Published online Jul 26, 2022. doi: 10.4330/wjc.v14.i7.403
Peer-review started: December 23, 2021
First decision: February 15, 2022
Revised: March 29, 2022
Accepted: June 17, 2022
Article in press: June 17, 2022
Published online: July 26, 2022
Processing time: 208 Days and 15 Hours
It remains unclear whether the current arbitrary screening recommendations of trastuzumab-related cardiotoxicity provides an adequate balance between preventing heart damage and curtailing a curative treatment.
To determine the incidence rate and consequences of trastuzumab-induced cardiotoxicity as adjuvant treatment in a real-world scenario.
We present a retrospective analysis of cardiac function measured by echocardiogram at baseline and every 3 mo during trastuzumab treatment. Cardiotoxicity was defined as a drop in left ventricular ejection fraction (LVEF) ≥ 10% from baseline and/or any drop < 50%.
Between January 2011 and December 2014, 407 patients were selected. Most (93.6%) were treated with an anthracycline followed by a taxane-based regimen and trastuzumab for 12 mo. Forty patients (9.8%) had cardiotoxicity. None of them were symptomatic, and 28 (72.5%) completely recovered LVEF. Cardiotoxicity happened early as shown by LVEF measured on echocardiogram 2 to 4 as compared to 5 to 7 (odds ratio = 2.47, 95% confidence interval: 1.09, 5.63, P = 0.024). There were 54 deaths (13.3%) during the 70-mo follow-up period; 1 (0.2%) was attributed to late cardiotoxicity (4 years after treatment). The absence of symptomatic cardiotoxicity during trastuzumab treatment and moreover the early occurrence on the treatment period may translate into a strategy to evaluate less frequently.
We observed a 10% rate of asymptomatic cardiotoxicity, which mirrors the results from the large adjuvant trials. Despite being transient, an LVEF drop led to frequent treatment delays and interruptions. It remains unclear whether LVEF decline is predictive of late cardiotoxicity, and treatment efficacy is compromised.
Core Tip: It remains unclear whether the current arbitrary screening recommendations for trastuzumab-related cardiotoxicity in early-stage HER2-positive breast cancer provides an adequate balance between preventing heart damage and curtailing a curative treatment. Real world data showed that despite a low rate of mainly early, asymptomatic and transient cardiotoxicity, treatment delays and interruptions occur due to these findings. The study results suggest optimization of cardiac monitoring after an initial period without a decrease in cardiac function.
- Citation: Rala de Paula BH, Costa METF, de Sousa CAM, Bines J. Is there a window of opportunity to optimize trastuzumab cardiac monitoring? World J Cardiol 2022; 14(7): 403-410
- URL: https://www.wjgnet.com/1949-8462/full/v14/i7/403.htm
- DOI: https://dx.doi.org/10.4330/wjc.v14.i7.403
Trastuzumab, a monoclonal antibody targeting HER2, represents a milestone in breast cancer treatment. The drug improves the progression-free and overall survival in metastatic and localized HER2-positive breast cancer[1,2]. Cardiotoxicity remains the most compromising side effect[3]. Myocardial HER2 receptors are associated with cardiac function protection physiologically[4]. Therefore, the drug administration could lead to a decrease in left ventricular ejection fraction (LVEF), usually reversible, although a few patients need to delay or permanently stop their ongoing treatment[4,5]. The incidence is around a quarter of patients receiving the drug, with a small percentage experiencing heart failure (1%-4%)[6]. Several factors can contribute, such as the chemotherapy regimen, particularly anthracycline, patient characteristics such as age, previous cardiovascular disease and low ejection fraction prior to treatment initiation[7].
Cardiotoxicity of anti-cancer treatment includes any toxicity affecting the heart[8] and suggested by biomarkers, such as decrease of LVEF or signs of heart failure[9]. Guidelines suggest a baseline pre-treatment evaluation and risk stratification, during treatment monitoring and post-treatment surveillance[2,10]. Although these recommendations mimic the schedules used in the clinical trials, the cardiac assessment was not supported by prospective data. Our study aimed to evaluate the cardiac function during trastuzumab treatment for early-stage breast cancer in a real-world scenario.
A retrospective chart review was performed using patient medical files from January 2010 to December 2014, at the Instituto Nacional de Cancer, Brazil. Patients had tissue confirmation of HER2-positive breast cancer, stage I to III, treatment with chemotherapy combined with and followed by trastuzumab. Exclusion criteria included loss to follow-up in less than 3 mo after treatment initiation.
Echocardiogram was performed at baseline and every 3 mo during trastuzumab treatment. It was performed by the same examiner and device [Siemens SONOLINE G 60, with P 4-2 cardiac probe (4.0-2.0 MHz)]. The analyses performed included the M-mode, 2D-mode, spectral doppler, color Doppler and tissue doppler imaging. The cavities dimensions were obtained according to the recommendation of the American Society of Echocardiography[11]. LVEF was calculated though the Teichholz Formulae. Cardiotoxicity was defined as: a 10% drop in LVEF from the baseline echo, a drop below 50% or symptoms according to the New York Heart Association class III or IV[12,30].
The study was approved by the institutional review board and conducted in accordance with the Good Clinical Practice Guidelines and the Helsinki declaration.
Numerical variables were reported by central tendency measures, and categorical variables were represented by absolute frequency and percentages. A bar plot containing the percentage of cardiotoxicity detected by echocardiograms at each scheduled measurement was performed to describe differences between patients that developed cardiotoxicity. A univariate analysis using the χ2 method for categorical variables and two sample t-test for continuous variables were initially performed to test the association between cardiotoxicity and potential confounders in clinical practice (age, comorbidities, body mass index).
To verify statistical differences in cardiotoxicity during the follow-up time, odds ratio was calculated to show differences in cardiac event odds in the beginning vs end of screening period. The prevalence ratio was calculated using the Wald[13] and Score[14] methods. The R statistical software was used to calculate the odds ratio and prevalence measures using the epiR package[15].
From 423 eligible patients, 16 were excluded (7 with metastatic disease and 9 lost to follow-up), with 407 remaining for the final analysis. The median age was 52 years, and the body mass index was 27.54 kg/m2. The stage at presentation was predominantly stage III: 59.47%. Most tumors were invasive ductal carcinoma (98.64%), and an anthracycline followed by taxane-based regimen was the most common treatment (93.6%). Almost all patients received trastuzumab for the whole 1-year period (97.0%) (Table 1).
| Variable | Absolute number | Percentage |
| Age | ||
| Minimum | 18 | - |
| Median | 52 | - |
| Maximum | 79 | - |
| Body mass index | ||
| Minimum | 14.27 | - |
| Median | 27.54 | - |
| Maximum | 50.58 | - |
| Comorbidities | ||
| Hypertension | 56 | 13.75 |
| Diabetes | 19 | 4.66 |
| Lack of other comorbidities reported | 330 | 81.10 |
| Menopausal status | ||
| Post-menopausal | 304 | 74.69 |
| Pre-menopausal | 103 | 25.31 |
| Histological subtypes | ||
| Invasive ductal carcinoma | 400 | 98.28 |
| Invasive lobular carcinoma | 5 | 1.22 |
| Others | 2 | 0.50 |
| Clinical stage | ||
| I | 36 | 8.86 |
| II | 129 | 31.69 |
| III | 242 | 59.45 |
| Chemotherapy regimen | ||
| Anthracycline and taxane based | 381 | 93.61 |
| Non-anthracycline based | 26 | 6.39 |
| Chemotherapy purpose | ||
| Neoadjuvant | 204 | 50.12 |
| Adjuvant | 203 | 48.88 |
| Adjuvant radiotherapy | ||
| Yes | 213 | 52.33 |
| No | 194 | 47.67 |
| Trastuzumab duration | ||
| 1 yr | 395 | 97.06 |
| Less than 1 yr | 12 | 2.94 |
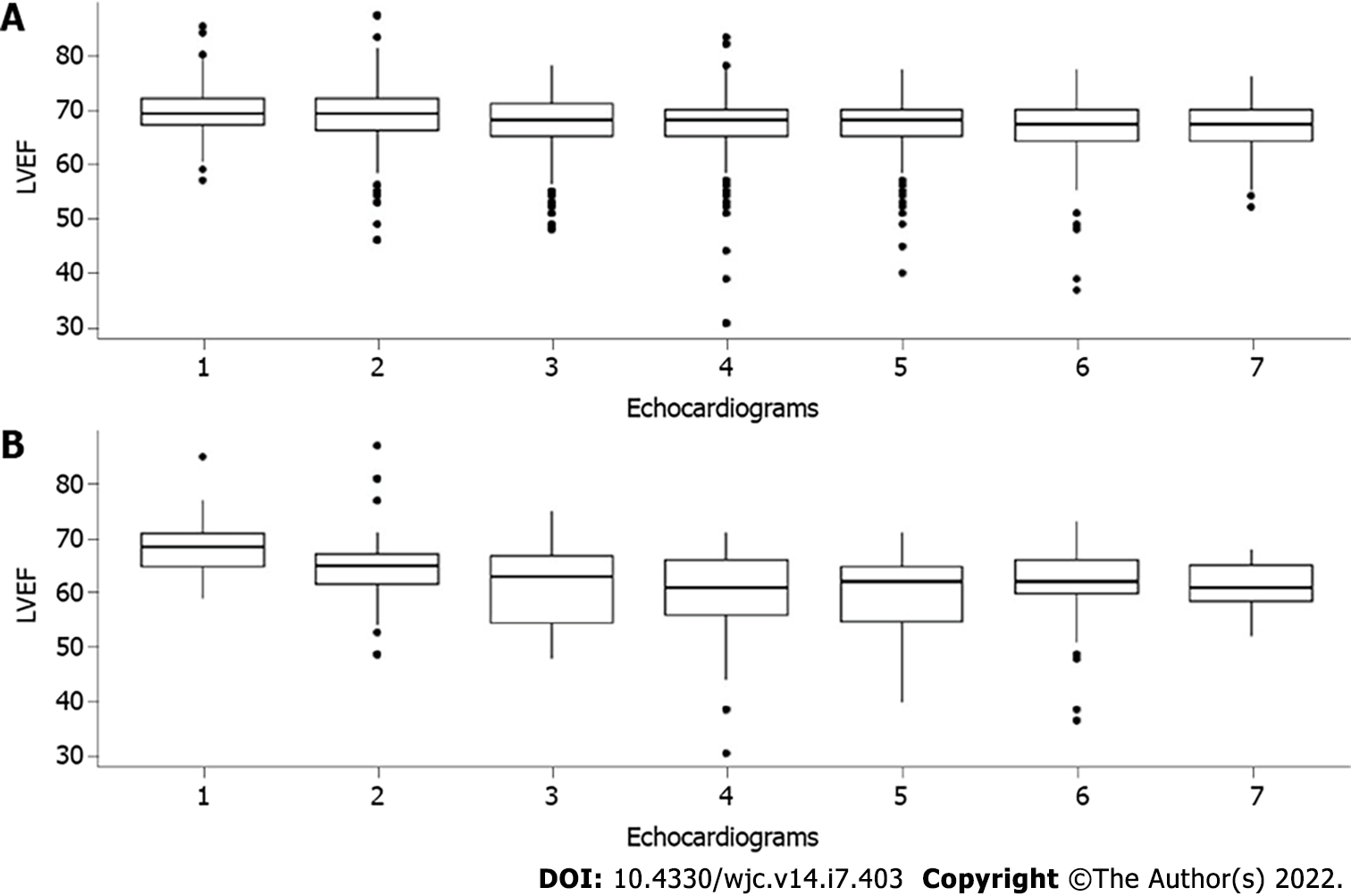
Forty patients (9.8%) had cardiotoxicity at a median time of 289 d (114-680 dc) from treatment initiation, and a wider variation of LVEF was seen in cardiotoxicity patients as shown by Figure 1. Although none of these patients were symptomatic, all of them had their treatment delayed due to the echocardiogram findings. Twenty-nine patients (72.5%) recovered the LVEF, for which the drug was restarted, and 11 (27.5%) had trastuzumab suspended. The rates of cardiotoxicity did not vary according to age (P = 0.58), comorbidities (P = 0.81) or body mass index (P = 0.64).
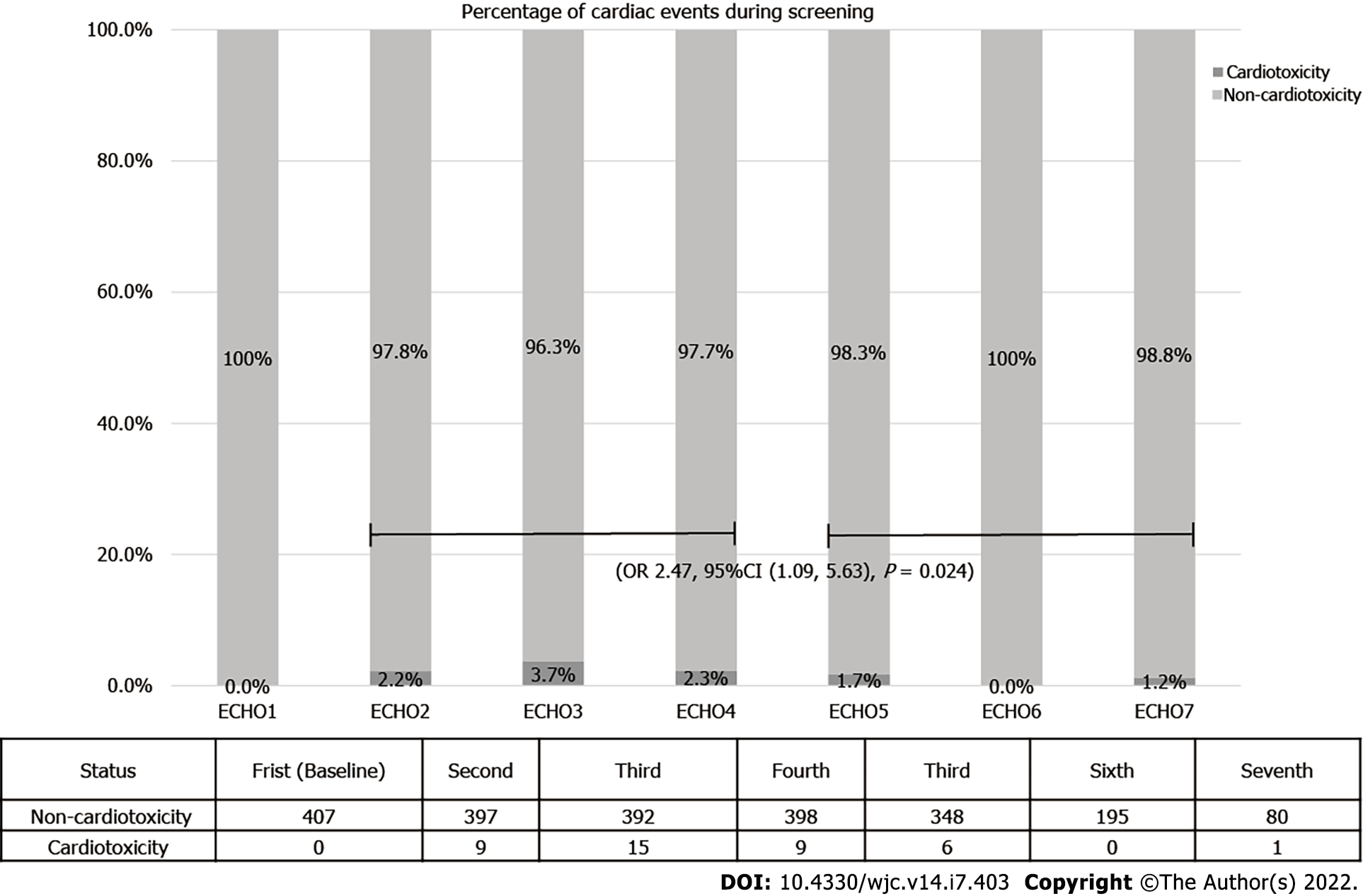
Cardiotoxicity occurred early, as shown in Figure 2. The prevalence in echocardiograms 2 to 4 was 2.7% against 1.1% prevalence ratio in echocardiograms 5 to 7. The odds of cardiotoxicity were 2.5 times higher when echocardiogram 2 to 4 were compared to echocardiogram 5 to 7 (odds ratio = 2.47, 95% confidence interval: 1.09, 5.63, P = 0.024). The median follow-up time was 70 mo, and there were 54 deaths (13.3%). Overall, survival did not vary according to cardiotoxicity (P = 0.08). One death (0.2%) was attributed to heart failure. However, it occurred 4 years after the end of trastuzumab treatment, possibly related to late anthracycline cardiotoxicity.
We showed a 9.8% cardiotoxicity rate detected by routine echocardiogram during trastuzumab-based treatment for early-stage breast cancer. To our knowledge, this is the largest real-world cohort reported in South America. Our results were similar to the ones presented by the large breast cancer adjuvant clinical trial 3, which varied from 6.0% to 35.4%[16,17]. This wide variation could be explained by the different chemotherapy regimens but more likely attributed to patient selection and diverse definitions of cardiotoxicity[18]. Whilst contemporary studies focus on predictive biomarkers (i.e. plasma levels of troponin and/or brain natriuretic peptide) and more costly imaging studies (i.e. cardiac magnetic resonance imaging), transthoracic echocardiogram is widely available with an affordable cost, which allows its widespread use[19]. Although the pathophysiology of cardiotoxicity is being elucidated, and players such as neuregulin[20] have been suggested. Reliable and validated biomarkers with a better cost-effectiveness than LVEF estimation are awaited[21].
Recommendations to withhold trastuzumab in Europe (absolute LVEF decrease > 20% or > 10% to < 50% or symptomatic heart failure)[22] and America (LVEF decrease ≥ 16% from baseline or LVEF below institutional limits of normality and ≥ 10% absolute LVEF decreased from baseline)[23] are roughly similar in not considering borderline asymptomatic decrease in LVEF. None of the patients in this cohort had symptoms at the time of the abnormal echocardiographic findings. Notwithstanding, it led to treatment delays and interruptions based on the guidelines available. The consequences of asymptomatic LVEF drop are unknown as well as whether early trastuzumab treatment interruption may compromise its efficacy[24,25]. More recent trials showed less clinical cardiac dysfunction in shorter trastuzumab treatment duration compared to longer trastuzumab treatment duration[26].
There are known risk factors associated with trastuzumab-related cardiotoxicity such as age above 65, Ile655Val HER2 polymorphism, previous cardiovascular disease, radiation therapy and the use of anthracycline, especially high cumulative doses[27,28]. In our cohort, we were unable to show such a correlation. The studies on the other hand are conflicting about other factors such as other comorbidities (diabetes or kidney function impaired) or baseline LVEF (high or low)[3]. We interpret these factors with caution once the standard of care population is significantly heterogeneous and frequently differs from the subjects included in clinical trials. Moreover, specific recommendations to adapt cardiac monitoring is lacking, unless the patient has a high cardiotoxicity risk[29].
Of note, we showed an increased incidence of cardiotoxicity in the early monitoring as compared to the later cardiac function evaluation through echocardiogram. As there is a lack of prospective randomized clinical trials for optimal cardiac monitoring[30], our results provide an opportunity to such an endeavor. Our study limitations included its retrospective nature, the limited number of patients and the lack of standard reporting of comorbidities. On the other hand, similar studies suggest that a population to optimize monitoring might exist[31].
The cardiotoxicity rates in a real-world population were similar to those reported by the large adjuvant trastuzumab trials. Most events occurred early during the initial monitoring examinations. As these findings led to treatment changes with unknown long-term consequences. These results deserve a prospective confirmation to assess the optimal way to monitor and manage trastuzumab-related cardiac events.
It remains unclear whether the current arbitrary screening recommendations of trastuzumab-related cardiotoxicity provides an adequate balance between preventing heart damage and curtailing a curative treatment.
There is an urgent need to optimize monitoring of cardiotoxicity.
This study aimed to determine the incidence rate and consequences of trastuzumab-induced cardiotoxicity as adjuvant treatment in a real-world scenario.
A retrospective chart review was performed using patient medical files during 5 years at a single institution in Brazil. Patients had tissue confirmation of HER2-positive breast cancer, stage I to III, treatment with chemotherapy combined with and followed by trastuzumab. Exclusion criteria included loss to follow-up in less than 3 mo after treatment initiation.
Forty patients (9.8%) had cardiotoxicity (out of 407 included). None of them were symptomatic, and 28 (72.5%) completely recovered left ventricular ejection fraction. Cardiotoxicity happened early as shown by left ventricular ejection fraction measured on echocardiogram 2 to 4 as compared to 5 to 7 (odds ratio = 2.47, 95% confidence interval: 1.09, 5.63, P = 0.024). There were 54 deaths (13.3%) during the 70-mo follow-up period; 1 (0.2%) was attributed to late cardiotoxicity (4 years after treatment).
The absence of symptomatic cardiotoxicity during trastuzumab treatment and moreover the early occurrence on the treatment period may translate into a strategy to evaluate less frequent cardiac monitoring.
This data alongside similar studies in the literature warrants a prospective evaluation of a de-escalation of cardiotoxicity monitoring in a selected population.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Pradhan A, India; Wang Y, China S-Editor: Wu YXJ L-Editor: Filipodia A P-Editor: Wu YXJ
| 1. | Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8204] [Cited by in RCA: 8139] [Article Influence: 339.1] [Reference Citation Analysis (0)] |
| 2. | National Comprehensive Cancer Network (nccn) NCCN Clinical Practice Guidelines in Oncology: Breast Cancer Ver. 1.2019. (2019) Fort Washington, PA: NCCN. Available from: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. |
| 3. | Onitilo AA, Engel JM, Stankowski RV. Cardiovascular toxicity associated with adjuvant trastuzumab therapy: prevalence, patient characteristics, and risk factors. Ther Adv Drug Saf. 2014;5:154-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 4. | Hudis CA. Trastuzumab--mechanism of action and use in clinical practice. N Engl J Med. 2007;357:39-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1728] [Cited by in RCA: 1925] [Article Influence: 106.9] [Reference Citation Analysis (0)] |
| 5. | Florescu M, Cinteza M, Vinereanu D. Chemotherapy-induced Cardiotoxicity. Maedica (Bucur). 2013;8:59-67. [PubMed] |
| 6. | Yu AF, Flynn JR, Moskowitz CS, Scott JM, Oeffinger KC, Dang CT, Liu JE, Jones LW, Steingart RM. Long-term Cardiopulmonary Consequences of Treatment-Induced Cardiotoxicity in Survivors of ERBB2-Positive Breast Cancer. JAMA Cardiol. 2020;5:309-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 7. | Armenian SH, Lacchetti C, Lenihan D. Prevention and Monitoring of Cardiac Dysfunction in Survivors of Adult Cancers: American Society of Clinical Oncology Clinical Practice Guideline Summary. J Oncol Pract. 2017;13:270-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 8. | National Cancer Institute. Bethesda, MD: NCI Dictionaries; [cited March 29 2022]. Available from: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/cardiotoxicity. |
| 9. | Bovelli D, Plataniotis G, Roila F; ESMO Guidelines Working Group. Cardiotoxicity of chemotherapeutic agents and radiotherapy-related heart disease: ESMO Clinical Practice Guidelines. Ann Oncol. 2010;21 Suppl 5:v277-v282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 282] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 10. | Zamorano JL, Lancellotti P, Rodriguez Muñoz D, Aboyans V, Asteggiano R, Galderisi M, Habib G, Lenihan DJ, Lip GY, Lyon AR, Lopez Fernandez T, Mohty D, Piepoli MF, Tamargo J, Torbicki A, Suter TM, Zamorano JL, Achenbach S, Agewall S, Badimon L, Barón-Esquivias G, Baumgartner H, Bax JJ, Bueno H, Carerj S, Dean V, Erol Ç, Fitzsimons D, Gaemperli O, Kirchhof P, Kolh P, Nihoyannopoulos P, Ponikowski P, Roffi M, Vaz Carneiro A, Windecker S; Authors/Task Force Members; ESC Committee for Practice Guidelines (CPG); Document Reviewers. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur J Heart Fail. 2017;19:9-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 285] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 11. | Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3897] [Cited by in RCA: 5495] [Article Influence: 549.5] [Reference Citation Analysis (0)] |
| 12. | Dolgin M; Association NYH; Fox AC, Gorlin R, Levin RI; New York Heart Association. Criteria Committee. Nomenclature and criteria for diagnosis of diseases of the heart and great vessels. 9th ed. Boston, MA: Lippincott Williams and Wilkins; 1994. |
| 13. | Wald A. Tests of statistical hypotheses concerning several parameters when the number of observations is large. Trans Am Math Soc. 1943;54:426-482. [DOI] [Full Text] |
| 14. | Miettinen O, Nurminen M. Comparative analysis of two rates. Stat Med. 1985;4:213-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 735] [Cited by in RCA: 812] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 15. | R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. (2019). Available from: https://www.Rproject.org/. |
| 16. | Joensuu H, Kellokumpu-Lehtinen PL, Bono P, Alanko T, Kataja V, Asola R, Utriainen T, Kokko R, Hemminki A, Tarkkanen M, Turpeenniemi-Hujanen T, Jyrkkiö S, Flander M, Helle L, Ingalsuo S, Johansson K, Jääskeläinen AS, Pajunen M, Rauhala M, Kaleva-Kerola J, Salminen T, Leinonen M, Elomaa I, Isola J; FinHer Study Investigators. Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med. 2006;354:809-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1069] [Cited by in RCA: 1006] [Article Influence: 52.9] [Reference Citation Analysis (0)] |
| 17. | Spielmann M, Roché H, Delozier T, Canon JL, Romieu G, Bourgeois H, Extra JM, Serin D, Kerbrat P, Machiels JP, Lortholary A, Orfeuvre H, Campone M, Hardy-Bessard AC, Coudert B, Maerevoet M, Piot G, Kramar A, Martin AL, Penault-Llorca F. Trastuzumab for patients with axillary-node-positive breast cancer: results of the FNCLCC-PACS 04 trial. J Clin Oncol. 2009;27:6129-6134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 230] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 18. | Chung R, Ghosh AK, Banerjee A. Cardiotoxicity: precision medicine with imprecise definitions. Open Heart. 2018;5:e000774. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 19. | Dang CT, Yu AF, Jones LW, Liu J, Steingart RM, Argolo DF, Norton L, Hudis CA. Cardiac Surveillance Guidelines for Trastuzumab-Containing Therapy in Early-Stage Breast Cancer: Getting to the Heart of the Matter. J Clin Oncol. 2016;34:1030-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 20. | Cote GM, Sawyer DB, Chabner BA. ERBB2 inhibition and heart failure. N Engl J Med. 2012;367:2150-2153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 21. | de Paula BDHR, Bandeira, LLB, Ramos AP, Pinheiro BLM, Ferreira RM, de Oliveira Araújo II, da Costa PCV, Crocamo S. Contemporaneous Screening Options of Cardiotoxicity Related to Oncological Treatment. Revista Brasileira de Cancerologia. 2019;65:07388. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 22. | Curigliano G, Lenihan D, Fradley M, Ganatra S, Barac A, Blaes A, Herrmann J, Porter C, Lyon AR, Lancellotti P, Patel A, DeCara J, Mitchell J, Harrison E, Moslehi J, Witteles R, Calabro MG, Orecchia R, de Azambuja E, Zamorano JL, Krone R, Iakobishvili Z, Carver J, Armenian S, Ky B, Cardinale D, Cipolla CM, Dent S, Jordan K; ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann Oncol. 2020;31:171-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 612] [Cited by in RCA: 649] [Article Influence: 129.8] [Reference Citation Analysis (0)] |
| 23. | Kondapalli L. Cardiotoxicity: an unexpected consequence of HER2-targeted therapies. 2021. Available from: https://www.acc.org/Latest-in-cardiology/articles/2016/06/06/09/32/cardiotoxicity. |
| 24. | Moilanen T, Jokimäki A, Tenhunen O, Koivunen JP. Trastuzumab-induced cardiotoxicity and its risk factors in real-world setting of breast cancer patients. J Cancer Res Clin Oncol. 2018;144:1613-1621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 25. | Gong IY, Verma S, Yan AT, Ko DT, Earle CC, Tomlinson GA, Trudeau ME, Krahn MD, Krzyzanowska MK, Brezden-Masley CB, Gavura S, Peacock S, Chan KK. Long-term cardiovascular outcomes and overall survival of early-stage breast cancer patients with early discontinuation of trastuzumab: a population-based study. Breast Cancer Res Treat. 2016;157:535-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Earl HM, Hiller L, Vallier AL, Loi S, McAdam K, Hughes-Davies L, Harnett AN, Ah-See ML, Simcock R, Rea D, Raj S, Woodings P, Harries M, Howe D, Raynes K, Higgins HB, Wilcox M, Plummer C, Mansi J, Gounaris I, Mahler-Araujo B, Provenzano E, Chhabra A, Abraham JE, Caldas C, Hall PS, McCabe C, Hulme C, Miles D, Wardley AM, Cameron DA, Dunn JA; PERSEPHONE Steering Committee and Trial Investigators. 6 vs 12 mo of adjuvant trastuzumab for HER2-positive early breast cancer (PERSEPHONE): 4-year disease-free survival results of a randomised phase 3 non-inferiority trial. Lancet. 2019;393:2599-2612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 213] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 27. | Pondé NF, Lambertini M, de Azambuja E. Twenty years of anti-HER2 therapy-associated cardiotoxicity. ESMO Open. 2016;1:e000073. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 28. | Miller L. Searching for the Key to Trastuzumab Cardiotoxicity in HER2+ Breast Cancer Targeted therapies in oncology February 2017, published online in March 2017. Available from: https://www.targetedonc.com/publications/targeted-therapynews/2017/february-2017/searching-for-the-key-totrastuzumab-cardiotoxicityin-her2-breast-cancer. |
| 29. | Kim DY, Park MS, Youn JC, Lee S, Choi JH, Jung MH, Kim LS, Kim SH, Han S, Ryu KH. Development and Validation of a Risk Score Model for Predicting the Cardiovascular Outcomes After Breast Cancer Therapy: The CHEMO-RADIAT Score. J Am Heart Assoc. 2021;10:e021931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 30. | Curigliano G, Cardinale D, Suter T, Plataniotis G, de Azambuja E, Sandri MT, Criscitiello C, Goldhirsch A, Cipolla C, Roila F; ESMO Guidelines Working Group. Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy: ESMO Clinical Practice Guidelines. Ann Oncol. 2012;23 Suppl 7:vii155-vii166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 523] [Cited by in RCA: 559] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 31. | Bouwer NI, Steenbruggen TG, van Rosmalen J, Rier HN, Kitzen JJEM, van Bekkum ML, Tije AJT, de Jong PC, Drooger JC, Holterhues C, Smorenburg CH, Kofflard MJM, Boersma E, Sonke GS, Levin MD, Jager A. Cardiotoxicity during long-term trastuzumab use in patients with HER2-positive metastatic breast cancer: who needs cardiac monitoring? Breast Cancer Res Treat. 2021;186:851-862. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |