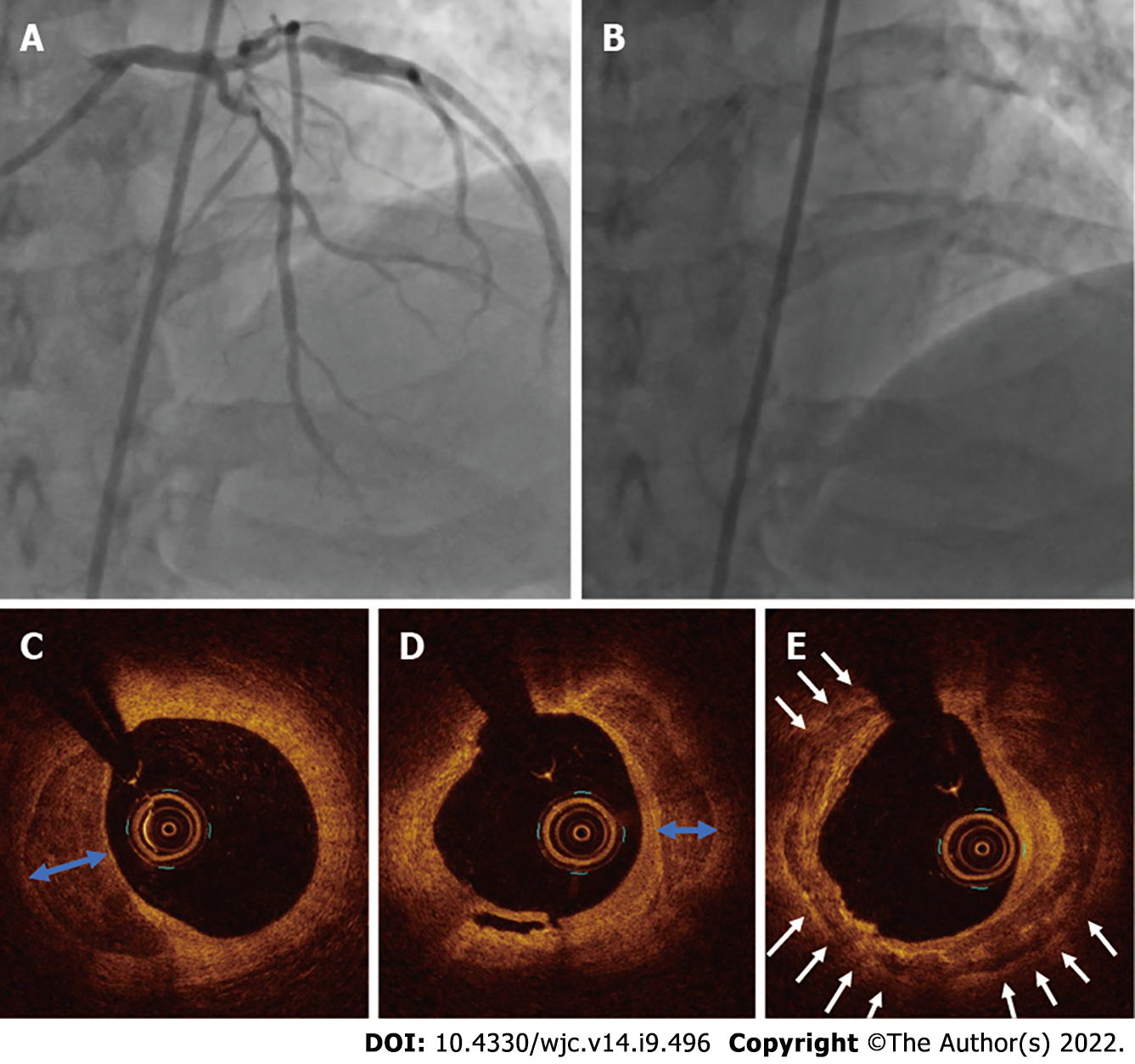
Case 1: He exhibited normal left ventricular functions without any regional wall motion abnormality on echocardiography. Coronary angiography was performed with an intention to revascularize, which revealed calcific 90% stenosis in the proximal left anterior descending (LAD) coronary artery, 70%-80% stenosis in the major obtuse marginal artery and 80% stenosis in the distal left circumflex coronary artery (Figure 1A and B).
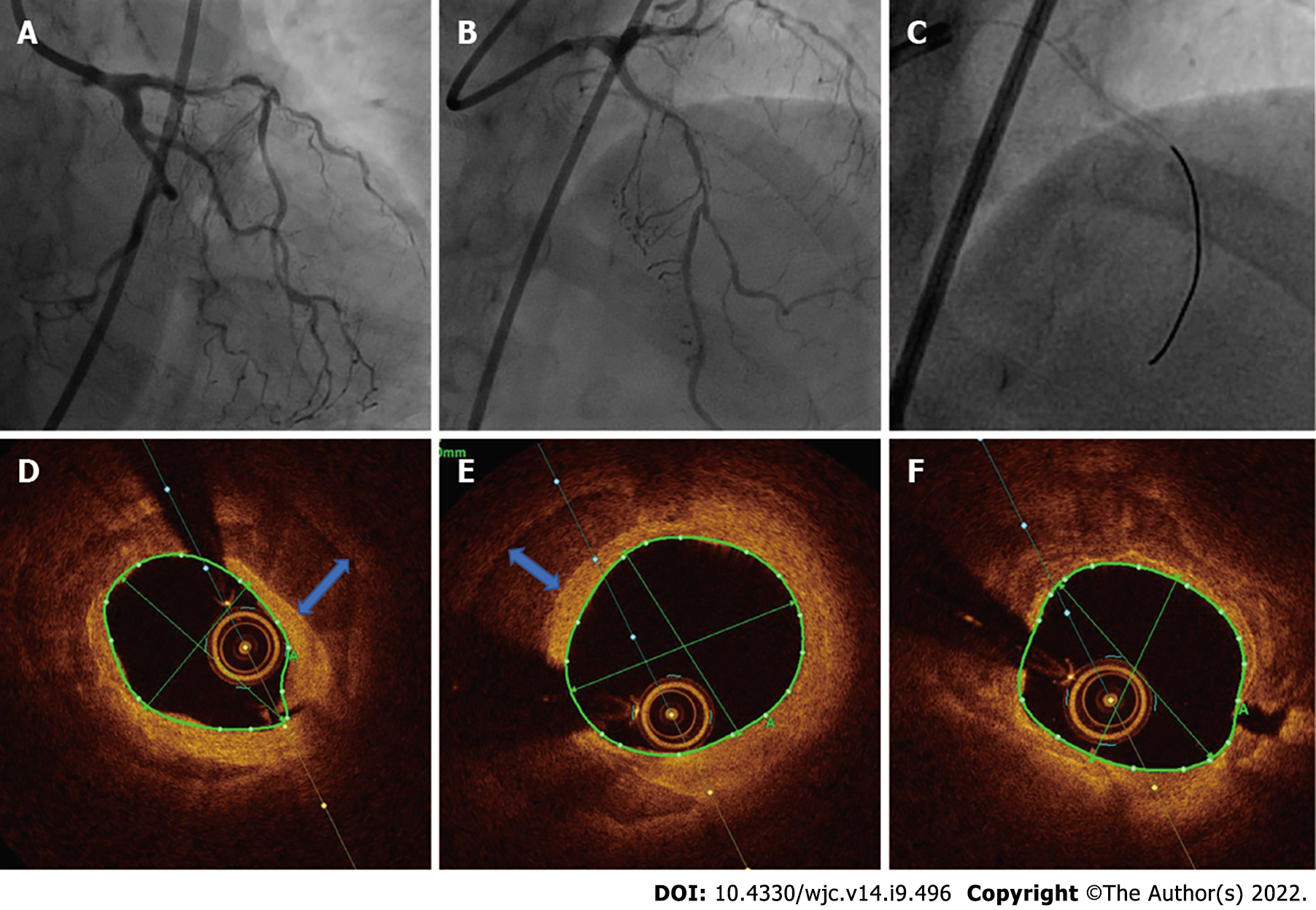
Case 2: The LAD showed severe calcified 90% stenosis in the proximal-mid part, whereas the left circumflex coronary artery exhibited 30%-50% disease in the distal part. There was mild disease in the left main coronary artery, whereas the non-dominant right coronary artery had severe stenosis. The patient was referred to our center for revascularization (Figure 2A-C). Her left ventricular ejection fraction was 62%.
Case 2: After the meeting of the cardiac team, she was given the options of coronary artery bypass graft surgery and percutaneous coronary intervention (PCI) to the LAD, and she opted for the latter.
Based on clinical, biochemical and angiographic features, a diagnosis of acute coronary syndrome-unstable angina with calcified double vessel disease was made in the first case. In the second case, chronic stable angina with calcified triple vessel disease was made.
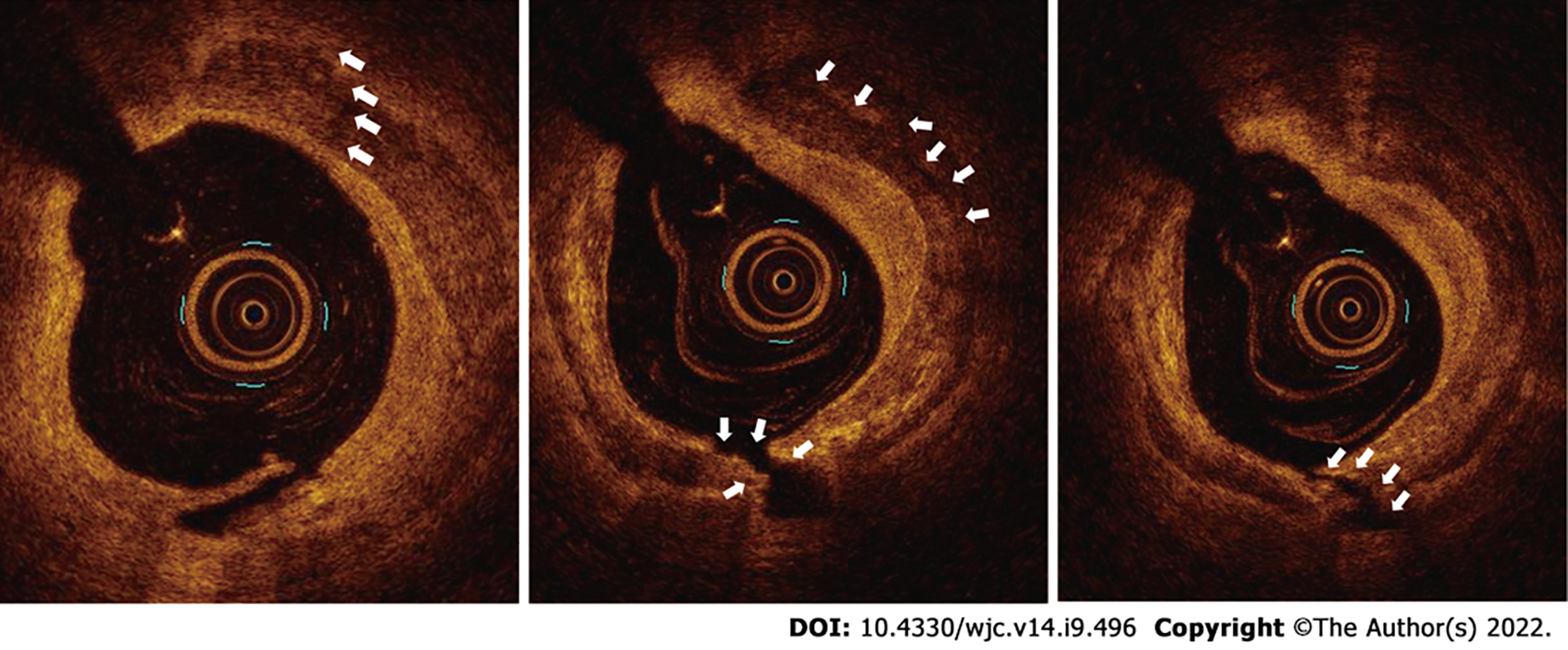
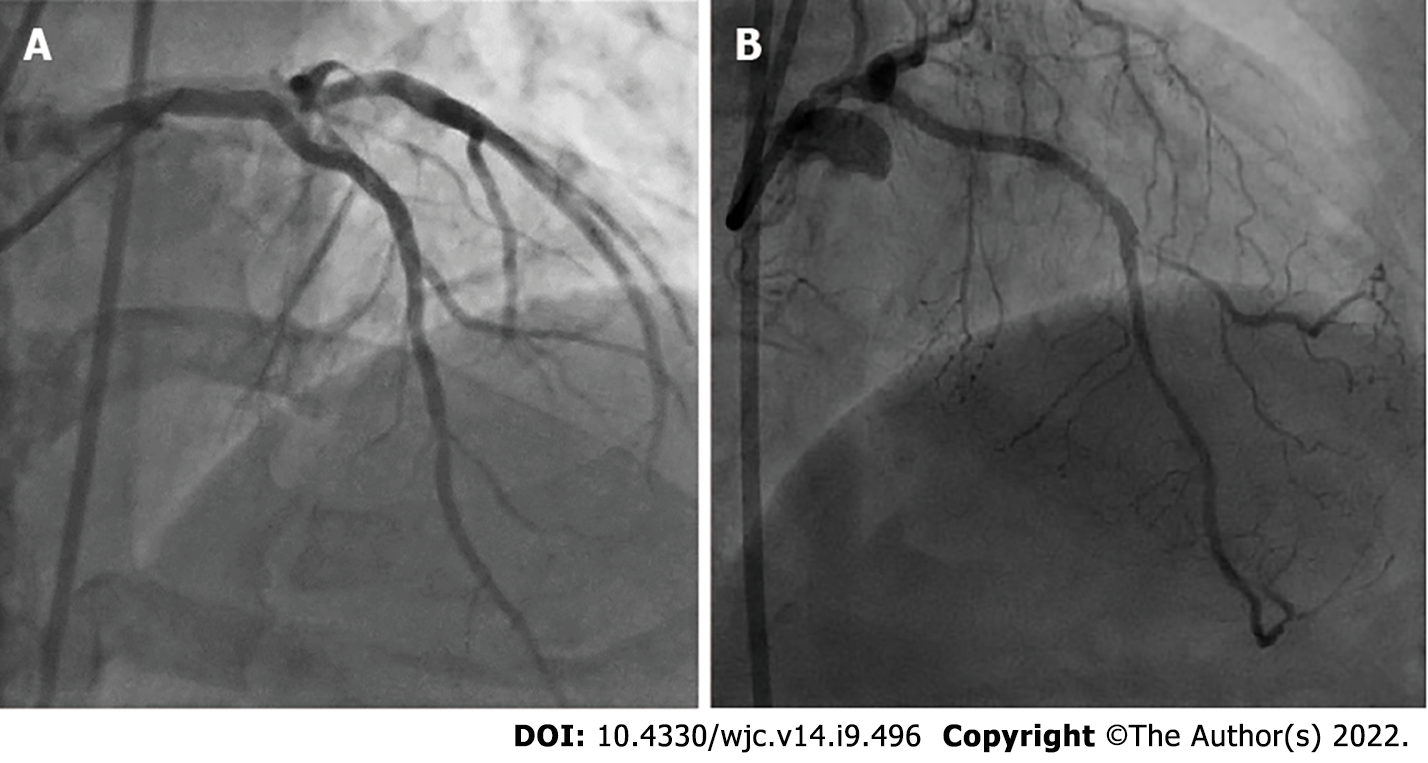
Case 1: The lesion in the proximal LAD was predilated using a 2.5 mm × 10 mm semicompliant balloon. Subsequently, OCT was performed to evaluate the degree of calcium, which revealed both superficial and deep circumferential calcium (Figure 1C-E). An IVL balloon catheter (C2IVL from Shockwave Medical Inc., Santa Clara, CA) measuring 3 mm × 12 mm was then placed across the lesion and dilated at 4 atmospheric pressure (atm). Ten pulses of shock wave were then delivered followed by IVL balloon dilatation at 6 atm. The cycle was repeated twice, which resulted in calcium fractures as seen in OCT performed after IVL (Figure 3). Next, a 3.0 mm × 40 mm sirolimus-eluting stent was deployed at 10 atm. The lesion was then post dilated with 3.5 mm × 12 mm noncompliant balloon. The final angiographic run revealed thrombolysis in myocardial infarction (TIMI) 3 flow and well expanded without any residual dissection (Figure 4A). A corresponding OCT run revealed a well-expanded and opposed stent, with a minimum stent area of 6.2 cm2.
Case 2: Because of severe calcification of the LAD, imaging-assisted PCI was planned. The lesion in the proximal LAD was predilated using a 2.5 mm × 12 mm noncompliant balloon to allow the passage of the OCT catheter, which revealed both superficial and deep circumferential calcium (Figure 2D-F). In view of the deep calcium, we selected S-IVL as the plaque modification strategy. An IVL balloon (C2IVL from Shockwave Medical Inc., Santa Clara, CA) measuring 2.5 mm × 12 mm was then placed across the distal lesion and dilated at 4 atm. Subsequently, 10 pulses of shock waves were delivered, followed by dilatation at 6 atm. The cycle was repeated twice in the proximal lesions too. Subsequently, two overlapping sirolimus drug-eluting stents, 2.5 mm × 30 mm (distal) followed by 3 mm × 21 mm (proximal), were deployed at 10-12 atm. The lesions were then post dilated with a noncompliant balloon (sequentially 2.5 mm and 2.75 mm and subsequently 3.0 mm). Unfortunately, the OCT catheter could not be maneuvered into the LAD for final imaging as the patient became transiently unstable after post dilatation because of the slow-flow/no-reflow phenomenon. However, following intracoronary pharmacotherapy, the final angiography revealed TIMI 3 flow without residual dissection or stenosis (Figure 4B).
Both patients were doing well at the 30-d and 3-mo follow-up without any symptoms.
Coronary calcification is a part of the aging process and is exacerbated by concomitant cardiovascular risk factors and comorbidities[4,5]. Approximately 20% of the coronary interventions are complicated by severe calcific lesions, which are independent predictors of procedural failure and adverse cardiac events in the future[6,7]. Moreover, calcific lesions heighten the risk for procedural complications and increase the procedural time. Characteristics of calcific lesions, viz. location inside the coronary arteries (superficial vs deep) and calcium burden (thickness of the calcified plaque, arc angle subtended, and longitudinal extension), are the factors that determine plaque compliance, stent delivery, adequate stent expansion and finally procedural success and long-term outcomes[8].
Several techniques are available for plaque modification in severely calcified coronary lesions, including high pressure noncompliant balloons, scoring balloons, cutting balloons, rotational/orbital atherectomy and excimer laser. These devices cause plaque compression or plaque debulking and are not without complications, such as distal embolization, slow flow, coronary dissection and perforation. Moreover, they are less successful if the calcification is deep, thick or eccentric, and the tissue injury occurring in the process may induce uncontrolled neointimal proliferation and restenosis[9].
