Published online May 26, 2022. doi: 10.4330/wjc.v14.i5.307
Peer-review started: November 9, 2021
First decision: February 8, 2022
Revised: March 10, 2022
Accepted: April 15, 2022
Article in press: April 15, 2022
Published online: May 26, 2022
Processing time: 189 Days and 11.5 Hours
For patients with cardiovascular disease, blood pressure variability (BPV), distinct from hypertension, is an important determinant of adverse cardiac events. Whether pre-operative BPV adversely affects outcomes after percutaneous coronary intervention (PCI) is to this point unclear.
To investigate the relationship between blood pressure variability and outcomes for patients post-PCI.
Patients undergoing PCI in a single state in 2017 were studied (n = 647). Systolic and diastolic BPV, defined as both the largest change and standard deviation for the 3-60 mo prior to PCI was calculated and patients with more than ten blood pressure measurements in that time were included for analysis (n = 471). Adverse outcomes were identified up to a year following the procedure, including major adverse cardiac events (MACE), myocardial infarction, cerebrovascular accident, death, and all-cause hospitalization.
Visit-to-visit systolic BPV, as measured by both standard deviation and largest change, was higher in patients who had myocardial infarction, were readmitted, or died within one year following PCI. Systolic BPV, as measured by largest change or standard deviation, was higher in patients who had MACE, or readmissions (P < 0.05). Diastolic BPV, as measured by largest change, was higher in patients with MACE and readmissions (P < 0.05).
As BPV is easily measured and captured in the electronic medical record, these findings describe a novel method of identifying at-risk patients who undergo PCI. Aggressive risk modification for patients with elevated BPV and known coronary artery disease is indicated.
Core Tip: Pre-procedural visit-to-visit blood pressure variability, as measured by either standard deviation or largest change between two consecutive visits, is higher in patients who are readmitted, have complications, or die after percutaneous coronary intervention. Aggressive risk modification is indicated for patients with elevated blood pressure variability and known coronary artery disease.
- Citation: Weisel CL, Dyke CM, Klug MG, Haldis TA, Basson MD. Day-to-day blood pressure variability predicts poor outcomes following percutaneous coronary intervention: A retrospective study. World J Cardiol 2022; 14(5): 307-318
- URL: https://www.wjgnet.com/1949-8462/full/v14/i5/307.htm
- DOI: https://dx.doi.org/10.4330/wjc.v14.i5.307
Percutaneous coronary intervention (PCI) has long been established as an effective method of coronary revascularization for patients with coronary artery disease and is performed over 965000 times each year in the United States[1]. When patients present with acute coronary syndrome, it is estimated that approximately 60% will undergo PCI, 10%-15% will require surgical revascularization with coronary artery bypass graft (CABG), and the remainder are treated with medical therapy alone[2]. Although PCI is generally safe, known subsets of patients are at elevated risk for procedural complications after PCI. These include patients in shock, chronic heart failure, complex vascular anatomy, and diabetes mellitus, among others[3]. In addition to acute complications (such as bleeding at the entry site, vascular injuries, and arrythmias), patients may suffer from delayed complications after the procedure. Post-procedural additional major adverse cardiac events (MACE), include myocardial infarction (MI), cerebrovascular accident (CVA), hospitalization, or death. Risk factors for these delayed outcomes are less well understood. Aside from diabetes mellitus, relatively little is known about non-cardiac factors impacting outcomes after PCI.
In particular, whether preoperative blood pressure variability (BPV) affects outcomes after PCI is unclear. BPV, which is distinct from hypertension, is a measure of the degree of instability of a patient’s blood pressure (BP) over time. BPV has been shown to be a risk factor for 90-day rates of complications after major surgical procedures, including coronary artery bypass graft (CABG)[4,5]. BPV may be calculated in a variety of ways, using standard deviation (SD), average change, or largest change between consecutive measurements (LC), and may be based upon either systolic or diastolic blood pressure readings[6]. BPV is most commonly reported in the literature by SD, but each method of reporting BPV may be similarly valid[7]. High outpatient BPV is associated with higher risk of all-cause hospitalization and death in ambulatory medical patients[8] and surgical patients[4], regardless of if the patient is hypertensive, normotensive, or hypotensive[9]. Indeed, BPV has recently been shown to predict cardiac events in patients with heart failure[10], and to be associated with development of end stage renal disease[11], and with cerebral small vessel disease leading to CVA[12]. The causes of BPV are likely highly multifactorial and may be due to physiological abnormalities (such as vascular wall stiffness and hypertrophy), autonomic dysfunction, “white coat syndrome”, and medication non-compliance[13,14]. For patients with cardiovascular disease, consistency of BP control has been shown to be an important determinant of adverse cardiac events[3,6,8]. BPV has also been shown to be associated with adverse outcomes in patients with cardiac failure[10], survivors of STEMI[15], in patients undergoing CABG[5], and other major surgical procedures[4]. We therefore sought to determine whether elevated BPV would be associated with adverse outcomes in patients undergoing less invasive cardiac procedures than CABG, such as PCI. In particular, we hypothesized that patients who had adverse outcomes would have higher mean BPV than those who did not have these outcomes, and that the likelihood of a poor outcome would be greater for patients with larger pre-procedural BPV.
Previously collected data was reviewed from a prospectively maintained registry of patients who underwent PCI at a single institution and whose outcomes were then prospectively tracked. Patients who had a minimum of 10 prior outpatient BP recordings 3 to 60 mo prior to the procedure were included in this study to assure accuracy of BPV calculation. Charts were retrospectively reviewed to calculate BPV as both standard deviation and largest change for both systolic and diastolic BPV. BPV in patients who had poor outcomes was compared to those who did not; logistic regressions were used to control for the indication of the procedure.
This study was approved by the Institutional Review Boards of the University of North Dakota and the Sanford Medical Center. The subjects for this study were retrospectively drawn from a prospectively maintained database of all patients who underwent a PCI at Sanford Medical Center in Fargo, North Dakota in 2017 (n = 647). Patients within the reach of this system generally receive most of their healthcare, both inpatient and outpatient, at either the same or an affiliated institution. The electronic medical record was queried and BP recordings (n = 25844) both from within and outside the hospital from patients prior to PCI were identified. Only individuals who had a minimum of 10 BP recordings 3-60 mo before PCI (n = 471) were included for analysis. The remaining 176 patients were excluded from the study. Of these excluded, 2 were missing demographics, 75.29% were male, and the average age was 66.3
A total of 22,253 BP recordings were analyzed for 471 patients. BPV was defined as systolic and diastolic SD and largest change (LC, mmHg) between consecutive patient encounters. MACE outcomes of MI, CVA, death, and all-cause hospitalization were identified up to a year after PCI. Readmission was defined as a recurrent admission to the hospital within 1 year of discharge after hospitalization from PCI procedure. The procedural indication was categorized as staged PCI (n = 48), non-STEMI (n = 249) or other (n = 174). Other variables including demographics, prior diagnoses, and medication use were retrieved from the records.
BPV and BP characteristics along with demographics, diagnoses, medications, and indications were described for patients by MACE outcome status. Independent t-tests and chi-square analyses were used to determine any relationships between patients with or without an outcome of MACE. Logistic regressions of BPV predicting MACE, readmission, and MI outcomes after 1-year were done while controlling for age, sex, smoking status, diagnoses of hypertension or diabetes, prior cardiovascular disease, prior MI, prior PCI, prior CABG, pre-procedure creatine level, prior PCI left ventricular ejection fraction, anginal class (no symptoms as reference value, Canadian Cardiovascular Society I, II, III, or IV), on anti-anginal medications, and indication (staged PCI was used as the reference value). Although the registry data did not indicate which patients had pre-existing chronic kidney disease, we did analyze pre-procedural serum creatinine level. This was categorized as values of less than or equal to 2, 2-5, or greater than 5 mg/dL. Odds ratios (ORs) with 95% confidence intervals (CIs) were estimated. Receiving operator characteristic (ROC) analysis was done to determine the best cutoff values for the four measures of BPV in determining MACE, readmission, and MI. Two-way analysis of variance (ANOVA) with interaction was done for the BPV measures between MACE and the categorical variables age, anginal class, and indication to test if the relationships between the BPVs and MACE differed for levels of those variables. SAS v. 9.4 was used for the analysis and alpha was set to 0.05.
Four hundred and seventy-one patients who had undergone a PCI and had 10 or more blood pressure readings 3-60 mo prior to PCI were studied. Table 1 presents the demographics of this patient sample. The average age was 68.8 (SD 11.5, range 35-95) and 72.1% were male. Five types of adverse outcomes were identified: 147 (31.2%) of the patients had MACE, 131 (27.8%) were readmitted, 47 (10.0%) had MI, 21 (4.5%) died, and 6 (1.3%) had CVA. Patients who had a MACE were an average of 2 years older (P = 0.016). Hypertension was very common in both groups, though more so in patients with no MACE (P = 0.013). 15% more of those with a MACE were on anti-anginal medication (P = 0.003), with the largest difference found in patients taking beta blockers (16%; P = 0.002) and long-acting nitrates (10%; P = 0.011). Patients with a MACE were 5% more likely to be in anginal class CSS I and 8% more in CSS II (P < 0.001). About half were non-STEMI but twice as many MACE patients were STEMI.
| No MACE (n = 324) | Had MACE (n = 147) | |||||
| n | mean | SD | n | mean | SD | |
| Systolic SD | 324 | 13.72 | 6.02 | 147 | 15.38 | 5.26 |
| Diastolic SD | 324 | 8.54 | 3.11 | 147 | 8.93 | 2.71 |
| Systolic LC | 324 | 37.11 | 14.79 | 147 | 44.31 | 15.42 |
| Diastolic LC | 324 | 23.60 | 7.78 | 147 | 26.37 | 9.09 |
| Systolic average | 324 | 131.83 | 11.47 | 147 | 132.20 | 11.63 |
| Diastolic average | 324 | 74.82 | 7.75 | 147 | 71.33 | 7.64 |
| Number of BP readings | 324 | 40.19 | 35.86 | 147 | 62.81 | 52.85 |
| Mean days between readings | 324 | 59.07 | 37.04 | 147 | 42.19 | 30.70 |
| Age | 322 | 67.87 | 11.21 | 147 | 70.69 | 11.86 |
| Pre PCI LVEF | 252 | 56.44 | 11.73 | 122 | 53.06 | 13.63 |
| Pre creatinine | 307 | 1.15 | 0.98 | 138 | 1.50 | 1.19 |
| n | % | n | % | |||
| Sex | 469 | |||||
| Male | 110 | 74.83 | 228 | 70.37 | ||
| Female | 37 | 25.17 | 94 | 29.01 | ||
| Race | 469 | |||||
| White | 142 | 96.60 | 312 | 96.30 | ||
| Other | 5 | 3.40 | 10 | 3.09 | ||
| Hispanic | 1 | 0.68 | 4 | 1.23 | ||
| Smokes | 467 | 22 | 14.97 | 51 | 15.74 | |
| Has hypertension | 469 | 135 | 91.84 | 266 | 82.10 | |
| Has diabetes | 469 | 67 | 45.58 | 122 | 37.65 | |
| Had prior CVD | 469 | 39 | 26.53 | 68 | 20.99 | |
| Had prior MI | 470 | 106 | 32.72 | 63 | 42.86 | |
| Had prior PCI | 470 | 125 | 38.58 | 67 | 45.58 | |
| Had prior CABG | 470 | 53 | 16.36 | 41 | 27.89 | |
| Prior creatinine | 445 | |||||
| 0 to 2 | 296 | 91.36 | 118 | 80.27 | ||
| > 2 to 5 | 8 | 2.47 | 18 | 12.24 | ||
| > 5 | 3 | 0.93 | 2 | 1.36 | ||
| Anginal class | 469 | |||||
| No symptoms | 33 | 22.45 | 24 | 7.41 | ||
| CCS I | 11 | 7.48 | 39 | 12.04 | ||
| CCS II | 27 | 18.37 | 87 | 26.85 | ||
| CCS III | 43 | 29.25 | 95 | 29.32 | ||
| CCS IV | 33 | 22.45 | 77 | 23.77 | ||
| On anti-anginal medication | 469 | 114 | 77.55 | 204 | 62.96 | |
| Beta-blockers | 98 | 66.67 | 164 | 50.62 | ||
| Calcium channel blockers | 37 | 25.17 | 76 | 23.46 | ||
| Long-acting nitrates | 33 | 22.45 | 41 | 12.65 | ||
| Ranolazine | 3 | 2.04 | 2 | 0.62 | ||
| Indication | 471 | |||||
| Non-STEMI | 82 | 55.78 | 167 | 51.54 | ||
| STEMI | 9 | 6.12 | 39 | 12.04 | ||
| Other stage | 56 | 38.10 | 118 | 36.42 | ||
| MACE within 1 yr | 471 | |||||
| Readmission | 131 | 27.81 | ||||
| MI | 47 | 9.98 | ||||
| Death | 21 | 4.46 | ||||
| CVA | 6 | 1.27 | ||||
BPV was measured in two ways, SD of all patient BPs in the study period and the largest change (LC) between two consecutive outpatient BP measurements. Table 1 shows the average values for the four BPV measures by MACE category. Systolic SD measures were significantly higher for patients with MACE (mean 15.38 ± 5.26) than patients with no MACE (mean 13.72 ± 6.02; P = 0.004). The diastolic SD were less than the systolic (8.54 and 8.93) but not significantly different between MACE categories (P = 0.188). Like the systolic SD, the systolic LC was significantly higher in MACE group (P < 0.001). The diastolic LC was on average 3 points higher for the MACE group (P = 0.002). Average systolic measures were comparable in each group, mean 131.83 ± 11.47 and mean 132.20 ± 11.63 (P = 0.748). The average diastolic measures of the MACE patients (mean 71.33 ± 7.64) were significantly lower than patients with no MACE (mean 74.82 ± 7.75) (P < 0.001). We also tested the metrics for gathering the BPs and found that those with MACE had 12 more BP readings on average (P < 0.001) though this variable was badly skewed. MACE patients had 17 fewer days between readings on average than non-MACE patients (P < 0.001).
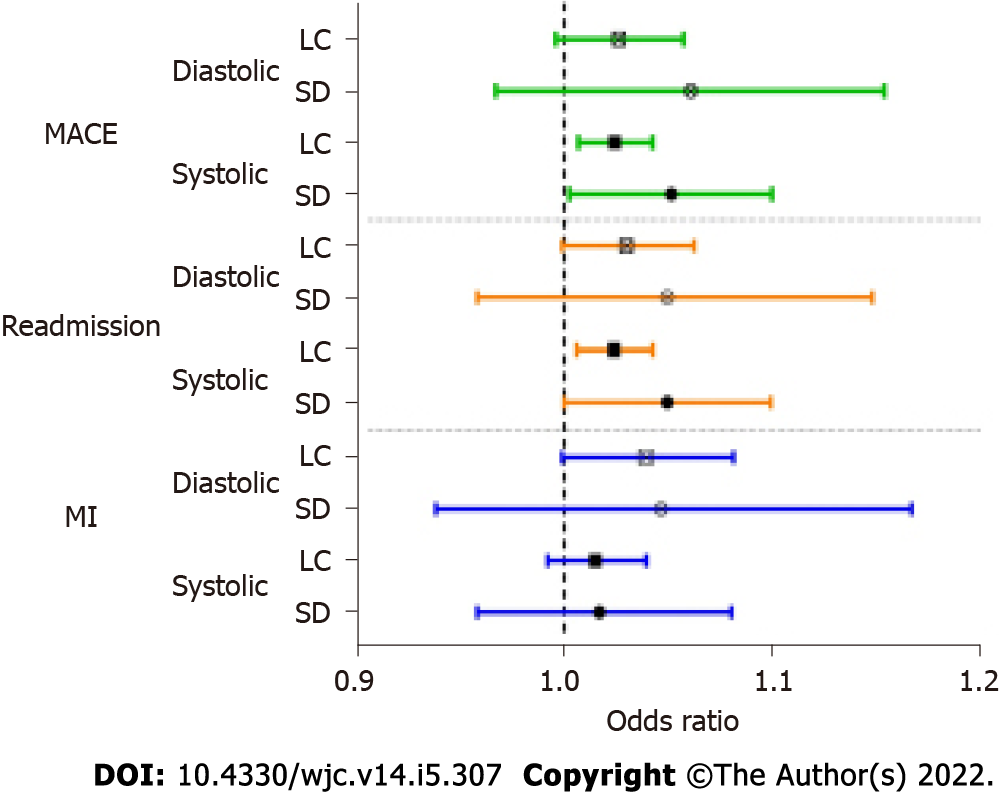
Logistic regressions (controlling for demographics and health status) were used to estimate the risk of higher BPV for adverse outcomes. Only the outcomes of MACE, all-cause hospitalization, and MI were used for these analyses due to relatively small number of patients who experienced the other specific outcomes. Figure 1 shows the ORs for BPV predicting the outcomes. No BPV measures significantly increased the risk of MI when controlling for demographics and health status. The risk of all-cause hospitalization was increased significantly by higher systolic BPV as calculated by both LC (OR = 1.024, 95%CI: 1.006-1.042) and SD (OR = 1.049, 95%CI: 1.000-1.099). The risk of MACE was also increased significantly by higher systolic BPV as calculated by LC (OR = 1.024, 95%CI: 1.007-1.042) and SD (OR = 1.049, 95%CI: 1.003-1.100). Although eight of the risks of these outcomes were not statistically significant, we noted a trend where patients with high BPV had increased risk of any outcome.
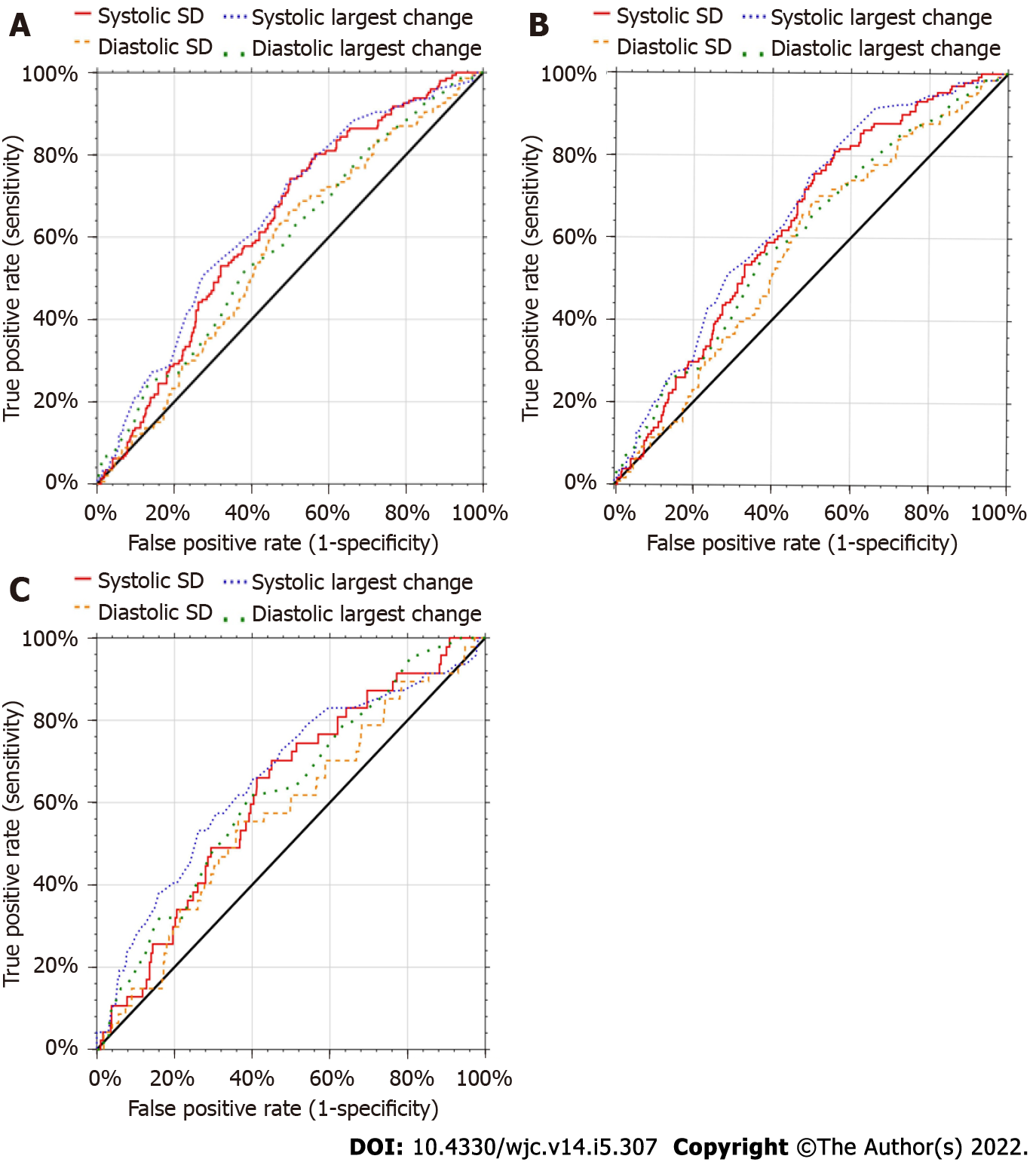
Receiving operator characteristic (ROC) curves were generated to determine cutoff values of the four BPV measures for predicting MACE, hospitalizations, and MI (Figure 2). The systolic BPVs appeared better at predicting outcomes. Table 2 shows the cutoff value used that maximized sensitivity and specificity, the area under the ROC curve (AUC) and corresponding 95% confidence intervals. The cutoff values for systolic SD determining MACE was 12.0, 14.0 for readmission, and 13.5 for MI. Diastolic SD ranged from 8 to 9, systolic LC was 33 to 48, and diastolic LC was 15 to 26. Sensitivities ranged from 45% to 82%, and specificities from 44% to 77%. All AUCs were significantly different from 50%.
| Cutoff | Sensitivity | Specificity | AUC | 95% confidence interval | ||
| Low level | Upper level | |||||
| MACE | ||||||
| Systolic SD | 12.0 | 0.7755 | 0.4475 | 0.6300 | 0.5752 | 0.6792 |
| Diastolic SD | 8.0 | 0.6395 | 0.5216 | 0.5674 | 0.5102 | 0.6195 |
| Systolic LC | 33.0 | 0.7891 | 0.4414 | 0.6510 | 0.5957 | 0.7001 |
| Diastolic LC | 26.0 | 0.5102 | 0.6235 | 0.5837 | 0.5262 | 0.6359 |
| Readmission | ||||||
| Systolic SD | 14.0 | 0.5573 | 0.6324 | 0.6348 | 0.5792 | 0.6846 |
| Diastolic SD | 8.0 | 0.6565 | 0.5206 | 0.5734 | 0.5149 | 0.6267 |
| Systolic LC | 33.0 | 0.8168 | 0.4412 | 0.6592 | 0.6039 | 0.7083 |
| Diastolic LC | 25.0 | 0.5573 | 0.6176 | 0.6018 | 0.5426 | 0.6549 |
| MI | ||||||
| Systolic SD | 13.5 | 0.6596 | 0.5684 | 0.6234 | 0.5371 | 0.6967 |
| Diastolic SD | 9.0 | 0.4894 | 0.6604 | 0.5730 | 0.4800 | 0.6533 |
| Systolic LC | 48.0 | 0.4468 | 0.7665 | 0.6609 | 0.5649 | 0.7393 |
| Diastolic LC | 26.0 | 0.6170 | 0.6038 | 0.6255 | 0.5370 | 0.7004 |
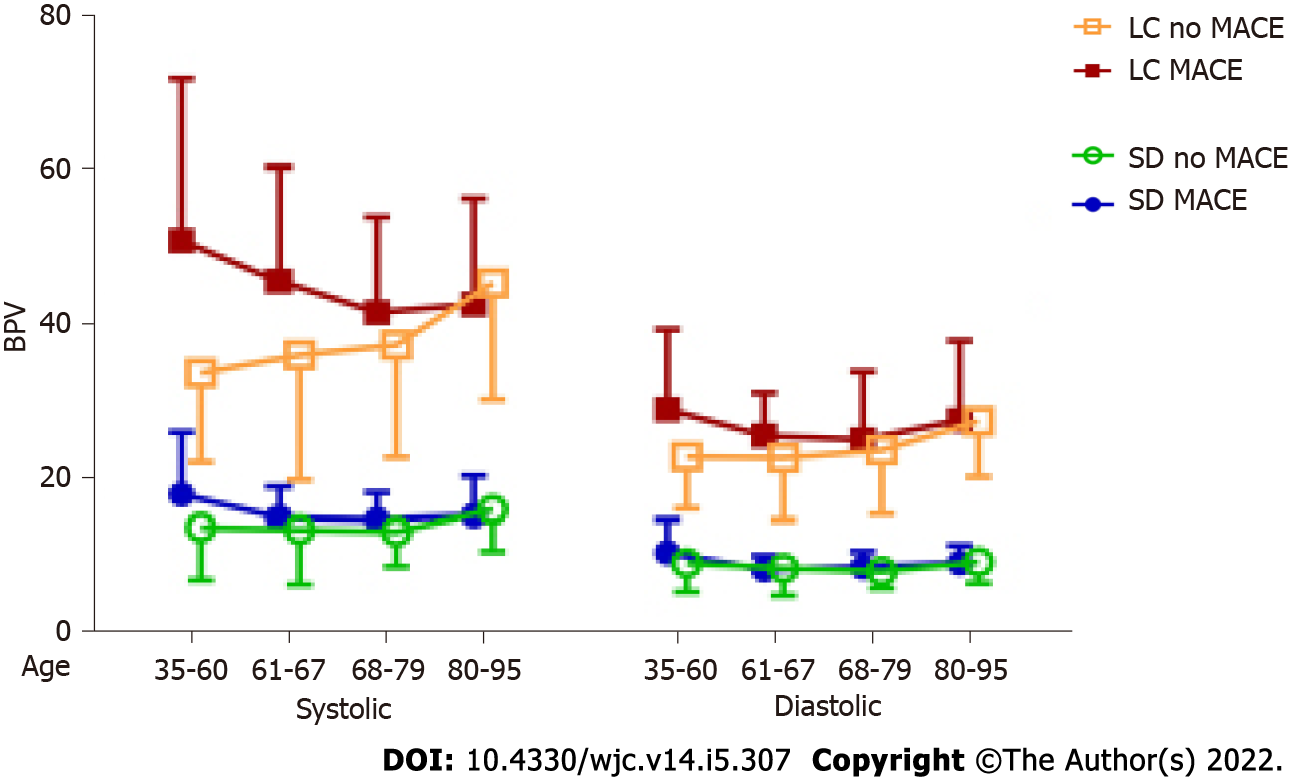
The relationships between the four BPVs and MACE were tested with subgroups of age, anginal class, and indication. Significant interaction in a two-way ANOVA indicated the relationship may differ according to groups. There were no significant interactions with anginal class and indication, suggesting the relationships between the BPV and MACE did not differ by those subgroups. Age (Figure 3) had significant interactions for systolic SD (P = 0.0429) and systolic largest change (P < 0.001).
Chronic outpatient BPV, distinct from hypertension, has been shown to be associated with poor patient outcomes, not only in the general population, but in those who undergo surgical procedures[4] including CABG[5]. BPV can be studied as either systolic or diastolic variability, and each can be calculated by standard deviation as well as the largest change between two consecutive measurements. A minimum of 10 outpatient BP recordings to measure BPV was previously used by other authors, ourselves included, because it was found to include enough measurements over a long enough timeframe to define BPV, yet short enough to be practical as patient physiology can drastically change with too large of a time interval[8]. Our key findings from this study were three. First, high pre-operative BPV gives patients elevated risk for poor outcomes following PCI. Second, systolic BPV may be a more sensitive indicator of adverse outcomes than diastolic BPV. Third, calculating BPV by LC seemed more indicative of adverse outcomes than calculating BPV by SD (following PCI).
While high BPV has been associated with worse post-operative outcomes after complex and highly invasive procedures such as CABG, colectomy, and total hip replacement[4,5], this is to our knowledge the first study investigating how BPV affects these outcomes after a much less invasive procedure such as PCI in patients who are known to have cardiac disease. This study confirms that patients with higher BPV are more likely to have poor outcomes after undergoing PCI. This is important because most patients who undergo PCI are already at higher baseline risk of adverse health outcomes, and thus preoperative BPV predisposes these individuals to an even higher risk. Patients who suffered from MI, all-cause hospitalization, and death within one year of the procedure had a significantly higher mean SD of their systolic BP. These patients also had a significantly larger mean greatest difference of both systolic and diastolic pressures. Moreover, when procedural indication was adjusted for, we found that risk for developing MI, all-cause hospitalization, and MACE was significantly increased when BPV was measured by LC.
Long term BPV has been shown to be a risk factor for MACE in several populations including type 2 diabetics, the elderly, younger populations, those with end stage renal disease, and post-operative patients[4,11,16-18]. Our results suggest that MACE occurs more frequently after PCI in patients with higher systolic BPV, and this remained true even when adjusted for indication. Regardless of how it is measured, even small changes in BPV can be clinically significant and associated with adverse outcomes for patients. Physicians should consider BPV while counseling patients who are considering elective PCI on the risks of the procedure. If a patient has high BPV, this may present an opportunity for physicians to educate their patients on their other cardiac risk factors. Perhaps patients could be more motivated to modify controllable risk factors, such as smoking or a sedentary lifestyle, if they know that they have additional non-modifiable risk factors such as BPV. Moreover, although all patients are followed carefully, when a patient with high BPV undergoes PCI it may be vital to conduct additional thorough follow-up and vigilant surveillance to identify and intervene if such outcomes may occur.
The etiology of BPV is not well understood, although a couple hypotheses exist on what contributes to BPV. One hypothesis is that BPV is related to differing coronary physiology due to vascular wall stiffness, hypertrophy, and cardiovascular plaque stability among others. Greater BPV in young people with an absence of cardiovascular disease has been shown to be related to central aortic stiffness[18]. BPV is associated with unstable coronary plaques in patients with stable angina[14] and with carotid arterial stiffness in elderly patients[17]. Blood pressure control may facilitate the regression of left ventricular hypertrophy, and it has been suggested that increased blood pressure variability may be a contributing cause of idiopathic cardiac hypertrophy[19,20]. BPV has been shown to increase arthroscopic plaque vulnerability[14,21] which could be a factor in some of these adverse outcomes. Surgical risks could be directly affected by autonomic instability which has been indicated in patients with BPV[20]. Any of these could cause a different response to PCI as compared to patients who have better blood pressure stability.
Others have suggested that BPV may be a proxy for differences in inflammatory responses to the physiologic stressors and the acute coronary illness that follows[22]. Components of both the innate and adaptive immune system, specifically various cytokine differences, toll-like receptors, and inflammasomes, have been shown to play a role in pathogenesis of elevated blood pressure[23]. Although this relationship has not yet been specifically studied in blood pressure variability, it is possible that inflammatory changes that are associated with hypertension might also lead to BPV within that hypertension. Such differences could alter the acute response to the trauma caused by PCI, thus putting patients at a higher risk for later adverse outcomes. Although further extrapolation of the etiology of BPV is certainly warranted, it seems likely that both intrinsic baseline biology and anatomy and differences in patients’ physiologic reactions to stress may all be associated with BPV in these patients and may contribute to their subsequent risk.
Although we[8] and others[7] have previously suggested that how BPV is calculated may be inconsequential, the results of this study seem to contrast with this idea. In this post-PCI population, systolic BPV seemed to be more sensitive of a predictor of adverse outcomes than diastolic BPV. Additionally, largest change may have been a more powerful predictor of adverse outcomes than standard deviation. This is potentially important because SD seems to be the most common way that BPV is analyzed in the literature. Increased systolic BPV showed statistical significance as a risk factor for each adverse outcome in this population as measured by LC. Systolic BPV as a more indicative measure of adverse outcomes after PCI may partially be due to the relatively high age of the patients who undergo this procedure. Systolic BP is known to have more use as a prognostic indicator with increasing age[22] and the average age of this population was 68.8 ± 11.5. Another factor to consider is that the association between elevated BPV and coronary atheroma progression was more strongly associated with systolic BPV[21].
Increased diastolic BPV also showed statistical significance for three outcomes but did not achieve statistical significance with any outcomes when calculated by SD. This suggests that diastolic BPV can also be a predictor of adverse outcomes when measured by LC. Although patients who experienced adverse outcomes were not shown to be significantly different than those who did not have adverse outcomes when measuring diastolic BPV by SD, we did observe a trend in this direction and it is possible that this might have become statistically significant with a larger sample size since we did observe statistical significance when diastolic BPV was assessed using LC. Additionally, although six of the risks of the outcomes measured by logistic regression were not statistically significant, a general trend was noted in that patients with high BPV had elevated risk of any outcome occurring. Although age is a potential hypothesis for the differences between systolic and diastolic BPV as a risk factor for adverse outcomes, work remains to be done to determine the etiologic differences that exist between systolic and diastolic BPV.
Although it is possible that LC may be a more sensitive indicator of risk than SD in patients undergoing PCI, this may also be an artifact of this particular sample. Regardless, it seems clear that LC is at least as useful, if not more useful than SD in risk estimation. This is important because until electronic medical records are programmed to automatically calculate BPV for every patient, the average physician will find LC to be much easier to calculate, less time consuming, and more intuitive than attempting to determine SD. The physician may simply scan a list of blood pressure readings and find the largest change between consecutive encounters to rapidly screen patients for BPV prior to selection for PCI. Further studies need to be done to determine if LC could indeed be a stronger predictor of adverse outcomes than SD.
In cardiovascular trials, different authors use various defined composite clinical endpoints, one of which is commonly MACE. 3P MACE and 4P MACE exist depending on whether 3 or 4 individual event endpoints are used, with some variability of what these endpoints are 3 endpoint MACE are commonly defined to include MI, death, and CVA[24]. Although not commonly reported as a MACE, hospitalization is a commonly used endpoint related to heart failure or other post-operative trials, so we believe that it is appropriate to use all cause hospitalization as a composite endpoint for a major adverse cardiac event[24]. Therefore, we used a somewhat original 4P MACE for our study which we defined as all-cause hospitalization, MI, death, and CVA.
This study has limitations. 27% of the patients who underwent angioplasty during the study period were excluded a priori because they did not have 10 outpatient BP readings 3-60 mo prior to PCI. We had made this decision in advance of collecting our data because our previous analysis[8] suggested that BPV can be very accurately calculated with at least 10 readings. These 171 patients otherwise had remarkably similar demographics to the patients who were included in the study, making it less likely that selection bias has affected our results. Another potential concern is whether we missed complications in patients who may have gone to a separate healthcare system with their post-procedural complications. However, Sanford Health has a large catchment area and shares access to surrounding health systems’ electronic records. Moreover, there seems no particular reason to postulate that patients with low or high BPV would have been more likely to seek attention at outside facilities which was indicative in that the outcomes we ascertained had 100% follow up prospectively. Another potential concern is that the BP readings that were used in this study were derived from chart review after routine clinical practice rather than being measured by pre-designed specified protocols. Clinical trials often utilize very precise practices to measure BP precisely because without such practices BP measurement may differ from how it is routinely measured in the clinical setting. Our BP measurements do lack standardization, which thus could be interpreted as a weakness in that measurements were not taken at fixed intervals with fixed protocols. However, the BP measurements used here do reflect how physicians would routinely assess patients’ BPV in the clinic. Thus, one might conversely propose that this apparent limitation actually makes our study results more relevant to the real world. While considering kidney disease simply by pre-procedural serum creatinine levels is not ideal and represents a limitation to this study, the diagnosis of chronic renal failure was not included in the data available for analysis. While it would have been interesting to calculate a Kaplan-Meier survival curve for MACE, the specific dates for these key complications were unfortunately not included in the registry and so these data were unfortunately unavailable for analysis.
High outpatient BPV predicts adverse outcomes after PCI, including all-cause hospitalization, death, MI, and CVA, regardless of whether the patient is chronically hypertensive or normotensive. Calculating BPV by largest change was a stronger predictor than standard deviation for MACE within 1 year of the procedure. This was true for both systolic and diastolic BPV, although systolic BPV seemed to be a more sensitive indicator of poor outcomes. Prior to PCI, patients with high BPV should be counseled by their physician about their increased risk for adverse outcomes and should be followed more vigilantly after their procedure. Most percutaneous coronary interventions are relatively urgent and cannot be postponed for long periods of time for patients to attempt to modify risk factors prior to PCI. Furthermore, further research is still required to identify changes or pharmacologic interventions that patients may undertake to usefully reduce their BPV. However, patients with higher BPV who are about to undergo PCI can and should be counselled that they are at a higher risk of post-procedural complications and that they should subsequently address any other modifiable risk factors that are also associated with poor post-operative outcomes to best optimize their individual post-procedural outcomes. Physicians performing PCI may also wish to consider BPV as they decide how aggressive to be in their procedures, while quality comparisons of PCI programs or research on future PCI interventions should consider as an additional risk factor in multivariate analyses of outcomes. Work remains to be done to discover the true etiology of BPV as well as why systolic and diastolic variability may have differing impacts on the patients’ outcomes.
Blood pressure variability (BPV), distinct from hypertension, is known to be a risk factor for long term complications, and has recently been shown to increase the acute risk of postoperative death, hospitalization, or other complications for patients undergoing major surgical procedures.
The impact of BPV on outcomes after the less invasive procedure of percutaneous coronary interventions (PCI) has not previously been explored despite the high risk nature of these patients.
To determine whether BPV represents an independent risk factor for poor outcomes after percutaneous coronary angioplasty.
Six hundred and forty-seven patients undergoing PCI in a single state in 2017 were prospectively enrolled in a patient registry which was then retrospectively analyzed. Systolic and diastolic BPV were calculated as both the largest consecutive change between blood pressure measurements and the standard deviation of all blood pressure measurements for the 30-60 mo prior to PCI, considering only the 471 patients with more than ten blood pressure measurements for analysis. Other variables including demographics, prior diagnoses and medication use were retrieved. Procedural indications were categorized as staged PCI, non-STEMI, or other. Adverse outcomes were identified for up to a year following the procedure, including MACE, myocardial infarction, cerebrovascular accident, death, and all-cause hospitalization.
Even after taking into account other patient characteristics, visit-to-visit systolic BPV, as measured by both standard deviation and largest change, was higher in patients who had myocardial infarctions, were readmitted, or died within one year following PCI. Systolic BPV was higher in patients who had major adverse cardiac events (MACE), or readmissions (P < 0.05). Diastolic BPV, as measured by largest change, was higher in patients with MACE and readmissions (P < 0.05).
BPV represents an independent risk factor for poor outcomes after PCI.
BPV is easily measured and captured from the electronic medical record. Cardiologists performing PCI should consider high BPV in choosing among procedural outcomes or observation, and should follow patients with high BPV more closely after PCI. Patients with high BPV should be counseled about this risk factor in the informed consent process and should be counseled to work more aggressively to reduce other more modifiable risk factors after PCI in the face of their BPV.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: El-Serafy AS, Egypt; Guo L, China S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Research iData. Over 965, 000 Angioplasties (PCIs) are Performed Each Year in the United States. [DOI] [Full Text] |
| 2. | Al-Omran M, Lindsay TF. Commentary: one-year cardiovascular event rates in outpatients with atherothrombosis. Steg PG, Bhatt DL, Wilson PW, et al; REACH Registry Investigators. JAMA. 2007;297: 1197-1206. Perspect Vasc Surg Endovasc Ther. 2007;19:416-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Sadrnia S, Pourmoghaddas M, Hadizadeh M, Maghamimehr A, Esmaeeli M, Amirpour A, Khosravi A. Factors affecting outcome of primary percutaneous coronary intervention for acute myocardial infarction. ARYA Atheroscler. 2013;9:241-246. [PubMed] |
| 4. | Basson MD, Klug MG, Newman WE, Dyke C. Preoperative outpatient blood pressure variability predicts postoperative mortality, readmission and morbidity after surgery. Am J Surg. 2020;220:1083-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Dyke CM, Benz CL, Taggart CM, Klug MG, Basson MD. Systolic and Diastolic Blood Pressure Variability as Risk Factors for Adverse Events After Coronary Artery Bypass Grafting. JAMA Surg. 2019;154:92-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Stevens SL, Wood S, Koshiaris C, Law K, Glasziou P, Stevens RJ, McManus RJ. Blood pressure variability and cardiovascular disease: systematic review and meta-analysis. BMJ. 2016;354:i4098. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 630] [Cited by in RCA: 585] [Article Influence: 65.0] [Reference Citation Analysis (0)] |
| 7. | Stergiou GS, Parati G, Vlachopoulos C, Achimastos A, Andreadis E, Asmar R, Avolio A, Benetos A, Bilo G, Boubouchairopoulou N, Boutouyrie P, Castiglioni P, de la Sierra A, Dolan E, Head G, Imai Y, Kario K, Kollias A, Kotsis V, Manios E, McManus R, Mengden T, Mihailidou A, Myers M, Niiranen T, Ochoa JE, Ohkubo T, Omboni S, Padfield P, Palatini P, Papaioannou T, Protogerou A, Redon J, Verdecchia P, Wang J, Zanchetti A, Mancia G, O'Brien E. Methodology and technology for peripheral and central blood pressure and blood pressure variability measurement: current status and future directions - Position statement of the European Society of Hypertension Working Group on blood pressure monitoring and cardiovascular variability. J Hypertens. 2016;34:1665-1677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 115] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 8. | Basson MD, Klug MG, Hostetter JE, Wynne J. Visit-to-Visit Variability of Blood Pressure Is Associated With Hospitalization and Mortality in an Unselected Adult Population. Am J Hypertens. 2018;31:1113-1119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Grassi G, Bombelli M, Brambilla G, Trevano FQ, Dell'oro R, Mancia G. Total cardiovascular risk, blood pressure variability and adrenergic overdrive in hypertension: evidence, mechanisms and clinical implications. Curr Hypertens Rep. 2012;14:333-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 10. | Berry M, Lairez O, Fourcade J, Roncalli J, Carrié D, Pathak A, Chamontin B, Galinier M. Prognostic value of systolic short-term blood pressure variability in systolic heart failure. Clin Hypertens. 2016;22:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Bae EH, Lim SY, Han KD, Oh TR, Choi HS, Kim CS, Ma SK, Kim SW. Association Between Systolic and Diastolic Blood Pressure Variability and the Risk of End-Stage Renal Disease. Hypertension. 2019;74:880-887. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 12. | Tully PJ, Yano Y, Launer LJ, Kario K, Nagai M, Mooijaart SP, Claassen JAHR, Lattanzi S, Vincent AD, Tzourio C; Variability in Blood Pressure and Brain Health Consortium †; Variability in Blood Pressure and Brain Health Consortium†. Association Between Blood Pressure Variability and Cerebral Small-Vessel Disease: A Systematic Review and Meta-Analysis. J Am Heart Assoc. 2020;9:e013841. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 88] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 13. | Nagai M, Dote K, Kato M, Sasaki S, Oda N, Kagawa E, Nakano Y, Yamane A, Higashihara T, Miyauchi S, Tsuchiya A. Visit-to-Visit Blood Pressure Variability and Alzheimer's Disease: Links and Risks. J Alzheimers Dis. 2017;59:515-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 14. | Aoyama R, Takano H, Suzuki K, Kubota Y, Inui K, Tokita Y, Shimizu W. The impact of blood pressure variability on coronary plaque vulnerability in stable angina: an analysis using optical coherence tomography. Coron Artery Dis. 2017;28:225-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Shah AM, Claggett B, Sweitzer NK, Shah SJ, Anand IS, Liu L, Pitt B, Pfeffer MA, Solomon SD. Prognostic Importance of Impaired Systolic Function in Heart Failure With Preserved Ejection Fraction and the Impact of Spironolactone. Circulation. 2015;132:402-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 368] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 16. | Chiriacò M, Pateras K, Virdis A, Charakida M, Kyriakopoulou D, Nannipieri M, Emdin M, Tsioufis K, Taddei S, Masi S, Georgiopoulos G. Association between blood pressure variability, cardiovascular disease and mortality in type 2 diabetes: A systematic review and meta-analysis. Diabetes Obes Metab. 2019;21:2587-2598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 17. | Nagai M, Dote K, Kato M, Sasaki S, Oda N, Kagawa E, Nakano Y, Yamane A, Kubo Y, Higashihara T, Miyauchi S, Harada W, Masuda H. Visit-to-visit blood pressure variability, average BP level and carotid arterial stiffness in the elderly: a prospective study. J Hum Hypertens. 2017;31:292-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Boardman H, Lewandowski AJ, Lazdam M, Kenworthy Y, Whitworth P, Zwager CL, Francis JM, Aye CY, Williamson W, Neubauer S, Leeson P. Aortic stiffness and blood pressure variability in young people: a multimodality investigation of central and peripheral vasculature. J Hypertens. 2017;35:513-522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 19. | Parati G, Faini A, Valentini M. Blood pressure variability: its measurement and significance in hypertension. Curr Hypertens Rep. 2006;8:199-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 84] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 20. | McGrane S, Atria NP, Barwise JA. Perioperative implications of the patient with autonomic dysfunction. Curr Opin Anaesthesiol. 2014;27:365-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Clark D 3rd, Nicholls SJ, St John J, Elshazly MB, Ahmed HM, Khraishah H, Nissen SE, Puri R. Visit-to-Visit Blood Pressure Variability, Coronary Atheroma Progression, and Clinical Outcomes. JAMA Cardiol. 2019;4:437-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 22. | Franklin SS, Larson MG, Khan SA, Wong ND, Leip EP, Kannel WB, Levy D. Does the relation of blood pressure to coronary heart disease risk change with aging? Circulation. 2001;103:1245-1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 841] [Cited by in RCA: 870] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 23. | De Miguel C, Rudemiller NP, Abais JM, Mattson DL. Inflammation and hypertension: new understandings and potential therapeutic targets. Curr Hypertens Rep. 2015;17:507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 173] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 24. | Zannad F, Garcia AA, Anker SD, Armstrong PW, Calvo G, Cleland JG, Cohn JN, Dickstein K, Domanski MJ, Ekman I, Filippatos GS, Gheorghiade M, Hernandez AF, Jaarsma T, Koglin J, Konstam M, Kupfer S, Maggioni AP, Mebazaa A, Metra M, Nowack C, Pieske B, Piña IL, Pocock SJ, Ponikowski P, Rosano G, Ruilope LM, Ruschitzka F, Severin T, Solomon S, Stein K, Stockbridge NL, Stough WG, Swedberg K, Tavazzi L, Voors AA, Wasserman SM, Woehrle H, Zalewski A, McMurray JJ. Clinical outcome endpoints in heart failure trials: a European Society of Cardiology Heart Failure Association consensus document. Eur J Heart Fail. 2013;15:1082-1094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 189] [Article Influence: 15.8] [Reference Citation Analysis (0)] |