Published online Sep 26, 2022. doi: 10.4330/wjc.v14.i9.483
Peer-review started: April 9, 2022
First decision: May 31, 2022
Revised: May 31, 2022
Accepted: August 17, 2022
Article in press: August 17, 2022
Published online: September 26, 2022
Processing time: 163 Days and 10.9 Hours
Cardiac magnetic resonance (CMR) is a unique tool for non-invasive tissue characterization, especially for identifying fibrosis.
To present the existing data regarding the association of electrocardiographic (ECG) markers with myocardial fibrosis identified by CMR - late gadolinium enhancement (LGE).
A systematic search was performed for identifying the relevant studies in Medline and Cochrane databases through February 2021. In addition, we conducted a relevant search by Reference Citation Analysis (RCA) (https://www.referencecitationanalysis.com).
A total of 32 studies were included. In hypertrophic cardiomyopathy (HCM), fragmented QRS (fQRS) is related to the presence and extent of myocardial fibrosis. fQRS and abnormal Q waves are associated with LGE in ischemic cardiomyopathy patients, while fQRS has also been related to fibrosis in myocarditis. Selvester score, abnormal Q waves, and notched QRS have also been associated with LGE. Repolarization abnormalities as reflected by increased Tp-Te, negative T-waves, and higher QT dispersion are related to myocardial fibrosis in HCM patients. In patients with Duchenne muscular dystrophy, a significant correlation between fQRS and the amount of myocardial fibrosis as assessed by LGE-CMR was observed. In atrial fibrillation patients, advanced inter-atrial block is defined as P-wave duration ≥ 120 ms, and biphasic morphology in inferior leads is related to left atrial fibrosis.
Myocardial fibrosis, a reliable marker of prognosis in a broad spectrum of cardiovascular diseases, can be easily understood with an easily applicable ECG. However, more data is needed on a specific disease basis to study the association of ECG markers and myocardial fibrosis as depicted by CMR.
Core Tip: Myocardial fibrosis, a reliable marker of prognosis in a broad spectrum of cardiovascular diseases, can be easily understood with an easily applicable electrocardiogram (ECG). However, more data is needed on a specific disease basis to study the association of ECG markers and myocardial fibrosis as depicted by cardiac magnetic resonance.
- Citation: Bazoukis G, Garcia-Zamora S, Çinier G, Lee S, Elvin Gul E, Álvarez-García J, Miana G, Hayıroğlu Mİ, Tse G, Liu T, Baranchuk A. Association of electrocardiographic markers with myocardial fibrosis as assessed by cardiac magnetic resonance in different clinical settings. World J Cardiol 2022; 14(9): 483-495
- URL: https://www.wjgnet.com/1949-8462/full/v14/i9/483.htm
- DOI: https://dx.doi.org/10.4330/wjc.v14.i9.483
Cardiac magnetic resonance (CMR) is a useful non-invasive and radiation-free imaging modality that is the gold standard for estimating left ventricular volumes and ejection function[1]. Furthermore, CMR is a unique tool for non-invasive tissue characterization, especially for identifying edema, infarction, scar, and fibrosis. Tissue characterization can provide useful data not only for diagnostic purposes but also for the risk stratification of patients in different clinical settings[2-6]. In this setting, late gadolinium enhancement (LGE) is a commonly used CMR technique to identify myocardial fibrosis. However, CMR is not a widely available imaging modality, and also the high cost limits its widespread use in clinical practice.
On the other hand, electrocardiogram (ECG) is a well-established, easily obtained, low-cost diagnostic tool that is the cornerstone of cardiological evaluation. ECG markers have been associated with the presence of myocardial fibrosis, as depicted from CMR evaluation. This systematic review aimed to present the existing data regarding the association of ECG markers with myocardial fibrosis identified by CMR-LGE.
This systematic review was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA Statement; PROSPERO ID: CRD42021225119)[7].
This study aimed to identify all relevant studies that provided data about the association of ECG markers with myocardial fibrosis as depicted by CMR. Two independent investigators searched Medline and Cochrane databases systematically through February 2021. The reference lists of all included studies, relevant review studies, systematic reviews, and meta-analyses were manually searched. The following keywords were used in the search strategy: “(CMR OR cardiac magnetic resonance) AND (LGE OR late gadolinium enhancement) AND (ECG OR electroc*)” without any limitations. We first screened the titles and abstracts of each retrieved study, and in case of considering a study relevant, we studied the full text. In addition, we conducted a relevant search by Reference Citation Analysis (RCA) (https://www.referencecitationanalysis.com).
We included studies that provided data regarding the association of any ECG markers with myocardial fibrosis as depicted by CMR in different clinical settings. We excluded studies that did not provide data about the studied outcome, studies that provided data about the association of endocardial electrograms with fibrosis, or data about the association of atrial LGE with atrial fibrillation, as well as review studies, case reports/series, and experimental studies.
The data extraction was performed independently by two authors. The following data were extracted: First author, year of publication, journal, type of study (single or multicenter), number of patients, gender, age, clinical setting, ECG markers that were studied, as well as the major outcomes reported in each study. The Newcastle-Ottawa Quality Assessment Scale was used for the quality assessment of the observational studies[8].
