Published online Apr 26, 2021. doi: 10.4330/wjc.v13.i4.103
Peer-review started: November 30, 2020
First decision: December 20, 2020
Revised: January 1, 2021
Accepted: March 18, 2021
Article in press: March 18, 2021
Published online: April 26, 2021
Processing time: 141 Days and 13.8 Hours
Pregnancy-associated spontaneous coronary artery dissection (PSCAD) is an important cause of chest pain and acute myocardial infarction in pregnant and postpartum women. Pregnancy is considered an isolated risk factor for spontaneous coronary artery dissection. The etiology, pathogenesis, and incidence of PSCAD are not known.
We present a case of a 33-year-old postpartum female who presented with sudden onset chest pain and was diagnosed with spontaneous coronary artery dissection and needed urgent catheterization revealing left anterior descending coronary artery dissection. She underwent emergent coronary artery bypass graft surgery with good post-operative recovery.
Most patients with PSCAD can be managed conservatively with medical management and have good outcomes. Patients with high-risk presentations benefit from the invasive approach. Coronary artery bypass graft may be required in select few patients based on angiography findings. Due to the risk of recurrent spontaneous coronary artery dissection, subsequent pregnancies are discouraged.
Core Tip: Chest pain during pregnancy and peripartum period need a comprehensive workup. Pregnancy is an isolated risk factor for spontaneous coronary artery dissection. Patients with pregnancy-associated spontaneous coronary artery dissection often have an elevated rate of high-risk presentations and may require invasive treatment or coronary artery bypass graft in few cases. Multidisciplinary care coordinated by a team of experts including interventional cardiologists, high-risk obstetricians, internists, cardiothoracic surgeons and Critical care specialists is essential in managing these patients in the peripartum period. Early diagnosis and timely intervention are lifesaving in cases involving Pregnancy associated spontaneous coronary artery dissection.
- Citation: Prudhvi K, Jonnadula J, Rokkam VRP, Kutti Sridharan G. Pregnancy associated spontaneous coronary artery dissection: A case report and review of literature. World J Cardiol 2021; 13(4): 103-110
- URL: https://www.wjgnet.com/1949-8462/full/v13/i4/103.htm
- DOI: https://dx.doi.org/10.4330/wjc.v13.i4.103
Chest pain is the second most common chief complaint for emergency room visits[1]. Common causes of chest pain presenting to the emergency department are gastrointestinal causes, musculoskeletal causes, acute coronary syndrome, pericarditis and pulmonary embolism[2,3]. Spontaneous coronary artery dissection (SCAD) leading to coronary ischemia is an uncommon cause of chest pain predominantly seen in women. SCAD is defined as an epicardial coronary artery dissection that is not associated with trauma, atherosclerosis or due to iatrogenic causes. Many cases of SCAD in women happen during the peripartum period, and this can be life-threatening events in young females[4,5].
Pregnancy-associated spontaneous coronary artery dissection (PSCAD) is an important cause of chest pain and acute myocardial infarction in pregnant and postpartum women. It remains rare in incidence overall, with a handful of case reports and few small case series reported in the literature. Pregnancy and puerperium are considered major risk factors for SCAD because of hormonal changes. The etiology of PSCAD is not clearly understood. Patients with PSCAD often have high-risk presentations and have a complicated course. Failure to diagnose and treat PSCAD timely may lead to worse outcomes.
Here, we report a case of a 33-year-old postpartum female who had a complicated course following SCAD requiring coronary artery bypass graft (CABG) and had an uneventful recovery. To further improve understanding of PSCAD, we conducted an extensive literature search of Medline database for articles published until October 2020 using the following search terms “Myocardial infarction”, “Pregnancy”, “Spontaneous Coronary artery dissection” and “postpartum” in various combinations. We have reviewed the etiology, clinical presentation, diagnosis and treatment strategy of PSCAD in this literature review.
Sudden onset chest pain.
A previously healthy 33-year-old Hispanic female with no significant medical history presented to the emergency room with sudden onset of chest pain while she was cooking dinner. The pain originated in the neck area on the left side and mid-back region, radiated to the anterior chest, was 10/10 intensity, pressure-like, lasted for 15 min, and resolved with aspirin. Chest pain was associated with nausea, vomiting, diaphoresis, shortness of breath, and lightheadedness. A month ago, she delivered her third child by normal vaginal delivery at 37 wk of gestation. The review of systems was otherwise unremarkable.
The patient had no prior history of coronary artery disease, aortic dissection, or pulmonary embolism.
She had no significant social history including smoking, alcohol or recreational substance use. She denied use of any over the counter medicines and denied use of any hormonal contraceptives before. Her only medications included prenatal vitamins and iron supplements. There was no history of sudden cardiac death or early myocardial infarction in the family.
Her physical examination was unremarkable. Blood pressure was 104/56 mmHg and heart rate was 82 beats per minute. Equal intensity pulses were palpable in both arms. There were no cardiac murmurs, jugular venous distension, crackles on auscultatory lung areas, and pedal edema.
Laboratory markers showed low hemoglobin of 11.6 g/dL (normal 12-15.5 g/dL) , white blood cell count of 6 × 109 cells/L, creatinine of 0.6 mg/dL, ESR of 16 mm/h (normal range, 0-20 mm/h) and CRP of 2.7 mg/L (normal range, 0-10 mg/L). Serial Troponins (Troponin T) were abnormal and trended up from 0.01 ng/mL and peaked at 15.5 ng/mL.
Her initial electrocardiogram (EKG) was normal. Subsequent EKGs showed dynamic T wave inversions. Chest radiography did not reveal mediastinal widening or any fractures and dislocations. Transthoracic echocardiogram revealed 48% LVEF, moderate-sized apical wall motion abnormality with akinesia of the anteroseptal segments.
A multidisciplinary team including cardiology, cardiothoracic surgery, critical care medicine was consulted, and they recommended starting heparin drip, high intensity statin therapy, and giving loading doses of aspirin and clopidogrel.
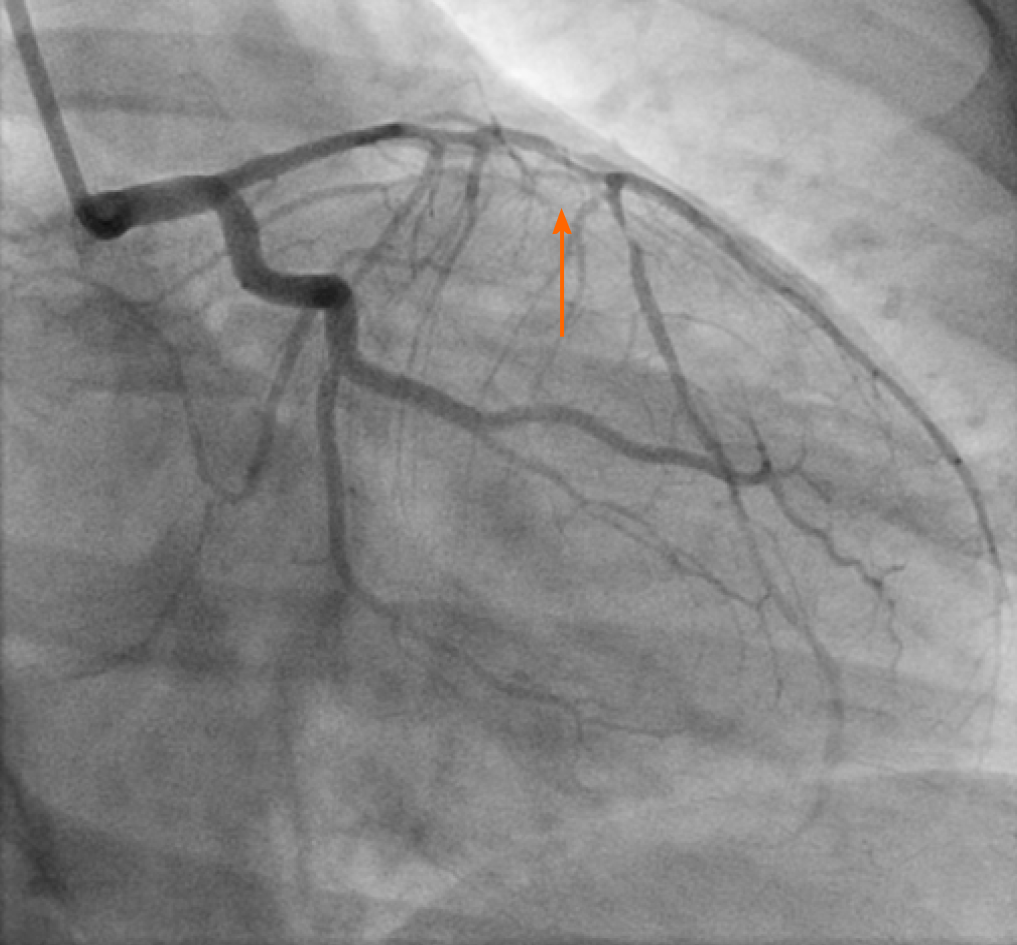
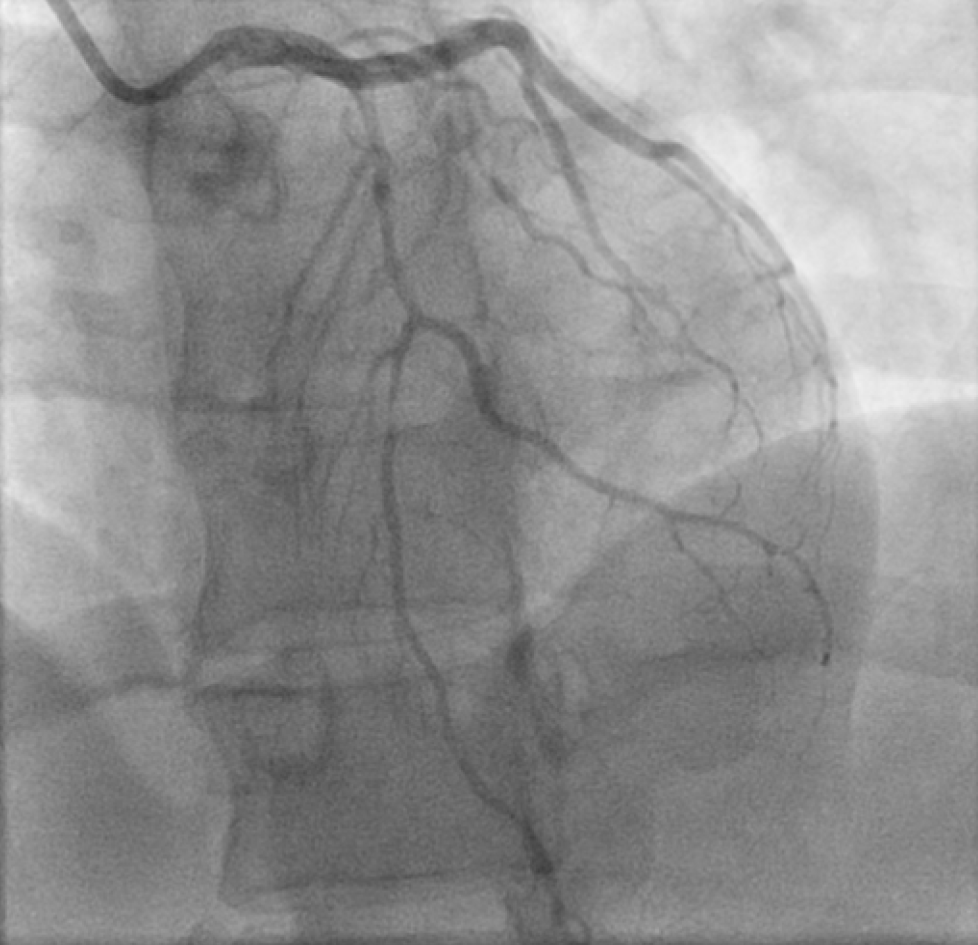
She underwent Left heart catheterization, which revealed spontaneous circumferential dissection extending from Ostia to mid-left anterior descending (LAD) with distal 90% stenosis (Figures 1 and 2). Diagnosis was made on the basis of symptoms of chest pain, elevated troponins, EKG findings of T-wave inversions, echocardiogram findings of wall motion abnormalities with low ejection fraction 48% and more importantly angiographic findings of dissection.
An intra-aortic balloon pump was placed, IV nitroglycerine and metoprolol were started. Conservative management was not an option for her owing to ongoing ischemia. Percutaneous coronary intervention (PCI) was considered extremely high risk for her due to extensive involvement of left anterior descending and CABG was favored. The patient underwent a CABG (free left internal mammary artery to left anterior descending and saphenous vein graft to diagonal 1 and 2) and was transferred to the cardiac intensive care unit with an intra-aortic balloon pump and dopamine infusion.
She had an uneventful post-operative recovery and was discharged home on Aspirin, Atorvastatin and Metoprolol with close clinic follow-up. Autoimmune workup including antinuclear antibody, anti-DS antibody, ANCA antibodies, anticardiolipin antibody, and diluted Russell Viper venom test was negative. Post-discharge clinic follow up in one and 3 mo were uneventful, and she was advised to continue medical management.
Pregnancy predisposes to an increased risk of myocardial infarction by three-fold compared to non-pregnant females in the reproductive age group[4]. PSCAD comprises about 40% of pregnancy-associated myocardial infarction[5]. PSCAD occurs in approximately 1.8 per 100000 pregnancies[6].
Given the rarity of this condition, the etiology of PSCAD is largely unknown. Black race, hyperlipidemia, hypertension and migraine have been associated with the development of PSCAD[6]. A meta-analysis of Genome wise studies has identified a common genetic locus PHACTR1-EDN1 implicated in the development of both fibromuscular dysplasia (FMD) and SCAD in affected patients[7]. In a PSCAD cohort, Fibromuscular dysplasia was noted in greater than 40% of cases who underwent imaging, revealing there may be a strong connection between FMD and PSCAD[8,9]. Autopsy studies have suggested that SCAD may be an initial manifestation of FMD[10,11]. Besides, several conditions that have been associated with SCAD including advanced maternal age, intense exercise, oral contraceptives, immunosuppressive therapy, menopause, sleep deprivation, cocaine abuse, connective tissue disorders such as Marfan syndrome and Ehlers-Danlos syndrome, polyarteritis nodosa and systemic lupus erythematosus may be related to PSCAD[12-14]. PSCAD patients also have higher rates of prior infertility treatments.
Pregnancy and puerperium are considered major risk factors for SCAD because of hormonal changes[15]. Changes in the levels of sex hormones leading to alterations in intima and media layers of arterial walls were proposed as a cause of PSCAD. Impaired collagen synthesis, myocyte proliferation and altered texture of mucopolysaccharides may weaken the layers of the coronary arterial wall. During pregnancy, a hyperdynamic circulatory state with increased shear stress on the labile coronary artery leads to coronary artery dissection[16,17]. PSCAD is more commonly observed in the left anterior descending coronary artery and Left main artery[15].
Clinical features are related to myocardial ischemia. Myocardial hypoperfusion is due to true luminal narrowing by false lumen compression caused by intramural hematoma. Clinical features depend on the anatomy of coronary involvement and the severity of involvement. The clinical spectrum varies from nonspecific chest pain, anginal equivalent, typical chest pain suggestive of unstable angina, cardiogenic shock leading to hemodynamic instability and sudden cardiac death.
Elevated cardiac biomarkers including troponins due to myocyte injury from ischemia may be seen. EKG may reflect changes corresponding to the involvement and extent of coronary territory and the severity of ischemia. Emergent coronary catheterization is essential for establishing a definitive diagnosis. Computed tomographic angiography coronary angiography may be useful in postpartum patients for diagnosing dissection and also during follow-up evaluation for resolution[18]. Unlike SCAD, where mid and distal coronary artery occlusions are common, PSCAD patients are more likely to have proximal vessel (mainly left main and left anterior descending) or multivessel involvement[19]. They present with worse LVEF compared with other SCAD patients. EKG could be normal but may also show a nonspecific ischemic pattern, T wave inversions, ST-segment depression, or ST-segment elevation.
The management strategy employed in PSCAD is similar to the management of SCAD with special attention to the stage of pregnancy and emphasis on fetal and maternal well-being. Both conservative treatment with medical therapy alone and percutaneous coronary intervention have been used in the management of PSCAD. There are no evidence-based guideline recommendations from professional societies yet as SCAD/PSCAD is rare. There have been no randomized controlled trials to guide management and so there is no established strategy to guide treatment. Emergent coronary angiography is the key in reviewing coronary arteries' anatomy and diagnosing SCAD and also to identify the severity and extent of the coronary artery dissection[5]. Intravascular ultrasound and optical coherence tomography may help in differentiating true lumen vs false lumen. Conservative management strategy may be followed in stable PSCAD patients as in one case series of 750 patients, about 85% were managed conservatively and had good long-term outcomes[20]. PCI may be considered in patients in patients with proximal occlusions, cardiogenic shock, unstable rhythm and those who fail conservative treatment strategy[15]. However, PCI in SCAD patients has higher rates of complications such as propagation of hematoma (noted in one-third of cases) and iatrogenic dissection[21,22]. Difficulty in identifying true lumen limits the success rate with stenting in establishing the coronary flow. Coronary arteries may be more friable due to hormonal changes in pregnancy and PCI may further worsen dissection in PSCAD patients[6].
CABG is reserved for patients with multivessel involvement or involvement of the left main coronary artery with ongoing ischemia/infarction[5,23-25]. Thrombolytics should be avoided due to the risk of reexpansion of hematoma, causing expanding dissection leading to compression of the true lumen with worsening ischemia. Besides, thrombolytics fall in risk category C for use in pregnancy[26,27]. Caution advised with medical management as certain drugs are contraindicated or not well studied during pregnancy. Antiplatelet agents such as aspirin, beta-blockers such as labetalol are safe during pregnancy. Available human data suggests the benefits of Clopidogrel use outweigh risks during pregnancy and risk of fetal harm is not expected[28,29]. Angiotensin converting enzyme inhibitors should be avoided during pregnancy.
Previously considered a universally fatal condition, survival outcomes of PSCAD have improved in the past decades due to advances in the management of acute myocardial infarction. Maternal mortality rates are still high and variable in PSCAD patients, with one recent study reporting in-hospital mortality rates of 4.5%[30,31]. In another recent series of 750 patients containing 34 peripartum patients, PSCAD was associated with a higher rate of major adverse events (20.6%) such as Stroke/TIA, all-cause mortality, re-infection, Cardiogenic shock, congestive heart failure and cardiac arrest[20]. Patients with PSCAD were found to have an elevated rate of high-risk presentations and also known to have low ejection fraction compared to females with acute myocardial infarction in the same age group. Recurrence after SCAD has been noted, although less common. There is a paucity of information on the risk of recurrent SCAD in patients with prior PSCAD. Due to the high risk of mortality and morbidity associated with PSCAD, pre-conceptional counseling is recommended for subsequent pregnancies. Coronary tortuosity, migraine headaches, fibromuscular dysplasia, hypertension are associated with recurrent PSCAD. Careful evaluation, low threshold for coronary angiography referral and early intervention may help reduce the rate of missed diagnosis in high-risk women during the peripartum period. Multidisciplinary care coordinated by a team of experts including interventional cardiologists, high-risk obstetricians, internists, cardiothoracic surgeons and critical care specialists is essential in managing these patients in the peripartum period[32]. Targeted cardiac rehabilitation programs are preferred over strenuous high-intensity exercise programs in postpartum women[33].
Strong suspicion and emergent catheterization should be considered when pregnant and postpartum women present with chest pain, electrocardiogram changes and elevated biomarkers. Emergent coronary catheterization is essential for establishing a definitive diagnosis. Although no standard guidelines exist, conservative therapy with medical management has been increasingly adopted in pregnancy-associated spontaneous coronary artery dissection with good outcomes. Selected cases with high-risk presentations like ours have definitive benefit from PCI and coronary artery bypass graft. Hence, PCI should be reserved only for high-risk patients and coronary artery bypass graft in extremely high-risk or PCI failure cases.
Manuscript source: Unsolicited manuscript
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ciccone MM, Kharlamov AN, Zhang JX S-Editor: Zhang L L-Editor: A P-Editor: Li JH
| 1. | McCaig LF, Burt CW. National Hospital Ambulatory Medical Care Survey: 2002 emergency department summary. Adv Data. 2004: 1-34. [PubMed] [Cited in This Article: ] |
| 2. | Kontos MC, Diercks DB, Kirk JD. Emergency department and office-based evaluation of patients with chest pain. Mayo Clin Proc. 2010;85:284-299. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 3. | Stepinska J, Lettino M, Ahrens I, Bueno H, Garcia-Castrillo L, Khoury A, Lancellotti P, Mueller C, Muenzel T, Oleksiak A, Petrino R, Guimenez MR, Zahger D, Vrints CJ, Halvorsen S, de Maria E, Lip GY, Rossini R, Claeys M, Huber K. Diagnosis and risk stratification of chest pain patients in the emergency department: focus on acute coronary syndromes. A position paper of the Acute Cardiovascular Care Association. Eur Heart J Acute Cardiovasc Care. 2020;9:76-89. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 4. | James AH, Jamison MG, Biswas MS, Brancazio LR, Swamy GK, Myers ER. Acute myocardial infarction in pregnancy: a United States population-based study. Circulation. 2006;113:1564-1571. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 353] [Cited by in F6Publishing: 323] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 5. | Hayes SN, Tweet MS, Adlam D, Kim ESH, Gulati R, Price JE, Rose CH. Spontaneous Coronary Artery Dissection: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;76:961-984. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 223] [Article Influence: 74.3] [Reference Citation Analysis (0)] |
| 6. | Faden MS, Bottega N, Benjamin A, Brown RN. A nationwide evaluation of spontaneous coronary artery dissection in pregnancy and the puerperium. Heart. 2016;102:1974-1979. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 7. | Adlam D, Olson TM, Combaret N, Kovacic JC, Iismaa SE, Al-Hussaini A, O'Byrne MM, Bouajila S, Georges A, Mishra K, Braund PS, d'Escamard V, Huang S, Margaritis M, Nelson CP, de Andrade M, Kadian-Dodov D, Welch CA, Mazurkiewicz S, Jeunemaitre X; DISCO Consortium; Wong CMY, Giannoulatou E, Sweeting M, Muller D, Wood A, McGrath-Cadell L, Fatkin D, Dunwoodie SL, Harvey R, Holloway C, Empana JP, Jouven X; CARDIoGRAMPlusC4D Study Group; Olin JW, Gulati R, Tweet MS, Hayes SN, Samani NJ, Graham RM, Motreff P, Bouatia-Naji N. Association of the PHACTR1/EDN1 Genetic Locus With Spontaneous Coronary Artery Dissection. J Am Coll Cardiol. 2019;73:58-66. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 135] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 8. | Saw J, Humphries K, Aymong E, Sedlak T, Prakash R, Starovoytov A, Mancini GBJ. Spontaneous Coronary Artery Dissection: Clinical Outcomes and Risk of Recurrence. J Am Coll Cardiol. 2017;70:1148-1158. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 277] [Cited by in F6Publishing: 381] [Article Influence: 54.4] [Reference Citation Analysis (0)] |
| 9. | Tweet MS, Young KA, Best PJM, Hyun M, Gulati R, Rose CH, Hayes SN. Association of Pregnancy With Recurrence of Spontaneous Coronary Artery Dissection Among Women With Prior Coronary Artery Dissection. JAMA Netw Open. 2020;3:e2018170. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 10. | Moulson N, Kelly J, Iqbal MB, Saw J. Histopathology of Coronary Fibromuscular Dysplasia Causing Spontaneous Coronary Artery Dissection. JACC Cardiovasc Interv. 2018;11:909-910. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Lie JT, Berg KK. Isolated fibromuscular dysplasia of the coronary arteries with spontaneous dissection and myocardial infarction. Hum Pathol. 1987;18:654-656. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 107] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | Lawal L, Lange R, Schulman S. Acute myocardial infarction in two young women without significant risk factors. J Invasive Cardiol. 2009;21:E3-E5. [PubMed] [Cited in This Article: ] |
| 13. | Azam MN, Roberts DH, Logan WF. Spontaneous coronary artery dissection associated with oral contraceptive use. Int J Cardiol. 1995;48:195-198. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 60] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Tsimikas S, Giordano FJ, Tarazi RY, Beyer RW. Spontaneous coronary artery dissection in patients with renal transplantation. J Invasive Cardiol. 1999;11:316-321. [PubMed] [Cited in This Article: ] |
| 15. | Cade JR, Szarf G, de Siqueira ME, Chaves Á, Andréa JC, Figueira HR, Gomes MM Jr, Freitas BP, Filgueiras Medeiros J, Dos Santos MR, Fiorotto WB, Daige A, Gonçalves R, Cantarelli M, Alves CM, Echenique L, de Brito FS Jr, Perin MA, Born D, Hecht H, Caixeta A. Pregnancy-associated spontaneous coronary artery dissection: insights from a case series of 13 patients. Eur Heart J Cardiovasc Imaging. 2017;18:54-61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 16. | Heefner WA. Dissecting hematoma of the coronary artery. A possible complication of oral contraceptive therapy. JAMA. 1973;223:550-551. [PubMed] [Cited in This Article: ] |
| 17. | Barger AC, Beeuwkes R 3rd, Lainey LL, Silverman KJ. Hypothesis: vasa vasorum and neovascularization of human coronary arteries. A possible role in the pathophysiology of atherosclerosis. N Engl J Med. 1984;310:175-177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 646] [Cited by in F6Publishing: 675] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 18. | Rose E, Gedela M, Miller N, Carpenter PL. Pregnancy-Related Spontaneous Coronary Artery Dissection: A Case Series and Literature Review. J Emerg Med. 2017;52:867-874. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Ito H, Taylor L, Bowman M, Fry ET, Hermiller JB, Van Tassel JW. Presentation and therapy of spontaneous coronary artery dissection and comparisons of postpartum vs nonpostpartum cases. Am J Cardiol. 2011;107:1590-1596. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 20. | Saw J, Starovoytov A, Humphries K, Sheth T, So D, Minhas K, Brass N, Lavoie A, Bishop H, Lavi S, Pearce C, Renner S, Madan M, Welsh RC, Lutchmedial S, Vijayaraghavan R, Aymong E, Har B, Ibrahim R, Gornik HL, Ganesh S, Buller C, Matteau A, Martucci G, Ko D, Mancini GBJ. Canadian spontaneous coronary artery dissection cohort study: in-hospital and 30-day outcomes. Eur Heart J. 2019;40:1188-1197. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 281] [Cited by in F6Publishing: 261] [Article Influence: 52.2] [Reference Citation Analysis (0)] |
| 21. | Prakash R, Starovoytov A, Heydari M, Mancini GB, Saw J. Catheter-Induced Iatrogenic Coronary Artery Dissection in Patients With Spontaneous Coronary Artery Dissection. JACC Cardiovasc Interv. 2016;9:1851-1853. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 100] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 22. | Tweet MS, Eleid MF, Best PJ, Lennon RJ, Lerman A, Rihal CS, Holmes DR Jr, Hayes SN, Gulati R. Spontaneous coronary artery dissection: revascularization vs conservative therapy. Circ Cardiovasc Interv. 2014;7:777-786. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 343] [Cited by in F6Publishing: 421] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 23. | Sarmento-Leite R, Machado PR, Garcia SL. Spontaneous coronary artery dissection: stent it or wait for healing? Heart. 2003;89:164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Jorgensen MB, Aharonian V, Mansukhani P, Mahrer PR. Spontaneous coronary dissection: a cluster of cases with this rare finding. Am Heart J. 1994;127:1382-1387. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 139] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 25. | Asuncion CM, Hyun J. Dissecting intramural hematoma of the coronary artery in pregnancy and the puerperium. Obstet Gynecol. 1972;40:202-210. [PubMed] [Cited in This Article: ] |
| 26. | Buys EM, Suttorp MJ, Morshuis WJ, Plokker HW. Extension of a spontaneous coronary artery dissection due to thrombolytic therapy. Cathet Cardiovasc Diagn. 1994;33:157-160. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 66] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 27. | Zupan I, Noc M, Trinkaus D, Popovic M. Double vessel extension of spontaneous left main coronary artery dissection in young women treated with thrombolytics. Catheter Cardiovasc Interv. 2001;52:226-230. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 5] [Reference Citation Analysis (0)] |
| 28. | King M, Bernstein PS. Clopidogrel During Pregnancy. In: Obstetrics and Maternal-Fetal Medicine, Medscape, 2005 [cited March 17, 2021]. Available from: https://www.medscape.com/viewarticle/516210. [Cited in This Article: ] |
| 29. | De Santis M, De Luca C, Mappa I, Cesari E, Mazza A, Quattrocchi T, Caruso A. Clopidogrel treatment during pregnancy: a case report and a review of literature. Intern Med. 2011;50:1769-1773. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 30. | Smilowitz NR, Gupta N, Guo Y, Zhong J, Weinberg CR, Reynolds HR, Bangalore S. Acute Myocardial Infarction During Pregnancy and the Puerperium in the United States. Mayo Clin Proc. 2018;93:1404-1414. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 69] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 31. | Paratz ED, Kao C, MacIsaac AI, Somaratne J, Whitbourn R. Evolving management and improving outcomes of pregnancy-associated spontaneous coronary artery dissection (P-SCAD): a systematic review. Int J Cardiol Heart Vasc. 2018;18:1-6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Kim ESH. Spontaneous Coronary-Artery Dissection. N Engl J Med. 2020;383:2358-2370. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 116] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 33. | Chou AY, Prakash R, Rajala J, Birnie T, Isserow S, Taylor CM, Ignaszewski A, Chan S, Starovoytov A, Saw J. The First Dedicated Cardiac Rehabilitation Program for Patients With Spontaneous Coronary Artery Dissection: Description and Initial Results. Can J Cardiol. 2016;32:554-560. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 91] [Article Influence: 11.4] [Reference Citation Analysis (0)] |