Published online Jul 26, 2022. doi: 10.4330/wjc.v14.i7.382
Peer-review started: January 16, 2022
First decision: March 16, 2022
Revised: March 30, 2022
Accepted: June 18, 2022
Article in press: June 18, 2022
Published online: July 26, 2022
Processing time: 185 Days and 0.9 Hours
Myocarditis is now recognized as a rare complication of coronavirus disease 2019 (COVID-19) mRNA vaccination, particularly in adolescent and young adult males. Since the authorization of the Pfizer-BioNTech™ and Moderna™ mRNA vaccines targeting the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) spike protein, the Centers for Disease Control and Prevention (CDC) has reported 1175 confirmed cases of myocarditis after COVID-19 vaccination in individuals ages 30 years and younger as of January 2022. According to CDC data in June 2021, the incidence of vaccine-mediated myocarditis in males ages 12-29 years old was estimated to be 40.6 cases per million second doses of COVID-19 mRNA vaccination administered. Individuals with cases of COVID-19 vaccine-mediated myocarditis typically present with acute chest pain and elevated serum troponin levels, often within one week of receiving the second dose of mRNA COVID-19 vaccination. Most cases follow a benign clinical course with prompt resolution of symptoms. Proposed mechanisms of COVID-19 vaccine myocarditis include molecular mimicry between SARS-CoV-2 spike protein and self-antigens and the triggering of preexisting dysregulated immune pathways in predisposed individuals. The higher incidence of COVID-19 vaccine myocarditis in young males may be explained by testosterone and its role in modulating the immune response in myocarditis. There is limited data on long-term outcomes in these cases given the recency of their occurrence. The CDC continues to recommend COVID-19 vaccination for everyone 5 years of age and older given the greater risk of serious complications related to natural COVID-19 infection including hospitalization, multisystem organ dysfunction, and death. Further study is needed to better understand the immunopathology and long-term outcomes behind COVID-19 mRNA vaccine-mediated myocarditis.
Core Tip: In this review article, we aim to synthesize the current literature surrounding coronavirus disease 2019 (COVID-19) vaccine-mediated myocarditis. COVID-19 mRNA vaccination has been associated with increased cases of myocarditis, particularly in the adolescent and young adult male population. Presentation typically occurs several days following administration of the second dose of a COVID-19 mRNA vaccination. As the world continues to vaccinate against COVID-19, understanding this vaccine-related adverse event is clinically important. Potential mechanisms are reviewed, and current clinical recommendations are discussed.
- Citation: Morgan MC, Atri L, Harrell S, Al-Jaroudi W, Berman A. COVID-19 vaccine-associated myocarditis. World J Cardiol 2022; 14(7): 382-391
- URL: https://www.wjgnet.com/1949-8462/full/v14/i7/382.htm
- DOI: https://dx.doi.org/10.4330/wjc.v14.i7.382
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), the novel virus responsible for the coronavirus disease 2019 (COVID-19) pandemic, has impacted the entire globe and continues to spread. On December 11, 2020, the United States Food and Drug Administration granted an emergency use authorization (EUA) for the Pfizer-BioNTech™ COVID-19 vaccine in individuals 16 years of age or older. Seven days later, another EUA was released for the Moderna™ vaccine in adults 18 years of age or older[1]. Since their introduction, the mRNA vaccines against the SARS-CoV-2 virus have been highly effective in preventing both symptomatic and asymptomatic infections along with COVID-19-related hospitalizations and death[2]. Despite the great success of these vaccines, they have not come onto the public stage without controversy. In May 2021, the first case of myocarditis following mRNA vaccination was identified, and as of January 12, 2022, the Vaccine Adverse Events Reporting System (VAERS) had received 2077 reports of myocarditis or pericarditis among people ages 30 and younger who received a COVID-19 vaccine with 1175 confirmed cases[3]. This article aims to review the current literature regarding COVID-19 vaccine-related myocarditis.
While the possibility for developing myocarditis or pericarditis following COVID-19 vaccination is concerning, it is important to emphasize that the incidence of this adverse effect is rare. Since January 2022 there have been over 502 million doses of COVID-19 mRNA vaccines administered across the United States with less than 1175 confirmed cases of myocarditis or pericarditis[3]. The primary group being impacted by this adverse event is the male adolescent and young adult population, ages 12-29[4-8]. The principal window of risk for the development of COVID-19 vaccine-mediated myocarditis appears to be within a week of receipt of the vaccine and occurs most commonly following the second dose of an mRNA vaccine[4,5,7,9]. Affected young men are predominately healthy individuals without a history of COVID-19 infection or comorbidities. Resolution of clinical symptoms usually occurs within 6 d with preservation of cardiac function, indicative of overall fast recovery with no short-term complications[4,5,8].
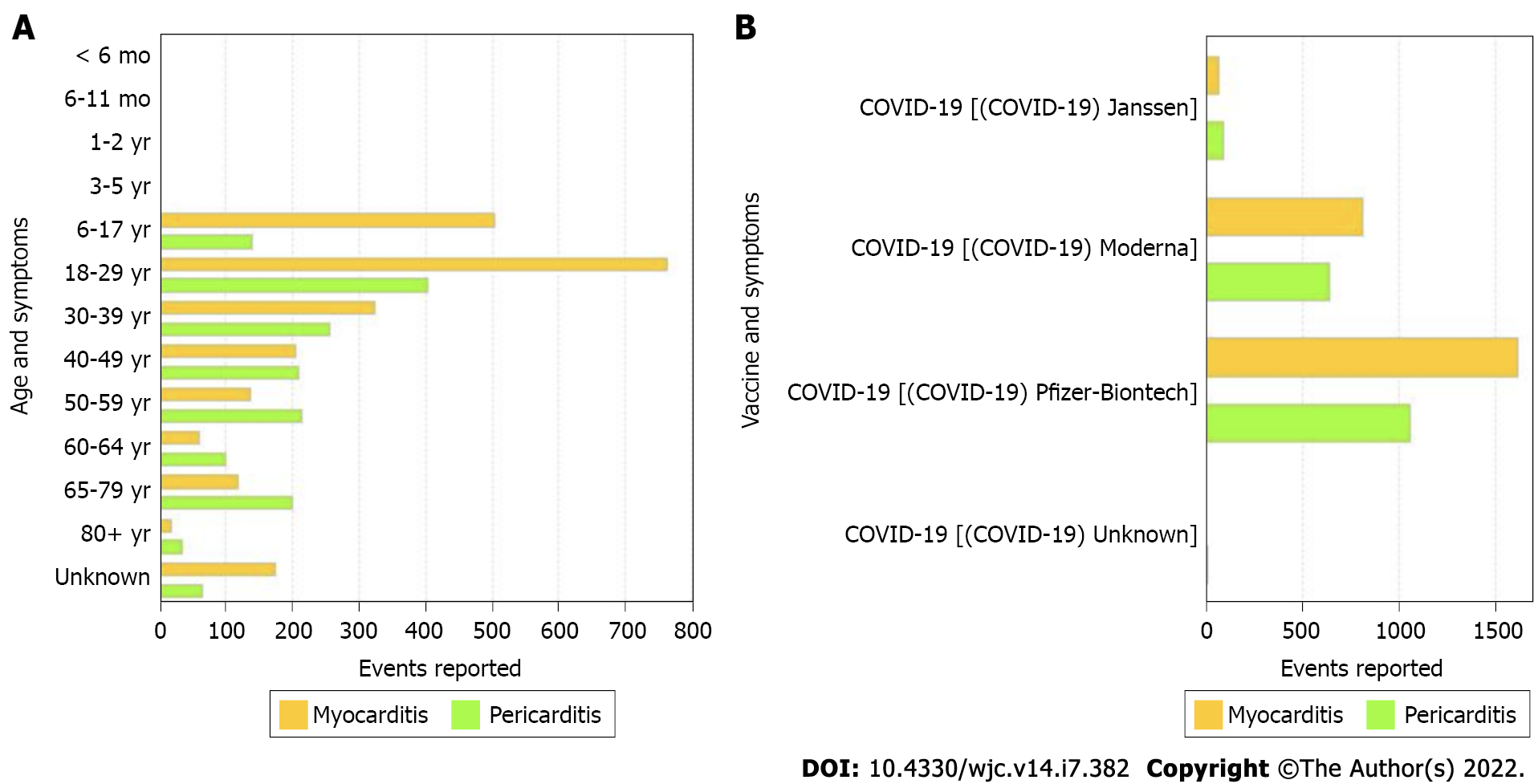
It is challenging to calculate the true incidence of vaccine-related myocarditis in the United States, as currently reported case series are not population-based. Based on crude data with both confirmed and unconfirmed cases reported to the VAERS, the CDC has estimated the incidence rates of myocarditis to be 40.6 cases per million second doses of mRNA COVID-19 vaccines administered to males between 12 and 29 years old[1]. Females in the same age group had an estimated incidence of 4.2 cases of myocarditis per million second doses. In adults 30 years and older, rates of myocarditis were reported as 2.4 cases per million second doses in males and 1.0 case per million second doses in females. As of December 31, 2021, VAERS has processed 4317 reported events of COVID-19 vaccine-associated myocarditis and pericarditis across all age groups with the highest number of cases reported for both myocarditis and pericarditis in the age group 18-29 (Figure 1A)[9].
Investigators from Israel queried the database of the largest Israeli healthcare organization that contains data related to 2.5 million vaccinated individuals. They determined that post-vaccine myocarditis had an estimated incidence rate of 2.13 cases [95% confidence interval (CI): 1.56-2.70] per 100000 individuals who had received at least one dose of the Pfizer-BioNTech™ vaccine[10]. Additionally, the incidence increased to 10.69 cases per 100000 individuals (95%CI: 6.93-14.46) among males between 16 and 29 years old.
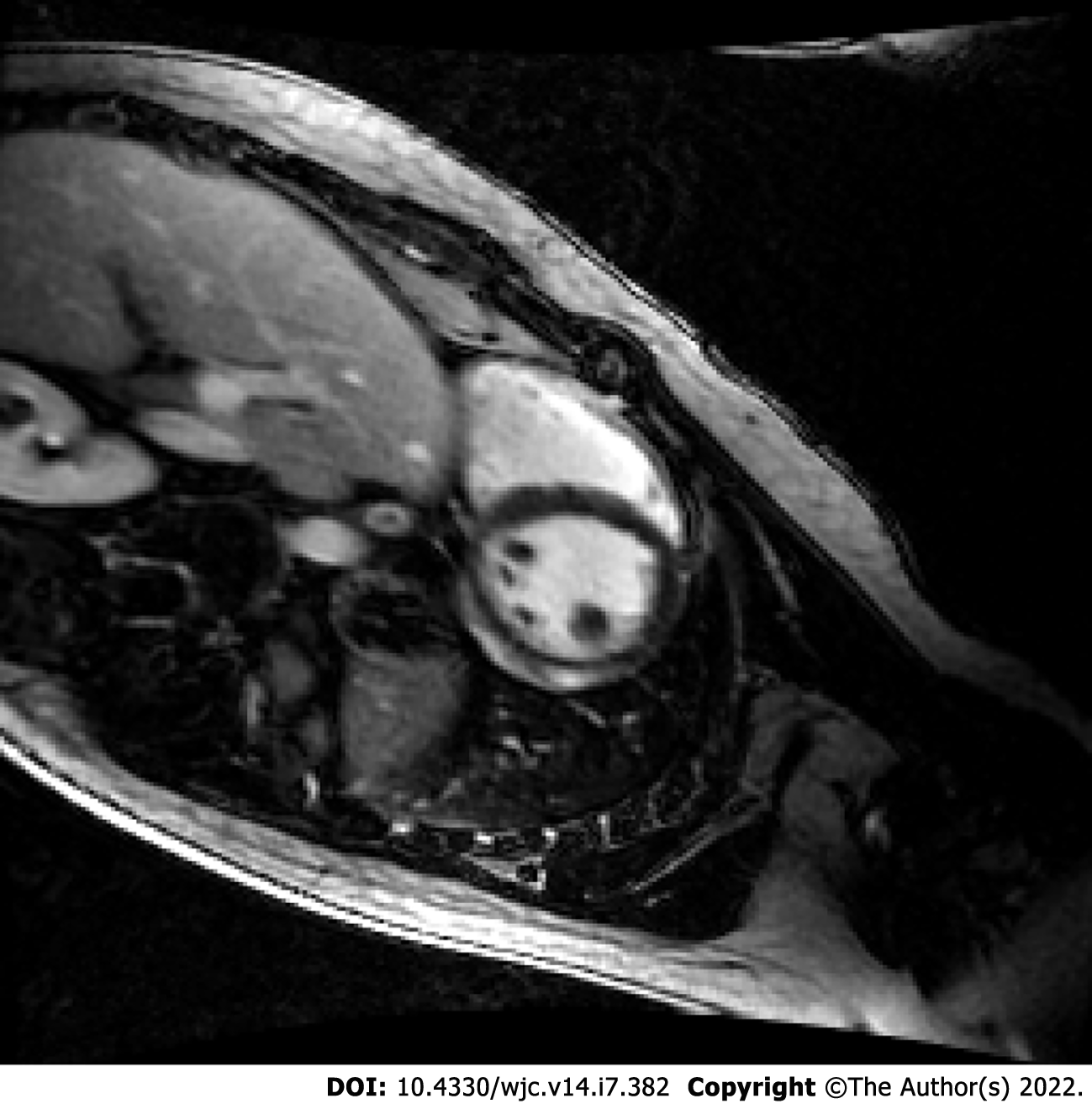
To compare the three types of vaccines, a systematic review of 6 case reports and 2 case series with a total of 15 patients reported that 60% of the myocarditis-related COVID-19 vaccine cases were associated with the Pfizer-BioNTech™ vaccine, 33% were associated with the Moderna™ vaccine, and 7% were associated with the Johnson & Johnson™ vaccine[5]. Similarly, as of December 31, 2021, VAERS indicated Pfizer-BioNTech™ had the highest number of cases reported for myocarditis and pericarditis, 1615 and 1063 cases respectively (Figure 1B)[11]. The clinical presentation of post-vaccine myocarditis is similar to other forms of myocarditis, most commonly featuring acute chest pain combined with other symptoms such as shortness of breath, fever, and palpitations[6,8,9,12,13]. Evidence of myocardial injury via serum troponin elevations was present in all cases. Electrocardiogram (EKG) findings were varied but often showed ST segment elevations. Echocardiogram findings ranged from preserved ejection fraction to varying degrees of wall motion abnormalities. When cardiac magnetic resonance imaging (MRI) was performed, findings were consistent with acute myocarditis with late gadolinium enhancement being the most commonly cited abnormality. Figure 2 displays a cardiac MRI consistent with myocarditis following COVID-19 vaccination in a 21-year-old male. Notably, most cases resulted in normalization of symptoms, troponin levels, and EKG/echocardiogram abnormalities upon discharge or at follow-up (Table 1). The CDC Vaccine Safety Technical Work Group Report on August 30, 2021, reviewed 98 cases with chest pain, pressure, and discomfort of which 56% of the cases met confirmatory criteria for myocarditis within 0-21 d of vaccination with elevated troponin, abnormal EKG findings, and abnormal MRI commonly found. It was determined that all of these included cases were discharged home, with 76% of them being discharged within 0-2 d[14].
| Ref. | Study design | Sample size | Main findings | Analysis |
| Salah and Mehta[5], 2021 | Systematic review of 6 case reports and 2 case series with a total of 15 patients | 15 individuals who developed myocarditis following a COVID-19 vaccine, regardless of the type or dose of the vaccine | (1) 60% of the myocarditis related COVID-19 vaccine cases were associated with the Pfizer-BioNTech™ vaccine, 33% were associated with the Moderna™ vaccine, and 7% were associated with the Johnson & Johnson™ vaccine; (2) All the myocarditis related to the Moderna™ vaccine (5/5) occurred following the second dose of the vaccine, whereas 6/9 (66.7%) of the myocarditis related to the Pfizer-BioNTech™ vaccine occurred following the second dose of the vaccine; (3) Peak cardiac troponin I level (ng/mL) was reported in 13/15 patients, and it ranged between 0.37 and 51.37 ng/mL (mean 12.9 ng/mL). Peak troponin T levels were reported in the other 2/15 patients and were 854 ng/L and 1693 ng/L; (4) Transthoracic echocardiogram in all these patients showed preserved LVEF; exact LVEF value was reported in 13/15 patients with a mean LVEF of 53.5% and a range of 48% to 65%. In the other 2/15 patients, the LVEF was reported as normal with no value; (5) There were no regional wall abnormalities in 14/15 of the patients; 1 patient had subtle apical septal and apical lateral hypokinesis with a LVEF of 52%; and (6) All patients recovered within 6 d of their presentation with complications reported | (1) Myocarditis related to COVID-19 vaccines mostly occurs in young male individuals following the 2nd dose of the vaccine; (2) Myocarditis related to COVID-19 vaccines mostly occurs with mRNA vaccines (i.e., Pfizer-BioNTech™ and Moderna™ COVID-19 vaccines); (3) In all the reported cases of myocarditis related to COVID-19 vaccine, clinical symptoms resolved within 6 d with preservation of the cardiac function; and (4) No complications were reported in any of these patients showing that myocarditis related to COVID-19 vaccine has an overall fast recovery with no short-term complications |
| Mevorach et al[4], 2021 | Retrospective review of myocarditis cases from the Israeli Ministry of Health database between December 2020 and May 2021 | 142 Israeli patients diagnosed with myocarditis within 21 d of receiving the first dose of Pfizer-BioNTech™ vaccine or 30 d of receiving the second dose | (1) In the 136 cases of definite or probable myocarditis with recent vaccination, the clinical presentation in 129 was generally mild, with resolution of myocarditis in most cases, as judged by clinical symptoms and inflammatory markers and troponin elevation, electrocardiographic and echocardiographic normalization, and a relatively short length of hospital stay; one fulminant case was fatal; (2) As compared with the expected incidence of myocarditis based on historical data, the standardized incidence ratio was 5.34 (95%CI: 4.48-6.40) and was highest after the second dose in male recipients between the ages of 16 and 19 yr (13.60; 95%CI: 9.30-19.20); and (3) Definite or probable cases of myocarditis among persons between the ages of 16 and 19 yr within 21 d after the second vaccine dose occurred in approximately 1 of 6637 male recipients and in 1 of 99853 female recipients | (1) There was a slight increase in the incidence of myocarditis after the Pfizer-BioNTech™ vaccine, particularly after the second dose among young male recipients; and (2) The incidence of myocarditis after two doses of the Pfizer-BioNTech™ mRNA vaccine was low but higher than the incidence among unvaccinated persons and among historical controls, driven primarily by young males after receiving their second dose |
| Rosner et al[6], 2021 | Case series of 7 patients hospitalized for acute myocarditis-like illness after COVID-19 vaccination from 2 United States medical centers | Seven males, all < 40 years old | (1) Six patients received an mRNA vaccine (Moderna™ or Pfizer-BioNTech™), and 1 received the adenovirus vaccine (Johnson and Johnson™); (2) All patients presented 3 to 7 d after vaccination with acute onset chest pain and troponin elevations; EKG varied from normal to 1 mm ST segment elevations; Echocardiograms showed left ventricular ejection fraction ranging from 35% to 62%, with 5 of 7 having some degree of hypokinesis; (3) Multifocal subepicardial late gadolinium enhancement was present in 7 of 7 patients and additional midmyocardial late gadolinium enhancement was found in 4 of 7 patients; and (4) Treatment included β-blocker and anti-inflammatory medication. Hospital length of stay was 3 ± 1 d, and all patients’ symptoms resolved by hospital discharge | (1) There is a potential causal association with vaccination given the temporal relationship, clinical presentation, and cardiac magnetic resonance imaging findings; (2) Vaccine-associated myocarditis appears to have a favorable clinical course; and (3) The benefits of vaccination outweigh the risks of vaccine-related myocarditis in younger adults given the potential morbidity of COVID-19 infection |
| Dionne et al[7], 2021 | Case series of 15 adolescents at single United States center | 15 adolescents ages 12-18 years old hospitalized with myocarditis after receiving Pfizer-BioNTech™ COVID-19 vaccine | (1) 14 children were male, and 1 child was female; (2) Symptoms started 1-6 d following vaccine administration (14 of 15 occurring after second dose); common symptoms included chest pain, fever, myalgia, and headache; (3) Elevated troponins found in all 15 cases; (4) Cardiac MRI findings were consistent with myocarditis in 13 patients, with 12 patients showing evidence of late gadolinium enhancement; (5) No patients required intensive care and the median length of hospital stay was 2 d (range 1-5 d); and (6) At 1-to-13-d follow-up after hospital discharge, 11 patients had full resolution, 1 patient had persistent borderline low LV systolic dysfunction (EF = 54%), 3 patients had mildly elevated troponins, 1 patient had nonsustained ventricular tachycardia on ambulatory monitor | (1) Following Pfizer-BioNTech™ vaccination, most cases of myocarditis were diagnosed in male children after the second dose; (2) All patients had a benign clinical course; and (3) Long-term risks of post-vaccine myocarditis in the child population remains unknown |
The mechanism underlying COVID-19 vaccine-mediated myocarditis is poorly understood. SARS-CoV-2 mRNA vaccines contain nucleoside-modified mRNA encoding for the virus’s spike protein encapsulated in lipid nanoparticles which aid in delivery of the mRNA into the cell. The cell then produces the spike protein, and a subsequent adaptive immune response ensues generating antibodies against the spike protein. The nucleoside modification of the mRNA aids in reducing the mRNA’s immunogenicity, however in some individuals with an unknown genetic predisposition, exposure to the mRNA may result in an overactivated immune response via dendritic cells and Toll-like receptors of the innate immune system leading to proinflammatory immune cascades and cytokine activation[15]. This inflammatory response is thought to play a role in COVID-19 vaccine-associated myocarditis. The role of mRNA in the development of vaccine-mediated myocarditis is further supported by the evidence that the incidence of myocarditis occurs at a much higher rate following mRNA vaccination compared to the adenovirus vector vaccine of Johnson and Johnson™[4].
Another potential mechanism for COVID-19 vaccine myocarditis is molecular mimicry between the spike protein of SARS-CoV-2 and self-antigens. Antibodies of the SARS-CoV-2 spike protein have been shown to cross-react with human proteins of similar structure including α-myosin in experimental studies[16]. It appears more likely that the immune-mediated adverse effects of mRNA vaccination are due to the triggering of preexisting dysregulated pathways in certain predisposed individuals rather than the inherent immunogenicity of the vaccine itself[15]. Polymorphisms in interleukin-6 have been suggested as an important genetic component for determining autoinflammatory dysregulation that may ensue upon exposure to SARS-CoV-2, however further study is needed to elucidate these theories[17].
Young men have been found to be most susceptible to the development of myocarditis outside the setting of COVID-19 vaccination as well. Kytö et al[18] have presented evidence that testosterone appears to play a major role in the pathogenesis of myocarditis identifying testosterone-mediated mechanisms such as inhibition of anti-inflammatory cell populations promoting cardiac inflammation, a preference towards a Th1 immune response, and increased transcription of cardiac fibrotic remodeling genes[18]. Conversely, estrogen appears to play a protective role via the preference of a Th2 immune response, stimulation of inhibitory regulatory T cells, and inhibition of proinflammatory T cells[18]. These mechanisms may contribute to why the young male population has the highest incidence of post-vaccine myocarditis.
Cases of myocarditis have also been reported in the childhood population since the Pfizer-BioNTech™ COVID-19 vaccine was authorized for emergency use on May 10, 2021, for children ages 12 and older. A case series of 15 adolescents who developed myocarditis following administration of the Pfizer-BioNTech™ vaccine found that, similar to the adult population, the most commonly affected group were young males ages 12-18 years old following administration of the second dose[7]. All patients in this study had an uncomplicated short-term clinical course, however the long-term prognosis of these adolescent patients remains unclear, emphasizing the importance of continued follow-up and monitoring.
A case series published by Marshall et al[19] reported myocarditis or pericarditis in 7 male adolescents ages 14-19 years old, all within 4 d of receiving the second dose of the Pfizer-BioNTech™ COVID-19 vaccine[19]. All 7 patients presented with elevated troponin levels. ST segment elevation was the most common EKG and was observed in 6/7 individuals. Echocardiogram results were normal in 5 of 7 patients; however, all patients had cardiac MRI findings consistent with acute myocarditis. Investigatory studies for other etiologies of myocarditis including respiratory pathogen panels, serum polymerase chain reaction (PCR) tests, and infectious serologies all returned negative, and multisystem inflammatory syndrome in children was excluded based on cardiac MRI findings.
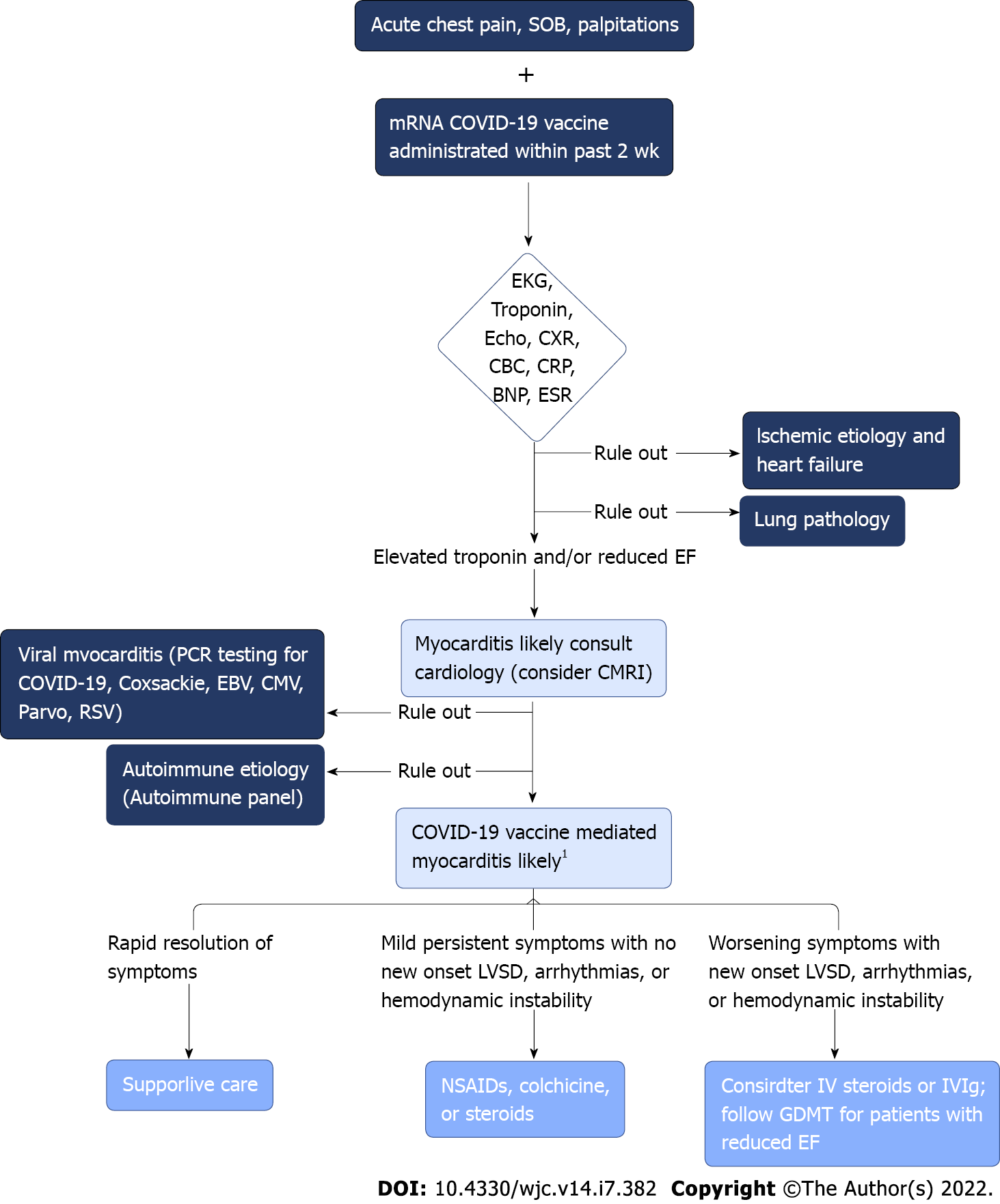
Given the increased incidence of myocarditis following mRNA vaccination in adolescent and young adult males, clinicians should have a high index of suspicion for myocarditis in this demographic who present with symptoms such as acute chest pain, shortness of breath, or palpitations. Initial evaluation should include obtaining an EKG, serum troponin levels, complete blood count with differential, chest x-ray, inflammatory markers such as C-reactive protein and erythrocyte sedimentation rate, brain natriuretic peptide, and an echocardiogram (Figure 3). If this initial workup supports a diagnosis of myocarditis, cardiology consultation should take place in conjunction with studies seeking to determine potential alternative etiologies of myocarditis. Consultation with infectious disease and/or rheumatology may also be considered to aid in this process[20]. PCR testing for acute COVID-19 infection and SARS-CoV-2 antibody testing for prior COVID-19 infection are of particular importance. Obtaining enterovirus PCR along with a respiratory pathogen panel can assist in ruling out other potential viral etiologies (e.g. Coxsackievirus, Epstein-Barr virus, cytomegalovirus, respiratory syncytial virus, parvovirus) and autoimmune serologies such as antinuclear antibodies may be indicated depending on clinical presentation. Cardiac MRI may be utilized to aid in diagnosing suspected myocarditis without the need for obtaining invasive endomyocardial biopsy.
The clinical management of COVID-19 vaccine-mediated myocarditis is largely supportive, and patients frequently exhibit rapid resolution of symptoms and normalization of cardiac biomarkers. Those with persistent mild symptoms and no signs of arrhythmia, left ventricular systolic dysfunction, or hemodynamic instability may benefit from therapy with nonsteroidal anti-inflammatory drugs, colchicine, or steroids. In more serious cases of myocarditis, such as those patients showing signs of hemodynamic instability, new-onset arrhythmia, or worsening systolic dysfunction, intravenous steroids or intravenous immunoglobulin may be considered[15]. Patients with reduced ejection fraction should be placed on beta-blockers and angiotensin-converting enzyme inhibitors according to guideline-directed medical therapy. The majority of cases from prior reports resulted in a resolution of symptoms and abnormal cardiac studies prior to discharge following a short hospital stay with supportive care or a short course of nonsteroidal anti-inflammatory. Close monitoring and avoidance of strenuous exercise until a complete resolution of symptoms and normalization of cardiac biomarkers, EKG, and echocardiogram is an important measure, especially in the young age group who may be eager to return to a normal exercise routine. If a patient develops myocarditis following a first dose of mRNA vaccination, the CDC recommends the second dose be delayed and reconsidered later following the complete resolution of signs and symptoms[20]. Of note, there are currently no randomized controlled trials examining the management of post-vaccine myocarditis which highlights the importance of the inclusion of cardiovascular specialists in the management and follow-up of these patients.
The concerted efforts of the biomedical community to develop safe and efficacious vaccinations in such a short time frame have been extraordinary. SARS-CoV-2 virus mRNA vaccines have been tremendously successful in curtailing the morbidity and mortality associated with COVID-19[21]. Healthy adolescents and young adults are not immune to serious complications from COVID-19 infection and rising adolescent hospitalization rates from COVID-19 infection have been observed[22]. Despite media attention regarding adverse effects of these vaccines, it is important to emphasize the low incidence in which these events occur. The benefits of vaccination to prevent both the spread and possible complications of COVID-19 infection including hospitalization, multisystem organ dysfunction, and death far outweigh the potential risk of post-vaccine myocarditis while this global pandemic persists.
The diagnosis of myocarditis is a serious one as cardiac myocytes do not regenerate, and an insult at a young age can lead to an increased risk of developing cardiac disease later on in life[23]. While myocarditis has been linked to COVID-19 mRNA vaccination, it is important to compare the risk of developing myocarditis following vaccination to the risk following natural COVID-19 infection. A large study in Israel used data from the nation’s largest healthcare organization to determine the risk of myocarditis following vaccination after adequately matching vaccinated individuals to unvaccinated individuals[24]. 42 d after vaccination, they found a risk ratio of 3.24; 95%CI: 1.55-12.44, and a risk difference of 2.7 events per 100000 persons; 95%CI: 1.0-4.6. To put this in context, they then determined the risk of myocarditis among SARS-CoV-2 infected individuals matched to uninfected individuals. COVID-19 infection was associated with a much higher risk of myocarditis with a risk ratio of 18.28; 95%CI: 3.95-25.12 and a risk difference of 11.0 events per 100000 persons; 95%CI: 5.6-15.8[24]. This large-scale study supports the notion that the risk of myocarditis is much higher in the setting of natural COVID-19 infection compared to myocarditis following vaccination. Given the widespread transmissibility of this virus, it stands to reason that an individual should receive a vaccine to safeguard against the increased risk of myocardial injury associated with COVID-19 infection.
Underreporting of myocarditis in the adolescent and young adult male population is possible, as there may be a low index of suspicion in this relatively healthy age group. Mild cases of post-vaccine myocarditis are likely to go unreported as there is currently no routine screening protocol in place. However, as public awareness of post-vaccine myocarditis continues to grow, there may also be a potential for overreporting as well, emphasizing the need for effective surveillance systems to confirm suspected cases.
Based on the current available data, the CDC is continuing to recommend that patients aged 5 years and older be vaccinated against COVID-19, stating that the known risks and potential complications associated with COVID-19 infection far outweigh the rare chance of developing an adverse reaction to vaccination including myocarditis. Vaccine-mediated myocarditis has a low incidence, and while clinicians should be vigilant for its occurrence, this adverse effect should not deter vaccination efforts during this pandemic based on current data. Continued monitoring and reporting to the Vaccine Adverse Event Reporting System is strongly encouraged. Additional guidance from the American Heart Association for follow-up of patients with myocarditis emphasizes the use of cardiac MRI to examine the heart in vivo[25].
One of the major limitations of many of the studies describing post-vaccine myocarditis is the lack of follow-up data given the recency of vaccine approval and administration. There is minimal long-term data available to date which limits our ability to interpret long-term outcomes of patients who receive a diagnosis of COVID-19 vaccine-associated myocarditis. However the availability of long-term data will accumulate with time. Additionally, many of these studies were case reports compiled via colleague communication rather than surveillance systems which allow for more complete diagnostic evaluation to exclude other potential etiologies of myocarditis. Many of the cases reported were presumed to be a result of the COVID-19 vaccine purely based on temporal association. While feasible, this assumption might result in the overestimation of myocarditis as a result of COVID-19 vaccination. Similarly, many studies used negative antibody tests to rule out active or prior COVID-19 infection which could be problematic due to false negatives or waning immunity.
With third dose eligibility for the Pfizer-BioNTech™ and Moderna™ mRNA vaccines recently expanding to include much of the adult general public, careful prospective observation of the rate of vaccine-mediated myocarditis following the third dose compared to rates following the second dose is needed. While there is limited data currently available, data obtained by the Israel Ministry of Health have reported lower rates of myocarditis following the third dose compared to the second dose. Proactive surveillance efforts have discovered 17 total myocarditis/perimyocarditis cases among ages 16-59 years following administration of over 2.5 million third doses of the Pfizer-BioNTech™ mRNA vaccine[26].
Vaccine-mediated myocarditis following vaccination against COVID-19 using mRNA vaccines is a rare, but potentially serious occurrence. Clinicians should be aware of the potential development of vaccine-mediated myocarditis, particularly in young males. Early consultation with cardiologists and further investigation via serum biomarkers and imaging with cardiac MRI may confirm the diagnosis. Long-term follow up of those patients developing vaccine-mediated myocarditis is necessary to assess the potential for chronic complications of this rare phenomenon. Despite the potential for vaccine-mediated myocarditis, vaccination continues to be recommended against COVID-19 in all eligible populations.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Apiratwarakul K, Thailand; Stepanova N, Ukraine A-Editor: Yao QG, China S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Gargano JW, Wallace M, Hadler SC, Langley G, Su JR, Oster ME, Broder KR, Gee J, Weintraub E, Shimabukuro T, Scobie HM, Moulia D, Markowitz LE, Wharton M, McNally VV, Romero JR, Talbot HK, Lee GM, Daley MF, Oliver SE. Use of mRNA COVID-19 Vaccine After Reports of Myocarditis Among Vaccine Recipients: Update from the Advisory Committee on Immunization Practices - United States, June 2021. MMWR Morb Mortal Wkly Rep. 2021;70:977-982. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 389] [Cited by in RCA: 399] [Article Influence: 99.8] [Reference Citation Analysis (0)] |
| 2. | Haas EJ, Angulo FJ, McLaughlin JM, Anis E, Singer SR, Khan F, Brooks N, Smaja M, Mircus G, Pan K, Southern J, Swerdlow DL, Jodar L, Levy Y, Alroy-Preis S. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397:1819-1829. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1377] [Cited by in RCA: 1138] [Article Influence: 284.5] [Reference Citation Analysis (0)] |
| 3. | Centers for Disease Control and Prevention (CDC). COVID-19. Selected Adverse Events Reported after COVID-19 Vaccination. [cited 2 December 2021]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/adverse-events.html. |
| 4. | Mevorach D, Anis E, Cedar N, Bromberg M, Haas EJ, Nadir E, Olsha-Castell S, Arad D, Hasin T, Levi N, Asleh R, Amir O, Meir K, Cohen D, Dichtiar R, Novick D, Hershkovitz Y, Dagan R, Leitersdorf I, Ben-Ami R, Miskin I, Saliba W, Muhsen K, Levi Y, Green MS, Keinan-Boker L, Alroy-Preis S. Myocarditis after BNT162b2 mRNA Vaccine against Covid-19 in Israel. N Engl J Med. 2021;385:2140-2149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 396] [Cited by in RCA: 426] [Article Influence: 106.5] [Reference Citation Analysis (0)] |
| 5. | Salah HM, Mehta JL. COVID-19 Vaccine and Myocarditis. Am J Cardiol. 2021;157:146-148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 68] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 6. | Rosner CM, Genovese L, Tehrani BN, Atkins M, Bakhshi H, Chaudhri S, Damluji AA, de Lemos JA, Desai SS, Emaminia A, Flanagan MC, Khera A, Maghsoudi A, Mekonnen G, Muthukumar A, Saeed IM, Sherwood MW, Sinha SS, O'Connor CM, deFilippi CR. Myocarditis Temporally Associated With COVID-19 Vaccination. Circulation. 2021;144:502-505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 160] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 7. | Dionne A, Sperotto F, Chamberlain S, Baker AL, Powell AJ, Prakash A, Castellanos DA, Saleeb SF, de Ferranti SD, Newburger JW, Friedman KG. Association of Myocarditis With BNT162b2 Messenger RNA COVID-19 Vaccine in a Case Series of Children. JAMA Cardiol. 2021;6:1446-1450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 134] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 8. | McLean K, Johnson TJ. Myopericarditis in a previously healthy adolescent male following COVID-19 vaccination: A case report. Acad Emerg Med. 2021;28:918-921. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 58] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 9. | United States Department of Health and Human Services (DHHS), Public Health Service (PHS). Centers for Disease Control (CDC)/Food and Drug Administration (FDA), Vaccine Adverse Event Reporting System (VAERS) 1990-12/31/2021, CDC WONDER On-line Database. [cited 14 January 2022]. Available from: http://wonder.cdc.gov/vaers.html. |
| 10. | Witberg G, Barda N, Hoss S, Richter I, Wiessman M, Aviv Y, Grinberg T, Auster O, Dagan N, Balicer RD, Kornowski R. Myocarditis after Covid-19 Vaccination in a Large Health Care Organization. N Engl J Med. 2021;385:2132-2139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 352] [Cited by in RCA: 443] [Article Influence: 110.8] [Reference Citation Analysis (0)] |
| 11. | United States Department of Health and Human Services (DHHS), Public Health Service (PHS). Centers for Disease Control (CDC)/Food and Drug Administration (FDA), Vaccine Adverse Event Reporting System (VAERS) 1990-12/31/2021, CDC WONDER On-line Database. [cited 14 January 2022]. Available from: http://wonder.cdc.gov/vaers.html. |
| 12. | Mansour J, Short RG, Bhalla S, Woodard PK, Verma A, Robinson X, Raptis DA. Acute myocarditis after a second dose of the mRNA COVID-19 vaccine: a report of two cases. Clin Imaging. 2021;78:247-249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 61] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 13. | Minocha PK, Better D, Singh RK, Hoque T. Recurrence of Acute Myocarditis Temporally Associated with Receipt of the mRNA Coronavirus Disease 2019 (COVID-19) Vaccine in a Male Adolescent. J Pediatr. 2021;238:321-323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 14. | Centers for Disease Control and Prevention COVID-19 VaST Work Group technical report—May 17, 2021. Advisory Committee on Immunization Practices (ACIP) May 2021. [cited 14 January 2022]. Available from: https://www.cdc.gov/vaccines/acip/index.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fvaccines%2Facip%2Fcommittee%2Fstructure-role.html. |
| 15. | Bozkurt B, Kamat I, Hotez PJ. Myocarditis With COVID-19 mRNA Vaccines. Circulation. 2021;144:471-484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 556] [Cited by in RCA: 619] [Article Influence: 154.8] [Reference Citation Analysis (0)] |
| 16. | Vojdani A, Kharrazian D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin Immunol. 2020;217:108480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 228] [Cited by in RCA: 421] [Article Influence: 84.2] [Reference Citation Analysis (0)] |
| 17. | Caso F, Costa L, Ruscitti P, Navarini L, Del Puente A, Giacomelli R, Scarpa R. Could Sars-coronavirus-2 trigger autoimmune and/or autoinflammatory mechanisms in genetically predisposed subjects? Autoimmun Rev. 2020;19:102524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 225] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 18. | Kytö V, Sipilä J, Rautava P. The effects of gender and age on occurrence of clinically suspected myocarditis in adulthood. Heart. 2013;99:1681-1684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 19. | Marshall M, Ferguson ID, Lewis P, Jaggi P, Gagliardo C, Collins JS, Shaughnessy R, Caron R, Fuss C, Corbin KJE, Emuren L, Faherty E, Hall EK, Di Pentima C, Oster ME, Paintsil E, Siddiqui S, Timchak DM, Guzman-Cottrill JA. Symptomatic Acute Myocarditis in 7 Adolescents After Pfizer-BioNTech COVID-19 Vaccination. Pediatrics. 2021;148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 265] [Article Influence: 66.3] [Reference Citation Analysis (2)] |
| 20. | Center for Disease Control and Prevention (CDC). COVID-19 vaccination. Clinical Considerations: Myocarditis and Pericarditis after Receipt of mRNA COVID-19 Vaccines Among Adolescents and Young Adults. [cited 11 December 2022]. Available from: https://www.cdc.gov/vaccines/covid-19/clinical-considerations/myocarditis.html. |
| 21. | Rotshild V, Hirsh-Raccah B, Miskin I, Muszkat M, Matok I. Comparing the clinical efficacy of COVID-19 vaccines: a systematic review and network meta-analysis. Sci Rep. 2021;11:22777. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 172] [Cited by in RCA: 160] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 22. | Havers FP, Whitaker M, Self JL, Chai SJ, Kirley PD, Alden NB, Kawasaki B, Meek J, Yousey-Hindes K, Anderson EJ, Openo KP, Weigel A, Teno K, Monroe ML, Ryan PA, Reeg L, Kohrman A, Lynfield R, Como-Sabetti K, Poblete M, McMullen C, Muse A, Spina N, Bennett NM, Gaitán M, Billing LM, Shiltz J, Sutton M, Abdullah N, Schaffner W, Talbot HK, Crossland M, George A, Patel K, Pham H, Milucky J, Anglin O, Ujamaa D, Hall AJ, Garg S, Taylor CA; COVID-NET Surveillance Team. Hospitalization of Adolescents Aged 12-17 Years with Laboratory-Confirmed COVID-19 - COVID-NET, 14 States, March 1, 2020-April 24, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:851-857. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 81] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 23. | Cocker MS, Abdel-Aty H, Strohm O, Friedrich MG. Age and gender effects on the extent of myocardial involvement in acute myocarditis: a cardiovascular magnetic resonance study. Heart. 2009;95:1925-1930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 85] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 24. | Barda N, Dagan N, Ben-Shlomo Y, Kepten E, Waxman J, Ohana R, Hernán MA, Lipsitch M, Kohane I, Netzer D, Reis BY, Balicer RD. Safety of the BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Setting. N Engl J Med. 2021;385:1078-1090. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 669] [Cited by in RCA: 720] [Article Influence: 180.0] [Reference Citation Analysis (0)] |
| 25. | Law YM, Lal AK, Chen S, Čiháková D, Cooper LT Jr, Deshpande S, Godown J, Grosse-Wortmann L, Robinson JD, Towbin JA; American Heart Association Pediatric Heart Failure and Transplantation Committee of the Council on Lifelong Congenital Heart Disease and Heart Health in the Young and Stroke Council. Diagnosis and Management of Myocarditis in Children: A Scientific Statement From the American Heart Association. Circulation. 2021;144:e123-e135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 177] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 26. | Centers for Disease Control and Prevention (CDC). Advisory Committee on Immunization Practices (ACIP). Coronavirus disease 2019 (COVID-19) vaccines. [cited 6 December 2022]. Available from: https://www.cdc.gov/vaccines/acip/meetings/slides-2021-11-19.html. |