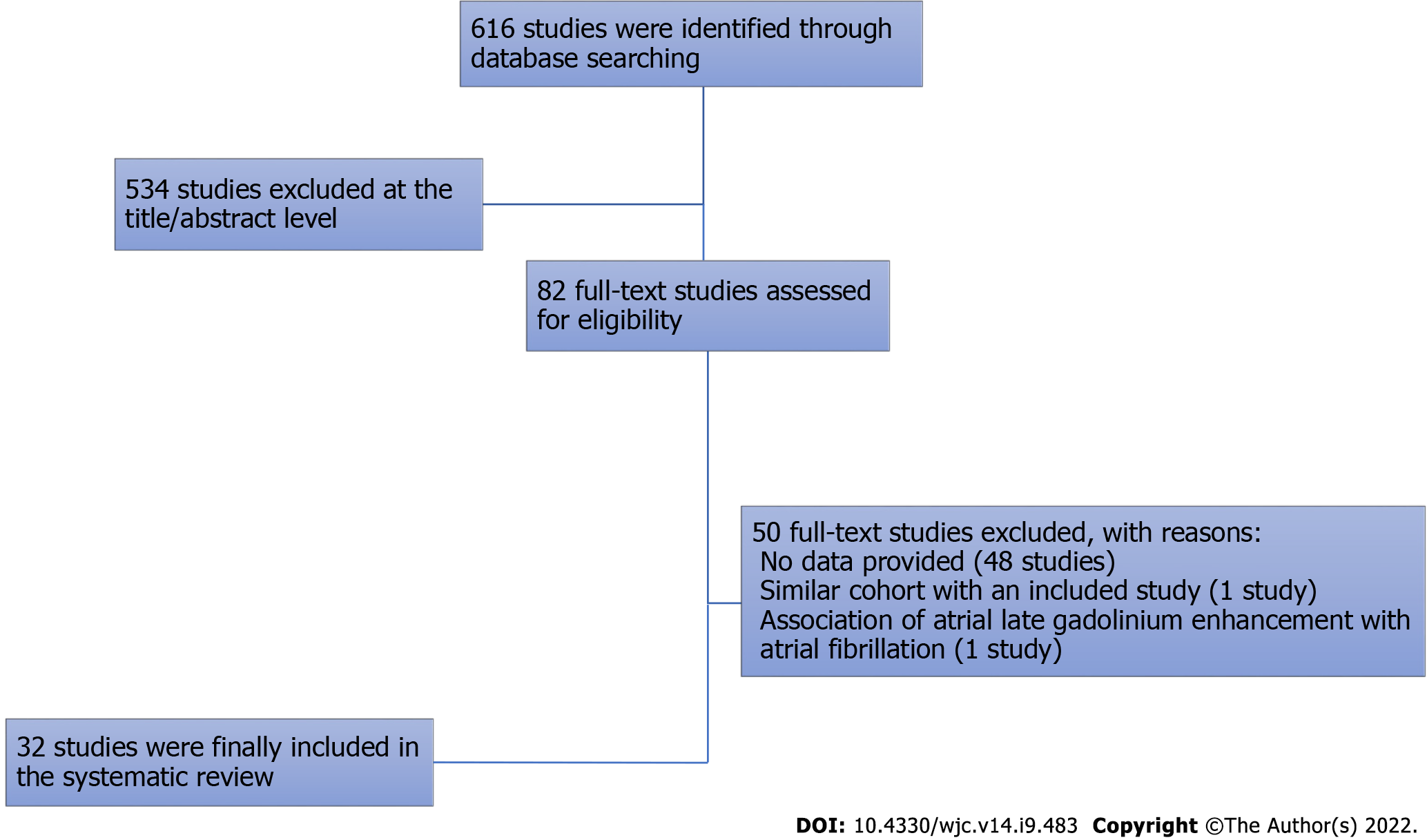
Of the 616 studies initially retrieved, 534 were excluded at the title/abstract level, and 50 were excluded at the full-text level. Finally, 32 studies were included in the systematic review[9-40]. The search strategy is shown in Figure 1.
The baseline characteristics and the main findings of the included studies are presented in Tables 1 and 2. Our search strategy identified 15 studies in hypertrophic cardiomyopathy patients[9-23], two with ventricular arrhythmias patients[24,25], two with non-ischemic cardiomyopathy patients[26,27], one with drug refractory AF patients[32], two with myotonic dystrophy patients[28,29], two with myocardial infarction patients[30,31], two about myocarditis[33,34], two including general population[35,36], one with arrhythmogenic cardiomyopathy patients[37], one with patients with preserved ejection fraction[38], one in cardiac sarcoidosis patients[39], and one in patients with left bundle branch block (LBBB)[40]. The quality assessment of the included studies is summarized in Supplemen
| Ref. | Setting | Country of origin | Multicenter | n | Enrolment period | Mean age | Male (%) | LVEF (%) |
| Oebel et al[25], 2017 | PVCs ablation | Germany | No | 101 | 2015-2016 | 57 | 59 | 46 |
| Sakamoto et al[24], 2015 | VT/VF | Japan | No | 34 | - | 60 | 71 | 45 |
| Piers et al[26], 2016 | NICM | Netherlands | No | 40 | 2011- | 57 | 83 | 30 |
| Becker et al[27], 2020 | DCM | Netherlands | No | 165 | 2016-2018 | 59 | 62 | 36 |
| Cho et al[29], 2017 | Duchenne muscular dystrophy | Korea | No | 37 | - | 16 | - | 55 |
| Cardona et al[28], 2019 | Myotonic dystrophy 1 | United States | No | 52 | 2012-2017 | 41 | 38 | 60 |
| Nadour et al[30], 2014 | MI | United States | No | 235 | 2006-2009 | 62 | 82 | 33 |
| Chew et al[31], 2018 | MI | Canada | No | 705 | 2011-2014 | 64 | 84 | 40 |
| Ciuffo et al[32], 2020 | AF | United States | No | 152 | 2010-2015 | 60 | 76 | 57 |
| Ferrero et al[33], 2020 | Myocarditis | Italy | Yes | 80 | 2008-2019 | 34 | 82 | 55 |
| Fischer et al[34], 2020 | Myocarditis | Switzerland | No | 587 | 2002-2015 | 48 | 59 | 48 |
| Inoue et al[35], 2017 | General population | United States | Yes | 1669 | 2000 - 2002 | 67 | 50 | 62 |
| De Lazzari et al[37], 2018 | AC | Italy | No | 79 | 2006-2016 | 33 | 60 | 58 |
| Mewton et al[38], 2016 | HFpEF | United States | No | 77 | 2009-2010 | 60 | 68 | 60 |
| Sobue et al[39], 2015 | Sarcoidosis | Japan | No | 59 | 2006-2010 | 29 | 51 | |
| Wieslander et al[36], 2015 | General population | United States | No | 193 | 2011-2013 | 63 | 66 | 49 |
| Wieslander et al[40], 2018 | LBBB | United States | Yes | 325 | - | 63 | 52 | 36 |
| Bi et al[9], 2020 | HCM | China | No | 69 | 2015-2020 | 46 | 62 | 65 |
| Chen et al[10], 2014 | HCM | China | No | 118 | 2005-2012 | 46 | 72 | 72 |
| Chen et al[11], 2020 | HCM | China | No | 135 | 2012-2016 | 51 | 51 | 62 |
| Riza-Demir et al [12], 2019 | HCM | Turkey | No | 74 | 2016-2018 | 51 | 65 | 66 |
| Dohy et al[13], 2020 | HCM | Hungary | No | 181 | - | 49 | 57 | 63 |
| Fronza et al[14], 2016 | HCM | Italy | No | 88 | 2004-2014 | 42 | 74 | 62 |
| Grall et al[15], 2014 | HCM | France | No | 42 | 2008-2012 | 47 | 72 | 62 |
| Guerrier et al[16], 2016 | Pediatric HCM | United States | No | 37 | 2006-2014 | 16 | 89 | 69 |
| Kawasaki et al[17], 2015 | HCM | Japan | No | 60 | 2010-2013 | 66 | 76 | 64 |
| Konno et al[18], 2015 | HCM | Japan | No | 108 | 2008 - 2014 | 62 | 65 | - |
| Matsuki et al[19], 2020 | HCM | Japan | No | 41 | - | 62 | 76 | 65 |
| Park et al[20], 2018 | HCM | Korea | No | 88 | - | 57 | 74 | 6 |
| Sakamoto et al[21], 2015 | HCM | Japan | No | 42 | 2004-2014 | 59 | 79 | 58 |
| Suwa et al[22], 2014 | HCM | Japan | Yes | 50 | 2004 - 2012 | - | - | - |
| Tangwiwat et al[23], 2019 | HCM | Thailand | No | 144 | 2005 - 2015 | 66 | 60 | 73 |
| Ref. | ECG markers studied | Main findings |
| Bi et al[9], 2020 | fQRS, AF, bundle branch block | Quantitative fQRS was an independent predictor for myocardial fibrosis in HCOM |
| Chen et al[10], 2014 | ST and T waves, LVH, Q waves, 1° AV block, 2° and 3° AV block, QRS duration | Abnormal Q waves were related to basal anteroseptal hypertrophy and extensive segmental LGE in HCM |
| Chen et al[11], 2020 | QRS duration, QTc, LVH, RBBB, LAFB, LBBB, Selvester score | Selvester score showed a significant positive correlation with the extent of LGE enhancement in HCM |
| Riza Demir et al[12], 2019 | QRS duration, QTc, TP-e interval, TP-e/QTc | Tp-e interval was an independent predictor of LGE in HCM |
| Dohy et al[13], 2020 | fQRS, Q wave, ST deviation, Sokolow, Cornell, and Romhilt-Estes score | fQRS and ST deviation (strain pattern) predicts myocardial fibrosis in HCM |
| Fronza et al[14], 2016 | Q waves, LBBB, signs of LV hypertrophy, negative T waves, ST depression | Negative T waves were correlated with LGE, whereas Q waves were associated with asymmetric hypertrophy in HCM |
| Grall et al[15], 2014 | AF, QRS duration, ST deviation, negative T wave, Q wave, Sokolow, Cornell, Romhilt-Estes score | Q waves were more prevalent in the presence of LGE but didn´t correlate with LGE location and extent in HCM |
| Guerrier et al[16], 2016 | QRS axis, QTc, PR interval, T wave inversion, ST depression, Q waves, LVH | Low left ventricle precordial voltages in ECG were associated with LGE in pediatric HCM patients |
| Kawasaki et al[17], 2016 | QRS duration and axis, QTc, AF, LVH, Q wave, ST deviation, T wave inversion, notched QRS | Notched QRS was correlated with LGE in HCM without LBBB |
| Konno et al[18], 2015 | Pathological Q waves and fQRS | fQRS was correlated with LGE in HCM, whereas Q waves were not correlated with LGE |
| Matsuki et al[19], 2020 | QT interval, QRS duration, Sum of R-wave amplitude, ventricular late potentials | Ventricular late potentials were not correlated with LGE in HCM |
| Park et al[20], 2018 | QRS, QTc, biphasic T wave, Q waves, sum S V1-3, Sokolow, Cornell, fQRS, AF, giant T wave inversion | The number of fQRS leads was significantly correlated to LGE in HCM |
| Sakamoto et al[21], 2015 | 24-hour ECG recordings and Time-domain T-wave alternans and QT dispersion | T-wave alternans and QT dispersion were associated with LGE in HCM |
| Suwa et al[22], 2014 | QRS, QTc, Sokolov, max ST, max T waves, fQRS | fQRS was associated with impaired apical contraction and apical LGE in HCM |
| Tangwiwat et al[23], 2019 | QRS duration, QTc, QRS axis, T-wave inversion, Sokolov, Cornell | fQRS in HCM was found to be associated with myocardial fibrosis in univariate analysis but not in the multivariate analysis |
| Sakamoto et al[24], 2015 | HR, QT, QTc, QTe/RR slope, QTa/RR slope, day/night slope, VT/FV | QTe day/night and QTa day/night ratios were significantly greater in patients with Ventricular Arrhythmias and LGE |
| Oebel et al[25], 2017 | PVC morphologies | RBBB, LBBB morphology and multiple PVC morphologies were associated with LGE in patients undergoing PVC ablation |
| Piers et al[26], 2016 | Prolongation of the paced QRS duration after premature stimulation | QRS duration was associated with ventricular tachycardia but not with LGE in non-ischemic cardiomyopathy |
| Becker et al[27], 2020 | HR, AV delay, 1° AV block, QRS duration, LBBB | QRS-prolongation was not correlated with LGE in non-ischemic dilated cardiomyopathy |
| Cardona et al[28], 2017 | PR, QRS, QT, QTc, Frontal QRS-T angle, LVH Cornell | Surface conduction abnormality was not associated with LGE in myotonic muscular dystrophy type 1 |
| Cho et al[29], 2019 | fQRS | f-QRS was correlated with LGE in Duchenne muscular dystrophy with low statistical significance levels |
| Nadour et al[30], 2014 | Q waves | Q waves in ECG have low value to detect a past myocardial infarction in the general population |
| Chew et al[31], 2018 | QRS 120 ms, QRS fragmentation, Axis, AF | fQRS was associated with increased peri-infarct zone LGE and unfavorable left ventricle remodeling |
| Ferrero et al[33], 2020 | fQRS | fQRS was correlated with LGE in patients with myocarditis |
| Fischer et al[34], 2020 | QTc, QRS-T angle, fQRS, BBB, ST deviation, PR depression, low voltage, Q and T wave | fQRS, low voltage and QRS-T angle > 90° were independently correlated with LGE in myocarditis |
| Inoue et al[35], 2020 | QRS duration, QTc, Sokolov and Cornell | QRS Cornell voltage, QRS duration, and QTc were significantly associated with LGE presence, while QRS Sokolow-Lyon voltage was not shown a significant correlation with LGE-CMR |
| Wieslander et al[36], 2015 | LBBB, RBBB, LAFB, RBBB + LAFB and Selvester score | Selvester score was not accurate to detect myocardial scar and LGE in patients with conduction abnormalities and BBB |
| De Lazzari et al[37], 2018 | Depolarization and repolarization abnormalities | Low QRS voltages in limb leads predicted LGE in Arrhythmogenic Cardiomyopathy |
| Mewton et al[38], 2016 | QRS d, QTc, QRS-T angle, QRS score, T wave alternans | A significant association between T-wave alternans value and total scar. Patients with a myocardial ischemic scar had greater QRS duration. QRS-T angle was not associated with total myocardial scar size, core of scar, and gray zone size in grams by LGE-CMR |
| Sobue et al[39], 2015 | QRS duration, atrioventricular block, LAFB, RBBB, Selvester QRS score | Selvester score was correlated with LGE in cardiac sarcoidosis |
| Wieslander et al[40], 2018 | LBBB | Selvester score was not accurate to detect myocardial scar and LGE in patients with LBBB |
| Ciuffo et al[32], 2020 | Inter-atrial block | Advanced IAB is associated with more fibrosis, while longer P-wave duration is also associated with more LA fibrosis. |
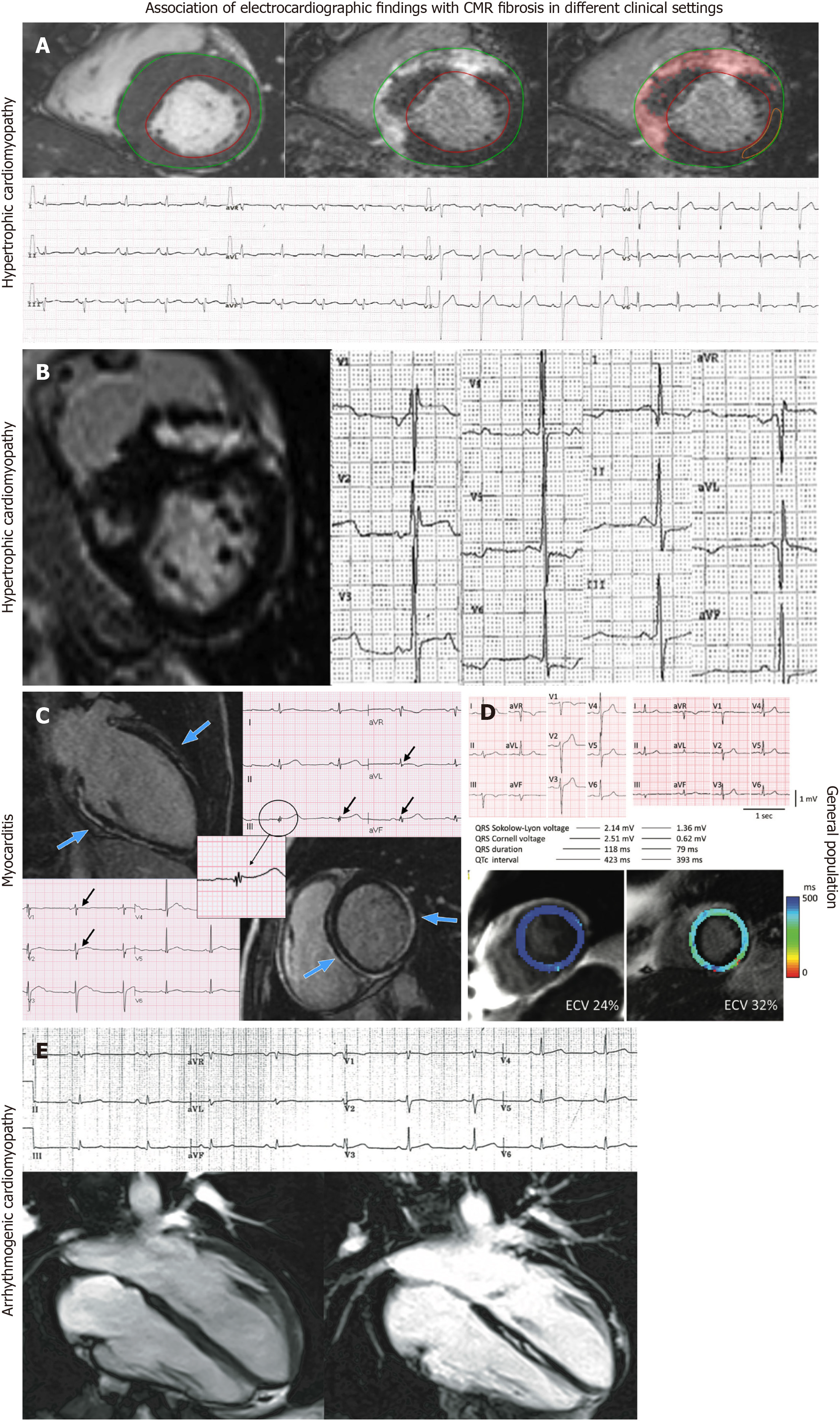
Hypertrophic cardiomyopathy: Fragmented QRS (fQRS) is defined as additional notches in the QRS complex. FQRS has been found to be related to more extensive myocardial fibrosis in HCM patients (Figure 2A)[9]. A recent study showed that quantitative fQRS, defined as the total amount of deflections in the QRS complex in all 12 routine ECG leads together, was an independent predictor of myocardial fibrosis and showed a good performance in identifying patients with a higher fibrotic burden[9]. Dohy et al[13] showed that fQRS and the strain pattern predicted more fibrosis, while the Cornell index was a negative predictor of myocardial fibrosis. The number of fQRS leads has been significantly correlated to %LGE, average ECV, and T2, while more than one lead with fQRS could predict > 5% of LGE mass with a 58% sensitivity and 63% specificity[20]. Suwa et al[22] showed that the presence of fQRS was associated with apical LGE. On the other hand, Tangwiwat et al[23] showed that fQRS was not associated with LGE. Chen et al[11] studied the role of Selvester QRS scoring criteria in diagnosing myocardial scar in HCM patients. The authors found that the Selvester score 1 showed a better performance in predicting LGE presence. Also, the same study showed a positive association between the Selvester score and the extent of LGE[11]. Abnormal Q waves are more prevalent in patients with LGE, but no correlation between the location of Q waves on ECG and territory of LGE on CMR was revealed (Figure 2B)[15]. Interestingly, quantitative analysis of LGE was not related to the presence of abnormal Q waves[15]. However, findings of another study showed that abnormal Q waves were associated with more ventricular segments with extensive LGE[10]. In a cohort study, LGE was associated with notched QRS, leftward QRS axis, and prolonged QRS duration, but not with abnormal Q waves, R-wave amplitude, or ST-T changes[17]. fQRS has been found to have higher diagnostic accuracy for detecting myocardial fibrosis compared to abnormal Q waves in HCM patients[18]. A cut-off of the number of leads with notched QRS ≥ 2 was found to predict the presence or absence of myocardial fibrosis, with a sensitivity of 70% and specificity of 81%[17]. Interestingly, the same study showed that the number of notched QRS leads was positively correlated with LGE volume, while a correlation between the lead distribution of notched QRS and the location of LGE was revealed[17]. Although giant negative T waves have been associated with apical HCM, no significant association was demonstrated with apical LGE[10]. On the other hand, in another observational study, repolarization disturbances, including negative T waves in lateral and anterior leads, have been correlated with “parietal” LGE scores, while QT dispersion has been associated with “global” LGE score[14]. Tp-Te has also been found to be an independent predictor of LGE, while a cut-off value of 99.4 ms can detect the LGE with a sensitivity of 64.3% and specificity of 84.2%[12]. In a small cohort of the pediatric population, the presence of LGE was associated with significantly decreased voltages in SV1, RV6, and SV1 + RV6 despite increased septal dimensions[16]. Furthermore, the slopes of the QTe/RR and QTa/RR have been found to be significantly steeper in the LGE positive patients, while both slopes have been significantly correlated with the total LGE scores[24]. The association of late potentials with myocardial fibrosis has also been studied in HCM patients. However, ventricular late potentials were not found to be a reliable marker for the detection of myocardial fibrosis as assessed by LGE on CMR[19].
Ischemic and non-ischemic cardiomyopathy: Two studies were identified through the search strategy regarding the association of ECG markers with fibrosis as identified by CMR. Nadour W et al[30] studied the comparative efficacy of Q waves and CMR-LGE to predict prior myocardial infarction. Interestingly, the authors found that ECG-defined scars had a lower sensitivity compared to CMR-LGE-defined scars. Specifically, it was found that a significant number of pathological Q waves had absent infarct etiology, indicating high false positivity[30]. Chew et al[31] showed that in myocardial infarction patients, fQRS has been found to be significantly associated with the peri-infarct zone but not with core infarct volume. In the setting of non-ischemic cardiomyopathy, two studies were identified. Specifically, Piers et al[26] found that prolongation of the paced QRS duration after premature stimulation was related to long, thick strands of fibrosis but not to focal LGE-CMR. CMR has been reported to have a complementary role to ECG findings in dilated cardiomyopathy patients[27]. Specifically, it has been found that while QRS prolongation and septal mid-wall LGE are often co-existed, no significant correlation between these markers was revealed[27].
Myocarditis: Two studies that provided data about ECG markers with CMR fibrosis were identified. In myocarditis patients, fQRS has been correlated with the distribution of LGE (Figure 2C and D)[33]. Interestingly, fQRS was also associated with ongoing inflammation and poor prognosis in terms of ventricular function and fatal arrhythmias[33]. Fischer et al[34] studied the association of ECG parameters with LGE-CMR in patients with clinical suspicion of acute or subacute myocarditis. In this population, a wide QRS-T angle, low voltage, and fQRS were found to be significantly associated with LGE-CMR[34].
Myotonic dystrophy: Two studies were found to provide data about ECG markers and myocardial fibrosis in patients with muscular dystrophy. Specifically, in patients with Duchenne muscular dystrophy, a significant correlation between fQRS and the amount of myocardial fibrosis as assessed by LGE-CMR was observed[29]. On the other hand, in patients with myotonic muscular dystrophy type 1, PR, QRS, and QTc duration, frontal QRS-T angle, absolute Cornell voltage, LVH-Cornell, LBBB, right bundle branch block (RBBB), fascicular block, bifascicular block, AH interval, and HV interval were not significantly different between LGE positive and LGE negative patients[28].
Other clinical settings: Ciuffo et al[32] studied the association between the interatrial block and atrial fibrosis using CMR imaging in patients with drug-refractory AF. It was found that advanced inter-atrial block, defined as P-wave duration ≥ 120 ms and biphasic morphology in inferior leads, was significantly associated with left atrial fibrosis[32]. Furthermore, P-wave duration was also independently associated with left atrial fibrosis in this clinical scenario[32]. Mewton et al[38] studied the association of ECG markers in patients with preserved ejection fraction. A significant independent and positive association between T-wave alternans value and total scar was revealed[38]. Furthermore, patients with a myocardial ischemic scar had significantly greater QRS duration as compared with patients with nonischemic scar and patients without a myocardial scar. On the other hand, QRS-T angle was not associated with total myocardial scar size, core of scar, and gray zone size in grams by LGE-CMR[38]. In the clinical setting of PVC, the presence of an RBBB pattern as the clinically dominant PVC morphology or the presence of multiple PVC morphologies were significantly correlated with the presence of LGE-defined fibrosis[25]. On the other hand, in patients with VT or VF, the slopes of the QTe/RR (QT measured at the apex of the T waves) and QTa/RR (QT measured at the end of T waves) were significantly steeper in the LGE positive patients while both slopes were significantly correlated with the total LGE scores[24]. Interestingly, the QTe day/night and QTa day/night ratios were significantly greater in LGE positive patients than in LGE negative patients, clearly demonstrating the correlation between fibrosis and QT dynamicity[24]. In the setting of cardiac sarcoidosis, QRS estimated scar using Selvester QRS score was significantly correlated with CMR-LGE scar while it was related with life-threatening arrhythmic events[39]. However, the Selvester QRS score intended for use in the presence of conduction abnormalities was not found to predict CMR-defined LV scar in a general population with suspected cardiovascular disease[36]. Similarly, the LBBB Selvester QRS score showed poor accuracy in the detection and quantification of myocardial scar in LBBB patients[40]. In ARVC patients, ε wave and terminal activation duration > 55 ms were not associated with either right or left ventricular LGE[37]. On the other hand, the presence of low QRS voltages in limb leads was associated with the presence of left ventricular LGE but not with right ventricular LGE (Figure 2E)[37]. In addition, the presence and extent of right precordial T-wave inversions were associated with the presence of right ventricular but not with left ventricular LGE[37]. Finally, in a prospective cross-sectional study that included individuals free of prior coronary heart disease, QRS Cornell voltage, QRS duration, and QTc were significantly associated with LGE presence, while QRS Sokolow-Lyon voltage was not shown to have a significant correlation with LGE-CMR (Figure 2D)[35].
In our systematic review, we examined in detail studies that have reported associations between ECG markers and CMR-reported myocardial fibrosis. In the literature, studies have reported controversial results regarding the association between pathological Q wave presence in ECG and LGE-CMR at first glance[10,17]. Moreover, another controversy on the association between fQRS and LGE in apical hypertrophic cardiomyopathy was reported[22,23]. These findings should be evaluated with caution because the study population, study design, ECG parameters used, and statistical approach have been heterogeneous among the included studies. Considering all included data, fQRS, QRS duration, Selvester QRS score, and ventricular repolarization variables have been detected to have great predictive value for myocardial fibrosis, which is validated by LGE-CMR in various cardiovascular diseases. The studies examining the association between ECG markers and CMR have been first evaluated in patients with HCM and ischemic cardiomyopathy. HCM has always been attracted attention due to its heterogenous electrocardiographic presentations, and it is rational to assess the fibrosis markers of ECG in HCM with the validation of CMR[41]. Since myocardial fibrosis has been associated with the arrhythmia burden in patients with HCM, early detection of myocardial fibrosis using 12-lead ECG has the potential to rapidly change management strategy in these patients[42,43]. LGE-CMR has been proposed as one of the predictors of clinical prognosis in patients with HCM[44]. Thus in the next step, ECG parameters correlated with LGE-CMR may be investigated in the risk scoring of HCM in addition to other well-known risk factors to provide more precise prediction in the follow-up of these patients. As the use of CMR is limited due to its high cost, ECG parameters found to represent myocardial fibrosis according to LGE-CMR may easily be used for the risk assessment.
In the evaluation of myocardial scar in patients with ischemic and non-ischemic cardiomyopathy, there appears to be a clear performance difference between CMR and ECG. The highly promising ECG parameters such as fQRS and pathological Q waves have not satisfied the expected performance compared to LGE-CMR[30]. The pathophysiological occurrence of myocardial scar in infarction may play an important role while explaining the poor performance of pathological Q waves in predicting myocardial fibrosis of LGE-CMR. Since Q waves symbolize a loss of electrical activity, not purely myocardial fibrosis, pathological Q waves without evident LGE-CMR may be explained for this reason[45]. However, fQRS, which has not been correlated with core infarct volume, has been associated with peri-infarct volume[31]. In myocarditis, fQRS has been demonstrated to have a good LGE-CMR prediction performance, similar to its significance in patients with HCM[33,34]. Since ECG variables, including fQRS, change dynamically during the disease course of myocarditis, more investigations are warranted to determine the time of obtained ECG, which should be examined to correlate LGE-CMR. On the other hand, ECG parameters regarding atrial tissue fibrosis have been closely related to LGE-CMR because there have been several investigations defending the association between P-wave duration and morphology and left atrial fibrosis. Therefore, P-wave duration and inter-atrial block have a great potential to present left atrial fibrosis, which has been validated by CMR[32].
Myocardial fibrosis, which is a reliable marker of prognosis in a wide spectrum of cardiovascular diseases, can be easily understood with an easily applicable ECG. More investigations are needed on a specific disease basis to fill the gap of evidence regarding the association of ECG markers and CMR, which may practically change our daily clinical practice.
Electrocardiogram (ECG) is a well-established, easily obtained, low-cost diagnostic tool that is the cornerstone of cardiological evaluation. ECG markers have been associated with the presence of myocardial fibrosis, as depicted from cardiac magnetic resonance (CMR) evaluation.
ECG can be a valuable tool for the risk stratification of sudden cardiac death in different clinical settings.
To elucidate the association of ECG markers with CMR-late gadolinium enhancement in different clinical settings.
Methodology of Systematic reviews in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA Statement).
Our results summarize the existing evidence about the association of ECG markers with fibrosis as identified by CMR. Existing data show that fragmented QRS, Q waves and repolarization abnormalities are some of the ECG indices that are associated with myocardial fibrosis.
Myocardial fibrosis, a marker of prognosis in a wide spectrum of clinical settings, can be easily identified by ECG indices.
Future research should be focused on the identification of ECG markers that are reliably associated with myocardial fibrosis in different clinical settings. Furthermore, the association of ECG markers with all-cause mortality and arrhythmic events is of great importance.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: Cyprus
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hamilton-Craig C, Australia; Tan X, China S-Editor: Wang LL L-Editor: Wang TQ P-Editor: Wang LL
| 1. | Hundley WG, Meshack BM, Willett DL, Sayad DE, Lange RA, Willard JE, Landau C, Hillis LD, Peshock RM. Comparison of quantitation of left ventricular volume, ejection fraction, and cardiac output in patients with atrial fibrillation by cine magnetic resonance imaging versus invasive measurements. Am J Cardiol. 1996;78:1119-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 2. | Raiker N, Vullaganti S, Collins JD, Allen BD, Choudhury L. Myocardial tissue characterization by gadolinium-enhanced cardiac magnetic resonance imaging for risk stratification of adverse events in hypertrophic cardiomyopathy. Int J Cardiovasc Imaging. 2020;36:1147-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Gräni C, Eichhorn C, Bière L, Murthy VL, Agarwal V, Kaneko K, Cuddy S, Aghayev A, Steigner M, Blankstein R, Jerosch-Herold M, Kwong RY. Prognostic Value of Cardiac Magnetic Resonance Tissue Characterization in Risk Stratifying Patients With Suspected Myocarditis. J Am Coll Cardiol. 2017;70:1964-1976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 320] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 4. | Gräni C, Benz DC, Gupta S, Windecker S, Kwong RY. Sudden Cardiac Death in Ischemic Heart Disease: From Imaging Arrhythmogenic Substrate to Guiding Therapies. JACC Cardiovasc Imaging. 2020;13:2223-2238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 5. | Aljaroudi WA, Flamm SD, Saliba W, Wilkoff BL, Kwon D. Role of CMR imaging in risk stratification for sudden cardiac death. JACC Cardiovasc Imaging. 2013;6:392-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 6. | Patel AR, Kramer CM. Role of Cardiac Magnetic Resonance in the Diagnosis and Prognosis of Nonischemic Cardiomyopathy. JACC Cardiovasc Imaging. 2017;10:1180-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 203] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 7. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52948] [Cited by in RCA: 47158] [Article Influence: 2947.4] [Reference Citation Analysis (0)] |
| 8. | Marshall SC, Molnar F, Man-Son-Hing M, Blair R, Brosseau L, Finestone HM, Lamothe C, Korner-Bitensky N, Wilson KG. Predictors of driving ability following stroke: a systematic review. Top Stroke Rehabil. 2007;14:98-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 137] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 9. | Bi X, Yang C, Song Y, Yuan J, Cui J, Hu F, Qiao S. Quantitative fragmented QRS has a good diagnostic value on myocardial fibrosis in hypertrophic obstructive cardiomyopathy based on clinical-pathological study. BMC Cardiovasc Disord. 2020;20:298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Chen X, Zhao T, Lu M, Yin G, Xiangli W, Jiang S, Prasad S, Zhao S. The relationship between electrocardiographic changes and CMR features in asymptomatic or mildly symptomatic patients with hypertrophic cardiomyopathy. Int J Cardiovasc Imaging. 2014;30 Suppl 1:55-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Chen S, Wang X, Huang L, Chen Y, Zhang Q. Performance of 12-lead electrocardiogram Selvester QRS scoring criteria to diagnose myocardial scar in patients with hypertrophic cardiomyopathy. Ann Noninvasive Electrocardiol. 2020;25:e12762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Riza Demir A, Celik Ö, Sevinç S, Uygur B, Kahraman S, Yilmaz E, Cemek M, Onal Y, Erturk M. The relationship between myocardial fibrosis detected by cardiac magnetic resonance and Tp-e interval, 5-year sudden cardiac death risk score in hypertrophic cardiomyopathy patients. Ann Noninvasive Electrocardiol. 2019;24:e12672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Dohy Z, Vereckei A, Horvath V, Czimbalmos C, Szabo L, Toth A, Suhai FI, Csecs I, Becker D, Merkely B, Vago H. How are ECG parameters related to cardiac magnetic resonance images? Ann Noninvasive Electrocardiol. 2020;25:e12763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Fronza M, Raineri C, Valentini A, Bassi EM, Scelsi L, Buscemi ML, Turco A, Castelli G, Ghio S, Visconti LO. Relationship between electrocardiographic findings and Cardiac Magnetic Resonance phenotypes in patients with Hypertrophic Cardiomyopathy. Int J Cardiol Heart Vasc. 2016;11:7-11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Grall S, Biere L, Clerfond G, Mateus V, Prunier F, Furber A. ECG characteristics according to the presence of late gadolinium enhancement on cardiac MRI in hypertrophic cardiomyopathy. Open Heart. 2014;1:e000101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Guerrier K, Madueme PC, Jefferies JL, Anderson JB, Spar DS, Knilans TK, Czosek RJ. Unexpectedly low left ventricular voltage on ECG in hypertrophic cardiomyopathy. Heart. 2016;102:292-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 17. | Kawasaki T, Harimoto K, Honda S, Sato Y, Yamano M, Miki S, Kamitani T. Notched QRS for the assessment of myocardial fibrosis in hypertrophic cardiomyopathy. Circ J. 2015;79:847-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Konno T, Hayashi K, Fujino N, Oka R, Nomura A, Nagata Y, Hodatsu A, Sakata K, Furusho H, Takamura M, Nakamura H, Kawashiri MA, Yamagishi M. Electrocardiographic QRS Fragmentation as a Marker for Myocardial Fibrosis in Hypertrophic Cardiomyopathy. J Cardiovasc Electrophysiol. 2015;26:1081-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 19. | Matsuki A, Kawasaki T, Kawamata H, Sakai C, Harimoto K, Kamitani T, Yamano M, Matoba S. Ventricular late potentials and myocardial fibrosis in hypertrophic cardiomyopathy. J Electrocardiol. 2020;58:87-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Park CH, Chung H, Kim Y, Kim JY, Min PK, Lee KA, Yoon YW, Kim TH, Lee BK, Hong BK, Rim SJ, Kwon HM, Choi EY. Electrocardiography based prediction of hypertrophy pattern and fibrosis amount in hypertrophic cardiomyopathy: comparative study with cardiac magnetic resonance imaging. Int J Cardiovasc Imaging. 2018;34:1619-1628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | Sakamoto N, Sato N, Oikawa K, Karim Talib A, Sugiyama E, Minoshima A, Tanabe Y, Takeuchi T, Akasaka K, Saijo Y, Kawamura Y, Hasebe N. Late gadolinium enhancement of cardiac magnetic resonance imaging indicates abnormalities of time-domain T-wave alternans in hypertrophic cardiomyopathy with ventricular tachycardia. Heart Rhythm. 2015;12:1747-1755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Suwa K, Satoh H, Sano M, Nobuhara M, Saitoh T, Saotome M, Urushida T, Katoh H, Tawarahara K, Ohtani H, Wakabayashi Y, Takase H, Terada H, Takehara Y, Sakahara H, Hayashi H. Functional, morphological and electrocardiographical abnormalities in patients with apical hypertrophic cardiomyopathy and apical aneurysm: correlation with cardiac MR. Open Heart. 2014;1:e000124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Tangwiwat C, Kaolawanich Y, Krittayaphong R. Electrocardiographic predictors of myocardial fibrosis and apical hypertrophic cardiomyopathy. Ann Noninvasive Electrocardiol. 2019;24:e12612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Sakamoto N, Sato N, Talib AK, Sugiyama E, Minoshima A, Tanabe Y, Fujino T, Takeuchi T, Akasaka K, Saijo Y, Kawamura Y, Hasebe N. Late Gadolinium Enhancement on Cardiac MRI Correlates with QT Dynamicity Represented by QT/RR Relationship in Patients with Ventricular Arrhythmias. Ann Noninvasive Electrocardiol. 2016;21:126-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 25. | Oebel S, Dinov B, Arya A, Hilbert S, Sommer P, Bollmann A, Hindricks G, Paetsch I, Jahnke C. ECG morphology of premature ventricular contractions predicts the presence of myocardial fibrotic substrate on cardiac magnetic resonance imaging in patients undergoing ablation. J Cardiovasc Electrophysiol. 2017;28:1316-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 26. | Piers SR, Askar SF, Venlet J, Androulakis AF, Kapel GF, de Riva Silva M, Jongbloed JJ, van Tintelen JP, Schalij MJ, Pijnappels DA, Zeppenfeld K. QRS prolongation after premature stimulation is associated with polymorphic ventricular tachycardia in nonischemic cardiomyopathy: Results from the Leiden Nonischemic Cardiomyopathy Study. Heart Rhythm. 2016;13:860-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Becker MAJ, Allaart CP, Zweerink A, Cornel JH, van de Ven PM, van Rossum AC, Germans T. Correlation between septal midwall late gadolinium enhancement on CMR and conduction delay on ECG in patients with nonischemic dilated cardiomyopathy. Int J Cardiol Heart Vasc. 2020;26:100474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Cardona A, Arnold WD, Kissel JT, Raman SV, Zareba KM. Myocardial fibrosis by late gadolinium enhancement cardiovascular magnetic resonance in myotonic muscular dystrophy type 1: highly prevalent but not associated with surface conduction abnormality. J Cardiovasc Magn Reson. 2019;21:26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 29. | Cho MJ, Lee JW, Lee J, Shin YB, Lee HD. Relationship Between Fragmented QRS Complexes and Cardiac Status in Duchenne Muscular Dystrophy: Multimodal Validation Using Echocardiography, Magnetic Resonance Imaging, and Holter Monitoring. Pediatr Cardiol. 2017;38:1042-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Nadour W, Doyle M, Williams RB, Rayarao G, Grant SB, Thompson DV, Yamrozik JA, Biederman RW. Does the presence of Q waves on the EKG accurately predict prior myocardial infarction when compared to cardiac magnetic resonance using late gadolinium enhancement? Heart Rhythm. 2014;11:2018-2026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 31. | Chew DS, Wilton SB, Kavanagh K, Vaid HM, Southern DA, Ellis L, Howarth AG, White JA, Exner DV. Fragmented QRS complexes after acute myocardial infarction are independently associated with unfavorable left ventricular remodeling. J Electrocardiol. 2018;51:607-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Ciuffo L, Bruña V, Martínez-Sellés M, de Vasconcellos HD, Tao S, Zghaib T, Nazarian S, Spragg DD, Marine J, Berger RD, Lima JAC, Calkins H, Bayés-de-Luna A, Ashikaga H. Association between interatrial block, left atrial fibrosis, and mechanical dyssynchrony: Electrocardiography-magnetic resonance imaging correlation. J Cardiovasc Electrophysiol. 2020;31:1719-1725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 33. | Ferrero P, Piazza I, Kühl U, Grosu A, Tschöpe C, Senni M. QRS fragmentation as a possible electrocardiographic diagnostic marker in patients with acute myocarditis: preliminary histopathological validation. ESC Heart Fail. 2020;7:2527-2533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 34. | Fischer K, Marggraf M, Stark AW, Kaneko K, Aghayev A, Guensch DP, Huber AT, Steigner M, Blankstein R, Reichlin T, Windecker S, Kwong RY, Gräni C. Association of ECG parameters with late gadolinium enhancement and outcome in patients with clinical suspicion of acute or subacute myocarditis referred for CMR imaging. PLoS One. 2020;15:e0227134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 35. | Inoue YY, Ambale-Venkatesh B, Mewton N, Volpe GJ, Ohyama Y, Sharma RK, Wu CO, Liu CY, Bluemke DA, Soliman EZ, Lima JA, Ashikaga H. Electrocardiographic Impact of Myocardial Diffuse Fibrosis and Scar: MESA (Multi-Ethnic Study of Atherosclerosis). Radiology. 2017;282:690-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 36. | Wieslander B, Nijveldt R, Klem I, Lokhnygina Y, Pura J, Wagner GS, Ugander M, Atwater BD. Evaluation of Selvester QRS score for use in presence of conduction abnormalities in a broad population. Am Heart J. 2015;170:346-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 37. | De Lazzari M, Zorzi A, Cipriani A, Susana A, Mastella G, Rizzo A, Rigato I, Bauce B, Giorgi B, Lacognata C, Iliceto S, Corrado D, Perazzolo Marra M. Relationship Between Electrocardiographic Findings and Cardiac Magnetic Resonance Phenotypes in Arrhythmogenic Cardiomyopathy. J Am Heart Assoc. 2018;7:e009855. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 38. | Mewton N, Strauss DG, Rizzi P, Verrier RL, Liu CY, Tereshchenko LG, Nearing B, Volpe GJ, Marchlinski FE, Moxley J, Killian T, Wu KC, Spooner P, Lima JA. Screening for Cardiac Magnetic Resonance Scar Features by 12-Lead ECG, in Patients with Preserved Ejection Fraction. Ann Noninvasive Electrocardiol. 2016;21:49-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 39. | Sobue Y, Harada M, Koshikawa M, Ichikawa T, Yamamoto M, Okuda K, Kato Y, Sarai M, Watanabe E, Ozaki Y. QRS-based assessment of myocardial damage and adverse events associated with cardiac sarcoidosis. Heart Rhythm. 2015;12:2499-2507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 40. | Wieslander B, Xia X, Jablonowski R, Axelsson J, Klem I, Nijveldt R, Maynard C, Schelbert EB, Sörensson P, Sigfridsson A, Chaudhry U, Platonov PG, Borgquist R, Engblom H, Couderc JP, Strauss DG, Atwater BD, Ugander M. The ability of the electrocardiogram in left bundle branch block to detect myocardial scar determined by cardiovascular magnetic resonance. J Electrocardiol. 2018;51:779-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 41. | Finocchiaro G, Sheikh N, Biagini E, Papadakis M, Maurizi N, Sinagra G, Pelliccia A, Rapezzi C, Sharma S, Olivotto I. The electrocardiogram in the diagnosis and management of patients with hypertrophic cardiomyopathy. Heart Rhythm. 2020;17:142-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 81] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 42. | Cui H, Schaff HV, Lentz Carvalho J, Nishimura RA, Geske JB, Dearani JA, Lahr BD, Lee AT, Bos JM, Ackerman MJ, Ommen SR, Maleszewski JJ. Myocardial Histopathology in Patients With Obstructive Hypertrophic Cardiomyopathy. J Am Coll Cardiol. 2021;77:2159-2170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 43. | Ariga R, Tunnicliffe EM, Manohar SG, Mahmod M, Raman B, Piechnik SK, Francis JM, Robson MD, Neubauer S, Watkins H. Identification of Myocardial Disarray in Patients With Hypertrophic Cardiomyopathy and Ventricular Arrhythmias. J Am Coll Cardiol. 2019;73:2493-2502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 93] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 44. | Li X, Lai L, Luo R, Yang H, Ma H, Yang Z, Zhao S, Su W, Hua W. The Clinical Prognosis of Presence and Location of Late Gadolinium Enhancement by Cardiac Magnetic Resonance Imaging in Patients with Hypertrophic Cardiomyopathy: a Single-Center Cohort Study. J Cardiovasc Transl Res. 2021;14:1001-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 45. | Hayıroğlu Mİ, Uzun AO, Keskin M, Börklü EB, Tekkeşin Aİ, Türkkan C, Kozan Ö. A simple independent prognostic electrocardiography parameter in first acute anterior myocardial infarction; Precordial total Q wave/precordial total R wave. J Electrocardiol. 2018;51:38-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |