Published online Mar 26, 2021. doi: 10.4330/wjc.v13.i3.55
Peer-review started: December 25, 2020
First decision: January 11, 2021
Revised: January 19, 2021
Accepted: February 11, 2021
Article in press: February 11, 2021
Published online: March 26, 2021
Processing time: 87 Days and 18.6 Hours
Elevated interleukin (IL)-6-levels have been described in familial variant transthyretin amyloidosis (ATTRv) associated polyneuropathy and heart failure. However, IL-6 in cardiac ATTR amyloidosis (ATTR-CM) and its prognostic value have not been investigated yet.
We aim to study the correlation between IL-6 levels with clinical presentation (Gillmore-class) and outcome [heart transplantation or death (htx/death)], or the combined endpoint of cardiac decompensation or htx/death in ATTR-CM.
IL-6 levels of 106 ATTR-CM patients [54 wild-type ATTRwt, 52 ATTRv-CM], 15 asymptomatic carriers of ATTR mutations (aATTRv-CM) and 27 healthy donors were quantified using Luminex technology. Statistical analysis was performed using parametric survival regression models.
We found that IL-6 levels from wild-type ATTR patients were significantly elevated compared to healthy controls, while aATTRv-CM carriers and ATTRv-CM patients did not show a significant difference. IL-6 levels showed significantly higher values in increasing Gillmore classes. Univariate analyses revealed association of low IL-6 levels with cardiac decompensation and htx/death [odds ratio: 0.26 (0.09-0.72), P = 0.01] and htx/death [odds ratio: 0.15 (0.04-0.58), P = 0.006]. However, in the multivariate model, no significant improvement of risk prediction was seen for IL-6, while established prognostic factors were significantly associated with outcome.
Raised IL-6 levels correlate with clinical presentation and are associated with worse outcome in ATTR-CM but do not improve stratification in addition to established risk factors.
Core Tip: This was a monocentric prospective trial with 106 patients suffering from transthyretin cardiomyopathy (ATTR-CM) seeking to evaluate the prognostic value of interleukin-6 in ATTR-CM. Interleukin-6 is associated with outcome in ATTR-CM but did not further ameliorate existing risk prediction models.
- Citation: Hein SJ, Knoll M, Aus dem Siepen F, Furkel J, Schoenland S, Hegenbart U, Katus HA, Kristen AV, Konstandin M. Elevated interleukin-6 levels are associated with impaired outcome in cardiac transthyretin amyloidosis. World J Cardiol 2021; 13(3): 55-67
- URL: https://www.wjgnet.com/1949-8462/full/v13/i3/55.htm
- DOI: https://dx.doi.org/10.4330/wjc.v13.i3.55
Systemic amyloidosis comprises a group of diseases leading to extracellular protein deposition in tissue resulting in organ dysfunction. Depending on the specific type of protein causing misfolding and amyloid formation, different organs are involved. In cardiac amyloidosis, protein deposition in myocardium is most frequently due to amyloid light chain or transthyretin (TTR) deposition[1]. In transthyretin cardiac amyloidosis (ATTR), two disease entities are found: Hereditary variant transthyretin amyloidosis (ATTRv) and wild-type transthyretin (ATTRwt) amyloidosis[2]. ATTRv results from a point mutation in the TTR gene. Until now, more than 100 different disease causing mutations are known. Depending on the mutation, patients present with leading neurological [familial transthyretin polyneuropathy (ATTRv-PN)] or cardiac symptoms (ATTRv-CM)[3]. Furthermore, thanks to increased awareness and also the establishment of new sensitive diagnostic methods, e.g., 99mTc-labelled bone scintigraphy and cardiac MRI, the incidence of ATTR amyloidosis in general and particularly of ATTRwt, has increased over the last years[4]. In this disease entity, no mutation in the transthyretin gene is found. ATTRwt mainly affects elderly, predominantly male patients with cardiac symptoms leading. The clinical course of ATTR varies significantly within patients with rapid progression of symptoms, within a few months in some patients, and stable course for many years in others[2]. Therefore, early identification of patients at high risk for a more aggressive course of the disease is crucial for the preservation of quality of life, exercise capacity and ultimately survival and early initiation of amyloid specific therapies.
In the last years the role of systemic inflammation for progression of cardiovascular disease in general has been established, especially in coronary heart disease. Elevated interleukin (IL)-6 levels are described in patients suffering from heart failure with preserved ejection fraction (HFpEF), atrial fibrillation and elevated N-terminal pro-brain natriuretic peptide (NTproBNP)[5]. Furthermore, on a molecular level increased cardiac IL-6 and IL-6 receptor messenger ribonucleic acid (mRNA) levels in myocardial tissue have been associated with worsening of heart failure[6]. In animal models of pressure overload and heart failure inhibition of the IL-6 axis was protective[7]. In very recent studies, association of inflammation and disease progression could be documented for amyloidosis too. Immunohistochemical detection of lymphocytes, macrophages and cytotoxic cells in cardiac specimen of light-chain amyloidosis (AL) and ATTR amyloidosis patients was associated with impaired outcome[8]. Gene expression profiling of peripheral blood leukocytes was valuable for the diagnosis of symptomatic patients with ATTR amyloidosis[9]. Moreover, systemic inflammatory state quantified as cytokine panels in patients’ blood plasma has been described in hereditary amyloid polyneuropathy[10,11]. In patients with ATTR-PN, data are inconsistent as IL-6 levels were found to be unchanged in ATTRv-PN patients and asymptomatic gene carriers compared to healthy controls[10]. In another study, increased IL-6 levels were seen in ATTRv-PN patients and asymptomatic mutation carriers as well[11].
Since nothing is known about IL-6 in patients with ATTR-CM to date, the aim of the present study was to quantify the levels of IL-6 in peripheral blood in this cohort and analyze the correlation with clinical presentation and prognosis.
Between July 2016 and October 2018, 138 patients who consecutively presented in our tertiary referral center for amyloidosis at Heidelberg University Hospital were screened and asked to donate blood for this study. One patient declined study participation. Inclusion criteria were age > 18 and < 90 years, diagnosis of ATTRwt, ATTRv or asymptomatic carrier of a mutation causing ATTRv. Patients who suffered from AL amyloidosis (n = 7) were excluded due to other pathophysiologic disease mechanism. Patients who underwent liver transplantation (n = 1) or diagnosis remained unclear (n = 3) were excluded from study participation. Also, patients receiving TTR-lowering therapies (n = 5) were excluded due to reduction of disease driving protein. Patients who received TTR-stabilizer therapy, however, were eligible to participate in the study (n = 26), as dysfunctional protein is still present. These patients already received tafamidis 20 mg daily at study inclusion for grade 1 ATTR polyneuropathy during the whole follow-up period. Therefore, a total of n = 121 patients were included in the present study and subscribed written informed consent approved by the ethical review committee Heidelberg (S-485-2016), in accordance to the declaration of Helsinki. To attain a control group, healthy volunteers were asked to donate blood, when echocardiography, clinical presentation and biomarkers were normal [high-sensitive troponin T, C-reactive protein (CRP) and NTproBNP] (n = 27).
Blood samples were attained with regular venipuncture during the medical visit. Therefore, one additional lithium heparin monovette (4.9 mL, Sarstedt, Nümbrecht, Germany) was collected and plasma was prepared by standard centrifugation. Samples were aliquoted and stored at -80 °C, conditions well established to have no impact upon IL-6 stability[12,13]. After the inclusion of the last patient, IL-6 levels of all patients were measured simultaneously.
For subgroup analysis in Figure 1, patients were divided by positive cardiac TroponinT (cTnT) levels according to the estimated cutoff in our recent study[14]. For subcohort definition regarding natriuretic peptide levels [glomerular filtration rate (GFR) adjusted NTproBNP], NTproBNP levels were adjusted to renal function as described by Luchner et al[15].
Echocardiography was conducted using 2D imaging, M-Mode, Doppler and Strain analyses. Ejection fraction was calculated from 2D echocardiography imaging and diastolic dysfunction was graded in accordance to current guidelines from the American Society of Echocardiography[16]. Grade II and higher were considered as significant diastolic dysfunction.
Endpoint follow-up was performed by interviewing patients directly during outpatient visits or via phone call after 12 mo. Additionally, patients’ files of subsequent hospitalization due to cardiac decompensation were analyzed. Pre-specified endpoints were heart transplantation or death (htx/death) and a combination of htx/death or cardiac decompensation (major cardiac events, MACE).
Plasma concentrations of IL-6 were quantified using the Luminex MAGPIX system (R&D systems, Minneapolis, MN, United States). IL-6 measurements were conducted in adherence to the manufacturer’s instructions.
Statistical analyses were conducted using R, v3.6.3[17]. IL-6 concentrations were calculated from a standard curve attained by the standards provided by the Luminex kit. Values were fitted using a sigmoidal, three parameter, hill fit equation [f = a*xb/(cb + xb)]. For further analysis, log transformed data were used. IL-6 values below the detection level were set to zero (n = 1). Measurements were transformed as described in[18] prior to log and z-transformation for subsequent analysis.
Time-to-event data were censored after 30 month. Median follow-up data were calculated with survreg using inverted event data. Confidence intervals were computed with the ciTools package[19]. Cutoff selection for prognostic stratification of patients (smallest P values, minimum group size of 10%) was performed with the dataAnalysisMisc package[20]. Cutoffs were calculated per endpoint.
Uni- and multivariate survival analyses were performed with parametric survival regression models assuming loglogistic distributed data.
Associations between diagnosis groups and patient characteristics were evaluated using analysis of variance or chi-squared tests for categorical and continuous variables, respectively. Significance level alpha was set to 5% (two-sided).
A total of 121 patients and 27 healthy controls participated in this study. In 50 patients, cardiac amyloidosis was confirmed by myocardial biopsy, 56 patients were diagnosed by specific myocardial storage in 99m-TC-DPD-bone scintigraphy and concomitant serological exclusion of AL amyloidosis. Furthermore, 15 asymptomatic gene carriers were diagnosed by familial history and genotyping. According to the examination results, patients were grouped into ATTRwt (n = 54), symptomatic ATTRv-CM (n = 52) and asymptomatic aATTRv-CM (n = 15).
The ATTRv-CM group consists of patients with mutations at Val30Met (n = 18), Val20Ile (n = 11), Ile107Val (n = 5), Leu58His (n = 6), Cys10Arg (n = 4), Val122Ile (n = 3), Ala45Thr (n = 1), Ile84Asn (n = 1), Ile107Phe (n = 1), Arg34Gly (n = 1), Thr126Arg (n = 1). Furthermore, the asymptomatic mutation carriers were Val20Ile (n = 5), Val30Met (n = 6), Val122Ile (n = 1), Cys10Arg (n = 1), Ile107Val (n = 1), Ile84Thr (n = 1). Table 1 presents clinical characteristics of study participants.
| Study population, n = 148 | Asympt ATTRv, n = 15 | ATTRv, n = 52 | Ctrl, n = 27 | ATTRwt, n = 54 | P value |
| Age (yr) | 46.9 ± 11.2 | 66.1 ± 8.1 | 53.3 ± 19.6 | 78.5 ± 6.8 | < 0.001 |
| Sex, n (%) | |||||
| Male | 9 (60.0) | 39 (75.0) | 16 (59.3) | 49 (90.7) | < 0.001 |
| Female | 6 (40.0) | 13 (25.0) | 11 (40.7) | 5 (9.3 ) | |
| BMI | 27.3 ± 5.6 | 26.1 ± 4.9 | 26.0 ± 5.5 | 25.5 ± 3.0 | 0.59 |
| Medication, n (%) | |||||
| Tafamidis | 0 (0) | 25 (48.1) | 0 (0) | 1 (1.9) | < 0.001 |
| Beta blocker | 2 (13.3) | 20 (38.5) | 7 (25.9) | 41 (75.9) | < 0.001 |
| ACE inhibitors/AT1 antagonists | 3 (25.0) | 16 (30.8) | 7 (25.9) | 37 (68.5) | < 0.001 |
| Diuretics | 2 (13.3) | 27 (51.9) | 4 (14.8) | 52 (96.3) | < 0.001 |
| Other antihypertensive medication (amlodipin, doxacor, nitrendipin) | 2 (13.3) | 1 (1.9) | 0 (0) | 7 (13.0) | 0.10 |
| Functional impairment | |||||
| Karnofsky performance index, n (%) | < 0.001 | ||||
| ≥ 80 | 15 (100) | 39 (75.0) | 27 (100) | 45 (83.3) | |
| < 80 | 0 (0.0) | 13 (25.0) | 0 (0 ) | 9 (16.7) | |
| NYHA class, n (%) | < 0.001 | ||||
| I | 15 (100) | 23 (44.2) | 23 (85.2) | 7 (12.9) | |
| II | 0 (0.0) | 15 (28.8) | 3 (11.1) | 16 (29.6) | |
| III/IV | 0 (0.0) | 14 (26.9) | 1 (3.7) | 31 (57.4) | |
| Risk classification, n (%) | |||||
| Gillmore | < 0.001 | ||||
| I | 15 (100) | 30 (57.7) | 16 (29.6) | ||
| II | 0 (0.0) | 14 (26.9) | 26 (48.1) | ||
| III | 0 (0.0) | 8 (15.4) | 12 (22.2) | ||
| Medical history, n (%) | |||||
| Pacemaker implantation | 0 (0.0) | 10 (19.2) | 1 (3.7) | 13(24.0) | 0.03 |
| Diabetes mellitus | 0 (0.0) | 3 (5.7) | 0 (0 ) | 9 (16.7) | 0.02 |
| Atrial fibrillation | 1 (6.7) | 16 (30.8) | 3 (11.1) | 35 (64.8) | < 0.001 |
| ECG findings | |||||
| Number of bundle branch blocks | 0.14 ± 0.36 | 0.70 ± 0.85 | 0.19 ± 0.49 | 1.1 ± 0.8 | < 0.001 |
| Sinus rhythm, n (%) | 14 (93.3) | 37 (71.2) | 24 (88.9) | 26 (48.1) | < 0.001 |
| Pace maker rhythm, n (%) | 0 (0.0) | 4 (7.7) | 0 (0) | 7 (13.0) | 0.11 |
| Low voltage pattern, n (%) | 2 (13.3) | 9 (17.3) | 0 (0) | 8 (14.8) | 0.18 |
| Heart frequency (bpm) | 68.8 ± 14.3 | 74.2 ± 14.5 | 69.4 ± 10.6 | 79.6 ± 13.9 | 0.006 |
| PQ interval (ms) | 142.1 ± 30.1 | 176.8 ± 39.2 | 158.3 ± 25.6 | 210.3 ± 41.9 | < 0.001 |
| QRS time (ms) | 99.8 ± 16.6 | 112.7 ± 30.2 | 97.3 ± 11.4 | 128.0 ± 33.3 | < 0.001 |
| QTc duration (ms) | 402.4 ± 15.9 | 432.8 ± 42.4 | 400.6 ± 12.5 | 445.7 ± 32.5 | < 0.001 |
| Echocardiography | |||||
| Posterior wall (mm) | 9.5 ± 1.9 | 14.0 ± 0.5 | 10.0 ± 0.3 | 15.4 ± 3.2 | < 0.001 |
| IVS (mm) | 10.9 ± 1.9 | 17.0 ± 0.7 | 11.0 ± 0.3 | 19.2 ± 3.9 | < 0.001 |
| Ejection fraction (%) | 58.7 ± 2.2 | 52.5 ± 1.4 | 60.0 ± 1.9 | 44.3 ± 11.3 | < 0.001 |
| Diastolic dysfunction, n (%) | 3 (20.0) | 40 (76.9) | 4 (14.8) | 49 (90.7) | < 0.001 |
| Global longitudinal strain | -14.6 ± 14.2 | -12.0 ± 0.7 | -9.8 ± 4.0 | < 0.001 | |
| MAPSE (mm) | 1.5 ± 0.3 | 1.1 ± 0.3 | 1.6 ± 0.09 | 0.9 ± 0.3 | 0.09 |
| TAPSE (mm) | 2.3 ± 0.4 | 1.8 ± 0.2 | 2.1 ± 0.1 | 1.5 ± 0.6 | 0.02 |
| Pericardial effusion, n (%) | 0 (0.0) | 3 (5.8) | 0 (0) | 6 (11.1) | 0.31 |
| PA pressure (mmHg) | 26.0 ± 4.1 | 31.0 ± 1.0 | 25.0 ± 1.3 | 35.8 ± 9.8 | 0.005 |
| Biomarkers | |||||
| NtproBNP(ng/L) | 173.6 ± 260.6 | 3457.3 ± 4321.9 | 258.8 ± 35.2 | 7219.4 ± 8213.5 | < 0.001 |
| hsTNT (pg/mL) | 4.7 ± 2.4 | 59.0 ± 95.4 | 8.1 ± 7.6 | 63.6 ± 40.7 | < 0.001 |
| GFR (mL/min) | 101.1 ± 14.6 | 75.9 ± 24.9 | 96.3 ± 22.1 | 55.8 ± 18.6 | < 0.001 |
ATTRwt patients were significantly older and primarily male patients. Furthermore, treatment with diuretics, beta blockers and angiotensin converting enzyme inhibitors was more common in the ATTRwt group. In electrocardiogram (ECG), atrial fibrillation was more common in ATTRwt patients compared to both other patient groups, and ATTRwt patients presented higher prevalence of bundle branch blocks and were significantly more often equipped with pacemakers. Furthermore, diabetes was more often recorded in their medical history as co-existing diseases. Serologically, ATTRwt patients presented higher cTnT and NTproBNP levels as well as lower GFR compared to both other groups. This resulted in higher clinical risk classes when classification was specified according to Gillmore et al[4]. In echocardiography, ATTRwt patients presented more pronounced myocardial hypertrophy and lower ejection fraction than patients from the two other groups.
During the recruitment period of the study, Tafamidis was only approved for ATTRv patients with concomitant polyneuropathy; therefore, treatment with Tafamidis was significantly more frequent in the ATTRv group. IL-6 levels did not show significant differences in patients treated with Tafamidis (Supplementary Figure 1). New York Heart Association (NYHA) class was higher in ATTRwt. All other variables tested (body mass index, antihypertensive medication, times in the ECG and echocardiographic parameters) were not significantly different between groups.
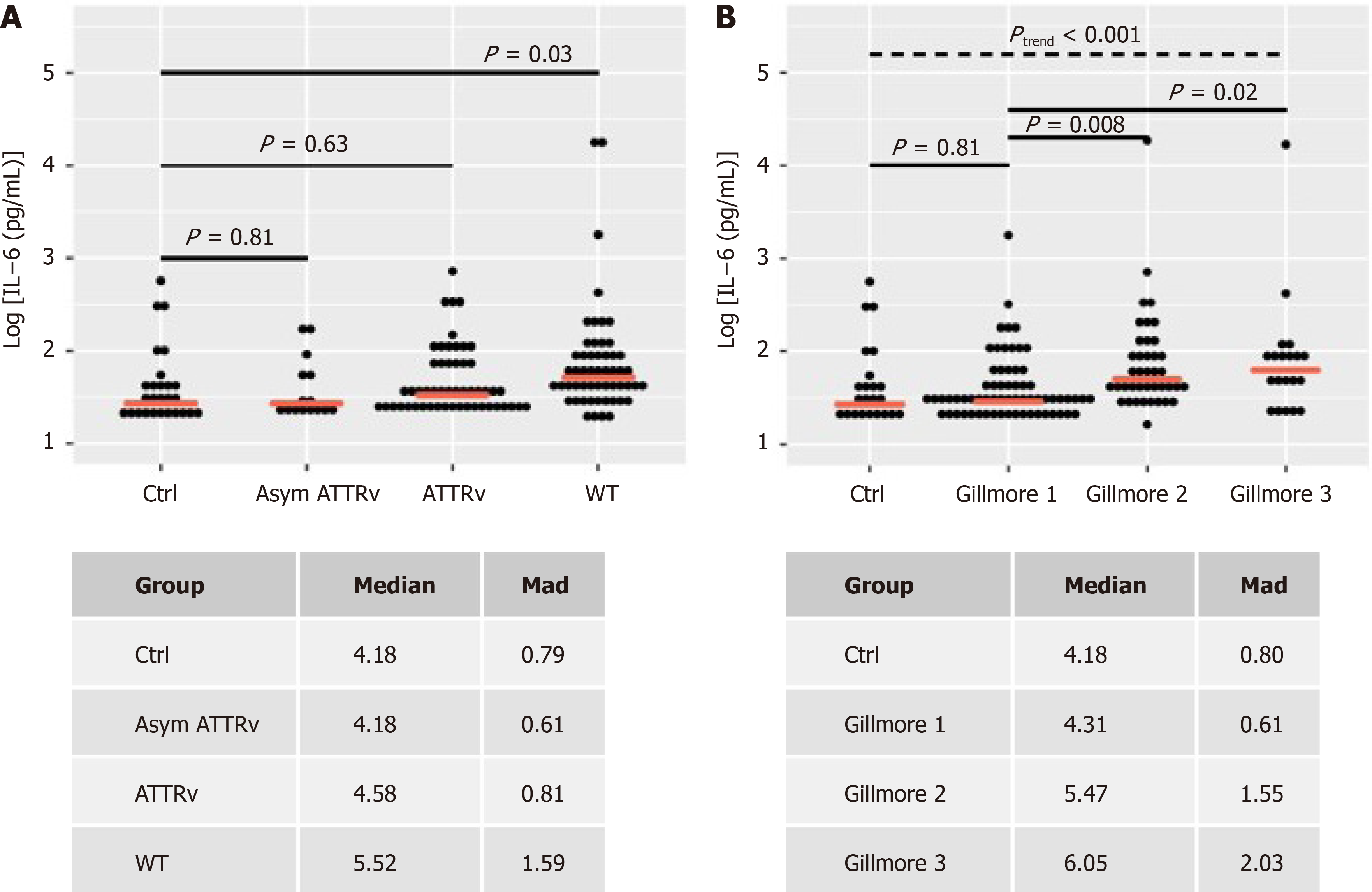
In healthy controls, median IL-6 level was 4.18 pg/mL (median absolute deviation (mad): 0.8) and in aATTRv-CM IL-6 was 4.18 (0.61) pg/mL as well (Figure 2A). Symptomatic patients with hereditary ATTRv-CM showed slightly increased levels of IL-6 with 4.58 pg/mL (0.81) not reaching significance (P = 0.63). In contrast, ATTRwt patients showed significant increased median IL-6 levels compared to controls [5.52pg/mL (1.59), P = 0.03]. IL-6 levels showed an increase from control and Gillmore class 1 to Gillmore classes 2 and 3 (trend-test, P < 0.001). Median IL-6 levels were significantly increased in Gillmore 2 and 3 vs Gillmore 1 (Figure 2B).
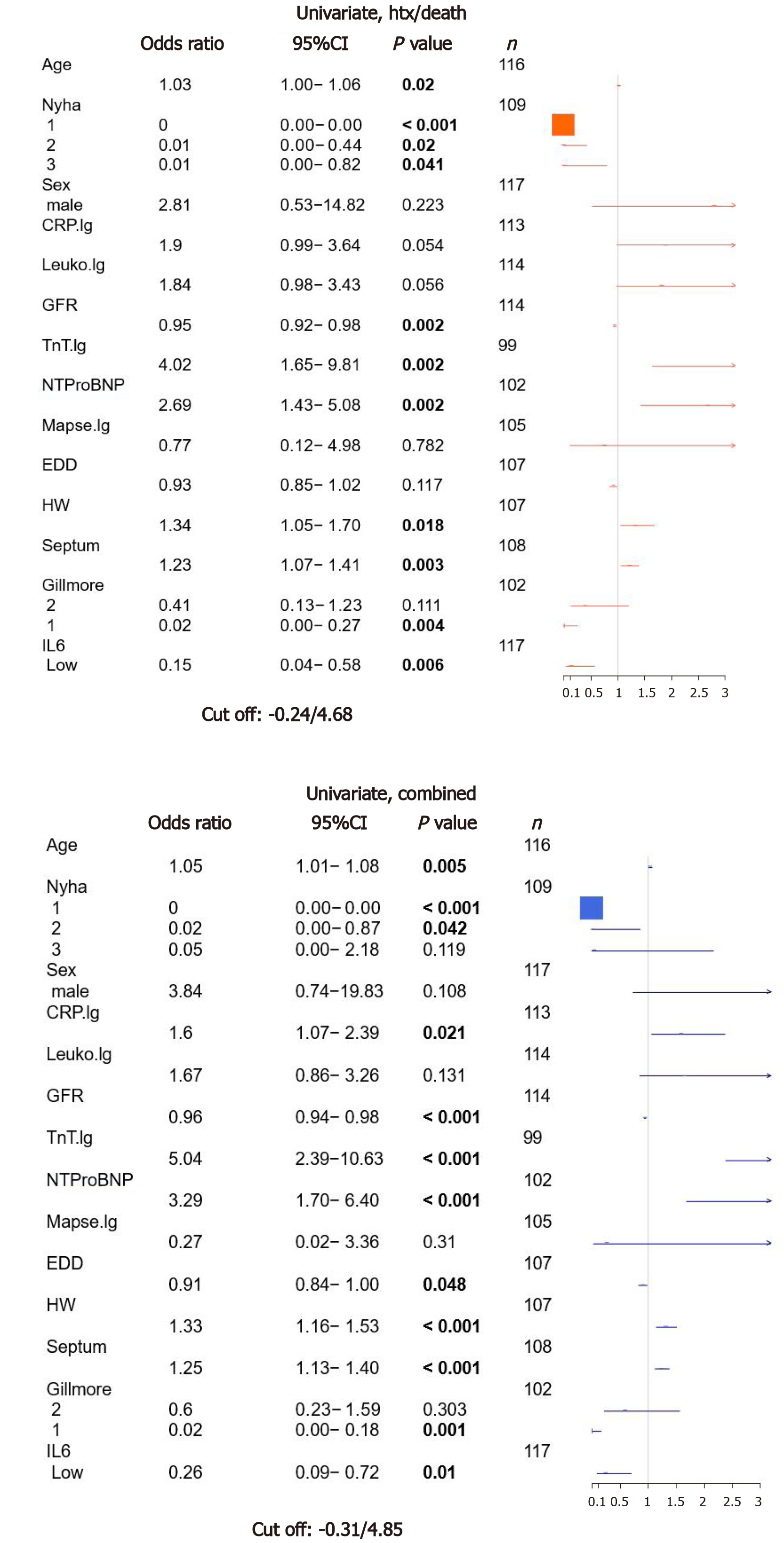
IL-6 levels were analyzed using parametric survival models to identify best separating cutoffs for the prognostic separation of patients for both evaluated endpoints. For death/htx endpoint, a cutoff of -0.24, corresponding to 4.68 pg/mL was identified (low: n = 78; high: n = 70).
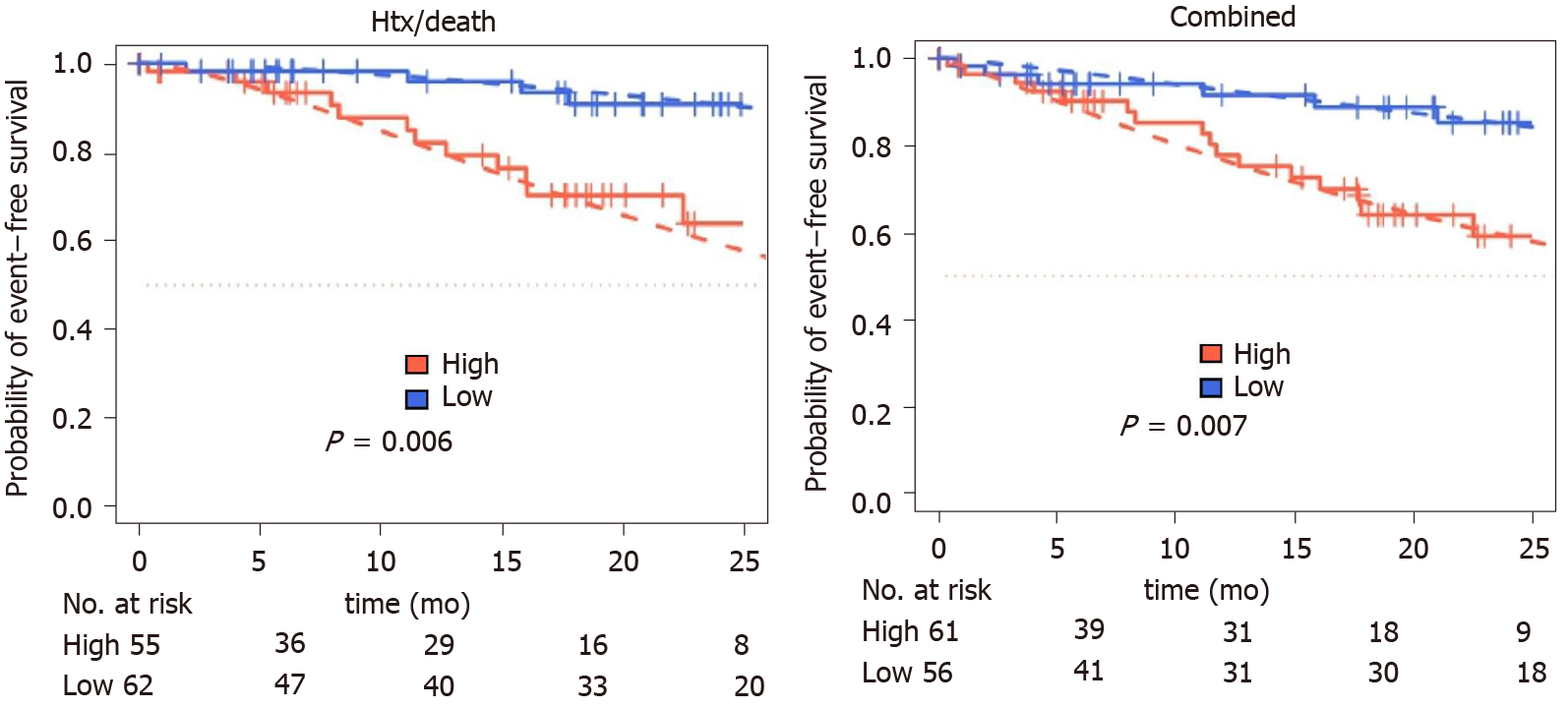
For MACE, a cutoff of -0.31, corresponding to 4.85 pg/mL, was identified (low: n = 72; high: n = 76). For 117 of 148 patients (79%), follow-up after index event was available. Median follow-up was 13.5 [95% confidence interval (CI): 11.6-15.7] months for death/htx and 13.6 (95%CI: 11.7-15.9) months for MACE.
During follow-up period, 19 patients died, six were listed for high urgent heart transplantation, two died waiting for the organ and two patients were successfully transplanted. Fourteen patients were hospitalized due to cardiac decompensation requiring additional treatment with diuretics for recompensation. Twenty-four patients reached at least one of the prespecified combined endpoints. Patients with increased IL-6 levels had a higher risk for MACE (P = 0.01) or death/htx (P = 0.006) (Figure 3).
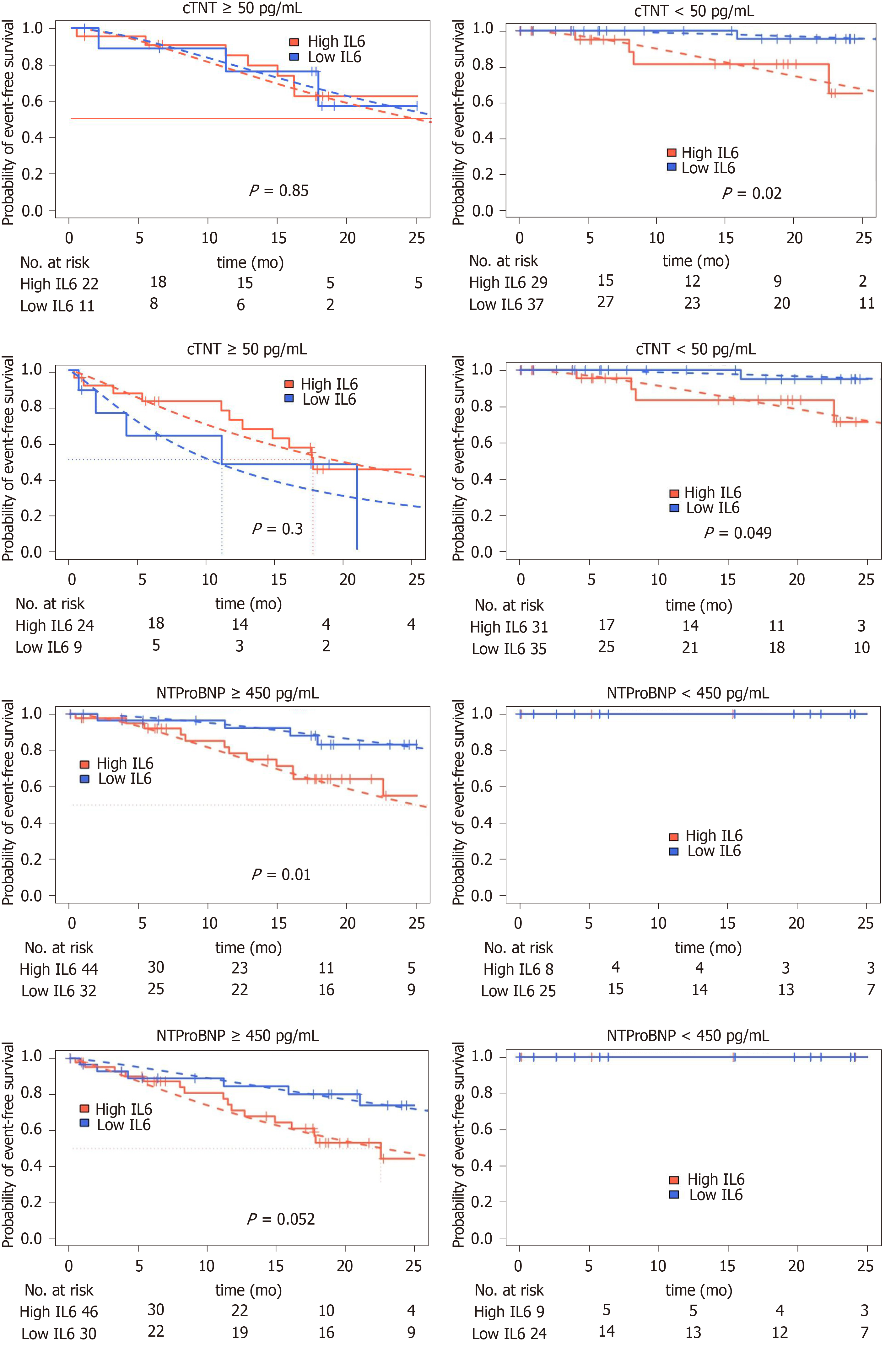
Two well established biomarkers for risk stratification in ATTR-CM are cTnT and NTproBNP levels[21-23]. Therefore, we repeated our risk assessment for patients in these two high-risk subgroups. In patients with low NTproBNP levels, no prespecified endpoint was observed during follow-up. In patients with elevated NTproBNP levels (above 450 ng/mL[24-26]), high IL-6 levels were significantly associated htx/death (P = 0.01) and a clear trend for increased incidence of MACE could be observed (P = 0.052). Dividing our cohort according to the established cutoff for cTnT of 50 ng/mL identified in the groups with cTnT negative group a prognostic separation for death/htx endpoint (P = 0.02) and a borderline significant prognostic separation for MACE (P = 0.049). In the cTnT negative group no significant prognostic separation was found (Figure 4).
To estimate the value of IL-6 for the prespecified endpoints htx/death as well as MACE, we performed an univariate survival analysis for established risk parameters as age, NYHA classification, gender, GFR, echocardiographic parameters (mitral annular plane systolic excursion, end diastolic diameter, septum thickness, estimated heart weight) and Gillmore class and biomarkers (cTnT, NTproBNP). Furthermore inflammatory markers as CRP and leukocyte count were included as well as IL-6. As shown in Figure 1, age, NYHA class, GFR, cTnT, NTproBNP, heart weight, septum thickness, Gillmore class and IL-6 showed significant association with htx/death. The same characteristics were also significantly associated with MACE, but also CRP levels and EDD showed a significant correlation with MACE. The odds ratio (OR) for low IL-6 and htx/death was 0.15 (P = 0.006), and for MACE, OR of low vs high IL-6 was 0.26 (P = 0.01).
To test for independent risk prediction of IL-6, a multivariate analysis was calculated including IL-6 and all other parameters significant in the univariate model except for the combined feature Gillmore. However, in the multivariate model, IL-6 did not improve risk stratification, neither for htx/death [OR: 0.42; 95%CI (0.06-2.83); P = 0.37] nor MACE [OR: 1.11; 95%CI (0.23-5.29); P = 0.89] (Supplementary Figure 2).
In the present monocentric prospective study, elevated IL-6 levels were found in patients with ATTRwt amyloidosis but not aATTRv-CM carriers or ATTRv-CM patients. IL-6 concentrations correlated with severity of clinical presentation as quantified in the Gillmore classification. Furthermore, IL-6 levels were significantly associated with clinical outcome as death/htx or MACE in the univariate analysis. However, in the multivariate analysis IL-6 did not show a significant additional value over established risk predictors.
Elevated IL-6 levels have been described in amyloid A (AA) amyloidosis and ATTRv-PN. AA amyloidosis is a secondary amyloidosis in chronic inflammatory diseases (e.g., rheumatoid arthritis)[27]. In these patients, application of the human monoclonal antibody tocilizumab directed against the IL-6 receptor induced rapid clinical improvement without influencing the amyloid deposits[28,29]. Therefore, for AA-amyloidosis the IL-6 axis is known to be causally involved in the pathogenesis of the inflammatory disease. In contrast, for ATTRv-PN data are inconsistent: Increased IL-6 levels were found independent from clinical presentation: Asymptomatic mutation carriers as well as symptomatic ATTRv-PN patients show increased IL-6 levels[11]. On the other hand no differences for IL-6 were seen in the study by Azevedo et al[10] in the same cohort. These data suggest that mutated TTR might be able to evoke an inflammatory state but not sufficient to establish the disease. Therefore, other factors have to be present, which are not identified yet. In contrast, in our cardiac cohort ATTRwt patients show elevated IL-6 levels, however, aATTRv-CM carriers or ATTRv-CM patients did not. Therefore, in patients with preferentially cardiac manifestation of ATTR amyloidosis, IL-6 rather seems to be secondary to the manifestation of the disease as sign of heart failure but not preceding the organ affection and, therefore, not causing the manifestation in the first hand.
In the large BIOSTAT-CHF cohort (n = 2329 patients) IL-6 levels were elevated in patients with heart failure and HFpEF, atrial fibrillation or elevated NTproBNP levels[5]. These clinical findings are often present in ATTR-cardiomyopathy patients as well. During the last years new diagnostic methods and novel therapeutic approaches raised awareness for ATTR cardiomyopathy enabling differentiation between cardiac amyloidosis and HFpEF from other reasons. On a molecular basis, activation of the IL-6 axis is known to induce concentric hypertrophy and diastolic dysfunction in rats, which might contribute to the pathology in ATTR-CM[30]. In line with these data, a correlation of IL-6 levels with clinical severity and prognosis of ATTR-CM could be observed in the herein project; however, in the multivariate analysis including well established risk factors no additional value was seen in the IL-6 quantification. Therefore, IL-6 rather seems to be a factor associated with heart failure irrespective of the underlying disease.
Taken together, raised IL-6 levels correlate with clinical presentation and are associated with worse outcome in ATTR-CM, but do not improve stratification in addition to established risk factors. Since molecular animal studies suggest a contribution of IL-6 to the manifestation of heart failure and pathological remodeling[7,31], a study interfering with the IL-6 axis is needed to prove this concept in patients with heart failure in general and ATTR-CM amyloidosis specifically. Our data show an association to severity and prognosis of the disease; evidence for causality in the pathogenesis cannot be provided here. Since the multivariate analysis did not show a significant association, further research is needed to improve risk stratification.
In transthyretin cardiac amyloidosis (ATTR), protein deposition leads to myocardial thickening and heart failure, which is defined as ATTR cardiomyopathy (ATTR-CM). Recently, evidence was raised that inflammation might be associated with disease progression in ATTR polyneuropathy and heart failure. But until now little is known about the inflammatory state in ATTR-CM. Therefore, we measured IL-6 levels in ATTR-CM and analyzed its predictive value for cardiac outcome.
In ATTR-CM stable disease over several years as well as rapidly progressive disease courses are described. This discrepancy might results from differences in immunological response to myocardial protein deposits in ATTR-CM.
The objective of the study was to investigate differences in IL-6 levels and evaluate its predictive value for cardiovascular outcome (death/heart transplantation, decom-pensation or a combined endpoint).
In this monocentric prospective study, 106 ATTR-CM patients were included, and IL-6 levels were measured using Luminex technology. Follow-up period was 12 mo, and statistical analysis was performed using parametric survival regression models.
IL-6 is associated with outcome in ATTR-CM but does not improve risk stratification in addition to established risk prediction parameters. The study thereby provides evidence that IL-6 axis might be involved in the pathogenesis of ATTR-CM. To investigate this hypothesis further, additional studies are needed.
The study showed that IL-6 is associated with outcome in ATTR-CM but does not add further risk stratification potential to established risk prediction models.
Further studies are needed to investigate inflammatory response in ATTR-CM.
We like to acknowledge our gratitude to Elisabeth Kliemank and Thomas Fleming for their support conducting the Luminex experiments. Furthermore we like to thank Shabana Din for editing the manuscript.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: European Society of Cardiology, No. 528873.
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: Germany
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ueda H S-Editor: Zhang H L-Editor: Filipodia P-Editor: Liu JH
| 1. | Maurizi N, Rella V, Fumagalli C, Salerno S, Castelletti S, Dagradi F, Torchio M, Marceca A, Meda M, Gasparini M, Boschi B, Girolami F, Parati G, Olivotto I, Crotti L, Cecchi F. Prevalence of cardiac amyloidosis among adult patients referred to tertiary centres with an initial diagnosis of hypertrophic cardiomyopathy. Int J Cardiol. 2020;300:191-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 2. | Ruberg FL, Berk JL. Transthyretin (TTR) cardiac amyloidosis. Circulation. 2012;126:1286-1300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 512] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 3. | Adams D, Koike H, Slama M, Coelho T. Hereditary transthyretin amyloidosis: a model of medical progress for a fatal disease. Nat Rev Neurol. 2019;15:387-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 304] [Article Influence: 50.7] [Reference Citation Analysis (0)] |
| 4. | Gillmore JD, Damy T, Fontana M, Hutchinson M, Lachmann HJ, Martinez-Naharro A, Quarta CC, Rezk T, Whelan CJ, Gonzalez-Lopez E, Lane T, Gilbertson JA, Rowczenio D, Petrie A, Hawkins PN. A new staging system for cardiac transthyretin amyloidosis. Eur Heart J. 2018;39:2799-2806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 515] [Article Influence: 85.8] [Reference Citation Analysis (0)] |
| 5. | Markousis-Mavrogenis G, Bournia VK, Panopoulos S, Koutsogeorgopoulou L, Kanoupakis G, Apostolou D, Katsifis G, Polychroniadis M, Dimitroulas T, Kolovou G, Kitas GD, Mavrogeni SI, Sfikakis PP. Cardiovascular Magnetic Resonance Identifies High-Risk Systemic Sclerosis Patients with Normal Echocardiograms and Provides Incremental Prognostic Value. Diagnostics (Basel). 2019;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 6. | Plenz G, Song ZF, Tjan TD, Koenig C, Baba HA, Erren M, Flesch M, Wichter T, Scheld HH, Deng MC. Activation of the cardiac interleukin-6 system in advanced heart failure. Eur J Heart Fail. 2001;3:415-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 64] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 7. | Fontes JA, Rose NR, Čiháková D. The varying faces of IL-6: From cardiac protection to cardiac failure. Cytokine. 2015;74:62-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 245] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 8. | Siegismund CS, Escher F, Lassner D, Kühl U, Gross U, Fruhwald F, Wenzel P, Münzel T, Frey N, Linke RP, Schultheiss HP. Intramyocardial inflammation predicts adverse outcome in patients with cardiac AL amyloidosis. Eur J Heart Fail. 2018;20:751-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 9. | Kurian SM, Novais M, Whisenant T, Gelbart T, Buxbaum JN, Kelly JW, Coelho T, Salomon DR. Peripheral Blood Cell Gene Expression Diagnostic for Identifying Symptomatic Transthyretin Amyloidosis Patients: Male and Female Specific Signatures. Theranostics. 2016;6:1792-1809. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Azevedo EP, Guimaraes-Costa AB, Bandeira-Melo C, Chimelli L, Waddington-Cruz M, Saraiva EM, Palhano FL, Foguel D. Inflammatory profiling of patients with familial amyloid polyneuropathy. BMC Neurol. 2019;19:146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 11. | Suenaga G, Ikeda T, Masuda T, Motokawa H, Yamashita T, Takamatsu K, Misumi Y, Ueda M, Matsui H, Senju S, Ando Y. Inflammatory state exists in familial amyloid polyneuropathy that may be triggered by mutated transthyretin. Sci Rep. 2017;7:1579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Flower L, Ahuja RH, Humphries SE, Mohamed-Ali V. Effects of sample handling on the stability of interleukin 6, tumour necrosis factor-alpha and leptin. Cytokine. 2000;12:1712-1716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 138] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 13. | Kenis G, Teunissen C, De Jongh R, Bosmans E, Steinbusch H, Maes M. Stability of interleukin 6, soluble interleukin 6 receptor, interleukin 10 and CC16 in human serum. Cytokine. 2002;19:228-235. [PubMed] |
| 14. | Kristen AV, Scherer K, Buss S, aus dem Siepen F, Haufe S, Bauer R, Hinderhofer K, Giannitsis E, Hardt S, Haberkorn U, Katus HA, Steen H. Noninvasive risk stratification of patients with transthyretin amyloidosis. JACC Cardiovasc Imaging. 2014;7:502-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 15. | Luchner A, Weidemann A, Willenbrock R, Philipp S, Heinicke N, Rambausek M, Mehdorn U, Frankenberger B, Heid IM, Eckardt KU, Holmer SR. Improvement of the cardiac marker N-terminal-pro brain natriuretic peptide through adjustment for renal function: a stratified multicenter trial. Clin Chem Lab Med. 2010;48:121-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Popescu BA, Waggoner AD. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29:277-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2879] [Cited by in RCA: 3844] [Article Influence: 427.1] [Reference Citation Analysis (0)] |
| 17. | R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundataion for Statistical Computing, 2018. Available from: https://www.R-project.org/. |
| 18. | Smithson M, Verkuilen J. A better lemon squeezer? Psychol Methods. 2006;11:54-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 770] [Cited by in RCA: 658] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 19. | John Haman MA, Institute for Defense Analyses. ciTools: Confidence or Prediction Intervals, Quantiles, and Probabilities for Statistical Models 2019. Available from: https://cran.r-project.org/web/packages/ciTools/index.html. |
| 20. | Knoll M. dataAnalysisMisc: Collection of functions for daily tasks. R package version 0.99.11 2020. Available from: http://github.com/mknoll/dataAnalysisMisc. |
| 21. | Dispenzieri A, Gertz MA, Kyle RA, Lacy MQ, Burritt MF, Therneau TM, Greipp PR, Witzig TE, Lust JA, Rajkumar SV, Fonseca R, Zeldenrust SR, McGregor CG, Jaffe AS. Serum cardiac troponins and N-terminal pro-brain natriuretic peptide: a staging system for primary systemic amyloidosis. J Clin Oncol. 2004;22:3751-3757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 588] [Cited by in RCA: 708] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 22. | Grogan M, Scott CG, Kyle RA, Zeldenrust SR, Gertz MA, Lin G, Klarich KW, Miller WL, Maleszewski JJ, Dispenzieri A. Natural History of Wild-Type Transthyretin Cardiac Amyloidosis and Risk Stratification Using a Novel Staging System. J Am Coll Cardiol. 2016;68:1014-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 530] [Article Influence: 66.3] [Reference Citation Analysis (0)] |
| 23. | Kristen AV, Biener M, Hegenbart U, Hardt S, Schnabel PA, Röcken C, Schonland SO, Katus HA, Giannitsis E. Evaluation of the clinical use of midregional pro-atrial natriuretic peptide (MR-proANP) in comparison to N-terminal pro-B-type natriuretic peptide (NT-proBNP) for risk stratification in patients with light-chain amyloidosis. Int J Cardiol. 2014;176:1113-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Lehrke S, Steen H, Kristen AV, Merten C, Lossnitzer D, Dengler TJ, Katus HA, Giannitsis E. Serum levels of NT-proBNP as surrogate for cardiac amyloid burden: new evidence from gadolinium-enhanced cardiac magnetic resonance imaging in patients with amyloidosis. Amyloid. 2009;16:187-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 25. | MacGowan GA, Neely D, Peaston R, Wrightson N, Parry G. Evaluation of NT-proBNP to predict outcomes in advanced heart failure. Int J Clin Pract. 2010;64:892-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Merlini G, Lousada I, Ando Y, Dispenzieri A, Gertz MA, Grogan M, Maurer MS, Sanchorawala V, Wechalekar A, Palladini G, Comenzo RL. Rationale, application and clinical qualification for NT-proBNP as a surrogate end point in pivotal clinical trials in patients with AL amyloidosis. Leukemia. 2016;30:1979-1986. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 27. | Okuda Y. AA amyloidosis - Benefits and prospects of IL-6 inhibitors. Mod Rheumatol. 2019;29:268-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 28. | Lane T, Gillmore JD, Wechalekar AD, Hawkins PN, Lachmann HJ. Therapeutic blockade of interleukin-6 by tocilizumab in the management of AA amyloidosis and chronic inflammatory disorders: a case series and review of the literature. Clin Exp Rheumatol. 2015;33:S46-S53. [PubMed] |
| 29. | Yamagata A, Uchida T, Yamada Y, Nakanishi T, Nagai K, Imakiire T, Oshima N, Kumagai H. Rapid clinical improvement of amyloid A amyloidosis following treatment with tocilizumab despite persisting amyloid deposition: a case report. BMC Nephrol. 2017;18:377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Meléndez GC, McLarty JL, Levick SP, Du Y, Janicki JS, Brower GL. Interleukin 6 mediates myocardial fibrosis, concentric hypertrophy, and diastolic dysfunction in rats. Hypertension. 2010;56:225-231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 359] [Cited by in RCA: 351] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 31. | Markousis-Mavrogenis G, Tromp J, Ouwerkerk W, Devalaraja M, Anker SD, Cleland JG, Dickstein K, Filippatos GS, van der Harst P, Lang CC, Metra M, Ng LL, Ponikowski P, Samani NJ, Zannad F, Zwinderman AH, Hillege HL, van Veldhuisen DJ, Kakkar R, Voors AA, van der Meer P. The clinical significance of interleukin-6 in heart failure: results from the BIOSTAT-CHF study. Eur J Heart Fail. 2019;21:965-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 200] [Article Influence: 33.3] [Reference Citation Analysis (0)] |