IVL is a novel technique for bed preparation in severely calcified lesions in coronary as well as peripheral arteries. Calcium fractures are achieved with pulsatile mechanical energy delivered via lithotripsy emitters mounted inside a low-pressure balloon catheter (max 4-6 atm). The electrohydraulic-generated high-speed sonic pressure waves pass through the soft vessel wall tissue and selectively modify the subendothelial calcium, which disrupts the calcified plaque. IVL has been approved by the United States Food and Drug Administration for the treatment of calcified peripheral lesions and has obtained the CE mark for coronary lesions.
The mechanism of IVL is similar to the commonly utilized electrohydraulic lithotripsy or commonly referred to as extracorporeal shockwave lithotripsy for fragmentation of urogenital tract stones[10]. IVL therapy uses the same technology with some modifications to address vascular calcium. The adaptations include the incorporation of lithotripsy emitters on the shaft of a balloon angioplasty catheter, which delivers localized pulsatile acoustic pressure waves circumferentially to modify vascular calcium.
In electrohydraulic lithotripsy, a spark gap discharge between two electrodes causes the formation of an acoustic pressure wave within the transmitting fluid medium. This pressure wave expands spherically outwards from the emitter. The energy discharged from the spark gap results in the formation of a plasma vapor bubble, and this immediately follows the initial acoustic shockwave. The rapid expansion and collapse of the vapor bubble, which is known as a cavitation bubble, and secondary shockwaves causes stone fracture on encountering differing acoustic impedances, such as the transition from soft tissue to calcified tissue.
Electrohydraulic lithotripsy spark gap technology was leveraged for use in IVL, but several modifications were done to ensure effective and safe intravascular treatment. Three key modifications were done for IVL: (1) IVL pressure waves deliver tissue-safe positive and minimal peak negative pressure pulses allowing sufficient compressive force to modify vascular calcium. This helps in mitigating soft tissue injury due to excessive cavitation or tensile stress; (2) Pressure wave emitters are enclosed within an inflated, fluid-filled balloon to prevent thermal injury; and (3) Multiple emitters are present in series along the shaft of the catheter for longitudinal treatment of the calcified vessel.
The IVL shockwaves are unfocused, which produces much lower energy flux density (the amount of acoustic energy per unit area) as compared to extracorporeal shockwave lithotripsy. Much less energy is required here as the IVL shockwaves are initiated in close proximity to the target vascular calcium. In addition, IVL integration of a semicompliant balloon with emitters on the shaft has several advantages. First, the mixture of contrast and saline in balloon dissipate heat generated during the formation of vapor bubbles and shields the emitters from direct contact with the vessel wall. Since the balloon is deflated in between two cycles, it helps in dissipating heat and residual gas bubbles allowing tissue perfusion and preventing ischemia. Second, it provides mechanical support, which minimizes any tissue deformation during IVL therapy. Last as the integrated balloon is thin and acoustically transparent, it provides efficient fluid to tissue transmission and effective coupling of IVL pressure pulse propagation from emitter to vascular wall. The appropriately sized IVL balloon catheter inflated at subnominal pressure (4 atm) produces efficient ring calcium fracture avoiding barotraumas.
The IVL system comprises the following components: (1) The IVL generator: The IVL generator is a portable and rechargeable orthogonal “box” weighing 6.8 kg and measuring 28.0 cm × 15.2 cm × 29.2 cm, which delivers small electrical pulses of up to 3000 V of electrical energy. The discharge frequency is one pulse per second (1 Hz), and the maximum number of continuous pulses per cycle is fixed and depends on the type of the IVL catheter used. The machine is user friendly and comprises two buttons, one for power and the other one for delivering energy. There are no external connections, except from the IVL connector cable. There is a color-coding that depicts the number of pulses remaining after each cycle and the size of the IVL catheter being used[9,11-13]; (2) The IVL connector cable: The IVL connector cable is 1.53 m in length and contains two magnetic poles. One pole is connected to the IVL generator, and the other one is specially designed with a push button that triggers energy emission and is connected directly to the proximal end of the IVL catheter. It forms the route and the gate for electrical energy transfer from the IVL generator to the IVL catheter[9,11-13]; and (3) The IVL catheters: The IVL catheters are balloon angioplasty catheters that possess a series of unfocused electrohydraulic lithotripsy emitters, which convert electrical energy into sonic pressure pulses. The sonic pressure waves travel circumferentially and create a spherical field at an effective pressure of approximately 50 atm. The waves selectively disrupt and fracture the calcium in situ and alter the vessel compliance while minimizing the injury and maintaining the integrity of the fibroelastic components of the vessel wall. The catheters are available in different sizes, internal design and maximum cycles and total pulses based on their use in coronary or peripheral vessels[9,11-13].
In general, the coronary IVL balloon is sized 1:1 to the reference artery diameter and is inflated to low pressure (4 atm). One cycle of ultrasound energy, i.e. 10 pulses over 10 s, is delivered. This process is followed by IVL balloon dilatation to the size of the reference vessel according to the balloon compliance chart. The procedure should be repeated to provide a minimum of 20 pulses in the target lesion, with a period of deflation in between the pulses to allow for distal perfusion. The required number of cycles depends upon the lesion resistance; however, a maximum of 80 pulses (8 cycles) can be emitted by a single catheter. If the lesion length exceeds the balloon length (12 mm), then the balloon can be repositioned and the lithotripsy repeated. Although the currently available IVL balloon is relatively bulky, contemporary rapid exchange guide extension catheters can accommodate it easily and aid in its smooth delivery.
Clinical studies on IVL are shown in Table 1.
| Trial | Year | No. of patients | Severe calcification at baseline | Calcified segment length in mm | Primary endpoints | Outcome | Luminal gain post IVL | Luminal gain post stenting | Residual stenoses | Calcium fracture on OCT |
| DISRUPT CAD I | 2019 | 60 | 100% | 22.3 ± 12.3 | 30 d MACE | 5% MACE observed | 1.7 mm | < 50% in 100 lesions; < 30% in 92% lesions; < 20% in 73% lesions | 78% | |
| DISRUPT CAD II | 2019 | 120 | 94.3% | 25.7 ± 12.4 | In-hospital MACE | MACE occurred 5.8% patients | 0.83 ± 0.47 mm | 7.8 ± 7.1% | 78.7% | |
| DISRUPT CAD III | 2020 | 431 | 100% | 47.9 ± 18.8 | Freedom from in-hospital MACE, procedural success | Freedom from in-hospital MACE occurred in 92.2%; Procedural success in 92.4% | 1.7 mm | 11.9% | 67.4% | |
| DISRUPT CAD IV | 2021 | 64 | 100% | 49.8 ± 15.5 | Freedom from in-hospital MACE, procedural success | Freedom from in-hospital MACE occurred in 93.8%, Procedural success in 93.8% | 1.42 ± 0.42 mm | 1.67 ± 0.37 mm | Residual diameter stenosis < 50% and < 30% in all | 53.5% |
Randomized trial data: (1) DISRUPT CAD I Trial. This trial was a prospective multicenter study designed to evaluate the safety of IVL in 60 patients with heavily calcified coronary lesions. The incidence of major adverse cardiac events (MACE), defined by cardiac death, myocardial infarction and target vessel revascularization, was low (5.0% at 30 d and 8.6% at 6 mo). Clinical success rate (defined by a residual stenosis of < 50% and no in-hospital MACE) and device success rate (defined by successful device delivery and IVL treatment at target lesion) were high (95.0% and 98.3%, respectively). The major mechanism for calcium modification was fracture, as evidenced on OCT, and it was independent of the depth[14].
(2) DISRUPT CAD II Trial. This trial was also a prospective multicenter study involving 120 patients with severe coronary artery calcium and an indication for revascularization. The primary endpoint was in-hospital MACE (cardiac death, myocardial infarction and target vessel revascularization). Furthermore, an OCT substudy was performed to elucidate the mechanism of calcium modification. The incidence of primary endpoint was 5.8%, and there was no evidence of abrupt closure, slow flow-no reflow or perforation. Post PCI OCT showed calcium fracture in 78.7% of the lesions[15].
(3) DISRUPT CAD III Trial. This trial was a larger multicenter international study that enrolled 431 patients with severely calcified de novo lesions undergoing PCI. The primary safety endpoint was freedom from MACE at 30 d, and the primary efficacy endpoint was procedural success. The overall primary safety endpoint achieved was 92.2%, whereas the procedural success rate was 92.4%. The study also noted that the procedure was well tolerated, with a low rate of periprocedural complications. An OCT substudy showed calcium fracture in 67.4% of the lesions[16].
(4) DISRUPT CAD IV Trial. This trial was a prospective multicenter study designed for Japanese regulatory approval for coronary interventions. Again, the primary safety endpoint was freedom from MACE at 30 d, and the primary efficacy endpoint was procedural success (residual stenosis < 50%). A propensity-matched historical control group was used for the comparison. The primary endpoints were noninferiority for freedom from MACE at 30 d (CAD IV 93.8% vs control 91.2%, P = 0.008) and procedural success (CAD IV 93.8% vs control 91.6%, P = 0.007). There were no complications in the form of perforations, abrupt closure or slow-flow/no-reflow during the procedure[17].
(5) Pooled Analysis of the DISRUPT Trials. A pooled analysis of the four above mentioned studies comprising 628 patients enrolled at 72 sites spread across 12 countries was performed[18]. Severe calcium was seen in almost all patients, with an average calcium segment size of 41.5 mm. The efficacy and safety outcomes were achieved in approximately 92% of the patients, whereas the 30-d cardiac death and stent thrombosis rates were < 1% each. Perforation, abrupt closure and slow flow were characteristically absent.
(6) Long-term follow-up. Long-term (1 year) follow-up data were published recently[19]. The MACE rate at 1 year was low at 13.8% (vs 7.8% at day 30 vide supra). The cardiac death and stent thrombosis rates were low at 1.1% each. Repeat revascularization (ischemia-driven) rates were also low at 4.3% for the target lesion and 6% for the target vessel.
Registry experience: A total of 71 patients from three centers who were eligible for IVL were taken into a prospective registry. Three patient groups participated, namely primary IVL therapy (Group A) with calcified de novo lesions (n = 39 lesions), secondary IVL therapy (Group B) for patients with failed dilatation of lesion with noncompliant balloon (n = 22 lesions) and tertiary IVL therapy (Group C) for patients with stent under-expansion after previous stenting (n = 17 lesions). The primary endpoints were procedural success (< 20% residual stenosis) and safety outcomes. The mean diameter of the pre-IVL stenosis was 71.8% ± 13.1%, which reduced to 45.1% ± 17.4% after IVL and 17.5% ± 15.2% after the stenting. Similarly, the mean minimal lumen diameter increased from 1.01 ± 0.49 mm at baseline to 1.90 ± 0.61mm after IVL and 2.88 ± 0.56 mm after the stenting. The procedural success rates were 84.6% (Group A), 77.3% (Group B) and 64.7% (Group C). There was no in-hospital MACE[20].
In another registry, patients treated with IVL were studied retrospectively to assess the clinical and angiographic outcomes of coronary IVL use in all-comers with moderate to severe calcified coronary lesions. The primary endpoint was in-hospital MACE (cardiac death, myocardial infarction and target vessel revascularization), and the secondary endpoints were clinical success [stent expansion with < 30% in-stent restenosis (ISR) and no in-hospital MACE] and angiographic success. A total of 50 calcified lesions were treated with IVL in 45 patients divided into three subgroups, similar to the above registry: primary IVL therapy (n = 23 lesions), secondary IVL (n = 15 lesions) and tertiary IVL (n = 12 lesions). The mean diameter of the stenosis decreased from 63.2% ± 10.2% at baseline to 33.5% ± 10.9% post IVL (P < 0.001) and 15.0% ± 7.1% post stenting (P < 0.001). The mean minimal lumen diameter increased from 1.1 ± 0.3 mm at baseline to 1.90 ± 0.5 mm post IVL and 2.80 ± 0.50 mm post stenting. The overall clinical success and angiographic success rates were 90% and 94%, respectively[21].
Systemic reviews and meta-analyses: In a meta-analysis performed by Sattar et al[22] involving 282 patients from four studies, IVL significantly improved the size of the vessel lumen to facilitate coronary stent delivery and deployment in severely calcified plaques. The mean pre-IVL diameter was 1.01 mm, whereas the post-IVL mean diameter was 2.70 mm. The post-IVL lumen diameter was significantly higher than the pre-IVL mean diameter, with a mean difference of -4.16 (95% confidence interval: from -5.08 to -3.24, P = 0.000001).
In another meta-analysis performed by Sattar et al[23] involving 24 patients from case reports and series, a success rate of 100% was achieved for stent implantation, with minimal complications. No significant differences were observed in the mortality rates of patients undergoing IVL for LAD, left circumflex coronary and left main coronary artery. The mortality rate was higher in patients who had prior comorbidities or required more than three cycles of IVL. Kaul et al[24] compared IVL with traditional rotational atherectomy in severely calcified coronary stenosis. Trials on rotablation and IVL revealed that the latter was safer than the former primarily because it reduced the risk for atheromatous embolization. The studies also revealed that IVL yielded better results for parameters such as acute lumen gain and residual stenosis; however, in-hospital MACE was better with rotablation.
One of the limitations of IVL is that it is contraindicated in angulated and tortuous coronary lesions because of its bulky design. The presence of uncrossable lesions may preclude its application; however, the use of hybrid techniques, such as “RotaTripsy,” can overcome this shortcoming.
RotaTripsy refers to the hybrid and tandem utilization of rotablation followed by IVL for lesions with extremely severe calcification in which a balloon cannot cross the lesion or fails to expand. The initial use of rotablation allows the passage of the IVL balloon while debulking the superficial calcium. The subsequent use of IVL fractures the deep calcium, thus leading to extraluminal gain. The technique aims to achieve the best of both contemporary avenues for managing calcium, i.e. combining the advantages of the balloon-based procedure and the ablation-based procedure. In an observational study of 34 patients in a real-world setting, the technique attained 100% procedural success without any in-hospital MACE[25]. Another case series also demonstrated the safety and feasibility of the “RotaTripsy” technique underscoring the frequent coexistence of superficial and deep calcium on intracoronary imaging[26].
Although not approved for use in ISR, there are many emerging reports on the successful use of IVL in calcific ISR with drug-eluting stents where the use of noncompliant balloons and rotablation failed to produce satisfactory luminal gain[27,28]. Recently, a report has been published on the use of IVL for treating neointimal hyperplasia after bare metal stent implantation where cutting balloon and nonslip element balloon had failed[29]. A single center retrospective study found that angiographic success was achieved in all but 1 patient with an IVL-guided management strategy for moderate to severe ISR (65%-88% stenosis)[30]. In the multicenter SMILE registry for ISR, IVL was utilized after the failure of noncompliant balloons. The use of IVL led to significant improvements in minimal stent area and minimal stent diameter in 87% of the patients[31]. There were no cases of death or stent thrombosis at 30 d.
The recently published IVL-DRAGON registry (n = 62) has also explored the role of S-IVL in stent under-expansion[32]. The primary end point (stent expansion > 80% as seen by qualitative coronary angiography) was seen in 72.5% cases. Significant increase in stent expansion following application of IVL was confirmed by contemporary imaging techniques: OCT (37.5 % to 86%) and intravascular ultrasound (57% to 89%). Device oriented composite end point was seen in only 1.6%. Furthermore, there is a report on the combined use of IVL and brachytherapy in such cases[33]. Hence, the use of IVL in cases of ISR appears promising.
Serious angiographic complications were witnessed in up to 2.1% of the patients in the pooled analysis of DISRUPT trials[18]. These complications were dominated by dissections and slow flow. Perforations, no reflow and abrupt closure were not reported. The data in the registries mirrored similar findings, with minimal complications and a predominance of dissections[20-21]. Premature ventricular ectopic beats or “shocktopics” and asynchronous ventricular pacing were observed in some subjects on electrocardiogram but were not associated with clinical consequences[34]. Bradycardia at baseline (heartrate < 65 bpm) was the most powerful predictor of ventricular capture although it also tended to occur in younger patients, those with higher creatinine levels and those with previous infarction. A few case reports on transient atrial arrhythmias have emerged, but the course was benign[35,36]. The use of S-IVL in proximal coronary arteries has been associated with supraventricular tachyarrhythmias because of the anatomical proximity. Synchronization of the shock waves with R waves on the electrocardiogram has been suggested as a precautionary measure to ameliorate these arrhythmic effects.
Currently, S-IVL is approved for severely calcified coronary lesions with clinical indications for revascularization. Intravascular imaging (especially OCT) can help identify severe lesions that will benefit from this novel technology thereby allowing judicious usage of this costly therapy. Calcium arc more than 180 degrees, calcium length more than 5 mm and thickness more than 0.5 mm indicate increased calcium burden that will necessitate specialized lesion modifying therapies[37]. Being a balloon based technology, this eliminated any specialized training and hence almost no learning curve. However, this makes balloon uncrossable lesions a contraindication for S-IVL. As previously described a combined approach with rotablation first followed by IVL - “Rotatripsy” can be applied for such cases. In addition, rotational or orbital atherectomy can be followed by S-IVL to address deeper and stubborn calcific lesions. A simplified algorithm by De Maria et al[1] for heavily calcified coronary lesions places S-IVL into context.
Current techniques for calcium modification can be broadly divided into “atherectomy” type and “balloon” based. S-IVL offers many advantages over many of these techniques[1,9,24]. It obviates the need for any proprietary wire like Rota wire (rotablation) or Viper Wire (orbital atherectomy), and the procedure is carried on a routine workhorse wire. This makes it cost and time effective. Again being a balloon-based technology, it does not require any additional equipment like an advancer, foot pedal and saline infusion as needed for atherectomy devices. The technique is also similar to the “run of the mill” monorail balloons used in daily interventional practice making it easy to master even for the beginners recusing the need for special training. It also offers uniform and circumferential calcium modification as the balloon emits acoustic waves all round compared to other local atherectomy techniques that might be subject to wire bias. There is also amelioration of any distal embolization as with atherectomy techniques like rotablation and orbital atherectomy making it a safe procedure.
The balloon inflation is done at low pressures compared to other balloon based methods (with some utilizing ultrahigh pressures), which has the potential to minimize vascular trauma and increase safety. There is no data of any reported case of perforation with S-IVL so far. There also is no need to remove additional wires in the vessel lumen like side branch wire or buddy wire as with other atherectomy techniques. Temporary pacemaker insertion again is not needed as with rotablation. Both superficial and deep calcium are taken care of by S-IVL, whereas all the contemporary calcium modification techniques target only superficial calcium due to their localization at the luminal surface of the vessel. Very severe lesions where a balloon cannot cross remains the Achilles’ heel for S-IVL therapy.
Calcific coronary stenosis is one of the biggest challenges for interventional cardiologists. If not treated properly, then the condition can lead to inadequate stent expansion and result in stent thrombosis and restenosis. Techniques such as rotablation, orbital atherectomy and excimer laser are commonly used to modify the coronary calcium; however, they are associated with high procedural complications and are less successful in cases of deep and eccentric calcium. S-IVL is a novel technique that selectively modifies the subendothelial calcium and is easy to perform. Based on the short-term and long-term success demonstrated in the DISRUPT-CAD trial series, S-IVL is being employed in hitherto unexplored areas, such as ISR, vein graft and balloon uncrossable lesions (coupled with rotablation). S-IVL is gradually becoming an indispensable tool for managing calcified lesions in the cardiac catheterization laboratory.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author’s Membership in Professional Societies: American Heart Association; American College of Cardiology; European society of cardiology.
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sun LX, China; Yu F, China S-Editor: Wu YXJ L-Editor: Filipodia P-Editor: Wu YXJ
| 1. | De Maria GL, Scarsini R, Banning AP. Management of Calcific Coronary Artery Lesions: Is it Time to Change Our Interventional Therapeutic Approach? JACC Cardiovasc Interv. 2019;12:1465-1478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 117] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 2. | Mehanna E, Abbott JD, Bezerra HG. Optimizing Percutaneous Coronary Intervention in Calcified Lesions: Insights From Optical Coherence Tomography of Atherectomy. Circ Cardiovasc Interv. 2018;11:e006813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 3. | Brinton TJ, Ali Z, Mario CD. Performance of the lithoplasty system in treating calcified coronary lesions prior to stenting: results from the DISRUPT-CAD OCT sub-study. J Am Coll Cardiol. 2017;69:11-21. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Généreux P, Redfors B, Witzenbichler B, Arsenault MP, Weisz G, Stuckey TD, Rinaldi MJ, Neumann FJ, Christopher Metzger D, Henry TD, Cox DA, Duffy PL, Mazzaferri EL Jr, Francese DP, Marquis-Gravel G, Mintz GS, Kirtane AJ, Maehara A, Mehran R, Stone GW. Two-year outcomes after percutaneous coronary intervention of calcified lesions with drug-eluting stents. Int J Cardiol. 2017;231:61-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 5. | Madhavan MV, Tarigopula M, Mintz GS, Maehara A, Stone GW, Généreux P. Coronary artery calcification: pathogenesis and prognostic implications. J Am Coll Cardiol. 2014;63:1703-1714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 399] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 6. | Bourantas CV, Zhang YJ, Garg S, Iqbal J, Valgimigli M, Windecker S, Mohr FW, Silber S, Vries Td, Onuma Y, Garcia-Garcia HM, Morel MA, Serruys PW. Prognostic implications of coronary calcification in patients with obstructive coronary artery disease treated by percutaneous coronary intervention: a patient-level pooled analysis of 7 contemporary stent trials. Heart. 2014;100:1158-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 227] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 7. | Lee MS, Shah N. The Impact and Pathophysiologic Consequences of Coronary Artery Calcium Deposition in Percutaneous Coronary Interventions. J Invasive Cardiol. 2016;28:160-167. [PubMed] |
| 8. | Kobayashi Y, Okura H, Kume T, Yamada R, Kobayashi Y, Fukuhara K, Koyama T, Nezuo S, Neishi Y, Hayashida A, Kawamoto T, Yoshida K. Impact of target lesion coronary calcification on stent expansion. Circ J. 2014;78:2209-2214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 150] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 9. | Kassimis G, Raina T, Kontogiannis N, Patri G, Abramik J, Zaphiriou A, Banning AP. How Should We Treat Heavily Calcified Coronary Artery Disease in Contemporary Practice? Cardiovasc Revasc Med. 2019;20:1172-1183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 10. | Kereiakes DJ, Virmani R, Hokama JY, Illindala U, Mena-Hurtado C, Holden A, Hill JM, Lyden SP, Ali ZA. Principles of Intravascular Lithotripsy for Calcific Plaque Modification. JACC Cardiovasc Interv. 2021;14:1275-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 101] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 11. | Wong B, El-Jack S, Newcombe R, Glenie T, Armstrong G, Khan A. Shockwave Intravascular Lithotripsy for Calcified Coronary Lesions: First Real-World Experience. J Invasive Cardiol. 2019;31:46-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Auberson D, Gray WA. A new Sherriff in town: Vascular calcium meets its match. Catheter Cardiovasc Interv. 2019;93:343-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Dini CS, Tomberli B, Mattesini A, Ristalli F, Valente S, Stolcova M, Meucci F, Baldereschi G, Fanelli F, Shlofmitz RA, Ali ZA, Di Mario C. Intravascular lithotripsy for calcific coronary and peripheral artery stenoses. EuroIntervention. 2019;15:714-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 14. | Brinton TJ, Ali ZA, Hill JM, Meredith IT, Maehara A, Illindala U, Lansky A, Götberg M, Van Mieghem NM, Whitbourn R, Fajadet J, Di Mario C. Feasibility of Shockwave Coronary Intravascular Lithotripsy for the Treatment of Calcified Coronary Stenoses. Circulation. 2019;139:834-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 232] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 15. | Ali ZA, Nef H, EscanedJ, WernerN, Hill JM. Safety and Effectiveness of Coronary Intravascular Lithotripsy for The treatment of Severely Calcified Coronary Stenoses: The DISRUPT CAD II Study. Ciec Cardiovasc Interv. 2019;12:e008434. |
| 16. | Hill JM, Kereiakes DJ, Shlofmitz RA, Klein AJ, Riley RF, Price MJ, Herrmann HC, Bachinsky W, Waksman R, Stone GW; Disrupt CAD III Investigators. Intravascular Lithotripsy for Treatment of Severely Calcified Coronary Artery Disease. J Am Coll Cardiol. 2020;76:2635-2646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 259] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 17. | Saito S, Yamazaki S, Takahashi A, Namiki A, Kawasaki T, Otsuji S, Nakamura S, Shibata Y; Disrupt CAD IV Investigators. Intravascular Lithotripsy for Vessel Preparation in Severely Calcified Coronary Arteries Prior to Stent Placement - Primary Outcomes From the Japanese Disrupt CAD IV Study. Circ J. 2021;85:826-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 18. | Kereiakes DJ, Di Mario C, Riley RF, Fajadet J, Shlofmitz RA, Saito S, Ali ZA, Klein AJ, Price MJ, Hill JM, Stone GW. Intravascular Lithotripsy for Treatment of Calcified Coronary Lesions: Patient-Level Pooled Analysis of the Disrupt CAD Studies. JACC Cardiovasc Interv. 2021;14:1337-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 108] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 19. | Kereiakes DJ, Hill JM, Shlofmitz RA, Klein AJ, Riley RF, Price MJ. Intravascular Lithotripsy for Treatment of Severely Calcified Coronary Lesions: 1-Year Results From the Disrupt CAD III Study. JSCAI 2022;1:10001. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 20. | Aksoy A, Salazar C, Becher MU, Tiyerili V, Weber M, Jansen F, Sedaghat A, Zimmer S, Leick J, Grube E, Gonzalo N, Sinning JM, Escaned J, Nickenig G, Werner N. Intravascular Lithotripsy in Calcified Coronary Lesions: A Prospective, Observational, Multicenter Registry. Circ Cardiovasc Interv. 2019;12:e008154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 72] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 21. | Umapathy S, Keh YS, Wong N, Ho KW, Tan JWC et. al. Real-world Experience of Coronary Intravascular Lithotripsy in an Asian Population: A retrospective, Observational , Single-Center, All-Comers Registry. J Invasive Cardiol.2021 June;33(6):E417-E424.. |
| 22. | Sattar Y, Ullah W, Mir T, Biswas S, Titus A, Darmoch F, Pacha HM, Mohamed MO, Kwok CS, Fischman DL, Bagur R, Mamas MA, Alraies MC. Safety and efficacy of coronary intravascular lithotripsy for calcified coronary arteries- a systematic review and meta-analysis. Expert Rev Cardiovasc Ther. 2021;19:89-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Sattar Y, Ullah W, Virk HUH, Doshi R, Rauf H, Desai H, Panchal A, Nasir M, Almas T, Ullah I, Pacha HM, Zaher N, Alraies MC. Coronary intravascular lithotripsy for coronary artery calcifications- systematic review of cases. J Community Hosp Intern Med Perspect. 2021;11:200-205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Kaul A, Dhalla PS, Bapatla A, Khalid R, Garcia J, Armenta-Quiroga AS, Khan S. Current Treatment Modalities for Calcified Coronary Artery Disease: A Review Article Comparing Novel Intravascular Lithotripsy and Traditional Rotational Atherectomy. Cureus. 2020;12:e10922. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 25. | Buono A, Basavarajaiah S, Choudhury A, Lee L, Bhatia G, Hailan A, Sharma V, Upadhyaya S, Naneishvili T, Ielasi A. "RotaTripsy" for Severe Calcified Coronary Artery Lesions: Insights From a Real-World Multicenter Cohort. Cardiovasc Revasc Med. 2022;37:78-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 26. | Gonzálvez-García A, Jiménez-Valero S, Galeote G, Moreno R, López de Sá E, Jurado-Román A. "RotaTripsy": Combination of Rotational Atherectomy and Intravascular Lithotripsy in Heavily Calcified Coronary Lesions: A Case Series. Cardiovasc Revasc Med. 2022;35:179-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (1)] |
| 27. | Karacsonyi J, Nikolakopoulos I, Vemmou E, Rangan BV, Brilakis ES. Intracoronary Lithotripsy: A New Solution for Undilatable In-Stent Chronic Total Occlusions. JACC Case Rep. 2021;3:780-785. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Watkins S, Good R, Hill J, Brinton TJ, Oldroyd KG. Intravascular lithotripsy to treat a severely underexpanded coronary stent. EuroIntervention. 2019;15:124-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 29. | Chan KH, Sia JE, Tan HC. Intravascular lithotripsy for the treatment of severe calcific neointimal hyperplasia in a bare metal stent 17 years after implantation. Coron Artery Dis. 2021;32:172-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Brunner FJ, Becher PM, Waldeyer C, Zengin-Sahm E, Schnabel RB, Clemmensen P, Westermann D, Blankenberg S, Seiffert M. Intravascular Lithotripsy for the Treatment of Calcium-Mediated Coronary In-Stent Restenoses. J Invasive Cardiol. 2021;33:E25-E31. [PubMed] |
| 31. | Ielasi A, Moscarella E, Testa L, Gioffrè G, Morabito G, Cortese B, Colangelo S, Tomai F, Arioli F, Maioli M, Leoncini M, Tumminello G, Benedetto S, Lucchina PG, Pennesi M, Ugo F, Viganò E, Bollati M, Missiroli B, Gaspardone A, Calabrò P, Bedogni F, Tespili M. IntravaScular Lithotripsy for the Management of UndILatable Coronary StEnt: The SMILE Registry. Cardiovasc Revasc Med. 2020;21:1555-1559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 32. | Wańha W, Tomaniak M, Wańczura P, Bil J, Januszek R, Wolny R, Opolski MP, Kuźma Ł, Janas A, Figatowski T, Gąsior P, Milewski M, Roleder-Dylewska M, Lewicki Ł, Kulczycki J, Włodarczak A, Tomasiewicz B, Iwańczyk S, Sacha J, Koltowski Ł, Dziarmaga M, Jaguszewski M, Kralisz P, Olajossy B, Sobieszek G, Dyrbuś K, Łebek M, Smolka G, Reczuch K, Gil RJ, Dobrzycki S, Kwiatkowski P, Rogala M, Gąsior M, Ochała A, Kochman J, Witkowski A, Lesiak M, D'Ascenzo F, Bartuś S, Wojakowski W. Intravascular Lithotripsy for the Treatment of Stent Underexpansion: The Multicenter IVL-DRAGON Registry. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 33. | Nikolakopoulos I, Vemmou E, Xenogiannis I, Brilakis ES. Combined use of intravascular lithotripsy and brachytherapy: A new approach for the treatment of recurrent coronary in-stent restenosis. Catheter Cardiovasc Interv. 2021;97:1402-1406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 34. | Wilson SJ, Spratt JC, Hill J, Spence MS, Cosgrove C, Jones J, Strange JW, Halperin H, Walsh SJ, Hanratty CG. Incidence of "shocktopics" and asynchronous cardiac pacing in patients undergoing coronary intravascular lithotripsy. EuroIntervention. 2020;15:1429-1435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 35. | Kechichian A, Allam C, Njeim M, Kadri Z, Badaoui G. Atrial Flutter Following Shockwave Intravascular Lithotripsy During Percutaneous Intervention of Left Anterior Descending Disease. Cardiovasc Revasc Med. 2022;40S:205-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 36. | Curtis E, Khan A, El-Jack S, Glenie T. Precipitation of de novo atrial fibrillation during Shockwave Intravascular Lithotripsy® after pacing capture during the treatment of proximal right coronary artery disease: a case report. Eur Heart J Case Rep. 2019;3:1-4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 37. | Fujino A, Mintz GS, Matsumura M, Lee T, Kim SY, Hoshino M, Usui E, Yonetsu T, Haag ES, Shlofmitz RA, Kakuta T, Maehara A. A new optical coherence tomography-based calcium scoring system to predict stent underexpansion. EuroIntervention. 2018;13:e2182-e2189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 168] [Article Influence: 24.0] [Reference Citation Analysis (0)] |