Published online Aug 26, 2021. doi: 10.4330/wjc.v13.i8.325
Peer-review started: March 18, 2021
First decision: June 7, 2021
Revised: June 9, 2021
Accepted: July 26, 2021
Article in press: July 26, 2021
Published online: August 26, 2021
Processing time: 158 Days and 10.4 Hours
Heart failure (HF) is a clinical syndrome that results from a structural or functional cardiac disorder that reduces the ability of the ventricle of the heart to fill with, or eject, blood. It is a multifaceted clinical condition that affects up to 2% of the population in the developed world, and is linked to significant morbidity and mortality; it is therefore considered a major concern for public health. Regarding the mechanism of HF, three neurohumoral factors - the renin-angiotensin-aldosterone system, the sympathetic nervous system, and natriuretic peptides — are related to the pathology of chronic HF (CHF), and the targets of treatment. Angiotensin receptor blocker and neprilysin inhibitor (angiotensin-receptor neprilysin inhibitor), namely sacubitril/valsartan (SAC/VAL), has been introduced as a treatment for CHF. SAC/VAL is an efficacious, safe, and cost-effective therapy that improves quality of life and longevity in patients with HF with reduced ejection fraction (HFrEF), and reduces hospital admissions. An in-hospital initiation strategy offers a potential new avenue to improve the clinical uptake of SAC/VAL. In the last five years, SAC/VAL has been established as a cornerstone component of comprehensive disease-modifying medical therapy in the management of chronic HFrEF. On the other hand, further work, with carefully designed and controlled preclinical studies, is necessary for un
Core Tip: Heart failure (HF) is a multi-faceted clinical condition that affects up to 2% of the population in the developed world, and is linked to significant morbidity and mortality; it is therefore considered a major concern for public health. In 2014, a newly developed angiotensin receptor blocker and neprilysin inhibitor (angiotensin-receptor neprilysin inhibitor), namely sacubitril/valsartan (SAC/VAL), was introduced as a treatment for chronic HF (CHF), and it proved to have the efficacy, safety, and cost-effectiveness to improve quality of life and longevity in patients with heart failure with reduced ejection fraction and reduces hospital admission. In this review, we first summarize the current knowledge regarding HF, then provide an overview of the current knowledge on SAC/VAL for CHF, together with relevant clinical trials and future perspectives.
- Citation: Usuda D, Higashikawa T, Hotchi Y, Usami K, Shimozawa S, Tokunaga S, Osugi I, Katou R, Ito S, Yoshizawa T, Asako S, Mishima K, Kondo A, Mizuno K, Takami H, Komatsu T, Oba J, Nomura T, Sugita M. Angiotensin receptor blocker neprilysin inhibitors. World J Cardiol 2021; 13(8): 325-339
- URL: https://www.wjgnet.com/1949-8462/full/v13/i8/325.htm
- DOI: https://dx.doi.org/10.4330/wjc.v13.i8.325
Heart failure (HF) is a clinical syndrome resulting from a structural or functional cardiac disorder, diminishing the ability of the cardiac ventricle to fill with, or eject, blood[1-3]. It is a multi-faceted clinical condition that affects up to 2% of the population in the developed world, and it is linked to both significant morbidity and mortality; it is therefore considered a major concern for public health[4].
According to several researches in Japan, HF results from myocardial injury due to a variety of causes, including age > 80 years old, male, underlying heart disease; ischemic, hypertensive, cardiomyopathy, vulver heart disease, medical history; prior hospitalization for HF, hypertension, dyslipidemia, diabetes mellitus (DM), smoking, atrial flutter/fibrillation, chronic respiratory disease, stroke/transient ischemic attack, continuous positive airway pressure, pacemaker, implantable cardioverter defi
| Arrhythmia |
| Cardiomyopathy |
| Cardiotoxic drug |
| CKD |
| Congenital heart disease |
| DM |
| Hypertensive heart disease |
| Hypertension |
| Infection |
| Ischemic heart disease |
| Myocardial disease |
| Pericardial disease |
| Pulmonary hypertension |
| Systemic toxins |
| Valvular disease |
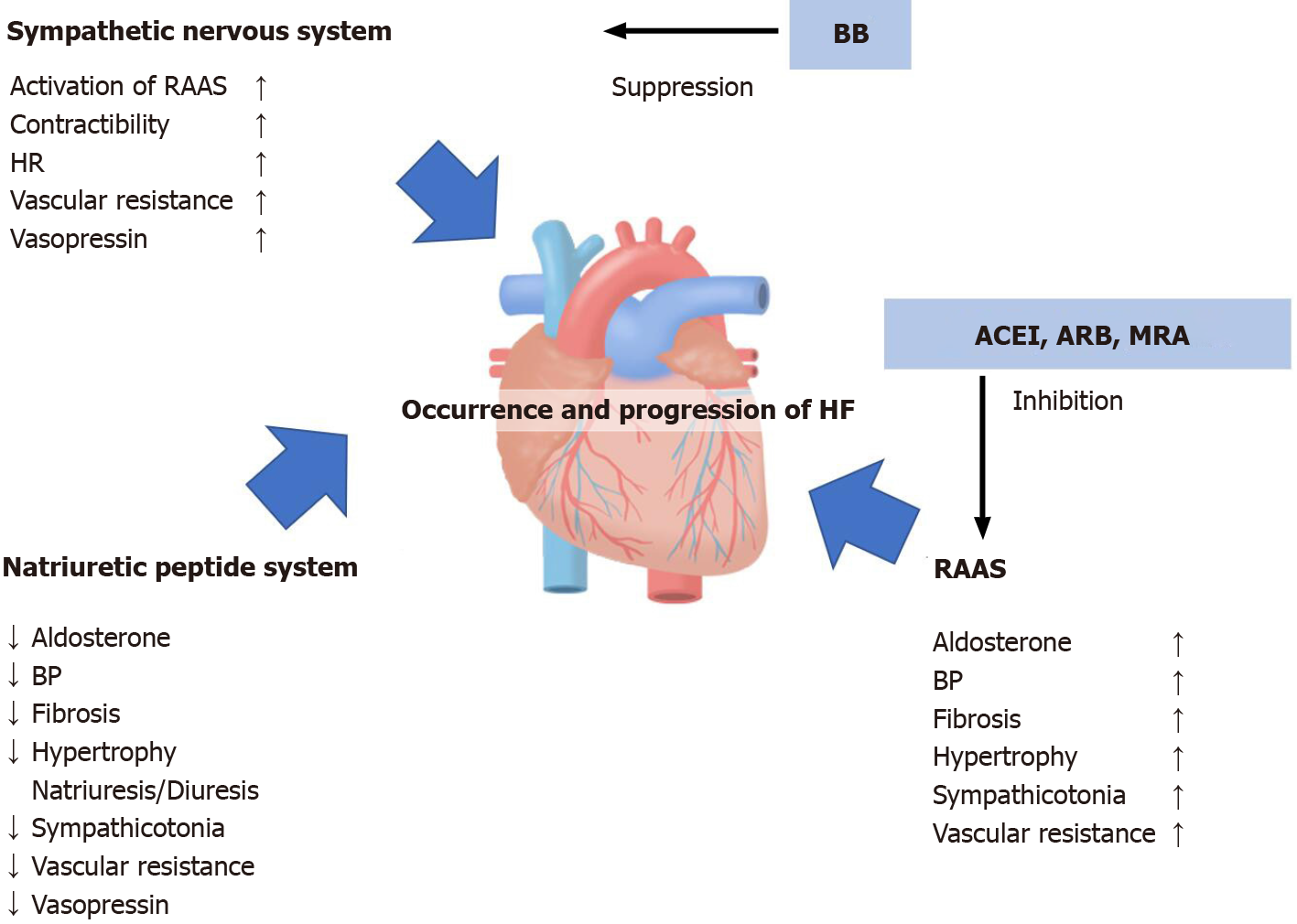
Regarding the mechanism of HF, vasoconstriction and fluid retention are caused by the renin-angiotensin-aldosterone system (RAAS) and the sympathetic nervous system (SNS), and the natriuretic peptides (NPs) secreted by the myocardium, which is itself both volume- and pressure-overloaded, promote vasodilation and diuresis[7,8]. The three neurohumoral factors related to the pathology of chronic heart failure, and the target of conventional remedies, are shown in Figure 1.
In this review, we first summarize the current knowledge of HF, then provide an overview of the current knowledge on angiotensin receptor blocker and neprilysin inhibitor [angiotensin-receptor neprilysin inhibitor (ARNI)], namely sacubi
HF is associated with a number of symptoms, including shortness of breath, breathing difficulties, nausea, diminished appetite, fatigue, intolerance to exercise, retention of fluid, coughing, weight gain from pulmonary congestion, and peripheral edema and ascites due to impaired venous return[1,3]. HF severity can be classified under the New York Heart Association (NYHA) classification system as follows: Class I, no symptoms; Class II, symptoms with ordinary activity; Class III, symptoms with less than ordinary activity; and Class IV, symptoms at rest or with any minimal activity[3].
HF can be further categorized based on ejection fraction (EF)[3]. In 2013, The American Heart Association (AHA) and American College of Cardiology (ACC) assigned an EF range to HFrEF and HFpEF[3,9]. This classification created a “gray area” of patients who have EF of 41–49%; this has ultimately come to be known as “HF with mid-range” (HFmrEF)[3,9]. “HF with preserved EF” (HFpEF) is defined as left ventricular (LV) EF (LVEF) of 50% or greater; HFmEF is defined as LVEF of 41%–49%, and HF with reduced EF (HFrEF) is defined as LVEF of up to 40%[3,9]. Of these, HFmrEF patients represent a group with heterogeneous clinical characteristics, sometimes resembling HFrEF, sometimes resembling HFpEF, and sometimes even resembling a unique phenotype entirely[9]. There are no randomized controlled trials (RCTs) for patients with HFmrEF, though HFrEF and HFpEF studies that include overlap suggest some potential benefits from β-blockers (BBs), angiotensin receptor blockers (ARBs), mineralocorticoid receptor antagonists (MRAs), and ARNI[9]. HFrEF occurs at LVEF of 40% or below, and is accompanied by progressive LV dilatation and adverse cardiac remodeling[2]. HF assessment begins with obtaining the patient’s medical history and performing a physical examination[2]. Other key factors for diagnosis are NPs that are elevated above age- and context-specific thresholds, and identifying LV systolic dysfunction with LVEF of 40% or less using echocardiography[2]. Worldwide, HF now affects an estimated 23 million people, approximately 50% of whom are HFrEF cases[2].
Management of HF depends on each individual’s NYHA classification and EF, but generally, treatment involves pharmacotherapies[3]. HF treatment strategies include the use of diuretics for symptom relief, and the application of an expanding armamentarium of disease-modifying drug and device therapies[2].
The foundation of HFrEF treatment is a number of pharmacotherapies that have been shown in large multinational RCTs to reduce morbidity and mortality[10]. With the exception of cases with specific contraindications, patients with HFrEF should be treated with BB, and one of ARNI, an angiotensin-converting enzyme (ACE) inhibitor (ACEI), or ARB, as foundational therapy, as well as diuretics, and additionally MRA which is recommended to reduce mortality and hospitalization in all the patients with HFrEF and EF ≤ 35%; until recently, however, it was unclear how to augment the beneficial effects of NPs in HF patients[2,7,10]. Of these, this review article covers ARNI in more detail below. In some cases, digoxin, ivabradine, ivabradine and hydralazine with isosorbide dinitrate, and hydralazine/isosorbide dinitrate also play roles in the care of some HFrEF patients[2]. More recently, sodium-glucose cotransporter 2 (SGLT2) inhibitors have led to further improvements in disease outcomes, bringing about significant reductions in cardiovascular and all-cause mortality, regardless of patient diabetes status; additionally, the soluble guanylate cyclase stimulator vericiguat has reduced hospitalization for HF in high-risk HFrEF patients[2]. Pharmacotherapy efficacy does not vary by age; therefore, each of these therapies should be considered for every patient, no matter their age[10]. Other factors, including co-morbidities such as renal dysfunction, may limit use of some of these drugs for elderly patients[10]. Building on this foundation, other, more advanced treatments, including implantable cardioverter defibrillators and cardiac resynchronization therapy, are recommended by HFrEF treatment guidelines; for a select few, mechanical circulatory support and cardiac transplantation also remain options[2,10]. Conversely, there are only limited options for HFpEF[10]. In the absence of robust outcome data from large randomized trials, MRA is a reasonable therapy for the reduction of hospitalization risk for HF in patients with HFpEF[10].
New therapeutic strategies that aim to tackle the rising socio-economic burden of HF have become a significant priority, and timely, efficient drug treatments play key roles in improving quality of life (QOL) and prognosis for HF patients[11,12]. Enhancing NP bioavailability through exogenous NP administration, and inhibiting neutral endopeptidase, are valuable therapeutic strategies for HF; current therapeutic concepts combine inhibition of the RAAS with blockage of the sympathetic system[8,12]. New therapeutic approaches, including selective heart rate reduction, attenuation of NP degradation through neutral endopeptidase inhibition, and treatment of comorbidities (such as iron deficiency, DM, or hyperkalemia) have led to further improvements to affected patient survival, time out of hospital, and QOL[8,12]. In addition, this approach has been proven to demonstrate beneficial effects, and reduce adverse events, in HF patients[8].
Typically, the natural course of HF is associated with repeated hospitalizations and the subsequent deterioration of patient prognosis[13,14]. In the past twenty years, the prognosis for HFrEF has steadily improved due to drug treatment advances and consistent implementation of evidence-based drug therapy as recommended by guidelines[12]. Therefore, a history of multiple previous admissions for HF was found to be a strong independent risk factor for adverse events following index admission, and number of hospitalizations could serve as a simple yet valuable surrogate indicating subsequent adverse events in HF patients[13]. Furthermore, another study conducted in Japan reported a 23.6%–26.2% HF readmission rate within one year after discharge for HF[15]. Overall, despite the underlying pathophysiological mechanisms of HF being well understood, the disease still has significant morbidity, with three-year mortality of 30% and five-year mortality of 50%[1,3,16].
As a classification of HF, HFrEF is a major public health concern that has substantial morbidity and mortality; however, recent developments such as SGLT2 inhibitors, vericiguat, and transcatheter mitral valve repair all incrementally improve prognosis beyond what was possible through foundational neurohormonal therapies[2]. On the other hand, one of the most common reasons for prolonged hospital admission is poor management of HF symptoms from decompensated HFpEF[17]. The high morbidity and mortality rates associated with HFpEF are compounded by poor understanding of the underpinning pathophysiology[17].
Though CHF is a common condition, if untreated, it will markedly impair QOL; it is associated with a high risk of recurrent hospitalization and death[18]. Availability of evidence-based treatment options is limited to congestive HF with low EF; the medication has been approved in the United Stated by the Food and Drug Administration (FDA) for the treatment of chronic HFrEF patients of NYHA class II, III, or IV[18,19]. Alongside the past decade’s marked progress in device therapy, more recent advances in CHF management have led to exciting new pharmacological options[20]. Pharmacotherapy is based on neurohumoral inhibition of the RAAS and the adrenergic system[18]. Previously, it has been reported that higher serum levels of both cortisol and aldosterone were independent predictors of increased mortality risk in CHF patients, and that these confer complementary and incremental prognostic value[21]. On the other hand, a recent article reported further prognostic improvements for patients with this condition by introducing ARNI[18]. Modern implantable devices serve as another component of treatment[18]. The use of implantable defibrillators and special pacemakers for cardiac resynchronization is well established; there is still a need for further studies to investigate the utility of alternative devices (such as baroreflex modulation or cardiac contractility modulation)[18]. The treatment of chronic systolic HF as recommended in relevant guidelines, using drugs and implanted devices as indicated, can greatly improve clinical outcomes[18].
In the early 1980s, the NP system was extensively characterized, with investigations into its potential influence on the development and progression of HF; in recent years, the NP system has drawn increasing attention[22]. Indeed, this new class of drugs for HF management is supported by recent results and a vast clinical development program, and may prompt a paradigm shift in HF treatment, moving from inhibition of RAAS and SNS, to more integrated targeting of rebalanced neurohormonal dysregulation in HF[22]. The study of NPs has become highly relevant, as they mediate beneficial effects at the cardiovascular level, such as diuresis, natriuresis, and decreased cardiac remodeling; their metabolism is mediated by neprilysin, a metalloproteinase that is widely expressed in humans, and which is capable of catalyzing various substrates[23]. One of these, neprilysin, is an endopeptidase that breaks down endogenous vasoactive peptides, including NP, bradykinin, and adrenomedullin[3]. Neprilysin inhibition increases the levels of vasoactive substances, helping to counter the neurohormonal overactivation that contributes to vasoconstriction, sodium retention, and other maladaptive processes of HF[3]. The modulation of these functions has been studied for decades, giving rise to the use of sacubitril, the first neprilysin inhibitor; in conjunction with ARB, it has demonstrated high efficacy and tolerability among HF patients[23].
The association of an angiotensin II receptor antagonist and a neprilysin inhibitor is a new actor in HF management; recently, a newer pharmacotherapy, SAC/VAL, has become available for this purpose[3,24]. The stimulation of counter-regulatory systems, in addition to neurohormonal blockade, constitutes a new paradigm, known as “neurohormonal modulation,” and SAC/VAL is the first example of this new approach[3,8,16,19]. This new pharmacological class of ARNI has prompted a substantial conceptual change in HF treatment, with a transition from only inhibition of the RAAS and SNS, to a strategy built around concomitant pharmacological enhan
The ARNI, namely SAC/VAL, is a single molecule that is synthesized through the co-crystallization of valsartan and the neprilysin inhibitor prodrug sacubitril (1:1 molar ratio)[25]. The substrates for neprilysin are multifarious, and include biologically active NPs, adrenomedullin, substance P, endothelin, and angiotensin II, among others; it is unclear which of those substrates, or combination(s) of substrates, might be responsible for the benefit observed[26]. In addition, it can exert an additive action, because it may increase the levels of compounds that can protect against lung and heart injury (NPs, adrenomedullin, substance P, bradykinin, and apelin)[27].
In humans, this peptidase is widely distributed throughout the body, expressed with broad substrate specificity that preferentially hydrolyses oligopeptide substrate[28,29]. It is also an endogenously induced peptidase, for modulation of the pro
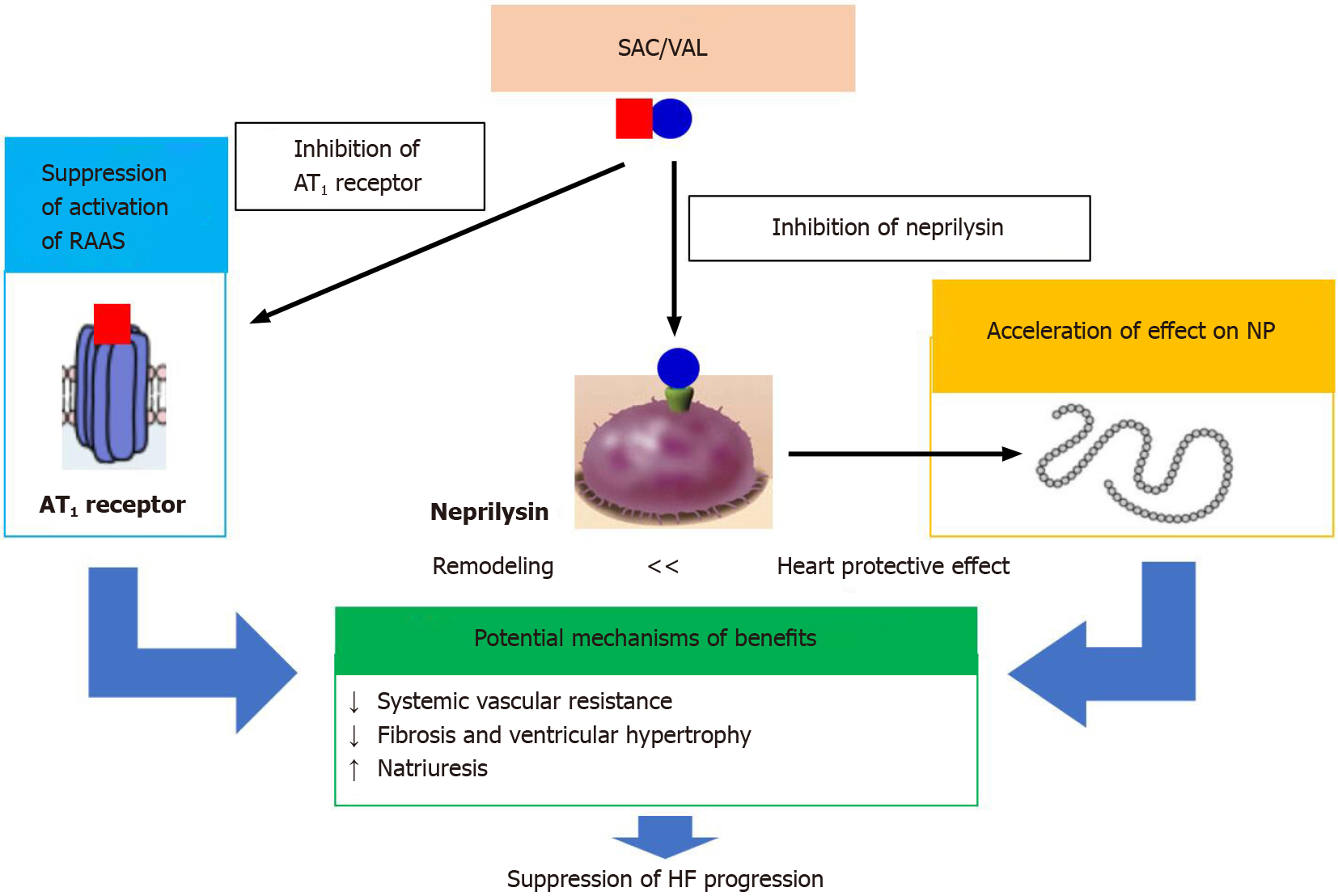
However, despite intensive research into neprilysin functions in various organisms, and into changes in how it is expressed and regulated during brain development and ageing, especially in age-related pathologies, concrete resolution is still not fully understood[28]. Currently, it is known that neprilysin regulates the cardiovascular, nervous, and immune systems[28,29]. SAC/VAL modulates the neurohormonal axis through inhibition of both angiotensin receptors and neprilysin, which additionally improves neurohormonal balance more than blocking the RAAS alone would[31]. Of these, unfavorable outcomes are attributed primarily to NP degradation[30]. NPs are involved in the RAAS inhibition and sympathetic system activation contributing to tubular and glomerular injury, and ARNI possesses the ability to counteract the effects of angiotensin II, as well as to increase NP activity[30,32]. Neprilysin exerts a beneficial effect by converting angiotensin-1 to angiotensin-(1-7), which activates the MAS-related G-protein coupled receptor[30]. Mas-related genes antagonize the angiotensin type 1 receptor (AT1R), reducing reactive oxygen species and inflammation, which ameliorates renal injury[30]. Neprilysin expression is increased by cytokines on the surface of the lung fibroblasts[27]. The current understanding of the mechanism of SAC/VAL, progressing to HF, is shown in Figure 2. According to the latest know
The approval of SAC/VAL, a first-in-class ARNI, marked the first novel pharmacological class in over a decade for HFrEF treatment[28,30,33]. Neprilysin plays a role as its mechanism, degrading the gross excess of circulating NPs in HF patients[7]. Compared to enalapril, SAC/VAL leads to reductions in symptoms of HF, cardiovascular death or HF hospitalization, sudden cardiac death, and disease progression, and improved QOL, in patients undergoing evidence-based contemporary medical therapy for HFrEF, and the NP assays for B-type NP (BNP) and N-terminal-proBNP (NT-proBNP) assays have been shown to have similar diagnostic accuracy for the differentiation of HF from other etiologies of shortness of breath[11,17,32,34-36]. In real-world settings, SAC/VAL was found to be associated with improved survival and reduced HF-related hospitalization compared to enalapril in Asian HF patients, with consistent effectiveness even in older populations[37]. SAC/VAL use has been shown to result in a modest, chronic elevation of BNP while reducing levels of NT-prBNP[35].
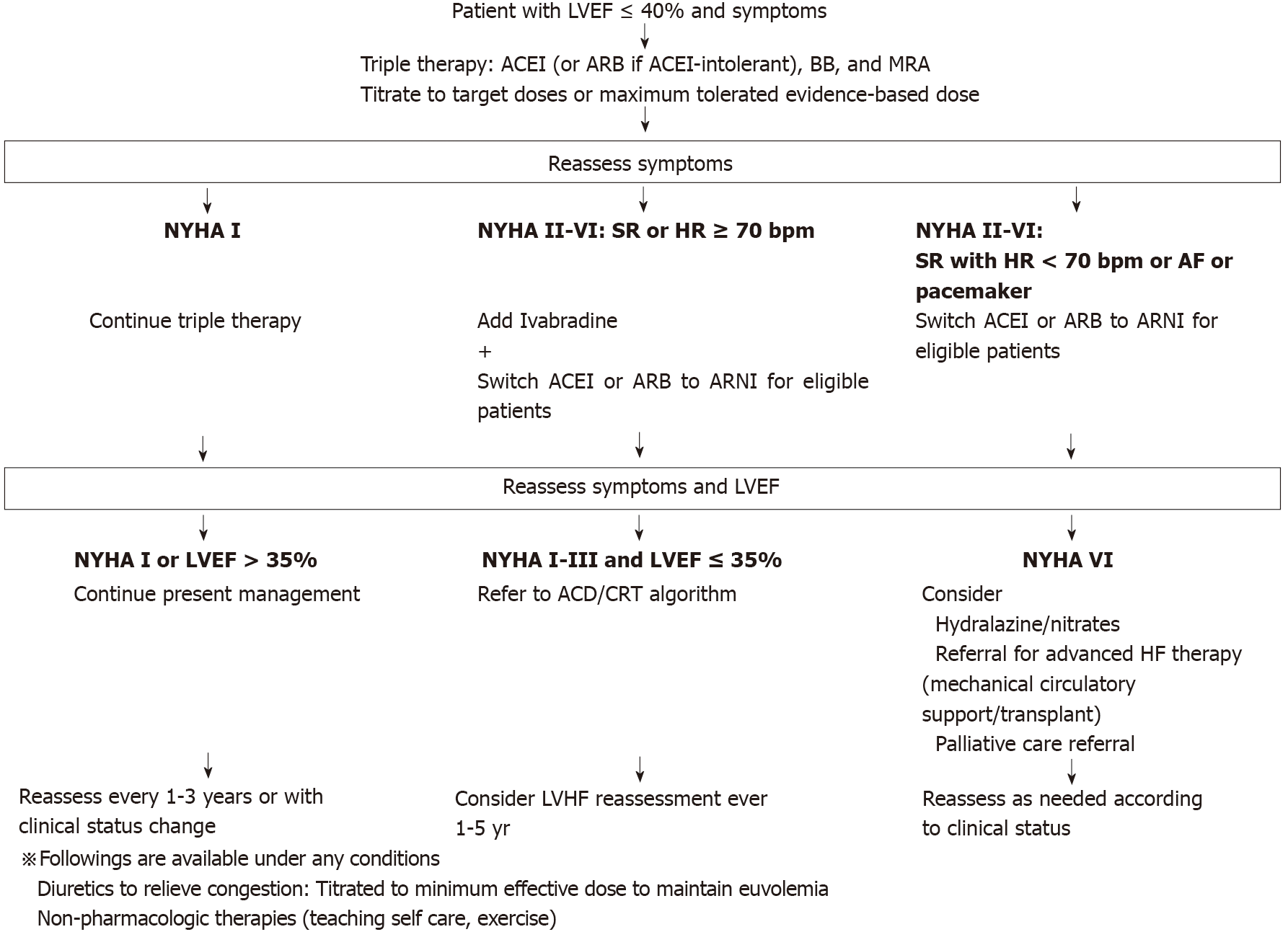
The European Society of Cardiology, the Canadian Cardiovascular Society, and the ACC HF guidelines all currently recommend the use of ACEI or ARB and BB in HFrEF treatment[38]. In addition, HFrEF patients should first be treated with a BB and an ACEI or ARB (or ARNI), followed by add-on therapy with MRA and a diuretic, based on volume status[19,38-40]. Due to the different mechanisms of action in SAC/VAL, this combination may be regarded as a potential treatment option for patients who remain symptomatic despite optimized therapy with other alternatives[3]. While SAC/VAL is indicated for HF NYHA class II or III severity, it is unclear whether there is sufficient evidence from clinical trials or observational studies to support their use in combination, from the perspectives of both effectiveness and safety[3]. On the other hand, although it remains unclear what the optimal timing is for initiation of SAC/VAL, early use seems likely to positively impact patient outcomes[3,33]. We present the therapeutic options and treatment lines of CHF, especially HFrEF, based on the European Society of Cardiology, the Canadian Cardiovascular Society, and the ACC HF guidelines, in Figure 3.
Other point of discussion regarding ARNI for HF include evaluating the prevalence and significance of hyperkalemia in HF patients, which is essential for optimized use of potassium sparing agents, such as RAAS inhibitors or ARNI and MRA, which represent a well-established cornerstone of life-saving therapy[41]. SAC/VAL has already proven highly effective for HFrEF, and there is convincing data available regarding the cardioprotective effects of dapagliflozin, an SGLT2 inhibitor[20,38]. These two treatments have earned class I and class II recommendations, respectively, in the European Society of Cardiology guidelines for the diagnosis and treatment of HF[20]. However, more research is necessary on the mechanisms of action of disease modification[38]. Another point of discussion, raised in 2017, is that it was recommended that “patients who are eligible for treatment with ivabradine may also be eligible for treatment with SAC/VAL”, but there was no evidence evaluating the combination of SAC/VAL and ivabradine, or assessing the comparative safety and efficacy of the two treatments[3]. An additional novel point of discussion is that SAC/VAL also has a positive impact on acute HF, as observed very frequently in deceased COVID-19 patients[27].
