Published online May 26, 2022. doi: 10.4330/wjc.v14.i5.319
Peer-review started: April 13, 2021
First decision: October 17, 2021
Revised: November 21, 2021
Accepted: April 26, 2022
Article in press: April 26, 2022
Published online: May 26, 2022
Processing time: 400 Days and 6.8 Hours
Adenosine is a coronary hyperemic agent used to measure invasive fractional flow reserve (FFR) of intermediate severity coronary stenosis.
To compare FFR assessment using adenosine with an alternate hyperemic agent, regadenoson.
PubMed, Google Scholar, CINAHL and Cochrane databases were queried for studies comparing adenosine and regadenoson for assessment of FFR. Data on FFR, correlation coefficient and adverse events from the selected studies were extracted and analyzed by means of random effects model. Two tailed P-value less than 0.05 was considered significant. Heterogeneity was assessed using I2 test.
Five studies with 248 patients were included in the final analysis. All included patients and coronary lesions underwent FFR assessment using both adenosine and regadenoson. There was no significant mean difference between FFR measurement by the two agents [odds ratio (OR) = -0.00; 95% confidence interval (CI): (-0.02)-0.01, P = 0.88]. The cumulative correlation coefficient was 0.98 (0.96-0.99, P < 0.01). Three of five studies reported time to FFR with cumulative results favoring regadenoson (mean difference 34.31 s; 25.14-43.48 s, P < 0.01). Risk of adverse events was higher with adenosine compared to regadenoson (OR = 2.39; 95%CI: 1.22-4.67, P = 0.01), which most commonly included bradycardia and hypotension. Vast majority of the adverse events associated with both agents were transient.
The performance of regadenoson in inducing maximal hyperemia was comparable to that of adenosine. There was excellent correlation between the FFR measurements by both the agents. The use of adenosine, was however associated with higher risk of adverse events and longer time to FFR compared to regadenoson.
Core Tip: Regadenoson has comparable efficacy in inducing maximal coronary hyperemia in patients undergoing invasive coronary angiography with lower risk of side effects compared to adenosine. To compare fractional flow reserve (FFR) assessment using adenosine with an alternate hyperemic agent, regadenoson. PubMed, Google Scholar, CINAHL and Cochrane databases were queried for studies comparing adenosine and regadenoson for assessment of FFR. There was excellent correlation between the FFR measurements by both the agents. The use of adenosine, was however associated with higher risk of adverse events and longer time to FFR compared to regadenoson.
- Citation: Gill GS, Gadre A, Kanmanthareddy A. Comparative efficacy and safety of adenosine and regadenoson for assessment of fractional flow reserve: A systematic review and meta-analysis. World J Cardiol 2022; 14(5): 319-328
- URL: https://www.wjgnet.com/1949-8462/full/v14/i5/319.htm
- DOI: https://dx.doi.org/10.4330/wjc.v14.i5.319
Coronary angiography has long been the gold standard for assessment of severity of coronary artery disease[1]. This modality is however limited to providing anatomic information with little physiologic and clinical correlation, and more notably has marked intra- and inter-observer variability with very little reproducibility[2,3]. Data has shown that reliability on angiography for assessment of stenosis to perform percutaneous coronary intervention may, in fact, result in higher rates of revascularization procedures without significant improvement in clinical outcomes[4]. Physiologic assessment of coronary stenosis is achieved through use of fractional flow reserve (FFR) which is the ratio of intracoronary pressures distal and proximal to the lesion. The distal pressure measurement can be recorded using a pressure wire sensor placed distal to the lesion and the proximal pressure can be recorded via the guide catheter. The resting FFR measurement may not unmask the true physiological significance of the lesion and is better unmasked under maximal coronary hyperemic conditions with the use of agents such as adenosine. This is especially useful in assessing hemodynamic significance of intermediate severity stenosis as shown by the FFR vs angiography for guiding percutaneous coronary intervention (FAME) and FFR-guided percutaneous coronary intervention plus optimal medical treatment versus optimal medical treatment alone in patients with stable coronary artery disease (FAME 2) trials[5,6].
Adenosine is the gold standard to achieve maximal hyperemia for adequate measurement of FFR, however due to non-selective receptor activation, it may cause transient shortness of breath, atrio-ventricular conduction blockade and chest pain. Regadenoson on the other hand is a selective A2A receptor agonist and has a more favorable side effect profile and straightforward dosing (400 μg vs variable weight-based infusion dosing for adenosine, i.e., 140 μg/kg/min for 2 min). Herein, we systematically reviewed published literature comparing the efficacy of regadenoson to adenosine for achieving maximal hyperemia, the correlation in FFR measurements, and the adverse effects to ascertain the safer and more cost-effective hyperemic agent.
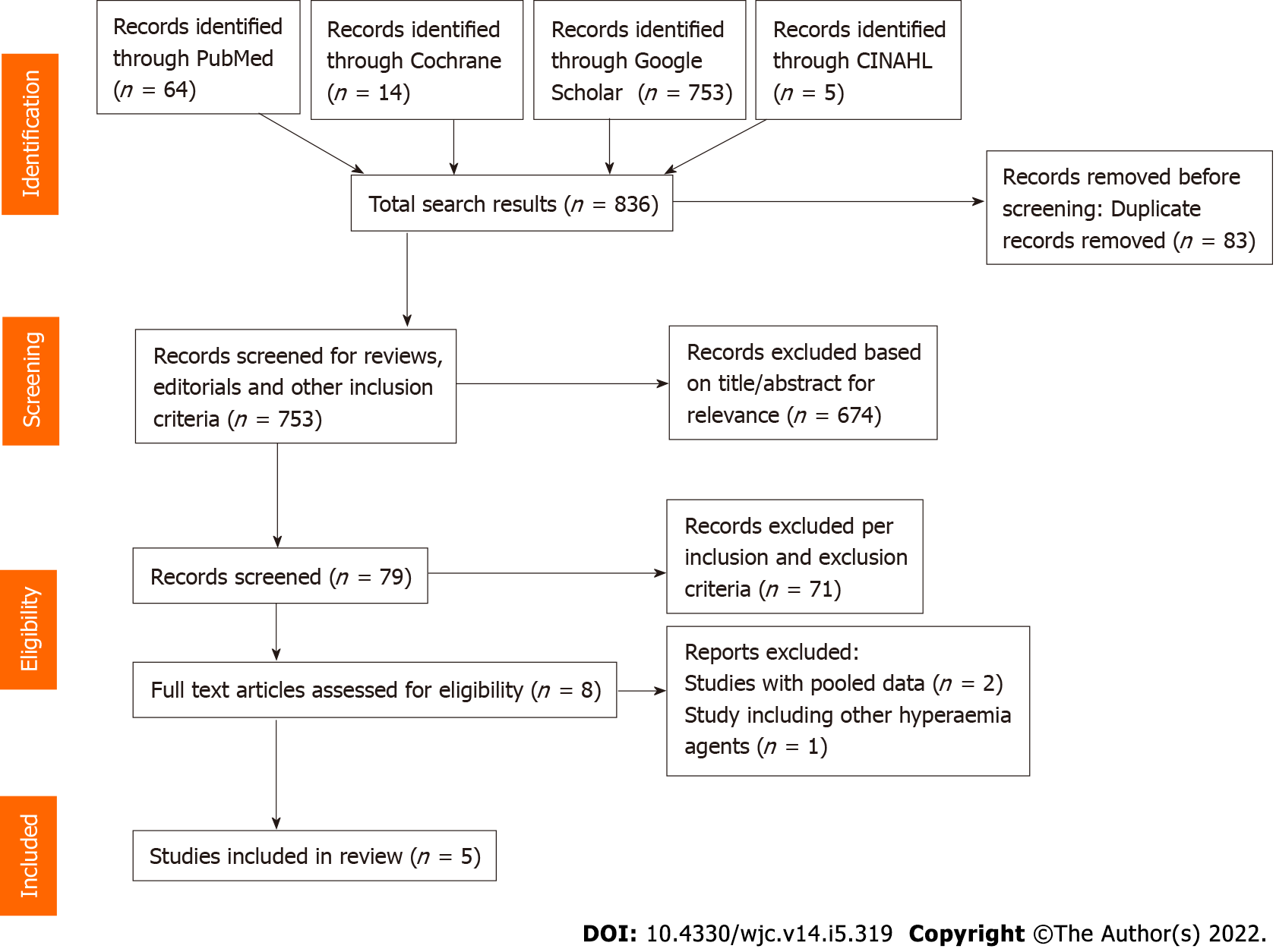
This investigation was conducted in accordance with the Cochrane collaboration guidelines and the results have been reported per the PRISMA statement[7]. Literature review was performed independently by two authors (Gill GS, Gadre A) in PubMed, Cochrane, CINAHL and Google Scholar databases using the keywords “adenosine”, “regadenoson”, “fractional flow reserve”, “FFR” and “hyperemia” in different combinations. 836 titles and abstracts were found, of which 83 were removed as duplicate. Of the remaining 753 titles, 674 were excluded based on title and abstract. 79 abstracts, original investigations, editorials and review articles were screened to assess for inclusion and exclusion criteria. Eight articles and their references were manually screened for any additional studies that could qualify for inclusion, of which, three were excluded from final analysis (systemic review, retrospective and pooled analyses) (Figure 1). The five included studies met following criteria: (1) Published as full manuscripts in English; (2) Involved patients with angiographic evidence of coronary artery stenosis; and (3) Assessed and reported at least one of the outcomes (FFR correlation, time to achieve FFR, adverse effects) with both adenosine and regadenoson. The following studies were excluded: (1) Duplicates of previous publications; (2) Pooled studies and systematic reviews; (3) Studies that included comparison of adenosine or regadenoson with nicorandil; (4) Abstracts, editorials, reviews, and commentaries; and (5) Animal studies. Any disagreement during the study screening and selection process was resolved by consensus among all authors.
The primary outcome of interest was FFR correlation, and secondary outcomes were time to achieve FFR, and a composite of all reported adverse effects. Data extraction was performed using a standardized data extraction form by two independent authors (Gill GS, Gadre A). Any disagreement on data was resolved by consensus among all authors. For all outcomes in our analyses, pooled odds ratio (OR) with their corresponding 95% confidence intervals (CIs) were calculated using the Mantel-Haenszel random-effects model for dichotomous variables and Inverse variance model for the continuous variables, and presented as Forest plots[8]. For the correlation coefficient Fisher’s Z transformation was performed and reverse transformation with restricted maximum likelihood method to obtain meta-summary correlation coefficient. Heterogeneity across the studies was assessed using the chi-square-based Cochrane Q test and quantified using I2statistics. I2index values of < 25%, 25%-50% and > 50% were considered low, intermediate, and high heterogeneity, respectively[9]. Exclusion sensitivity analysis was performed by excluding one study at a time and repeating the analysis. Publication bias was assessed using Begg’s dissemination selectivity test and visually inspected using funnel plots. All analyses were conducted using the Review Manager Version 5.4 software, STATA software version 16 (StataCorp 2019, College Station, TX, United States) and the IBM SPSS software Version 26 (IBM Corp, Armonk, NY, United States). There was no duplicate data within included studies. A two-sided P value of < 0.05 was considered statistically significant.
A total of eight investigations were reviewed to include five prospective studies involving 248 patients undergoing FFR analysis for 251 lesions in the final meta-analysis (Figure 1 and Table 1)[10-14]. A flowsheet of the study selection process in accordance with the PRISMA guidelines is shown in Figure 1. Study designs, infusion protocols, inclusion and exclusion criteria, and reported outcomes are represented in Table 1; and baseline characteristics of patients are reported in Table 2. Mean age of the study population was 63 years with women accounting for 25% of the participants. All studies were prospective with all 248 patients receiving both IV adenosine and IV regadenoson in a sequential manner.
| Ref. | Country | Patients (n) | Population | Enrollment period | Adenosine dosing | Regadenoson dosing | Inclusion/exclusion criteria | Measured outcomes |
| Nair et al[10], 2011 | United States | 25 | Prospective, single-center | July 2009-December 2010 | IV adenosine infusion at 140 μg/kg/min | IV regadenoson bolus 400 μg | Inclusion: Elective angiography, intermediate stenosis (40%-70%), remainder per ADVANCE trial (2) | FFR correlation, flushing, dyspnea, headache, chest discomfort, nausea, diaphoresis, metallic taste |
| Arumugham et al[11], 2013 | United States | 20 | Prospective, single-center | October 2009-September 2010 | IV adenosine infusion at 175 μg/kg/min | IV regadenoson bolus 400 μg | Inclusion: Intermediate stenosis (50%-80%). Exclusion: STEMI within 5 d, significant left main coronary artery stenosis, heart block, pregnancy, asthma or hypersensitive to either adenosine or regadenoson | FFR correlation, time to achieve FFR, effect on blood pressure and heart rate, heart block, bronchospasm, severe chest pain |
| Prasad et al[12], 2014 | United States | 571 | Prospective, multi-center | May 2011-November 2011 | IV adenosine at 140 μg/kg/min | IV regadenoson bolus 400 μg | Inclusion: Intermediate stenosis (50%-70%). Exclusion: Age < 18 years old, 3-vessel CAD, ACS within 1 wk, prior MI in territory supplied by target lesion, hypersensitivity to adenosine or regadenoson, reactive airway disease, 2nd or 3rd heart block, currently receiving dipyridamole, hemodynamic instability | FFR correlation, blood pressure, change in heart rate, dizziness, shortness of breath, heart block, flushing, arrhythmias |
| Van Nunen et al[13], 2015 | Netherlands | 100 | Prospective, single-center | NA | IV adenosine at 140 μg/kg/min | IV regadenoson bolus 400 μg | Inclusion: Ages 18-80 years old, lesions in proximal to mid coronary artery segments, at least 2 mm diameter, > 30% stenosis. Exclusion: Severe AS, 2nd-3rd heart block, acute MI within 5 d, bradycardia, severe hypotension, tortuous/calcified coronary vessels, severe asthma, pregnancy, inability to obtain femoral approach, dipyridamole within 48 h and methylxanthines within 12 h | FFR correlation, heart block, chest discomfort, blood pressure, heart rate, shortness of breath, nausea |
| Edward et al[14], 2018 | United States | 46 | Prospective, single-center | April 2012-May 2014 | IV adenosine at 140 μg/kg/min | IV regadenoson bolus 400 μg | Inclusion: Elective angiography, < 30%, >90% stenosis. Exclusion: Sinus node dysfunction, 2nd-3rd degree AV block without pacemaker, severe hypotension, acute MI within 30 d, severe AS, pregnancy, aberrant coronary anatomy or calcification | FFR correlation, time to reversal with aminophylline, side effects |
| Nair et al[10] | Arumugham et al[11] | Prasad et al[12] | van Nunen et al[13] | Edward et al[14] | |
| Number | 25 | 20 | 57 (60 lesions) | 100 | 46 |
| Age [yrs ± SD or yrs (CIs)] | 63 ± 11 | 63.9 ± 9 | 57 ± 8 | 66 ± 8 | 63 ± 10 |
| Women, n (%) | 12 (48) | 4 (20) | 10 (18) | 25 (25) | 9 (20) |
| Body mass index (mean ± SD) | 30.0 ± 5.7 | NA | 27.7 ± 4.1 | 26.7; H kg | 33 ± 7 |
| Hypertension, n (%) | 21 (84) | NA | 51 (90) | 54 | 44 (96) |
| Diabetes mellitus, n (%) | 6 (24) | NA | 26 (46) | 21 | 26 (57) |
| Hyperlipidemia, n (%) | 21 (84) | NA | 39 (68) | 36 | 44 (96) |
| Tobacco use, n (%) | 8 (32) | NA | 24 (42) | 20 | 7 (15) |
| CKD, n (%) | NA | NA | NA | NA | 9 (20) |
| Prior MI, n (%) | 5 (20) | NA | 23 (40) | 36 | NA |
| Prior PCI, n (%) | 8 (32) | NA | NA | 43 | NA |
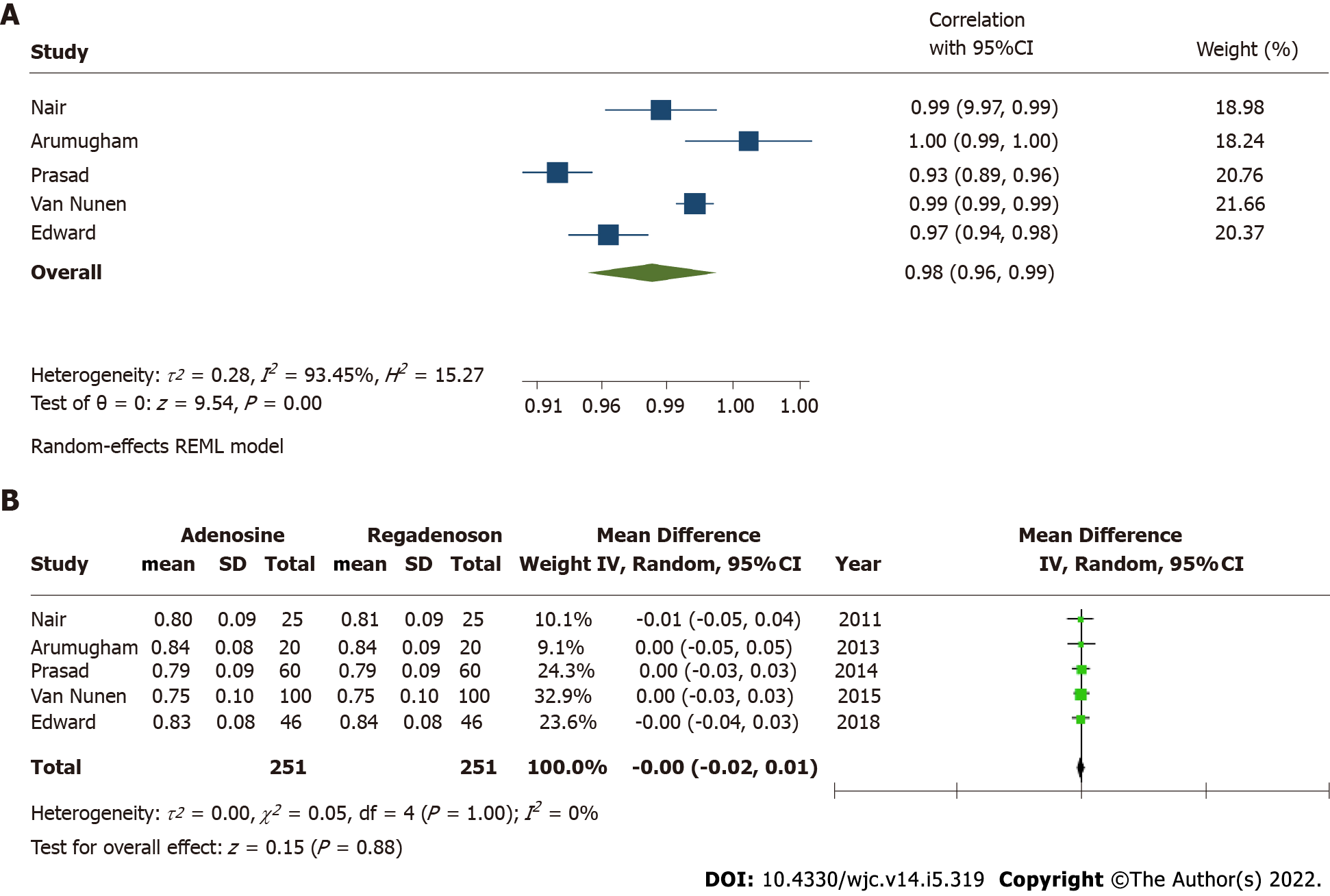
FFR correlation was reported in all five studies. The cumulative correlation coefficient for FFR measurement was 0.98 (95%CI: 0.96-0.99) with I2estimate for heterogeneity at 93% (Figure 2A). Mean difference in measured FFR values with adenosine and regadenoson in pooled analysis was -0.00 [95%CI: (-0.02)-0.01; P = 0.88], with I2estimate for heterogeneity 0%. An exclusionary sensitivity analysis was performed with exclusion of studies with lesions including low- and high-grade stenosis (Van Nunen et al[13] and Edward et al[14]), where results remained consistent with mean difference -0.00 [
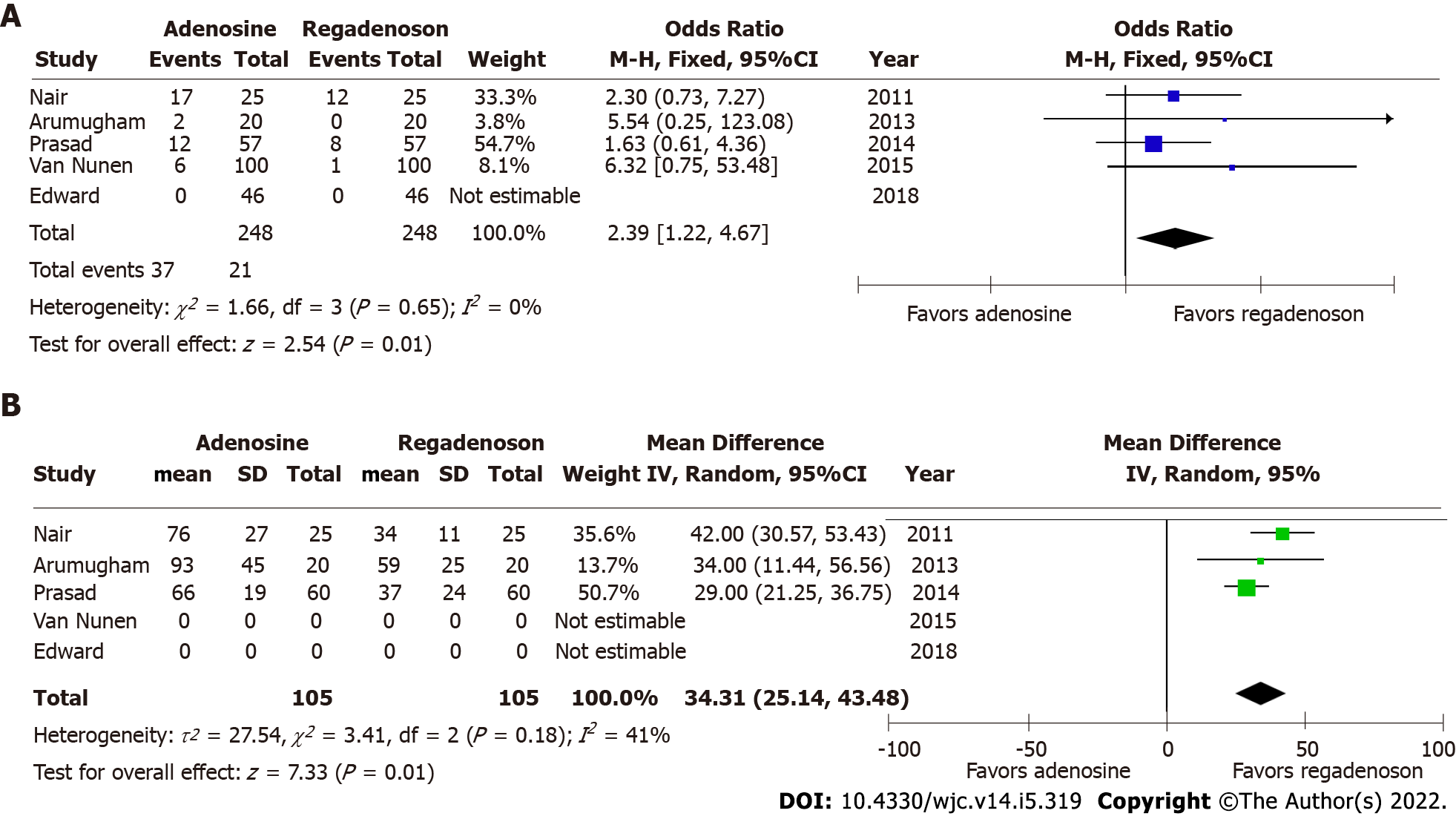
Pooled odds for any adverse effect were significantly higher for patients after administration of adenosine, than after receiving regadenoson (OR = 2.39, 95%CI: 1.22-4.67; P = 0.01) (Figure 3B). There was no evident heterogeneity among included studies with I2estimated at 0%. There was no evidence of dissemination bias on visual inspection of the funnel plot and Begg’s test. There were no adverse effects reported in either arm of the study by Edward et al[14] among forty-six participants in the analysis where aminophylline were administered after regadenoson, while side effects reported in other studies were only transient and did not necessitate discontinuation of infusion or FFR measurement. In the study by Arumugham et al[11], a higher dose of IV adenosine was infused, while significant adverse event rates were not higher.
The major findings of this analysis are: (1) FFR correlation was excellent among both IV adenosine and IV regadenoson; (2) IV regadenoson achieved maximal hyperemia in a shorter interval; and (3) Adverse effects, including transient atrioventricular conduction block, chest pain, shortness of breath, hypotension, flushing and headache were higher with adenosine. Anatomic as well as physiologic assessment of coronary vasculature are integral elements to defining coronary artery disease in a patient. Over the past few decades, there have been advances in anatomic evaluation beyond traditional angiography, and have included developments in intravascular imaging, such as, intravascular ultrasound and optical coherence tomography. Studies comparing translation of anatomic vs physiologic assessment of coronary artery disease into clinical outcomes have concluded in comparable results[15]. Furthermore, investigations attempting to correlate optimum minimal lumen area to FFR values have concluded that their reciprocity may be vessel dependent[16]. This can be explained by the independent role of these modalities in assessing flow and identifying vulnerable plaques, respectively[17]. Due to easy availability and low cost, FFR based assessment of intermediate stenosis has been widely incorporated to practice.
Adenosine has traditionally been the gold standard for inducing maximal hyperemia while measuring FFR, although different agents have been investigated for efficacy and side effects with comparable results[10-14,18-22]. There is excellent correlation in FFR estimated by adenosine and regadenoson which is consistent with prior studies that have shown both agents achieve comparable hyperemia in animal as well as human models[23,24]. Adenosine is a non-selective activator of 4 receptors, A1, A2A, A2B and A3[25]. The activation of A1 and A3 receptor subtypes decreases cyclic adenosine monophosphate (cAMP) levels while activation of receptors A2A and A2B increases the cAMP levels[26]. cAMP is an important mediator of smooth muscle relaxation and therefore, activation of A2A and A2B receptors leads to coronary and peripheral arterial vasodilation and hyperemia. The non-selective receptor activation by adenosine also causes bronchoconstriction and other side effects. Regadenoson, however, is a selective A2A receptor agonist, and thus causes preferential coronary vasodilation with fewer side effects when compared to adenosine[27]. In cases where patients may experience adverse effects from regadenoson, they can be reversed using intravenous aminophylline[14,28]. This easy reversibility makes physiologic evaluation feasible in patients with mild-to-moderate reactive airway disease and obstructive airway disease[27]. Another secondary outcome, time to FFR, was also shorter in patients who received regadenoson, thus favoring its use. This can potentially be explained by the non-weight-based bolus dosing of intravenous regadenoson, accommodated by the longer half-life (2-4 min) when compared to weight-based infusion of intravenous adenosine, which is administered preferably through central venous access due to its extremely short half-life (0.6-10 s). Because of its short half-life, administration of adenosine is challenging and could be time consuming. Our study results show that time to maximal hyperemia is shorter by about 30 s with the use of regadenoson. Further, this time does not take in to account the time taken for preparing the adenosine infusion which could take up to several minutes. This could potentially increase the duration of the procedure, and therefore, use of regadenoson may save valuable time for the catheterization laboratory and its staff while potentially leading to cost benefits despite the higher price of regadenoson.
As discussed above, our study demonstrates lower risk of adverse effects with regadenoson use. In study by Arumugham et al[11], a higher adenosine infusion rate was used (175 μg/kg/min), and to negate its effects, we conducted an exclusionary sensitivity analysis, where results remained similar (P = 0.02). We then conducted sensitivity analysis using jackknife approach for another secondary outcome, time to FFR, where heterogeneity was moderate with I2= 41% in the overall analysis. Here, we systematically excluded one study at a time, and noted a reduction in I2to 0% with exclusion of study by Nair et al[10], the oldest investigation. Outcome favoring regadenoson use remained unchanged (P < 0.01). Lastly, two of the five included studies did not limit the lesions to intermediate stenosis and a sensitivity analysis was conducted by excluding these investigations. The results remained unchanged with significant correlation between estimated FFRs (P = 0.92, I2= 0%).
The decision to use one agent over the other is further influenced by factors such as requirement of central venous access and cost. Although adenosine can be administered both via central or peripheral access, the onset of action is earlier and steady state hyperemia is more stable with central venous catheter. Regadenoson, on the other hand, has limitations including cost and unpredictability in the duration of hyperemia[13]. For this reason, regadenoson may not be the ideal agent, especially when evaluating multiple coronary arteries, and will require multiple dose administrations with incremental increase in the costs[29]. Regadenoson has near completely replaced adenosine for nuclear stress testing because of the ease of its administration and relatively fewer side effects and therefore is more appealing to be used for invasive FFR assessment. Our meta-analysis results clearly demonstrate that regadenoson is an acceptable alternative to adenosine for invasive FFR assessment of intermediate severity coronary stenosis.
Our meta-analysis has inherent limitations as well as those inherited from the included studies. First, among the studies included in this meta-analysis, there is considerable variability in the definition of intermediate severity stenosis. Second, the study by Arumugham et al[11] used a higher adenosine infusion rate when compared to other investigations in an attempt to mitigate effects of deactivation of peripherally administered adenosine. This variability in dosing may have affected all outcomes, and potentially, strengthened the association between adenosine use and adverse effects. Third, the studies included in our analysis varied in drug infusion protocol in terms of peripheral vs central access which may be of importance when using an agent with short half-life, and investigating a time sensitive outcome, such as time to achieve FFR. Fourth, since these studies included patients undergoing elective angiography, these results may not be extrapolated to populations with unstable angina or other acute coronary syndromes. Lastly, in an attempt to conduct the analysis in consistency with PRISMA guidelines, this study may be subject to publication bias[30]. Inherently however, our investigation had limited risk of residual bias since all included studies had a prospective design with patients receiving both hyperemic agents. We performed several sensitivity analyses to document the consistency of our results despite the aforementioned limitations. We employed jackknife approach and excluded individual studies to investigate outcomes and re-calculate I2. Given the lack of large multicenter studies addressing these differences in a well-designed prospective fashion, our analysis provides valuable insights into the differential outcomes of FFR measurement in patients with adenosine vs regadenoson use.
Our meta-analysis demonstrates that use of regadenoson for FFR measurement among patients undergoing elective angiography is associated with shorter time to achieve FFR, and lower risk of side effects while providing excellent correlation with the results obtained with adenosine and therefore may be an acceptable alternative for FFR measurement in the cardiac catheterization laboratory. The ease of its use, and a relatively favorable side effect profile make regadenoson a very appealing alternative to adenosine.
Regadenoson is a selective adenosine receptor agonist that causes coronary hyperemia and in limited studies has been shown to have comparative efficacy to adenosine in evaluating coronary fractional flow reserve (FFR).
Considering the evidence is limited in supporting the use of regadenoson as an alternative to adenosine in evaluating FFR, we hypothesized that using meta-analysis we can improve the strength of evidence comparing regadenoson vs adenosine in evaluating FFR in intermediate severity coronary stenosis.
To perform meta-analysis to evaluate regadenoson vs adenosine for efficacy and safety.
Pooled meta-analysis of published studies. Comparing correlation coefficient and adverse events using random effects model. Visual inspection for bias and heterogeneity assessment using I2 test.
The FFR correlation coefficient between regadenoson and adenosine was 0.98 [95% confidence interval (CI): 0.96-0.99, P < 0.01]. Time to achieve FFR was shorter by 34.31 s (95%CI: 25.14-43.48) in the regadenoson group. The risk of adverse events was higher with adenosine with odds ratio of 2.39 (95%CI: 1.22-4.67, P = 0.01).
Regadenoson had comparable efficacy in obtaining FFR compared to adenosine and this was achieved in a shorter duration of time and with lower incidence of adverse effects.
Regadenoson presents an alternative to adenosine in evaluating FFR in patients with intermediate severity coronary artery stenosis with lower risk of side effects and also saves time.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kharlamov AN, Netherlands S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Collet C, Grundeken MJ, Asano T, Onuma Y, Wijns W, Serruys PW. State of the art: coronary angiography. EuroIntervention. 2017;13:634-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 2. | Zir LM, Miller SW, Dinsmore RE, Gilbert JP, Harthorne JW. Interobserver variability in coronary angiography. Circulation. 1976;53:627-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 618] [Cited by in RCA: 541] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 3. | Galbraith JE, Murphy ML, de Soyza N. Coronary angiogram interpretation. Interobserver variability. JAMA. 1978;240:2053-2056. [PubMed] |
| 4. | Pinto DS, Stone GW, Ellis SG, Cox DA, Hermiller J, O'Shaughnessy C, Mann JT, Mehran R, Na Y, Turco M, Caputo R, Popma JJ, Cutlip DE, Russell ME, Cohen DJ; TAXUS-IV Investigators. Impact of routine angiographic follow-up on the clinical benefits of paclitaxel-eluting stents: results from the TAXUS-IV trial. J Am Coll Cardiol. 2006;48:32-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 117] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 5. | Tonino PA, De Bruyne B, Pijls NH, Siebert U, Ikeno F, van' t Veer M, Klauss V, Manoharan G, Engstrøm T, Oldroyd KG, Ver Lee PN, MacCarthy PA, Fearon WF; FAME Study Investigators. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360:213-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2974] [Cited by in RCA: 3061] [Article Influence: 191.3] [Reference Citation Analysis (0)] |
| 6. | De Bruyne B, Pijls NH, Kalesan B, Barbato E, Tonino PA, Piroth Z, Jagic N, Möbius-Winkler S, Rioufol G, Witt N, Kala P, MacCarthy P, Engström T, Oldroyd KG, Mavromatis K, Manoharan G, Verlee P, Frobert O, Curzen N, Johnson JB, Jüni P, Fearon WF; FAME 2 Trial Investigators. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med. 2012;367:991-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1837] [Cited by in RCA: 1995] [Article Influence: 153.5] [Reference Citation Analysis (0)] |
| 7. | Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13930] [Cited by in RCA: 13358] [Article Influence: 834.9] [Reference Citation Analysis (0)] |
| 8. | DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26739] [Cited by in RCA: 30428] [Article Influence: 780.2] [Reference Citation Analysis (0)] |
| 9. | Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39087] [Cited by in RCA: 46553] [Article Influence: 2116.0] [Reference Citation Analysis (3)] |
| 10. | Nair PK, Marroquin OC, Mulukutla SR, Khandhar S, Gulati V, Schindler JT, Lee JS. Clinical utility of regadenoson for assessing fractional flow reserve. JACC Cardiovasc Interv. 2011;4:1085-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 11. | Arumugham P, Figueredo VM, Patel PB, Morris DL. Comparison of intravenous adenosine and intravenous regadenoson for the measurement of pressure-derived coronary fractional flow reserve. EuroIntervention. 2013;8:1166-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Prasad A, Zareh M, Doherty R, Gopal A, Vora H, Somma K, Mehra A, Clavijo LC, Matthews RV, Shavelle DM. Use of regadenoson for measurement of fractional flow reserve. Catheter Cardiovasc Interv. 2014;83:369-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | van Nunen LX, Lenders GD, Schampaert S, van 't Veer M, Wijnbergen I, Brueren GR, Tonino PA, Pijls NH. Single bolus intravenous regadenoson injection versus central venous infusion of adenosine for maximum coronary hyperaemia in fractional flow reserve measurement. EuroIntervention. 2015;11:905-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Edward JA, Lee JH, White CJ, Morin DP, Bober R. Intravenous regadenoson with aminophylline reversal is safe and equivalent to intravenous adenosine infusion for fractional flow reserve measurements. Clin Cardiol. 2018;41:1348-1352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Burzotta F, Leone AM, Aurigemma C, Zambrano A, Zimbardo G, Arioti M, Vergallo R, De Maria GL, Cerracchio E, Romagnoli E, Trani C, Crea F. Fractional Flow Reserve or Optical Coherence Tomography to Guide Management of Angiographically Intermediate Coronary Stenosis: A Single-Center Trial. JACC Cardiovasc Interv. 2020;13:49-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 96] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 16. | Waksman R, Legutko J, Singh J, Orlando Q, Marso S, Schloss T, Tugaoen J, DeVries J, Palmer N, Haude M, Swymelar S, Torguson R. FIRST: Fractional Flow Reserve and Intravascular Ultrasound Relationship Study. J Am Coll Cardiol. 2013;61:917-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 170] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 17. | Kennedy MW, Fabris E, Ijsselmuiden AJ, Nef H, Reith S, Escaned J, Alfonso F, van Royen N, Wojakowski W, Witkowski A, Indolfi C, Ottervanger JP, Suryapranata H, Kedhi E. Combined optical coherence tomography morphologic and fractional flow reserve hemodynamic assessment of non- culprit lesions to better predict adverse event outcomes in diabetes mellitus patients: COMBINE (OCT-FFR) prospective study. Rationale and design. Cardiovasc Diabetol. 2016;15:144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (1)] |
| 18. | Lim WH, Koo BK, Nam CW, Doh JH, Park JJ, Yang HM, Park KW, Kim HS, Takashima H, Waseda K, Amano T, Kato D, Kurita A, Oi M, Toyofuku M, van Nunen L, Pijls NH. Variability of fractional flow reserve according to the methods of hyperemia induction. Catheter Cardiovasc Interv. 2015;85:970-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Stolker JM, Lim MJ, Shavelle DM, Morris DL, Angiolillo DJ, Guzman LA, Kennedy KF, Weber E, Zareh M, Neumayr RH, Zenni MM. Pooled comparison of regadenoson versus adenosine for measuring fractional flow reserve and coronary flow in the catheterization laboratory. Cardiovasc Revasc Med. 2015;16:266-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Rudzinski W, Waller AH, Rusovici A, Dehnee A, Nasur A, Benz M, Sanchez S, Klapholz M, Kaluski E. Comparison of efficacy and safety of intracoronary sodium nitroprusside and intravenous adenosine for assessing fractional flow reserve. Catheter Cardiovasc Interv. 2013;81:540-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Leone AM, Porto I, De Caterina AR, Basile E, Aurelio A, Gardi A, Russo D, Laezza D, Niccoli G, Burzotta F, Trani C, Mazzari MA, Mongiardo R, Rebuzzi AG, Crea F. Maximal hyperemia in the assessment of fractional flow reserve: intracoronary adenosine versus intracoronary sodium nitroprusside versus intravenous adenosine: the NASCI (Nitroprussiato versus Adenosina nelle Stenosi Coronariche Intermedie) study. JACC Cardiovasc Interv. 2012;5:402-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 22. | Li S, Deng J, Wang X, Zhao X, Han Y. Efficiencies of intracoronary sodium nitroprusside on fractional flow reserve measurement. Int J Clin Exp Med. 2015;8:2679-2683. [PubMed] |
| 23. | Trochu JN, Zhao G, Post H, Xu X, Belardinelli L, Belloni FL, Hintze TH. Selective A2A adenosine receptor agonist as a coronary vasodilator in conscious dogs: potential for use in myocardial perfusion imaging. J Cardiovasc Pharmacol. 2003;41:132-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Lieu HD, Shryock JC, von Mering GO, Gordi T, Blackburn B, Olmsted AW, Belardinelli L, Kerensky RA. Regadenoson, a selective A2A adenosine receptor agonist, causes dose-dependent increases in coronary blood flow velocity in humans. J Nucl Cardiol. 2007;14:514-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 103] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 25. | Borea PA, Gessi S, Merighi S, Vincenzi F, Varani K. Pharmacology of Adenosine Receptors: The State of the Art. Physiol Rev. 2018;98:1591-1625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 542] [Article Influence: 77.4] [Reference Citation Analysis (0)] |
| 26. | Olah ME, Stiles GL. Adenosine receptor subtypes: characterization and therapeutic regulation. Annu Rev Pharmacol Toxicol. 1995;35:581-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 416] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 27. | Ghimire G, Hage FG, Heo J, Iskandrian AE. Regadenoson: a focused update. J Nucl Cardiol. 2013;20:284-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 28. | Doran JA, Sajjad W, Schneider MD, Gupta R, Mackin ML, Schwartz RG. Aminophylline and caffeine for reversal of adverse symptoms associated with regadenoson SPECT MPI. J Nucl Cardiol. 2017;24:1062-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Pijls NH, van Nunen LX. Fractional flow reserve, maximum hyperemia, adenosine, and regadenoson. Cardiovasc Revasc Med. 2015;16:263-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 30. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52948] [Cited by in RCA: 47200] [Article Influence: 2950.0] [Reference Citation Analysis (0)] |