On the other hand, there seems to be no evidence of a difference between SAC/VAL and valsartan in patients with HFpEF[17,39]. Therefore, there are, at present, no universal treatment strategies recommended for HFpEF; instead, management should take an individualized approach, with consideration given to each patient’s comorbidities[17]. Additionally, modern guidelines should emphasize this lack of evidence for the combined use of ARB and BB for HFrEF, with the exception of candesartan[42]. Even as practice moves towards widespread adoption of ARNI (which contain the ARB valsartan) for HF, this distinction has significant implications for the ongoing role of combination therapy with BB, which has, to date, only been assumed, but not proven[42].
ARNI plays an important role in proteolytic processes in the kidney, as well as in cardiovascular regulation, immune response, cell proliferation, fetal development, and more[28]. First, in an exploratory study of patients with HFrEF who were treated with SAC/VAL using echocardiography, it was demonstrated to significantly decrease the ratio of early transmitral doppler velocity to early diastolic annular velocity (E/e′) ratio, a simple, straightforward parameter of heart diastolic function[43]. Further, SAC/VAL may improve cardiac volume and function markers at twelve months[43]. Secondly, SAC/VAL is effective in treatment of hypertension, and short-term RCTs have found that the highest doses of SAC/VAL (200 and 400 mg q.d.) are more effective at lowering both office and ambulatory blood pressure than either ACEI or ARB alone; it should particularly be used as a first-line therapy for hypertensive patients with HFrEF[25,44]. They seem promising as antihypertensive agents for HFpEF, but investigation is ongoing[44]. Thirdly, although no effect was found on kidney function (compared to the irbesartan control), allocation to SAC/VAL did cause more reduction in cardiac biomarkers than irbesartan did, which suggests that this treatment could improve cardiovascular outcomes for this population[5]. Fourthly, there is growing evidence of neprilysin’s role in glucose homeostasis: Because its activity in type 2 DM (T2DM) and obesity may potentially negatively impact metabolic processes in various tissues, it therefore plays a preventive role in the development of obesity and T2DM[28,29]. Thus, by raising the levels of various peptides that exert beneficial effects on glucose metabolism, such as glucagon-like peptide-1 (GLP-1), NPs, and bradykinin, the inhibition of neprilysin in nutrient excess conditions could prove to be a powerful strategy for improving glucose homeostasis[29]. However, because of the action of other enzymes (such as DPP-4) on neprilysin substrates, which results in reduced inhibitor efficacy, as well as the concomitant elevation of neprilysin substrates that can impair sensitivity to insulin and function of beta cells, the use of a combination of drugs is preferable to the use of a neprilysin inhibitor alone for the treatment of T2DM[29]. Moreover, the increased angiotensin II levels that are associated with neprilysin inhibition limit its utility as a monotherapy for T2DM patients; a neprilysin inhibitor should always be prescribed along with an ARB, which is preferred over ACEI in order to avoid angioedema[29]. Fifthly, in some cases, administering SAC/VAL at appropriate doses has allowed for recovery of the sinus rhythm; consequently, upstream therapy of atrial fibrillation may demonstrate good results[45]. Sixthly, it may play a preventive role in cancer development[28]. Seventhly, viral dependence on ACE-2, as entry receptors, has been a recent focus, driving research into the impact of RAAS on COVID-19 pathogenesis[46]. Several pieces of evidence have pointed to neprilysin as a pulmonary RAAS components[46]. Considering neprilysin’s protective effects against pulmonary inflammatory reactions and fibrosis, this suggests that future efforts should be directed towards its potential role in the pathophysiology of COVID-19[28,46].
On the other hand, the most frequently reported adverse events are hypotension and hyperkalemia[47]. Other adverse effects include teratogenicity from the ARB component; this medication should therefore be avoided during pregnancy[48]. In addition, though it was reported in 2018 that SAC/VAL could increase the risk for dementia, the risk was lower than the proportions reported for other medications[48].
To date, there have been a number of global clinical trials regarding SAC/VAL: PARAMOUNT, PARADIGM-HF, TRANSITION, PIONEER-HF, PARAGON-HF, and PARALLEL-HF. The detail is shown in Supplement material.
PARAMOUNT was a phase-2, randomized, parallel-group, double-blind multicenter trial in patients of NYHA class II-III HF, LVEF 45% or higher, and NT-proBNP greater than 400 pg/mL. Participants were randomly assigned (1:1), by a central interactive voice response system, to either ARNI LCZ696 titrated to 200 mg twice daily, or valsartan titrated to 160 mg twice daily, and treated for 36 wk[49]. The primary endpoint was changes in NT-proBNP, a marker of LV wall stress, from baseline to twelve weeks; the analysis included all patients randomly assigned to treatment groups who had a baseline and at least one postbaseline assessment[49]. The trial concluded that, in patients with HFpEF, SAC/VAL reduced NT-proBNP to a greater extent at twelve weeks than valsartan, and that it was well tolerated[49]. In this trial, the most common adverse event reported with SAC/VAL was symptomatic hypotension, with 19% frequency[48].
In the 2014 PARADIGM-HF (Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure) trial of 8399 outpatient subjects with HFrEF, SAC/VAL was found to be more effective than enalapril for slowing disease progression by decreasing the risk of worsening HF leading to the need for hospitalization or emergency admission and the need for intensified therapy; it also reduced the rates of 30-day HF readmission, as well as all-cause readmission after HF hospitalization, HF devices, or cardiac transplantation[24,31,37]. In addition, treatment with SAC/VAL was associated with statistically important reductions in cardiovascular death, a 16% reduction in all-cause mortality, and a 20% reduction in the composite of cardiovascular-related death or HF-related hospitalization (composite primary endpoint) compared to treatment with enalapril[16,26,33,37,47,48,50-52].
Accordingly, in 2016, the European and American cardiology societies (ACC/ AHA/Heart Failure Society of America) simultaneously issued a class I recom
Biomarker-based mechanistic studies have also provided further insight into potential pathways that may prove relevant to the benefits that have been observed with ARNI[26]. In this trial, treatment with SAC/VAL was associated with greater increases in BNP and urinary levels of cyclic guanosine monophosphate compared to treatment with enalapril; the latter reflects the increased intracellular second-messenger levels that result from NP action, as well as the other direct and indirect effects of mediators increased by inhibition of neprilysin[26]. However, most of the patients treated showed only a modest increase in BNP levels after initiation of SAC/VAL[26]. In contrast, neprilysin has a greater affinity for A-type NP (ANP) than for BNP, and after SAC/VAL initiation, ANP increased more consistently and robustly[26]. It is conceivable that ANP or perhaps other neprilysin substrates (such as C-type NP, urodilatin, adrenomedullin, substance P, apelin, bradykinin, vasoactive intestinal peptide, calcitonin gene-related peptide, or GLP-1) may play a predominant role in the mechanism of action of SAC/VAL; indeed, further mechanistic studies are currently ongoing, in order to elucidate the processes that underlie the clinical benefits that were observed in this study[26]. In addition, treatment with SAC/VAL led to significantly reduced levels of aldosterone, soluble ST2, matrix metalloproteinase-9, and its specific inhibitor (tissue inhibitor of metalloproteinases-1), reflecting reduced profibrotic signaling[26]. The levels of procollagen amino-terminal propeptide types I and III also were lower than with enalapril, reflecting reduced synthesis of collagen[26].
This study also compared safety outcomes: the SAC/VAL group had a higher risk of hypotension compared to conventional therapy (OR, 3.14; 95%CI, 0.94–10.55), with 18% frequency[48,53]. Thus, in order to prevent serious adverse advents, clinicians must monitor for hypotension, dizziness, cough, angioedema, hyperkalemia, and renal dysfunction[53]. The risk of other adverse effects of ARNI use, such as hyperkalemia, cough, and diminished renal function, have been demonstrated to be lower than when using ACEI on its own[48,53].
The TRANSITION trial was a randomized, multi-center, open-label study comparing two treatment initiation modalities of SAC/VAL, to assess tolerability and optimal time point for initiation of SAC/VAL in patients stabilized after acute HF: Either at least twelve hours pre-discharge, or days 1–14 post-discharge[54]. In summary, approximately half of the HFrEF patients who had stabilized after an acute HF decompensation event were able to achieve the recommended target dose of SAC/VAL within ten weeks, and at least 86% were able to maintain any dose of SAC/VAL for more than two weeks, following the label recommendations for initiation and up-titration[54]. There were few adverse events or permanent treatment discontinuations, particularly given the extreme vulnerability of the post-acute decompensated HF population[54]. The findings from this study complement those from the PIONEER-HF study, showing that early initiation of SAC/VAL in a wide range of HFrEF patients who have recently been admitted for acute decompensated HF is feasible, either as hospital patients or shortly after discharge[54].
The PIONEER-HF trial (Comparison of SAC/VAL Versus Enalapril on Effect on NT-proBNP in Patients Stabilized From an Acute HF Episode) was a multicenter, randomized, double-blind trial of in-hospital initiation of SAC/VAL (n = 440) compared to enalapril (n = 441), in patients who were stabilized during hospitalization for acute decompensated HF[55]. In this trial, a heterogeneity in the effect of SAC/VAL on these efficacy and safety outcomes were evaluated in the following selected subgroups of clinical concern: Patients with baseline systolic blood pressure ≤ 118 mmHg, baseline NT-pro BNP > 2701 pg/mL, estimated glomerular filtration rate < 60 mL/min per 1.73 m2, ≥ 1 additional hospitalization for HF within the prior year, admission to the ICU during the index hospitalization, inotrope use during the index hospitalization, and severe congestion[55]. As a result, the trial found that treatment with SAC/VAL after initial stabilization led to a consistent reduction in cardiovascular death or rehospitalization for HF in high-risk subpopulations admitted for acute decompensated HF, and that SAC/VAL was well tolerated[55].
The recently completed PARAGON-HF trial found that SAC/VAL modestly reduced total HF hospitalization and cardiovascular death risks, compared to valsartan, in patients with HFpEF, although this finding fell just short of being statistically significant[7,26,56]. Clinical benefits were observed in secondary endpoints, including QOL and kidney endpoints; more specifically women and patients who are at the lower end of the LVEF spectrum appeared to preferentially benefit[26]. In addition, the safety profile of SAC/VAL was found to be largely consistent with prior trials[26]. In this trial, 15.4% of the SAC/VAL group discontinued use of the trial drug due to an adverse event, and 58.9% patients had at least one serious adverse event; the most common serious adverse events (n ≥ 2% in the group) during the double-blind period, regardless of study drug relationship, by preferred term and SAC/VAL group, were cardiac failure (14.6%), atrial fibrillation (6.7%), pneumonia (6.7%), acute kidney injury (6.7%), congestive cardiac failure (3.6%), acute cardiac failure (3.5%), anemia (2.8%), acute myocardial infarction (2.5%), urinary tract infection (2.2%), hypotension (2.2%), and unstable angina (2.1%)[56]. By the time of the final visit, among the patients continuing therapy, the target dose was being taken by 82.0% of the SAC/VAL group[56].
SAC/VAL group patients had a greater likeliness of having hypotension, but were less likely to demonstrate creatinine and potassium level increases than valsartan group patients, and the mean systolic blood pressure at eight months was 4.5 mmHg (95%CI, 3.6–5.4), or lower in the SAC/VAL group than in the valsartan group; however, this difference did not correlate with the potential treatment effect[56].
The objective of the PARALLEL-HF trial was to describe the baseline characteristics and treatment of Japanese HFrEF patients[57]. The trial concluded that the patients studied were largely representative of contemporary ambulatory HFrEF patients who were well treated using evidence-based therapies[57]. In addition, PARALLEL-HF will assist in determining whether SAC/VAL provides clinical outcome improvements in Japanese HFrEF patients similar to those that were observed in the PARADIGM-HF study[57].
Though guidelines have changed worldwide to include SAC/VAL for HFrEF patients, even now, some seven years after PARADIGM-HF trial, there remains some uncertainty regarding when to start SAC/VAL, and in whom[7]. A treatment’s estimated long-term effects can serve as a helpful adjunct to clinical trial results, in order to provide patients with easily understood information regarding one treatment’s potential benefits compared to those of another[26]. Furthermore, both HFpEF diagnosis and treatment remain challenging, as do the management of advanced and acute HF[7,34]. Though progress remains slow with respect to HFpEF, both ARNI and SGLT2 inhibitors also hold great promise for this condition, and there are currently large clinical trials underway (PARALLAX)[20,26,32]. In addition, the recent development of new diagnostic algorithms, to improve HFpEF diagnostic accuracy, will assist in future clinical trials’ efforts to find effective therapies[20].
There are currently several other ongoing trials that aim to clarify and explore the benefits of SAC/VAL for HF management, as well[48]. It is unclear whether inhibition of neprilysin has a direct effect on extracellular matrix homeostasis, or if these profibrotic benefits reflect hemodynamic improvement; the completed PROVE-HF trial (prospective study of biomarkers, symptom improvement, and ventricular remodeling during SAC/VAL therapy for HF) will continue to examine a wide variety of biomarkers, including collagen homeostasis markers, in 795 HFrEF patients being treated with open-label SAC/VAL[26]. The currently ongoing PARADISE-MI trial (prospective ARNI vs ACEI trial to determine superiority in reducing HF events after myocardial infarction (MI)) aims to evaluate the effects of inpatient SAC/VAL compared to ramipril, for reducing cardiovascular death and HF hospitalization in post-acute MI patients who have evidence of LV systolic dysfunction (EF < 40%) and/or pulmonary congestion, and who have no known prior history of CHF[26,48]. Another dedicated, randomized, cardiac-magnetic-resonance-based trial, comparing SAC/VAL to valsartan in patients who have asymptomatic LV systolic dysfunction and a history of MI, RECOVER-LV (effects of SAC/VAL compared to valsartan on LV remodeling in asymptomatic LV systolic dysfunction after MI), is also expected to provide further insight into ARNI’s potential remodeling effects[26].
The effects of SAC/VAL on hypertensive organ damage have only been sparsely investigated; to date, no studies have established SAC/VAL’s impact on cardio
Finally, the barriers that prevent SAC/VAL from being prescribed for eligible patients may include practitioners' unfamiliarity with ARNI, safety concerns, and payer reimbursement issues[53].
SAC/VAL is an efficacious, safe, and cost-effective therapy that improves QOL and longevity in patients with chronic HFrEF, and reduces hospital admission. An in-hospital initiation strategy offers a potentially new avenue to improve clinical uptake of SAC/VAL. In the last five years, SAC/VAL has been established as a cornerstone component of comprehensive disease-modifying medical therapy in the management of chronic HFrEF. In the next five years, we should see SAC/VAL being brought into wider implementation in practice, with potential expansion of its therapeutic indications. Further work is necessary, with carefully designed and controlled preclinical studies, in order to better understand its molecular mechanisms and effects, and to confirm issues such as long-term safety in both human and animal models.
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lakusic N, Ugo O S-Editor: Ma YJ L-Editor: A P-Editor: Liu JH
| 1. | Kemp CD, Conte JV. The pathophysiology of heart failure. Cardiovasc Pathol. 2012;21:365-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 368] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 2. | Murphy SP, Ibrahim NE, Januzzi JL Jr. Heart Failure With Reduced Ejection Fraction: A Review. JAMA. 2020;324:488-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 455] [Article Influence: 91.0] [Reference Citation Analysis (0)] |
| 3. | Pohar R, MacDougall D. Combination Use of Ivabradine with Sacubitril/Valsartan: A Review of Clinical Effectiveness and Guidelines [Internet]. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2020 Feb 13. CADTH Rapid Response Reports. [PubMed] |
| 4. | Bratsos S. Efficacy of Angiotensin Converting Enzyme Inhibitors and Angiotensin Receptor-Neprilysin Inhibitors in the Treatment of Chronic Heart Failure: A Review of Landmark Trials. Cureus. 2019;11:e3913. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Kawashiro N, Kasanuki H, Ogawa H, Matsuda N, Hagiwara N; Heart Institute of Japan--Department of Cardiology (HIJC) Investigators. Clinical characteristics and outcome of hospitalized patients with congestive heart failure: results of the HIJC-HF registry. Circ J. 2008;72:2015-2020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 6. | Sato N, Kajimoto K, Keida T, Mizuno M, Minami Y, Yumino D, Asai K, Murai K, Muanakata R, Aokage T, Sakata Y, Mizuno K, Takano T; TEND Investigators. Clinical features and outcome in hospitalized heart failure in Japan (from the ATTEND Registry). Circ J. 2013;77:944-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 203] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 7. | Cuthbert JJ, Pellicori P, Clark AL. Cardiovascular Outcomes with Sacubitril-Valsartan in Heart Failure: Emerging Clinical Data. Ther Clin Risk Manag. 2020;16:715-726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Fu S, Chang Z, Luo L, Deng J. Therapeutic Progress and Knowledge Basis on the Natriuretic Peptide System in Heart Failure. Curr Top Med Chem. 2019;19:1850-1866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Srivastava PK, Hsu JJ, Ziaeian B, Fonarow GC. Heart Failure With Mid-range Ejection Fraction. Curr Heart Fail Rep. 2020;17:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 10. | Osmanska J, Jhund PS. Contemporary Management of Heart Failure in the Elderly. Drugs Aging. 2019;36:137-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Fabris E, Merlo M, Rapezzi C, Ferrari R, Metra M, Frigerio M, Sinagra G. Sacubitril/Valsartan: Updates and Clinical Evidence for a Disease-Modifying Approach. Drugs. 2019;79:1543-1556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Berliner D, Bauersachs J. New drugs: big changes in conservative heart failure therapy? Eur J Cardiothorac Surg. 2019;55:i3-i10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Akita K, Kohno T, Kohsaka S, Shiraishi Y, Nagatomo Y, Goda A, Mizuno A, Sujino Y, Fukuda K, Yoshikawa T; West Tokyo Heart Failure Registry Investigators. Prognostic Impact of Previous Hospitalization in Acute Heart Failure Patients. Circ J. 2019;83:1261-1268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 14. | Rocha BML, Menezes Falcão L. Acute decompensated heart failure (ADHF): A comprehensive contemporary review on preventing early readmissions and postdischarge death. Int J Cardiol. 2016;223:1035-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Shiraishi Y, Kohsaka S, Sato N, Takano T, Kitai T, Yoshikawa T, Matsue Y. 9-Year Trend in the Management of Acute Heart Failure in Japan: A Report From the National Consortium of Acute Heart Failure Registries. J Am Heart Assoc. 2018;7:e008687. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 177] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 16. | Silva-Cardoso J, Brás D, Canário-Almeida F, Andrade A, Oliveira L, Pádua F, Fonseca C, Bragança N, Carvalho S, Soares R, Santos JF. Neurohormonal modulation: The new paradigm of pharmacological treatment of heart failure. Rev Port Cardiol (Engl Ed). 2019;38:175-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Davidson A, Raviendran N, Murali CN, Myint PK. Managing heart failure with preserved ejection fraction. Ann Transl Med. 2020;8:395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Berliner D, Hänselmann A, Bauersachs J. The Treatment of Heart Failure with Reduced Ejection Fraction. Dtsch Arztebl Int. 2020;117:376-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 19. | Nicolas D, Kerndt CC, Reed M. Sacubitril/Valsartan. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 May 23. [PubMed] |
| 20. | Kuster GM, Pfister O. Chronic heart failure: advances in pharmacological treatment and future perspectives. Swiss Med Wkly. 2019;149:w20036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Güder G, Bauersachs J, Frantz S, Weismann D, Allolio B, Ertl G, Angermann CE, Störk S. Complementary and incremental mortality risk prediction by cortisol and aldosterone in chronic heart failure. Circulation. 2007;115:1754-1761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 223] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 22. | Volpe M, Carnovali M, Mastromarino V. The natriuretic peptides system in the pathophysiology of heart failure: from molecular basis to treatment. Clin Sci (Lond). 2016;130:57-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 211] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 23. | Salazar J, Rojas-Quintero J, Cano C, Pérez JL, Ramírez P, Carrasquero R, Torres W, Espinoza C, Chacín-González M, Bermúdez V. Neprilysin: A Potential Therapeutic Target of Arterial Hypertension? Curr Cardiol Rev. 2020;16:25-35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 24. | Russo-Vorms L, Meyer P, Reny JL. [« ARNI » (Angiotensin Receptor-Neprilysin Inhibitor): when, for whom and how? Rev Med Suisse. 2019;15:1882-1886. [PubMed] |
| 25. | Wehland M, Simonsen U, Buus NH, Krüger M, Grimm D. An evaluation of the fixed-dose combination sacubitril/valsartan for the treatment of arterial hypertension. Expert Opin Pharmacother. 2020;21:1133-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Docherty KF, Vaduganathan M, Solomon SD, McMurray JJV. Sacubitril/Valsartan: Neprilysin Inhibition 5 Years After PARADIGM-HF. JACC Heart Fail. 2020;8:800-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 95] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 27. | Bellis A, Mauro C, Barbato E, Trimarco B, Morisco C. The Rationale for Angiotensin Receptor Neprilysin Inhibitors in a Multi-Targeted Therapeutic Approach to COVID-19. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Nalivaeva NN, Zhuravin IA, Turner AJ. Neprilysin expression and functions in development, ageing and disease. Mech Ageing Dev. 2020;192:111363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 100] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 29. | Esser N, Zraika S. Neprilysin inhibition: a new therapeutic option for type 2 diabetes? Diabetologia. 2019;62:1113-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 30. | Sankhe R, Kinra M, Mudgal J, Arora D, Nampoothiri M. Neprilysin, the kidney brush border neutral proteinase: a possible potential target for ischemic renal injury. Toxicol Mech Methods. 2020;30:88-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 31. | Fonseca C, Brito D, Ferreira J, Franco F, Morais J, Silva Cardoso J; Experts opinion; endorsed by the Working Group on Heart Failure of the Portuguese Society of cardiology. Sacubitril/valsartan: A practical guide. Rev Port Cardiol (Engl Ed). 2019;38:309-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
| 32. | Volpe M, Rubattu S, Battistoni A. ARNi: A Novel Approach to Counteract Cardiovascular Diseases. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 33. | Sokos GG, Raina A. Understanding the early mortality benefit observed in the PARADIGM-HF trial: considerations for the management of heart failure with sacubitril/valsartan. Vasc Health Risk Manag. 2020;16:41-51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 34. | Tomasoni D, Adamo M, Anker MS, von Haehling S, Coats AJS, Metra M. Heart failure in the last year: progress and perspective. ESC Heart Fail. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 35. | Sbolli M, deFilippi C. BNP and NT-proBNP Interpretation in the Neprilysin Inhibitor Era. Curr Cardiol Rep. 2020;22:150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 36. | Alvarez CK, Cronin E, Baker WL, Kluger J. Heart failure as a substrate and trigger for ventricular tachycardia. J Interv Card Electrophysiol. 2019;56:229-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 37. | Pathadka S, Yan VKC, Li X, Tse G, Wan EYF, Lau H, Lau WCY, Siu DCW, Chan EW, Wong ICK. Hospitalization and Mortality in Patients With Heart Failure Treated With Sacubitril/Valsartan vs. Enalapril: A Real-World, Population-Based Study. Front Cardiovasc Med. 2020;7:602363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 38. | Sotirakos S, Wheen P, Spiers J, Armstrong R. New pharmacotherapy for heart failure with reduced ejection fraction. Expert Rev Cardiovasc Ther. 2020;18:405-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 39. | Nielsen EE, Feinberg JB, Bu FL, Hecht Olsen M, Raymond I, Steensgaard-Hansen F, Jakobsen JC. Beneficial and harmful effects of sacubitril/valsartan in patients with heart failure: a systematic review of randomised clinical trials with meta-analysis and trial sequential analysis. Open Heart. 2020;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 40. | Smith DK, Lennon RP, Carlsgaard PB. Managing Hypertension Using Combination Therapy. Am Fam Physician. 2020;101:341-349. [PubMed] |
| 41. | Rakisheva A, Marketou M, Klimenko A, Troyanova-Shchutskaia T, Vardas P. Hyperkalemia in heart failure: Foe or friend? Clin Cardiol. 2020;43:666-675. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 42. | Hyman DA, Siebert VR, Birnbaum GD, Alam M, Birnbaum Y. A Modern History RAAS Inhibition and Beta Blockade for Heart Failure to Underscore the Non-equivalency of ACEIs and ARBs. Cardiovasc Drugs Ther. 2020;34:215-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 43. | Januzzi JL Jr, Prescott MF, Butler J, Felker GM, Maisel AS, McCague K, Camacho A, Piña IL, Rocha RA, Shah AM, Williamson KM, Solomon SD; PROVE-HF Investigators. Association of Change in N-Terminal Pro-B-Type Natriuretic Peptide Following Initiation of Sacubitril-Valsartan Treatment With Cardiac Structure and Function in Patients With Heart Failure With Reduced Ejection Fraction. JAMA. 2019;322:1085-1095. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 469] [Cited by in RCA: 428] [Article Influence: 71.3] [Reference Citation Analysis (0)] |
| 44. | Gupta T, Rezan T, Krim SR. Managing hypertension in patients with heart failure: an ongoing quandary. Curr Opin Cardiol. 2019;34:359-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 45. | De Vecchis R, Paccone A, Di Maio M. Upstream Therapy for Atrial Fibrillation Prevention: The Role of Sacubitril/Valsartan. Cardiol Res. 2020;11:213-218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 46. | Mohammed El Tabaa M, Mohammed El Tabaa M. Targeting Neprilysin (NEP) pathways: A potential new hope to defeat COVID-19 ghost. Biochem Pharmacol. 2020;178:114057. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 47. | Proudfoot C, Studer R, Rajput T, Jindal R, Agrawal R, Corda S, Senni M. Real-world effectiveness and safety of sacubitril/valsartan in heart failure: A systematic review. Int J Cardiol. 2021;331:164-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 48. | Akbar S, Kabra N, Aronow WS. Impact of Sacubitril/Valsartan on Patient Outcomes in Heart Failure: Evidence to Date. Ther Clin Risk Manag. 2020;16:681-688. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 49. | Solomon SD, Zile M, Pieske B, Voors A, Shah A, Kraigher-Krainer E, Shi V, Bransford T, Takeuchi M, Gong J, Lefkowitz M, Packer M, McMurray JJ; Prospective comparison of ARNI with ARB on Management Of heart failUre with preserved ejectioN fracTion (PARAMOUNT) Investigators. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. Lancet. 2012;380:1387-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 831] [Cited by in RCA: 893] [Article Influence: 68.7] [Reference Citation Analysis (0)] |
| 50. | Vaduganathan M, Desai AS. Angiotensin-Neprilysin Inhibition as a Paradigm for All? Curr Cardiol Rep. 2016;18:115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 51. | McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR; PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition vs enalapril in heart failure. N Engl J Med. 2014;371:993-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4078] [Cited by in RCA: 4738] [Article Influence: 430.7] [Reference Citation Analysis (0)] |
| 52. | Dewan P, Docherty KF, McMurray JJV. Sacubitril/Valsartan in Asian Patients with Heart Failure with Reduced Ejection Fraction. Korean Circ J. 2019;49:469-484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 53. | Sauer AJ, Cole R, Jensen BC, Pal J, Sharma N, Yehya A, Vader J. Practical guidance on the use of sacubitril/valsartan for heart failure. Heart Fail Rev. 2019;24:167-176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 54. | Wachter R, Senni M, Belohlavek J, Straburzynska-Migaj E, Witte KK, Kobalava Z, Fonseca C, Goncalvesova E, Cavusoglu Y, Fernandez A, Chaaban S, Bøhmer E, Pouleur AC, Mueller C, Tribouilloy C, Lonn E, A L Buraiki J, Gniot J, Mozheiko M, Lelonek M, Noè A, Schwende H, Bao W, Butylin D, Pascual-Figal D; TRANSITION Investigators. Initiation of sacubitril/valsartan in haemodynamically stabilised heart failure patients in hospital or early after discharge: primary results of the randomised TRANSITION study. Eur J Heart Fail. 2019;21:998-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 237] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 55. | Berg DD, Samsky MD, Velazquez EJ, Duffy CI, Gurmu Y, Braunwald E, Morrow DA, DeVore AD. Efficacy and Safety of Sacubitril/Valsartan in High-Risk Patients in the PIONEER-HF Trial. Circ Heart Fail. 2021;14:e007034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 56. | Solomon SD, McMurray JJV, Anand IS, Ge J, Lam CSP, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B, Redfield MM, Rouleau JL, van Veldhuisen DJ, Zannad F, Zile MR, Desai AS, Claggett B, Jhund PS, Boytsov SA, Comin-Colet J, Cleland J, Düngen HD, Goncalvesova E, Katova T, Kerr Saraiva JF, Lelonek M, Merkely B, Senni M, Shah SJ, Zhou J, Rizkala AR, Gong J, Shi VC, Lefkowitz MP; PARAGON-HF Investigators and Committees. Angiotensin-Neprilysin Inhibition in Heart Failure with Preserved Ejection Fraction. N Engl J Med. 2019;381:1609-1620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1097] [Cited by in RCA: 1601] [Article Influence: 266.8] [Reference Citation Analysis (0)] |
| 57. | Tsutsui H, Momomura SI, Saito Y, Ito H, Yamamoto K, Ohishi T, Okino N, Kitamura T, Guo W. Angiotensin Receptor Neprilysin Inhibitor in Japanese Patients With Heart Failure and Reduced Ejection Fraction - Baseline Characteristics and Treatment of PARALLEL-HF Trial. Circ J. 2018;82:2575-2583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |