Published online Nov 26, 2021. doi: 10.4330/wjc.v13.i11.608
Peer-review started: May 2, 2021
First decision: June 17, 2021
Revised: June 27, 2021
Accepted: October 31, 2021
Article in press: October 31, 2021
Published online: November 26, 2021
Processing time: 206 Days and 14.9 Hours
Palpitations are one of the most common reasons for medical consultation. They tend to worry patients and can affect their quality of life. They are often a symptom associated with cardiac rhythm disorders, although there are other etiologies. For diagnosis, it is essential to be able to reliably correlate the sym
Core Tip: In recent years, electrocardiographic monitoring systems have incorporated many technical improvements that solve several of the 24-h Holter monitor limitations. This review provides an update on the different electrocardiographic cardiac monitoring methods currently available, remarking their indications and limitations, to help healthcare professionals appropriately select and use them in the work-up of patients with palpitations.
- Citation: Francisco-Pascual J, Cantalapiedra-Romero J, Pérez-Rodon J, Benito B, Santos-Ortega A, Maldonado J, Ferreira-Gonzalez I, Rivas-Gándara N. Cardiac monitoring for patients with palpitations. World J Cardiol 2021; 13(11): 608-627
- URL: https://www.wjgnet.com/1949-8462/full/v13/i11/608.htm
- DOI: https://dx.doi.org/10.4330/wjc.v13.i11.608
Palpitations are one of the most common reasons for medical consultation and can occur in up to 10% of the population at some point in their lives[1-3]. They tend to worry patients and can affect their quality of life. They are a symptom that is frequently associated with cardiac rhythm disorders, although there are other etiologies[4] (Table 1). In fact, in up to half of the cases, palpitations are not due to a significative arrhythmia[2].
| Cardiac and noncardiac causes | |
| Cardiac arrhythmias | Premature contractions (supraventricular or ventricular) |
| Supraventricular tachycardia (AF, flutter, AVRNT, etc.) | |
| Ventricular tachycardia | |
| Severe bradyarrhythmia/AV block | |
| Pacemaker mediated tachycardia | |
| Structural heart disease | Severe aortic regurgitation |
| Hypertrophic cardiomyopathy | |
| Congenital heart disease with significant shunt | |
| Mechanical prosthetic valves | |
| Systemic causes | Thyroid dysfunction |
| Pheochromocytoma | |
| Anaemia | |
| Fever | |
| Hypoglycaemia | |
| Arteriovenous fistula | |
| Autonomic dysfunction | |
| Psychosomatic disorders | Anxiety |
| Somatisation disorder | |
| Drugs | Sympathomimetic agents (bronchodilators, antidepressants) |
| Vasodilators (hydralazine, doxazosin) | |
| Recreational: Cocaine, alcohol, amphetamines, cannabis |
Having an accurate diagnosis is important to determinate the prognosis, to guide treatment and to plan the patient’s follow-up. In order to have a diagnosis, it is essential to be able to reliably correlate the symptoms with an electrocardiographic record allowing the identification or ruling out of a possible rhythm disorder. However, reaching a diagnosis is not always simple, given that they tend to be transitory symptoms and the patient is asymptomatic at the time of assessment[1,5].
Electrocardiographic monitoring systems are a first-line tool in assessing these patients. The introduction of the 24-h Holter monitor within 60 s was a true revolution and its use rapidly became widespread[6]. Other than diagnosing palpitations, it has also been an important tool in understanding the physiological cardiac rhythm behavior[7,8] and in the follow-up of patients at risk of cardiovascular disease[8], in syncope work-up[9-12], in risk stratification of certain patients[13-16] or in the detection of silent arrhythmias[8,17-21]. Due to its high availability and probably due to a certain degree of tradition, it is the monitoring system most commonly used by most doctors. However, it has several limitations that reduce its efficiency and diagnostic yield[2,22].
Fortunately, in recent years, in line with the overall technological development that our society has experienced, electrocardiographic monitoring systems have incorporated many technical upgrades allowing for improvement in several of the limitations presented by the 24-h Holter monitor. This evolution of electrocardiogram (ECG) recording systems has occurred in various directions. On the one hand, we have the improvement in the quality and quantity of recordings, such that, at the present time, it is possible to obtain 12-lead ECG traces of excellent quality with increased monitoring time, be it in the form of a continuous recording (which can last up to a month), or in the form of intermittent recordings which can last up to 3 years in the case of implantable recorders[8,23-25]. On the other hand, various recording and analysis algorithms have been developed, which allow for many improvements; for example, the automatic analysis of arrhythmias[18,23]. Remote data transmission systems have also been incorporated with multiple designs able to adapt to each patient’s different needs[8,24,26].
Unfortunately, these new devices are still not universally incorporated into daily clinical practice, either because they are not included or reimbursed in the healthcare systems, or due to the inertia of healthcare professionals[27]. In the work-up for palpitations, it is still usual to apply a traditional strategy that is fundamentally based on the use of a 24-h Holter monitor, which has low diagnostic yield on many occasions[2,22]. This lack of yield is due to the fact that the majority of the events to be diagnosed are paroxysmal ones that occur occasionally and unexpectedly. The use of standardized diagnostic protocols with the application of new electrocardiographic monitoring devices has been shown not only to markedly improve the diagnostic yield, but also to be clearly cost efficient[2,28-30].
However, it should also be noted that the new devices are not exempt from limitations. Some are related to patient tolerance, either due to the possibility of relative discomfort, the need to wear external devices with electrodes stuck to the skin, or because the implantable devices require a minor surgical procedure, meaning that certain patients may not accept them[8,24,27]. Also, the devices continue to record a significant number of artifacts or rhythmic abnormalities that are not pathological or significant, which inefficiently lengthen the interpretation time of the studies and can even saturate the memory of certain devices. In addition, it should be noted that for a suitable diagnosis to be made, not only is it important to have recorded a quality electrocardiographic trace; the latter must also be properly interpreted in the patient’s clinical context, which requires sufficient skill on the part of the healthcare professional interpreting the trace[31].
The objective of this review is to offer an update on the different monitoring methods currently available, their indications and limitations, to help healthcare professionals appropriately select and use them in the work-up of patients with palpitations.
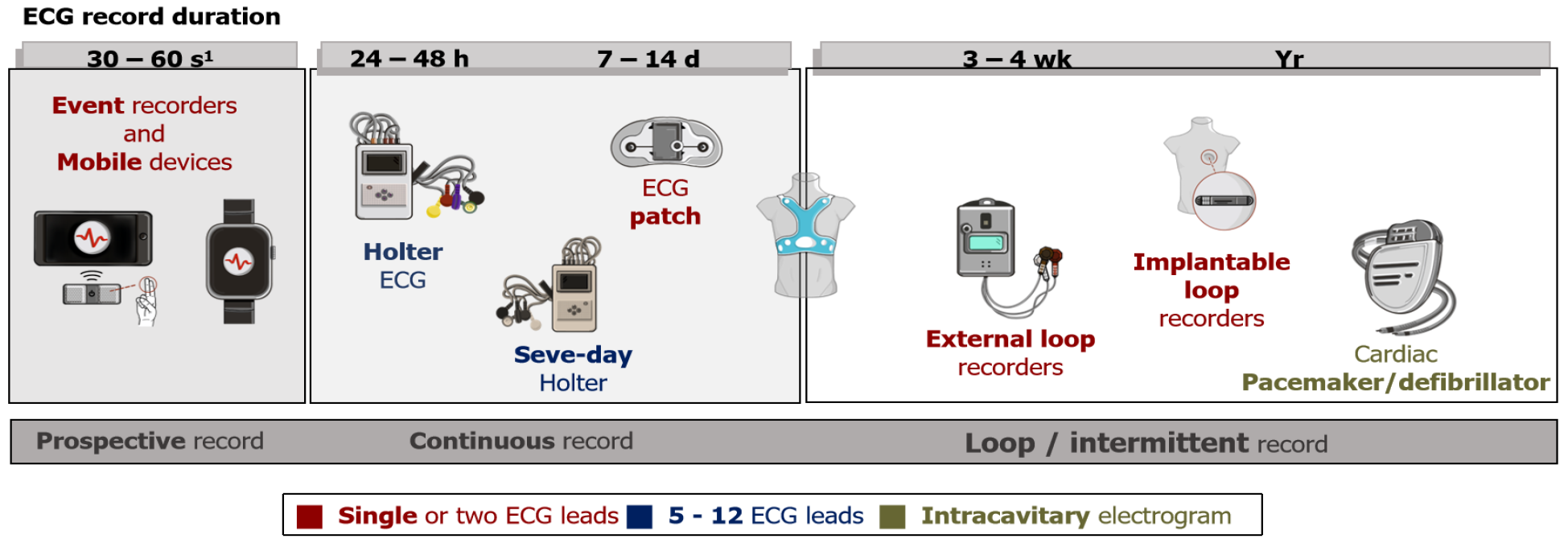
The monitoring devices available on the market have differing characteristics. Traditionally, these devices have been classified into three or four groups [24/48-h Holter, external prospective event recorders (PERs), loop recorders (LRs)] based on a series of shared characteristics[8,27] (Table 2). However, thanks to technical develop
| Advantages | Disadvantages | Main indications | |
| 24 h Holter | Continuous recording | Discomfort for the patient | Very frequent (daily) symptoms |
| 12 leads with good correlation with surface ECG | Artefacts | Permanent AF rate monitoring | |
| Low economic cost | Maximum recording of 24-48 h (low diagnostic yield) | Frequent ventricular premature beats | |
| Risk stratification of (hypertrophic) cardiomyopathies | |||
| Skin patches | Continuous recording of 7–14 d | Single use and greater economic cost | Frequent (weekly) symptoms |
| Good tolerability for patients | Analysis by external companies | AF detection in cryptogenic stroke (2 wk) | |
| Only one lead1 | |||
| External loop recorders | Loop recording (includes beginning and end of arrhythmic event) | Patient discomfort | Occasional symptoms (monthly) |
| 4 wk monitoring | Requires education from healthcare professional on how to correctly place the electrodes | AF detection in cryptogenic stroke (2–4 wk) | |
| High yield and efficiency in the assessment of palpitations | |||
| Implantable loop recorder | Loop recording | Invasiveness and associated complications (infection, bleeding, etc.) | Very infrequent symptoms |
| Up to 3-yr monitoring (good diagnostic yield) | Individual economic cost | AF detection in at-risk patients (cryptogenic stroke, post-ablation, etc.) | |
| Patient does not have to do anything | Single lead | Syncope | |
| Remote monitoring | |||
| External event recorders/mobile devices | Easy access for the general population | Single lead1 | Palpitations work-up |
| Possibility of prolonged use (years) | Data management | Population AF screening (not validated) | |
| Screening for asymptomatic events (AF screening) | Patient has to be involved (not suitable for syncope work-up) | ||
| Remote monitoring |
They may, thus, be classified according to the following.
(1) Monitoring time. There are short recording devices (24–48 h) that are useful for the examination of symptoms that occur every day or frequently; mid-range recording devices (up to 4 wk) and long recording devices (up to 3–4 years)[32]. Due to limitations of data storage, when we increase the monitoring time of the devices, they switch from continuous to intermittent recording, as will be discussed later. To achieve appropriate diagnostic yield in the work-up for palpitations, it is essential to adjust the monitoring time to the frequency of the symptoms. This is, without a doubt, one of the main characteristics when selecting a device.
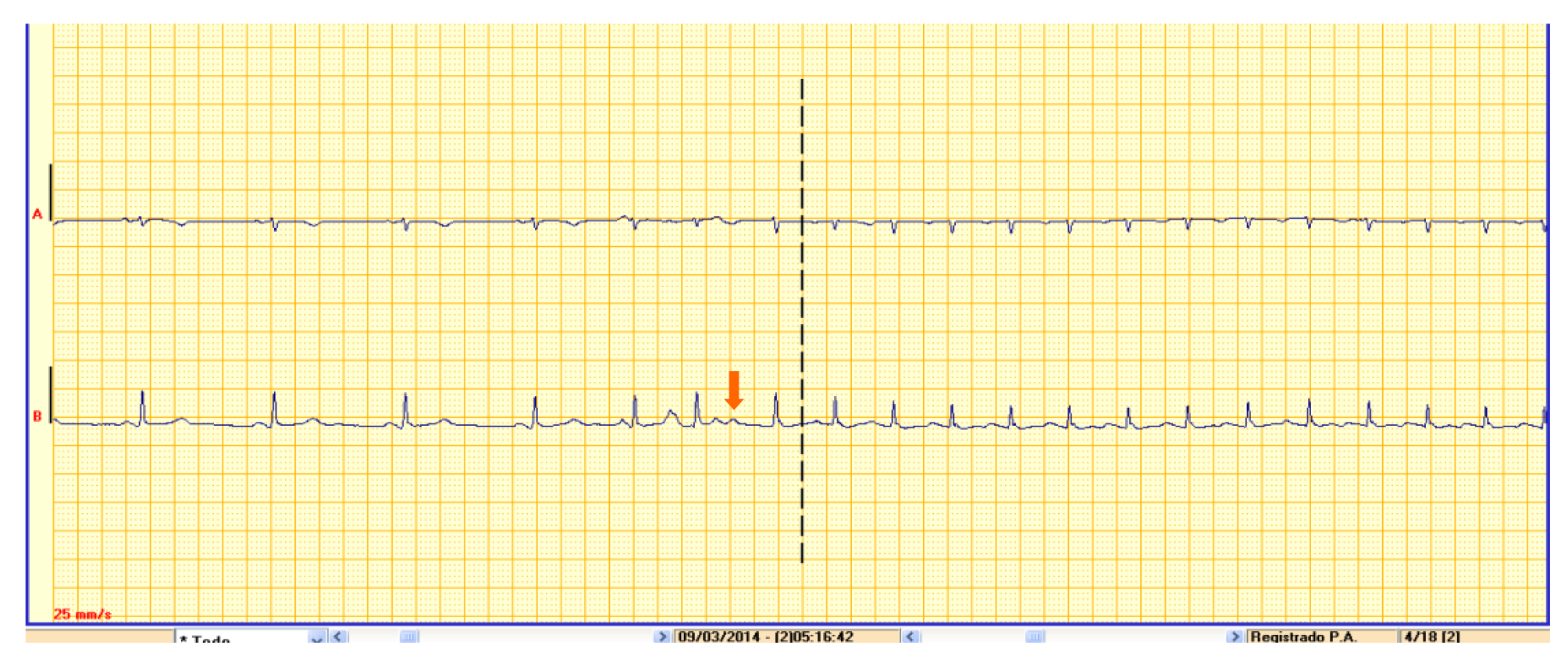
(2) Recording type. This is another important characteristic to be kept in mind when selecting the most suitable test for each patient type. On the one hand, continuous recording devices such as Holter monitors make a constant ECG recording that can later be reviewed in its entirety. Continuous recordings avoid information loss since they do not depend on activation by the patient or on arrhythmia detection algorithms. By contrast, tests using intermittent recording are patient-activated or activate automatically according to different arrhythmia detection algorithms. There are two distinct types of device with this characteristic: Loop recorders [external LRs (ELRs) or implantable LRs (ILRs)] and PERs. The main difference between them is the ability of the LR to record the trace both prospectively and retrospectively, allowing us to obtain the trace from the start of the event (which may be important for the precise diagnosis of certain arrhythmias) (Figure 2). Similarly, LRs have various algorithms allowing for the automatic recording of certain asymptomatic arrhythmias, which prospective recorders do not allow for, since they are only activated by the patient in the event of symptoms (as such, they are also not useful for syncope work-up)[17,32,33]. It should be noted that there are currently certain devices available with continuous recording capacity as well as off-line analysis software with detection algorithms for arrhythmias that present the information in a similar manner to event recorders (although the healthcare professional can review the rest of the recording if this is considered important)[19,34,35].
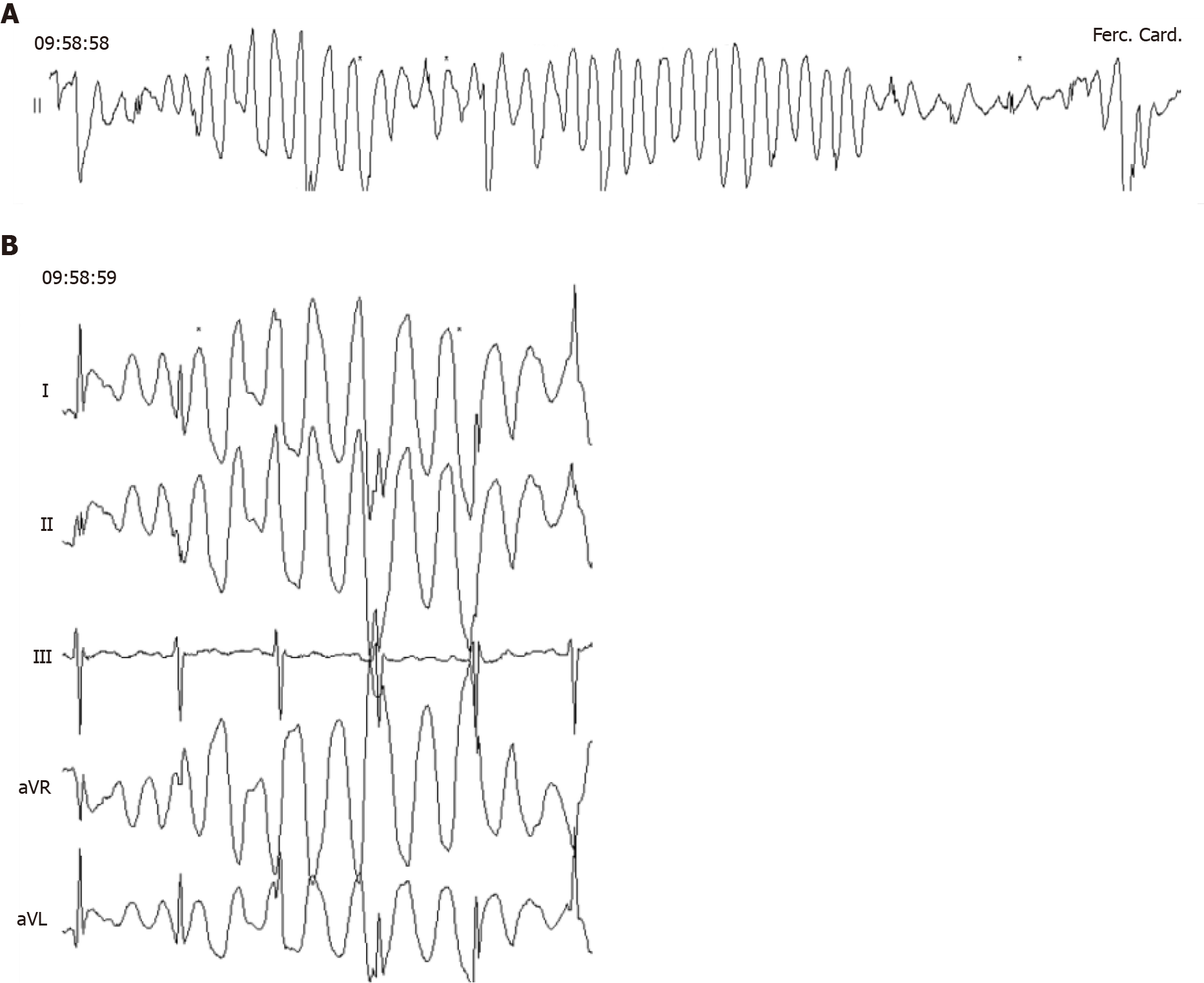
(3) Number of leads. Recordings range from systems with just one lead to full 12-lead ECGs in the devices with the most leads. It is important to keep in mind that, the greater the number of leads, the more memory the device will use. In addition, there will be a greater number of electrodes to be applied by the patient in order for the recording to be made. As such, as a general rule, the devices allowing for long monitoring times offer a limited number of leads. However, having more than one lead is often useful. For example, on occasion, it allows for the clearing up of doubts as to whether a lead is subject to artifacts (Figure 3) or to better assess the wave morphology, which is important on certain occasions[8,36,37].
(4) Degree of invasiveness. There are implantable and external devices. ILRs require a minor surgical procedure, but once the wound has healed, patients do not usually notice them, and they allow for prolonged monitoring[17,25].
(5) Connectivity. The most up-to-date devices incorporate connectivity systems that allow for remote monitoring. Some can even be linked to smartphones, allowing for the easy sharing and sending of recordings via the Internet or Bluetooth[26,32,38,39]. In addition, some applications have algorithms that offer the patient an instantaneous diagnosis of their arrhythmia (although it is recommended that this is ratified by a healthcare professional)[18,40,41].
(6) Automatic algorithms. Different recording and analysis algorithms have been developed in recent years and they have been incorporated into some devices. Most of these algorithms allow for the automatic analysis of arrhythmias such as atrial fibrillation (AF), bradycardia, or asystole, but some of them also allow for the filtering out of noise to try to improve recording quality. There are differences between the algorithms that different brands incorporate into their devices. It is important to evaluate not only the algorithms that they provide, but also their sensitivity and specificity for detecting each type of arrhythmia. All of them should have type I and type II errors, but there may be significant differences depending on the device. Therefore, when selecting a monitoring device, physicians should also consider the accuracy of these algorithms.
(7) Recording system type used. Initially, most devices used adhesives with electrodes. This is still the most common used format, although others have been developed that can record the cardiac signal, such as patches, belts or vests[8,24]. There are also devices that do not need to be in constant contact with the patient which incorporate electrodes into the recorder’s own case, which are brought close to the skin when a recording is desired[42-44].
(8) Availability of other biological signals. In addition to recording the electrocardiographic trace, some devices have sensors that allow other biological signals to be monitored, such as physical activity, bodily position, oxygen saturation and even the presence of apnea[39,45,46].
As noted above, there is currently a wide variety of monitoring devices offering a combination of characteristics, which makes it difficult to perform a strict classification. However, it is still useful to differentiate certain major groups (Figure 1 and Table 2), in the knowledge that certain models may have mixed characteristics between two groups or may be used in different ways as appropriate (e.g., there are devices that can function as a 24-h Holter monitor, a continuous 3-wk Holter or a loop recorder) .
Continuous Holter (24/48-h): This takes its name from the cardiologist Norman J. Holter, who developed this technology in 1961. It is currently the most common technique used in clinical practice[47]. Currently, most Holter devices consist of a lightweight recorder with a digital memory card and a series of cutaneous electrodes that obtain a continuous ECG recording. They allow for 24-48-h monitoring time in most cases, although there are devices with recording capacities of up to 7 d.
Originally, the recording was made up of only two or three leads and, still today, many tests are carried out in this way. However, as early as 1966, the first 12-lead Holter monitor was developed with a system of 10 electrodes arranged over the patient’s torso. Later on, a simplified five-electrode system was developed (the EASI system) which allowed for the reconstruction of 12 leads with good clinical reliability[48]. Having 12 leads may be especially important when assessing certain arrhythmias, such as the morphology of ventricular extrasystoles or certain repolarization patterns which may only appear in one specific lead. Torso electrodes avoid possible artifacts and discomfort associated with the positioning of the electrodes on the limbs. However, it should be noted that this arrangement attenuates the amplitude of the inferior leads and generates a slight right cardiac axis deviation[48], and some recordings obtained with torso leads cannot be considered completely equivalent to standard ECG leads[8].
After completing the recording, it is transferred into software installed on a computer (and/or a server in the cloud in some of the more advanced devices), which usually allows for an initial automatic analysis identifying the QRS complexes and the R-R interval to offer values such as maximum/minimum rate, histograms of rate or ST segment analysis. Finally, the healthcare professional must review the data to rule out the presence of possible artifacts and interpret the results of the ECG taking into account the patient’s symptom diary[47].
Holter monitors have demonstrated their utility in identifying arrhythmias in patients with palpitations or syncope with different efficacy values in line with the pretest probability of the population studied and the frequency of the symptoms[2,22,28]. In recent years, various case series, including our own[2,32], have shown that the global diagnostic yield of Holter monitors is limited and that, despite the fact that its cost is generally relatively low, its per-diagnosis cost is high[22,27,47]. For example, in a transversal study in usual clinical practice carried out by our group, Holter only offered a diagnostic yield of 3.5% for syncope and 16.5% for palpitations. This yield is even lower when the objective is screening for asymptomatic arrhythmias, such as AF in the context of cryptogenic stroke, where the yield can be < 1%[32].
Among other indications that have been considered, there was risk stratification in certain groups of patients, either through the detection of nonsustained arrhythmias and abnormalities of heart rate variability parameters or dispersion of the QT interval[15,49-53]. However, at the present time, few therapeutic decisions are based on Holter findings, perhaps with the exception of the presence of nonsustained ventricular tachycardia in patients with hypertrophic cardiomyopathy[15], which may be a decisive factor for the implantation of an implantable cardiac defibrillator.
In our opinion, in the work-up for palpitations at the present time, the 24/48-h Holter monitor should only be considered in patients with daily or very frequent symptoms. When this is not the case, there are other tools available that offer not only a greater diagnostic yield, but also better cost efficiency[2,39,54].
Skin patches: These systems, which were developed over the past decade, enjoy a certain degree of popularity in some countries. However, in others, such as Spain, their use remains marginal. They consist of patches of different materials, which adhere to the skin and contain electrodes to obtain one (the most common) or two ECG leads for 7–14 d’ monitoring. The device itself acquires, amplifies and filters the ECG signal, which is then telematically transmitted for analysis (usually through an external company)[8,24,55].
There are different patch models with different recording characteristics. In some cases, they are similar to a Holter monitor and in others to an ELR. For example, the ZioPatch creates a continuous ECG recording with a single lead that is interpreted via an analysis platform. It also has a symptom button on the patch itself[56,57]. Another different example is the NUVANT-Piix. It also has only one lead, but it does not perform a continuous recording; rather, it only records the traces identified as arrhythmias or when the patient experiences symptoms and activates the device with a special magnet[58].
There are studies comparing the efficacy of these new patches with traditional Holter monitors (Table 3), showing good tolerability on the part of patients and a greater diagnostic yield, particularly for the purposes of identifying paroxysmal AF[56,59]. However, these devices have a higher cost and are usually single use[8,56].
| Ref. | No. of patients | Study design | Study population | Duration of monitoring | Diagnostic yield | Other findings |
| Holter 24 h | ||||||
| Sulfi et al[22], 2008 | 2688 | Retrospective cohort | Palpitations and basal sinus rhythm | 24 h | 16% | Even less diagnostic yield in patients aged < 50 yr |
| Paudel et al[103], 2013 | 335 | Single-center prospective cohort | Palpitations | 24 h | 75% | 40% of patients with ventricular ectopy considered as diagnostic finding (possible selection bias) |
| ECG patchs | ||||||
| Barrett et al[59], 2014 | 146 | Prospective cohort comparing Patch vs 24 h Holter | Palpitations | 15 d | 60% more diagnostics than 24 h Holter | Over 90% of patients were comfortable with it. Best diagnostic yield during first week |
| Event recorders | ||||||
| Narasimha et al[104], 2018 | 38 | Prospective cohort comparing Kardia Mobile vs ELR (simultaneously) | Palpitations (less often than daily but more than monthly) | 14–30 d | 89.5% vs 68% in ELR group | Better compliance with Kardia Mobile |
| Hall et al[63], 2020 | 11 studies (> 20000 patients) | Systematic review | AF screening in general population | Heterogeneous | Up to 36% (depending of population’s AF burden) | More diagnostic yield in people aged > 65 yr. Approximately 4% of uninterruptable registries |
| ELR | ||||||
| Locati et al[54], 2016 | 392 (282 with palpitations) | Prospective cohort | > 2 episodes in last year | 4 wk | 71.6% | Early recorder use increase diagnostic yield. Diagnostic yield for syncope: 24.5% |
| Francisco-Pascual et al[2], 2019 | 149 (91 in ELR group) | Prospective ELR cohort compared with historical Holter cohort | > 2 episodes in last year | 21 d | 86.8% | Holter diagnostic yield: 20.7%. ELR reduce the cost per diagnosis |
| ILR | ||||||
| Giada et al[29], 2007 | 50 (26 in ILR group) | Prospective cohort comparing ILR with conventional strategy | 1 episode per month or less (longer than 1 min) | 321 d (mean) | 73% | Mean time to diagnosis: 279 d. Lower cost per diagnosis in ILR group |
| Padmanabhan et al[83], 2019 | 312 (51 with palpitations) | Prospective cohort of consecutive patients with an ILR implanted | Any indication form monitoring (16.3% due to palpitations) | 579 d (mean) | 64.7% | 38.7% useful in ruling out an arrhythmic cause for symptoms (all indications). 12% AF. |
In this section, we can also include the so-called “textrodes”, which are electrodes included in garments[34]. These devices are currently in a more experimental phase. One of the main problems they entail is the quantity of artifacts due to movement of the fabric. In this regard, studies are being carried out to select the best material and electrode positioning within the fabric[60,61].
Prospective external event recorders (without loop memory) and mobile devices: These tend to be small devices with a couple of electrodes incorporated into them, which allow, when activated by the patient, for a real-time recording of 30–60 s of a single ECG lead[8,24,41,44]. These devices allow for the recording of episodes of palpitations that are sufficiently long for the patient to have time to apply the recorder. However, they have the limitation of not allowing for the recording of the start of the episodes, which is frequently important for diagnosis when it comes to interpreting the mechanism of arrhythmias[32,39] (Figure 2). Similarly, their use is not appropriate for syncope work-up, because if the patient applies the device having recovered from the syncopal episode, in most cases, the possible cause of the syncope will have disappeared[10].
In recent years, these devices have become popular, since they are small devices that do not have to be constantly in contact with the patient and allow for prolonged use (even years, since the batteries can be recharged and they only record when activated), as well as having a relatively low cost. There are currently numerous models from different brands, with various designs. In addition, some of these models link to or are even included as tools belonging to smartphones or smartwatches (such as the Apple Watch), which allows for greater and easier access for the general public[24,40]. Indeed, a significant number of users, especially those of smartwatches, have no medical indication, which may also be controversial[62].
In most models, the recordings are stored in the device itself or in a linked mobile phone. Many incorporate their own algorithms which allow for the identification of certain types of arrhythmia (especially AF), and offer the option of being sent to health centers for interpretation[40,41,44].
Due to their simple use and accessibility, these applications’ utility for other indications such as population AF screening (most useful in patients aged > 65 years)[63,64] or also for the detection of cardiac ischemia has been analyzed[65-67], which could facilitate diagnosis in patients who live far away from health centers. New mobile devices are also being developed (they are not yet on the market) which obtain an ECG signal from the patient on an involuntary basis while the devices are being used as normal, which would allow for the intermittent detection of asymptomatic cardiac rhythm disorders[44,63,64,68].
LRs: These allow for more prolonged monitoring, since they do not store a continuous recording[2,49,69]. Even though they continuously monitor the ECG, the device only stores it in its memory for a few minutes before subsequently overwriting it with a newer recording (initially it was an endless circular tape, although the devices are now digital). Only when the device is activated (either via manual activation or an automatic arrhythmia detection algorithm), it records on another part of the memory (where it will not be deleted and can be reviewed) the recording from a few minutes before the start of the event until its end. In this way, since several minutes before activation are stored in the device memory, the likelihood of recording the trace at the time of the syncope episode (if it is activated for syncope), or the start of the episode of palpitations, is high[2,54,70,71].
At the present time, most LRs can record symptomatic arrhythmias after activation by the patient, usually in the context of symptoms, or silent arrhythmias on an automatic basis[25]. Within this category, we differentiated between external and implantable devices.
(1) ELRs: External event recorders are characterized by a loop memory, which uses cutaneous electrodes to record, be this in the form of independent electrodes or those integrated into a t-shirt. The patients themselves position the electrodes daily[8,25].
Due to the characteristics of these devices, these systems tend to be worn by patients for no more than a few weeks (usually 3–4 wk, although there are reports of more prolonged periods). They are useful for the investigation of symptoms that occur every 2 or 3 wk. It should be noted that palpitations usually occur more frequently than syncopal episodes, hence the diagnostic yield for palpitations is approximately 80%[2], whereas in cases where it is indicated for syncope, it is no greater than 10%[54,72-74] (Table 3).
The quality of the recordings tends to be good, although there may be a not insignificant number of recordings corresponding to an artifact. Another limitation may be patient adherence to the daily positioning of the electrodes, even though this is generally good, as well as the possibility that the patient may develop an allergy to the electrodes, even though this is rare[54,72].
In the investigation of palpitations, in addition to a high diagnostic yield, it allows for improved cost efficiency in the work-up of these patients[2,73]. A recent study by our group[2] compared the use of ELRs with a conventional Holter strategy in patients with more than two episodes of palpitations per year. We were able to demonstrate that investigation in the form of a diagnostic protocol, including that the use of an ELR, had a notably superior diagnostic yield (86% vs 21%) and offered a significant reduction in per-diagnosis cost (€375.13 in the ELR group and €5184.75 in the control group (P < 0.001). The cost-effectiveness study revealed that the systematic use of ELRs resulted in a cost reduction of €11.30 for each percentage point of increase in diagnostic yield.
Another usual clinical application of these devices is in patients with stroke of unknown etiology. It has been demonstrated that the use of these devices in patients with cryptogenic stroke, compared with the usual strategy of conventional follow-up and 24- or 48-h Holter monitoring, increases the detection rate of silent AF, allowing oral anticoagulation to be started in a greater proportion of patients and at an earlier stage[19,49,75-77].
(2) ILRs: These are small devices that are implanted subcutaneously, usually in the left parasternal region[8,25]. These devices allow for a more prolonged continuous monitoring of up to 3 to 4 years. They offer a single-lead ECG recording. The patient can activate the device when symptoms are experienced using a small remote control or through a smartphone application. Like the external devices, they use automatic arrhythmia detection algorithms[18,33]. The devices available on the market also offer a platform for remote monitoring of the events recorded, sometimes also with data transmission to the patient’s mobile[20,26,32].
They have the disadvantage of being minimally invasive, since the latest models have been made significantly smaller. They require a brief surgical procedure for their implantation. There are complications in a small percentage of patients requiring the device to be withdrawn, ranging from local infection/hematoma to intolerance[78,79]. Another limitation is the price of the device, which is significantly greater than that of ELRs[25,29].
In recent years, there has been abundant literature on the diagnostic yield of implantable recording devices. The greatest experience with this kind of device concerns patients with syncope of unknown etiology[10-12], given the long monitoring time that they offer, with diagnostic yield figures around 35%[9,18,30,70]. There are also numerous papers analyzing the role of ILRs in patients with stroke of unknown etiology, and, as with ELRs, it has been shown that the strategy of implanting an ILR leads to a greater and earlier diagnosis rate than following a conventional strategy[49,80]. They are also used in risk stratification and follow-up of certain patients[13-16,81,82]
In the field of palpitations, their use has been more limited due to their cost and the availability of noninvasive and cheaper alternatives[83]. The recurrent unexplained palpitations study[29] compared an ILR with a conventional strategy, confirming a notably superior diagnostic yield in the ILR group (73% vs 21%). Despite the higher initial cost, the cost per diagnosis in the ILR group was lower than in the conventional strategy group (€3056 ± 363 vs €6768 ± 6672, P = 0.012). However, with the appearance on the market of new ELR devices with good diagnostic yield figures, the use of ILRs is reserved for select cases.
Outpatient telemetry monitoring: These are external monitoring devices similar to Holter monitors or cutaneous patches, but which send a continuous recording via telemetry to a central site where the ECG trace can be reviewed in real-time in the event of the onset of symptoms[38,58]. Subsequently, the information can be sent to medical centers with a greater or lesser degree of urgency[8]. Like the skin patches, their use varies according to the region and they are not available in all countries. The most common indication tends to be to monitor for the presence of AF after ablation procedures or to monitor the presence of significant arrhythmias following cardiac surgery or transcatheter aortic valve implantation[8]. Some records have demonstrated the greater efficacy and efficiency of this method compared with conventional Holter monitors[84].
Intracardiac devices: Despite intracardiac devices (pacemakers, defibrillators or resynchronizers) not being indicated for the purpose of monitoring, it should be mentioned that they are also useful for the work-up of patients with palpitations in cases where patients have one of these devices for another indication, since they offer a recording of the intracavitary electrogram during the episode. Many devices have algorithms that automatically record these events allowing for their subsequent analysis. As such, it is not an ECG recording, but in the case of dual chamber devices, information can be obtained regarding the start of the event and the AV synchrony during tachycardia[85-87].
The aim of this section is to provide some clues to help physicians manage patients with palpitations, focusing on the proper use of electrocardiographic monitoring systems. However, it is mandatory to make a brief reference to other important aspects for the evaluation and management of these patients.
The sensation of palpitations is a nonspecific symptom with multiple causes, which are not only cardiological. As such, it is essential to take an appropriate medical history to guide us towards a given suspected diagnosis, allowing us to choose the most suitable tests.
It is important to ask about the patient’s medical history (systemic diseases, cardiological history, drug-abuse history, family history of sudden death, etc.). Similarly, it is relevant to ask about the characteristics of the palpitations: Are they sustained over time or not, or are they regular (sinus tachycardia, paroxysmal supraventricular tachycardia) or irregular (AF). The triggers (onset at rest or during physical exertion or with stressors) and the presence of certain accompanying characteristics (such as autonomic symptoms, syncope or anginal chest pain) are helpful in identifying at-risk patients requiring admission or more urgent monitoring[1,3].
The physical examination and other easily accessed complementary tests such as electrocardiography can offer specific data but with low sensitivity[1,88]. For example, the presence of a normal ECG does not exclude the presence of causes or arrhythmias, but any pathological findings do greatly increase the likelihood that the cause of the palpitations was cardiological. In up to 27% of patients, the ECG is the key to the diagnosis[88].
Thus, the initial assessment usually includes a detailed clinical history, a focused physical examination, a baseline ECG and usually also a general blood test including thyroid hormones. It is not uncommon in cardiology consultations to also systematically request an echocardiogram to rule out structural heart disease, although this may not be necessary in patients with no other risk factors for heart disease with symptoms highly suggestive of a nonrhythmic origin.
A key point after risk stratification is appropriately selecting the type of monitoring to be used among all the available devices, in order to achieve optimized diagnostic yield and efficiency in the patient in question.
In the field of palpitations, it is essential to correlate the patient’s symptoms with the electrocardiographic recordings to reach an objective diagnosis[2,6]. This point is worthy of special mention, because it is not uncommon for patients with palpitations to experience different sensations that may correspond to different disorders. For example, it is not unusual for patients to have a sensation of a single palpitation lasting a few seconds almost every day, but to also report occasional episodes of sustained rapid palpitations that start and end suddenly. If, using a Holter monitor, we record an atrial extrasystole and relate this to the single palpitation sensation, we cannot rule out the possibility that these extrasystoles trigger episodes of paroxysmal supraventricular tachycardia (which would explain the second, less frequent, symptom). It would be an error to attribute the entirety of the patient’s clinical presentation to the extrasystole, and we should select a monitoring method allowing us to record the less frequent symptom (which is the one suggestive of greater clinical importance). As such, it is essential to take a meticulous history of the symptoms experienced by the patient during monitoring before establishing a certain diagnosis[1,29].
As noted in the previous section, we currently have a wide range of devices at our disposal with significant differentiating characteristics. Various aspects must be considered. The first, and probably the most important, is the frequency with which the symptoms are experienced. The monitoring time must be in line with this frequency. As such, ideally, a 24-h Holter monitor should only be indicated in patients with frequent and almost daily symptoms[22,48]. If the symptoms are monthly or bimonthly, ELRs have shown excellent diagnostic cost-effectiveness and have certain advantages over other devices[2,54], such as obtaining information regarding the start of the episode. In the event of more infrequent symptoms, at present, external PER are probably the device of choice.
Another important factor is device availability. Many of the new devices are not yet offered as a usual diagnostic tool at healthcare centers or are not covered by insurers. If the patient cannot fund the device, we must choose from among those we have available.
Patient comfort, cost, the accuracy of the automatic algorithms, the possibility of carrying out telemetry monitoring, and the need or otherwise to be able to record the start of the episode are other factors that may influence our decision[8].
Finally, it should be pointed out that, within a specific group of devices, it is important to ensure that the model selected has suitable technical characteristics providing a good quality recording.
Although it is not the reason for this review, it should be mentioned that electrophysiological study (EPS) is an important diagnostic tool in managing patients with palpitations[1,89,90]. In addition to allowing for a precise diagnosis of certain arrhythmias (such as paroxysmal supraventricular tachycardias), it is possible to treat the arrhythmia with ablation during the procedure in those cases where it is indicated[91-93]. It also allows to evaluate other causes of syncope if present[9,10,94,95], and performing risk stratification in patients with structural heart disease[14,71]. Since it is an invasive test, it tends to be considered at the end of the diagnostic process, either in patients with a high probability of significant arrhythmia when the monitoring methods have not allowed for it to be documented, or in patients where, after documenting the clinical arrhythmia, ablation treatment is planned. Nonetheless, it may be indicated at an early stage in patients with recurrent palpitations whose clinical characteristics are highly suggestive of paroxysmal supraventricular tachycardia and who, as such, can benefit from ablation[1,88,89,96].
The diagnostic yield of electrophysiology study is greater in patients with structural heart disease and in those with clinical symptoms highly suggestive of paroxysmal supraventricular tachycardia. For example, in a study carried out by Valles et al[90] on patients with sustained palpitations that did not appear on monitoring, the diagnostic yield was 50%. Other papers have reported yields between 40% and 66%[1,89].
In any case, in our opinion, prescribing an electrophysiology study should not preclude the need for electrocardiographic monitoring during the waiting time, be it via telemetry monitoring systems if the patient is admitted, or with an external device if not.
Exercise-stress testing can be useful and should be considered from the outset when patients report palpitations on exertion[1,97]. Magnetic resonance imaging, coronary computed tomography, specific hormonal studies, etc. should be tailored to the patient in line with clinical suspicion and will be necessary in a minority of cases.
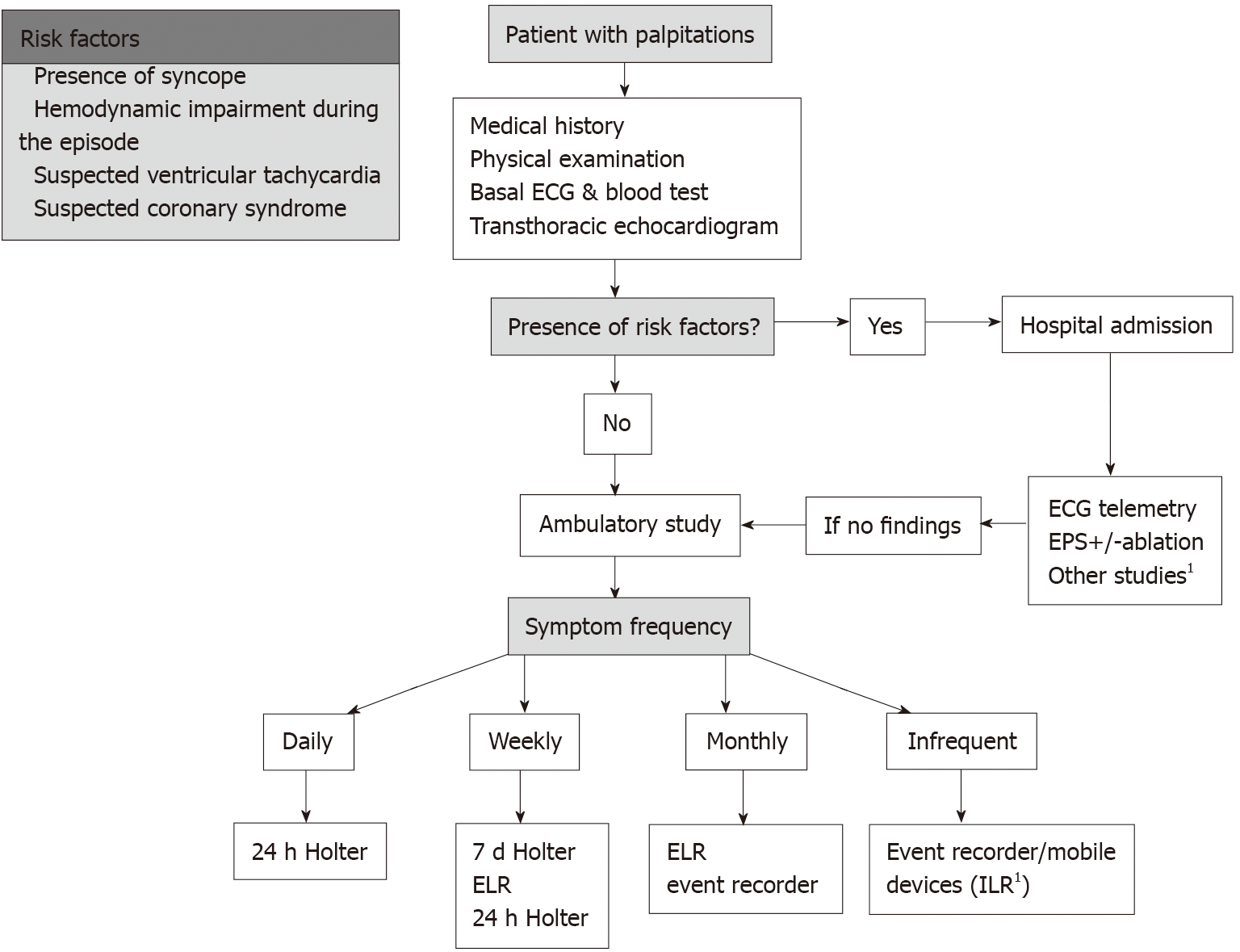
Figure 4 summarizes the proposed general algorithm for the management of patients with palpitations of unknown etiology. This algorithm offers an overview of the issue, although it can and should be adapted in specific cases and according to the availability of tests at the center.
The first step in all patients is to perform an initial clinical assessment and stratify the risk (as stated in section Clinical evaluation and risk stratification). In those patients with high-risk criteria and/or priority for EPS, it is necessary to prioritize tests and indicate the study in most cases. During the waiting period, a monitoring system should be maintained (telemetry if the patient is admitted or an ELR if it is an outpatient), because this may provide the diagnosis faster and obtain relevant information for the rest of the work-up.
In cases without risk factors (which form the majority of cases), and without a diagnosis, it is necessary to select a monitoring system, following the indications in section Selection of monitoring type.
Patients without heart disease and with sporadic symptoms not suggestive of an arrhythmic origin may not require further cardiological tests. However, it is not uncommon for the clinical symptoms to continue to generate a high degree of anxiety, hence in some cases we may consider monitoring to ensure there are no arrhythmias, to reassure the patient and to avoid other futile investigations and consultations.
Technological evolution shows no signs of slowing down and it is likely that monitoring devices will continue to be developed at high speed. We expect more widespread use of wearable devices, which incorporate sensors for other vital signs, to open the doors to other indications for monitoring. With regard to electrocardiographic monitoring specifically, we expect further work to be done to improve the current limitations. On the one hand, developing reliable devices that allow for quality external, comfortable, and long-lasting monitoring. On the other hand, the current recorders continue to produce a high number of artifacts and nonsignificant disorders, hence the development of software with algorithms to improve this area will be of clinical utility. The incorporation of artificial intelligence technology allowing for the prediction of future events is another of the most pioneering lines of research[98-102].
Without a doubt, technological development will help us improve the diagnosis and follow-up of patients with palpitations and other cardiac conditions. However, we must be cautious about the increasingly frequent nonmedical use of these devices. We healthcare professionals are faced with the challenge of how to manage, interpret and integrate into the healthcare system all the information that these new devices are providing.
Electrocardiographic cardiac monitoring devices are a useful diagnostic tool in confirming or excluding arrhythmias in patients with palpitations. In recent years, electrocardiographic monitoring systems have incorporated many technical improvements and many new devices are now available on the market. To achieve the best diagnostic yield and efficiency, a key point is to properly select the type of monitoring to be used among all available devices. This review provides an update on the different monitoring methods currently available, highlighting their indications and limitations, to help healthcare professionals to appropriately select and use them in the work-up of patients with palpitations.
The authors would like to thank Mr. S. Venegas for his help with the illustrations and graphics.
Provenance and peer review: Invited article; Externally peer reviewed
Corresponding Author's Membership in Professional Societies: European Society of Cardiology; European Heart Rhythm Asociation; Sociedad Española de Cardiologia; Societat Catalana de Cardiologia.
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: Spain
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Li X, Li A S-Editor: Fan JR L-Editor: Kerr C P-Editor: Fan JR
| 1. | Raviele A, Giada F, Bergfeldt L, Blanc JJ, Blomstrom-Lundqvist C, Mont L, Morgan JM, Raatikainen MJ, Steinbeck G, Viskin S, Kirchhof P, Braunschweig F, Borggrefe M, Hocini M, Della Bella P, Shah DC; European Heart Rhythm Association. Management of patients with palpitations: a position paper from the European Heart Rhythm Association. Europace. 2011;13:920-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 2. | Francisco-Pascual J, Santos-Ortega A, Roca-Luque I, Rivas-Gándara N, Pérez-Rodón J, Milà-Pascual L, García-Dorado D, Moya-Mitjans À. Diagnostic Yield and Economic Assessment of a Diagnostic Protocol With Systematic Use of an External Loop Recorder for Patients With Palpitations. Rev Esp Cardiol (Engl Ed). 2019;72:473-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 3. | Weinstock C, Wagner H, Snuckel M, Katz M. Evidence-Based Approach to Palpitations. Med Clin North Am. 2021;105:93-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Weber BE, Kapoor WN. Evaluation and outcomes of patients with palpitations. Am J Med. 1996;100:138-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 99] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 5. | Hoefman E, Boer KR, van Weert HC, Reitsma JB, Koster RW, Bindels PJ. Predictive value of history taking and physical examination in diagnosing arrhythmias in general practice. Fam Pract. 2007;24:636-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Hoefman E, Bindels PJ, van Weert HC. Efficacy of diagnostic tools for detecting cardiac arrhythmias: systematic literature search. Neth Heart J. 2010;18:543-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93:1043-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9482] [Cited by in RCA: 9032] [Article Influence: 311.4] [Reference Citation Analysis (0)] |
| 8. | Steinberg JS, Varma N, Cygankiewicz I, Aziz P, Balsam P, Baranchuk A, Cantillon DJ, Dilaveris P, Dubner SJ, El-Sherif N, Krol J, Kurpesa M, La Rovere MT, Lobodzinski SS, Locati ET, Mittal S, Olshansky B, Piotrowicz E, Saxon L, Stone PH, Tereshchenko L, Turitto G, Wimmer NJ, Verrier RL, Zareba W, Piotrowicz R. 2017 ISHNE-HRS expert consensus statement on ambulatory ECG and external cardiac monitoring/telemetry. Heart Rhythm. 2017;14:e55-e96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 156] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 9. | Moya A, Rivas-Gandara N, Perez-Rodón J, Franciso-Pascual J, Santos-Ortega A, Fumero P, Roca-Luque I. Syncope and bundle branch block : Diagnostic approach. Herzschrittmacherther Elektrophysiol. 2018;29:161-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Brignole M, Moya A, de Lange FJ, Deharo JC, Elliott PM, Fanciulli A, Fedorowski A, Furlan R, Kenny RA, Martín A, Probst V, Reed MJ, Rice CP, Sutton R, Ungar A, van Dijk JG; ESC Scientific Document Group. 2018 ESC Guidelines for the diagnosis and management of syncope. Eur Heart J. 2018;39:1883-1948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1294] [Cited by in RCA: 1140] [Article Influence: 162.9] [Reference Citation Analysis (0)] |
| 11. | Francisco-Pascual J, Rodenas E, Belahnech Y, Rivas-Gándara N, Pérez-Rodon J, Santos-Ortega A, Benito B, Roca-Luque I, Cossio-Gil Y, Serra Garcia V, Llerena-Butron S, Rodríguez-García J, Moya-Mitjans A, García-Dorado D, Ferreira-González I. Syncope in Patients With Severe Aortic Stenosis: More Than Just an Obstruction Issue. Can J Cardiol. 2021;37:284-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (1)] |
| 12. | Roca-Luque I, Francisco-Pascual J, Oristrell G, Rodríguez-García J, Santos-Ortega A, Martin-Sanchez G, Rivas-Gandara N, Perez-Rodon J, Ferreira-Gonzalez I, García-Dorado D, Moya-Mitjans A. Syncope, conduction disturbance, and negative electrophysiological test: Predictive factors and risk score to predict pacemaker implantation during follow-up. Heart Rhythm. 2019;16:905-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (1)] |
| 13. | Rodés-Cabau J, Urena M, Nombela-Franco L, Amat-Santos I, Kleiman N, Munoz-Garcia A, Atienza F, Serra V, Deyell MW, Veiga-Fernandez G, Masson JB, Canadas-Godoy V, Himbert D, Castrodeza J, Elizaga J, Francisco Pascual J, Webb JG, de la Torre JM, Asmarats L, Pelletier-Beaumont E, Philippon F. Arrhythmic Burden as Determined by Ambulatory Continuous Cardiac Monitoring in Patients With New-Onset Persistent Left Bundle Branch Block Following Transcatheter Aortic Valve Replacement: The MARE Study. JACC Cardiovasc Interv. 2018;11:1495-1505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 121] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 14. | Rivas-Gándara N, Francisco-Pascual J, Pijuan-Domenech A, Ribera-Solé A, Dos-Subirá L, Benito B, Terricabras M, Pérez-Rodon J, Subirana MT, Santos-Ortega A, Roses-Noguer F, Miranda B, Moya-Mitjans À, Ferreira-González I. Risk stratification of ventricular arrhythmias in repaired tetralogy of Fallot. Rev Esp Cardiol (Engl Ed). 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Nielsen JC, Lin YJ, de Oliveira Figueiredo MJ, Sepehri Shamloo A, Alfie A, Boveda S, Dagres N, Di Toro D, Eckhardt LL, Ellenbogen K, Hardy C, Ikeda T, Jaswal A, Kaufman E, Krahn A, Kusano K, Kutyifa V, S Lim H, Lip GYH, Nava-Townsend S, Pak HN, Rodríguez Diez G, Sauer W, Saxena A, Svendsen JH, Vanegas D, Vaseghi M, Wilde A, Bunch TJ, Buxton AE, Calvimontes G, Chao TF, Eckardt L, Estner H, Gillis AM, Isa R, Kautzner J, Maury P, Moss JD, Nam GB, Olshansky B, Molano LFP, Pimentel M, Prabhu M, Tzou WS, Sommer P, Swampillai J, Vidal A, Deneke T, Hindricks G, Leclercq C. European Heart Rhythm Association (EHRA)/Heart Rhythm Society (HRS)/Asia Pacific Heart Rhythm Society (APHRS)/Latin American Heart Rhythm Society (LAHRS) expert consensus on risk assessment in cardiac arrhythmias: use the right tool for the right outcome, in the right population. J Arrhythm. 2020;36:553-607. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 16. | Palà E, Pagola J, Juega J, Francisco-Pascual J, Bustamante A, Penalba A, Comas I, Rodriguez M, De Lera Alfonso M, Arenillas JF, de Torres R, Pérez-Sánchez S, Cabezas JA, Moniche F, González-Alujas T, Molina CA, Montaner J. B-type natriuretic peptide over N-terminal pro-brain natriuretic peptide to predict incident atrial fibrillation after cryptogenic stroke. Eur J Neurol. 2021;28:540-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 17. | Galli A, Ambrosini F, Lombardi F. Holter Monitoring and Loop Recorders: From Research to Clinical Practice. Arrhythm Electrophysiol Rev. 2016;5:136-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 18. | Francisco-Pascual J, Olivella San Emeterio A, Rivas-Gándara N, Pérez-Rodón J, Benito B, Santos-Ortega A, Moya-Mitjans À, Rodríguez García J, Llerena Butrón SI, Cantalapiedra Romero J, Ferreira González I. High incidence of subclinical atrial fibrillation in patients with syncope monitored with implantable cardiac monitor. Int J Cardiol. 2020;316:110-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Pagola J, Juega J, Francisco-Pascual J, Moya A, Sanchis M, Bustamante A, Penalba A, Usero M, Cortijo E, Arenillas JF, Calleja AI, Sandin-Fuentes M, Rubio J, Mancha F, Escudero-Martinez I, Moniche F, de Torres R, Pérez-Sánchez S, González-Matos CE, Vega Á, Pedrote AA, Arana-Rueda E, Montaner J, Molina CA; CryptoAF investigators. Yield of atrial fibrillation detection with Textile Wearable Holter from the acute phase of stroke: Pilot study of Crypto-AF registry. Int J Cardiol. 2018;251:45-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 20. | Francisco-Pascual J, Rivas-Gándara N, Santos-Ortega A, Pérez-Rodón J, Benito B, Belahnech Y, Ferreira-González I. [Cardiac biometric variables and arrhythmic events during COVID-19 pandemic lockdown in patients with an implantable cardiac monitor for syncope work-up]. Med Clin (Barc). 2021;156:496-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Muntané-Carol G, Urena M, Nombela-Franco L, Amat-Santos I, Kleiman N, Munoz-Garcia A, Atienza F, Serra V, Deyell MW, Veiga-Fernandez G, Masson JB, Canadas-Godoy V, Himbert D, Castrodeza J, Elizaga J, Francisco Pascual J, Webb JG, de la Torre Hernandez JM, Asmarats L, Pelletier-Beaumont E, Philippon F, Rodés-Cabau J. Arrhythmic burden in patients with new-onset persistent left bundle branch block after transcatheter aortic valve replacement: 2-year results of the MARE study. Europace. 2021;23:254-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Sulfi S, Balami D, Sekhri N, Suliman A, Kapur A, Archbold RA, Ranjadayalan K, Timmis AD. Limited clinical utility of Holter monitoring in patients with palpitations or altered consciousness: analysis of 8973 recordings in 7394 patients. Ann Noninvasive Electrocardiol. 2008;13:39-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Mittal S, Movsowitz C, Steinberg JS. Ambulatory external electrocardiographic monitoring: focus on atrial fibrillation. J Am Coll Cardiol. 2011;58:1741-1749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 24. | Sana F, Isselbacher EM, Singh JP, Heist EK, Pathik B, Armoundas AA. Wearable Devices for Ambulatory Cardiac Monitoring: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;75:1582-1592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 161] [Article Influence: 32.2] [Reference Citation Analysis (1)] |
| 25. | Task Force members. , Brignole M, Vardas P, Hoffman E, Huikuri H, Moya A, Ricci R, Sulke N, Wieling W; EHRA Scientific Documents Committee, Auricchio A, Lip GY, Almendral J, Kirchhof P, Aliot E, Gasparini M, Braunschweig F; Document Reviewers, Lip GY, Almendral J, Kirchhof P, Botto GL; EHRA Scientific Documents Committee. Indications for the use of diagnostic implantable and external ECG loop recorders. Europace. 2009;11:671-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 229] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 26. | Kohno R, Nantsupawat T, Benditt DG. Trends in Subcutaneous Cardiac Monitoring Technology. J Innov Card Rhythm Manag. 2018;9:3247-3255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Zimetbaum P, Goldman A. Ambulatory arrhythmia monitoring: choosing the right device. Circulation. 2010;122:1629-1636. [PubMed] [DOI] [Full Text] |
| 28. | de Asmundis C, Conte G, Sieira J, Chierchia GB, Rodriguez-Manero M, Di Giovanni G, Ciconte G, Levinstein M, Baltogiannis G, Saitoh Y, Casado-Arroyo R, Brugada P. Comparison of the patient-activated event recording system vs. traditional 24 h Holter electrocardiography in individuals with paroxysmal palpitations or dizziness. Europace. 2014;16:1231-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 29. | Giada F, Gulizia M, Francese M, Croci F, Santangelo L, Santomauro M, Occhetta E, Menozzi C, Raviele A. Recurrent unexplained palpitations (RUP) study comparison of implantable loop recorder versus conventional diagnostic strategy. J Am Coll Cardiol. 2007;49:1951-1956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 102] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 30. | Fogel RI, Evans JJ, Prystowsky EN. Utility and cost of event recorders in the diagnosis of palpitations, presyncope, and syncope. Am J Cardiol. 1997;79:207-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 47] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 31. | Mason JW, Hancock EW, Gettes LS; American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; American College of Cardiology Foundation; Heart Rhythm Society, Bailey JJ, Childers R, Deal BJ, Josephson M, Kligfield P, Kors JA, Macfarlane P, Pahlm O, Mirvis DM, Okin P, Rautaharju P, Surawicz B, van Herpen G, Wagner GS, Wellens H. Recommendations for the standardization and interpretation of the electrocardiogram: part II: Electrocardiography diagnostic statement list: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society: endorsed by the International Society for Computerized Electrocardiology. Circulation. 2007;115:1325-1332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 32. | Moya Mitjans A, Francisco Pascual J, Pérez Rodón J, Rivas Gándara N, Garcia-Dorado D. Nuevos avances en la monitorización electrocardiográfica prolongada: Reveal LINQ TM. [cited 10 April 2021]. Available from: https://www.researchgate.net/publication/281176697_Nuevos_avances_en_la_monitorizacion_electrocardiografica_prolongada_Reveal_LINQ_TM. |
| 33. | Sanders P, Pürerfellner H, Pokushalov E, Sarkar S, Di Bacco M, Maus B, Dekker LR; Reveal LINQ Usability Investigators. Performance of a new atrial fibrillation detection algorithm in a miniaturized insertable cardiac monitor: Results from the Reveal LINQ Usability Study. Heart Rhythm. 2016;13:1425-1430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 127] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 34. | Balsam P, Lodziński P, Tymińska A, Ozierański K, Januszkiewicz Ł, Główczyńska R, Wesołowska K, Peller M, Pietrzak R, Książczyk T, Borodzicz S, Kołtowski Ł, Borkowski M, Werner B, Opolski G, Grabowski M. Study design and rationale for biomedical shirt-based electrocardiography monitoring in relevant clinical situations: ECG-shirt study. Cardiol J. 2018;25:52-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 35. | Steinberg C, Philippon F, Sanchez M, Fortier-Poisson P, O'Hara G, Molin F, Sarrazin JF, Nault I, Blier L, Roy K, Plourde B, Champagne J. A Novel Wearable Device for Continuous Ambulatory ECG Recording: Proof of Concept and Assessment of Signal Quality. Biosensors (Basel). 2019;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 36. | Welinder A, Wagner GS, Maynard C, Pahlm O. Differences in QRS axis measurements, classification of inferior myocardial infarction, and noise tolerance for 12-lead electrocardiograms acquired from monitoring electrode positions compared to standard locations. Am J Cardiol. 2010;106:581-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 37. | Brockway R, Brockway M, Brockway B, Hamlin R. Comparison of one- and three-lead ECG to measure cardiac intervals and differentiate drug-induced multi-channel block. J Pharmacol Toxicol Methods. 2018;93:80-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 38. | Engel JM, Chakravarthy N, Katra RP, Mazar S, Libbus I, Chavan A. Estimation of patient compliance in application of adherent mobile cardiac telemetry device. Annu Int Conf IEEE Eng Med Biol Soc. 2011;2011:1536-1539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 39. | Heilbron EL. Advances in modern electrocardiographic equipment for long-term ambulatory monitoring. Card Electrophysiol Rev. 2002;6:185-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 40. | Perez MV, Mahaffey KW, Hedlin H, Rumsfeld JS, Garcia A, Ferris T, Balasubramanian V, Russo AM, Rajmane A, Cheung L, Hung G, Lee J, Kowey P, Talati N, Nag D, Gummidipundi SE, Beatty A, Hills MT, Desai S, Granger CB, Desai M, Turakhia MP; Apple Heart Study Investigators. Large-Scale Assessment of a Smartwatch to Identify Atrial Fibrillation. N Engl J Med. 2019;381:1909-1917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 810] [Cited by in RCA: 1107] [Article Influence: 184.5] [Reference Citation Analysis (0)] |
| 41. | Wegner FK, Kochhäuser S, Ellermann C, Lange PS, Frommeyer G, Leitz P, Eckardt L, Dechering DG. Prospective blinded Evaluation of the smartphone-based AliveCor Kardia ECG monitor for Atrial Fibrillation detection: The PEAK-AF study. Eur J Intern Med. 2020;73:72-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 42. | Kinlay S, Leitch JW, Neil A, Chapman BL, Hardy DB, Fletcher PJ. Cardiac event recorders yield more diagnoses and are more cost-effective than 48-hour Holter monitoring in patients with palpitations. A controlled clinical trial. Ann Intern Med. 1996;124:16-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 129] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 43. | Attanasio P, Huemer M, Loehr L, Parwani AS, Boldt LH, Haverkamp W, Wutzler A. Use of a Patient-Activated Event Recording System in Patients with Tachycardic Palpitations: How Long to Follow Up? Ann Noninvasive Electrocardiol. 2015;20:566-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 44. | Rischard J, Waldmann V, Moulin T, Sharifzadehgan A, Lee R, Narayanan K, Garcia R, Marijon E. Assessment of Heart Rhythm Disorders Using the AliveCor Heart Monitor: Beyond the Detection of Atrial Fibrillation. JACC Clin Electrophysiol. 2020;6:1313-1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 45. | Beltrame T, Amelard R, Wong A, Hughson RL. Extracting aerobic system dynamics during unsupervised activities of daily living using wearable sensor machine learning models. J Appl Physiol (1985). 2018;124:473-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 46. | Hu Y, Kim EG, Cao G, Liu S, Xu Y. Physiological acoustic sensing based on accelerometers: a survey for mobile healthcare. Ann Biomed Eng. 2014;42:2264-2277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 47. | Palma Gámiz JL, Arribas Jiménez A, González Juanatey JR, Marín Huerta E, Martín-Ambrosio ES. [Spanish Society of Cardiology practice guidelines on ambulatory monitoring of electrocardiogram and blood pressure]. Rev Esp Cardiol. 2000;53:91-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 48. | Su L, Borov S, Zrenner B. 12-lead Holter electrocardiography. Review of the literature and clinical application update. Herzschrittmacherther Elektrophysiol. 2013;24:92-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 49. | PérezRodon J, FranciscoPascual J, RivasGándara N, RocaLuque I, Bellera N, MoyaMitjans À. Cryptogenic Stroke And Role Of Loop Recorder. J Atr Fibrillation. 2014;7:1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 50. | Stojkovic S, Ristl R, Moser FT, Wolzt M, Wojta J, Schmidinger H, Pezawas T. T-wave variability for the prediction of fast ventricular arrhythmias – prospective, observer-blind study. Circ J. 2015;79:318-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 51. | Follansbee CW, Beerman L, Arora G. Automated QT analysis on Holter monitors in pediatric patients can differentiate long QT syndrome from controls. Pacing Clin Electrophysiol. 2018;41:50-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 52. | Casas G, Rivas-Gándara N, Francisco-Pascual J, Moya-Mitjans À, García-Dorado D. Postural Orthostatic Tachycardia Syndrome and Vasospastic Angina: Therapeutic Approach to a Previously Unreported Association. Rev Esp Cardiol (Engl Ed). 2019;72:509-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 53. | Roca-Luque I, Rivas-Gándara N, Dos Subirà L, Francisco Pascual J, Pijuan-Domenech A, Pérez-Rodon J, Subirana-Domenech MT, Santos-Ortega A, Rosés-Noguer F, Miranda-Barrio B, Ferreira-Gonzalez I, Casaldàliga Ferrer J, García-Dorado García D, Moya Mitjans A. Long-Term Follow-Up After Ablation of Intra-Atrial Re-Entrant Tachycardia in Patients With Congenital Heart Disease: Types and Predictors of Recurrence. JACC Clin Electrophysiol. 2018;4:771-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 54. | Locati ET, Moya A, Oliveira M, Tanner H, Willems R, Lunati M, Brignole M. External prolonged electrocardiogram monitoring in unexplained syncope and palpitations: results of the SYNARR-Flash study. Europace. 2016;18:1265-1272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 55. | Lobodzinski SS. ECG patch monitors for assessment of cardiac rhythm abnormalities. Prog Cardiovasc Dis. 2013;56:224-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 56. | Yenikomshian M, Jarvis J, Patton C, Yee C, Mortimer R, Birnbaum H, Topash M. Cardiac arrhythmia detection outcomes among patients monitored with the Zio patch system: a systematic literature review. Curr Med Res Opin. 2019;35:1659-1670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 57. | Murali S, Brugger N, Rincon F, Mashru M, Cook S, Goy JJ. Cardiac Ambulatory Monitoring: New Wireless Device Validated Against Conventional Holter Monitoring in a Case Series. Front Cardiovasc Med. 2020;7:587945. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 58. | Engel JM, Mehta V, Fogoros R, Chavan A. Study of arrhythmia prevalence in NUVANT Mobile Cardiac Telemetry system patients. Annu Int Conf IEEE Eng Med Biol Soc. 2012;2012:2440-2443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 59. | Barrett PM, Komatireddy R, Haaser S, Topol S, Sheard J, Encinas J, Fought AJ, Topol EJ. Comparison of 24-hour Holter monitoring with 14-day novel adhesive patch electrocardiographic monitoring. Am J Med. 2014;127:95.e11-95.e17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 311] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 60. | Soroudi A, Hernández N, Berglin L, Nierstrasz V. Electrode placement in electrocardiography smart garments: A review. J Electrocardiol. 2019;57:27-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 61. | Cho H, Lee JH. A Study on the Optimal Positions of ECG Electrodes in a Garment for the Design of ECG-Monitoring Clothing for Male. J Med Syst. 2015;39:95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 62. | Myerburg RJ. The Screening ECG and Cardiac Risks. JAMA. 2018;319:2277-2279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 63. | Hall A, Mitchell ARJ, Wood L, Holland C. Effectiveness of a single lead AliveCor electrocardiogram application for the screening of atrial fibrillation: A systematic review. Medicine (Baltimore). 2020;99:e21388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 64. | Bumgarner JM, Lambert CT, Hussein AA, Cantillon DJ, Baranowski B, Wolski K, Lindsay BD, Wazni OM, Tarakji KG. Smartwatch Algorithm for Automated Detection of Atrial Fibrillation. J Am Coll Cardiol. 2018;71:2381-2388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 290] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 65. | Cobos Gil MÁ. Standard and Precordial Leads Obtained With an Apple Watch. Ann Intern Med. 2020;173:249-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 66. | Samol A, Bischof K, Luani B, Pascut D, Wiemer M, Kaese S. Recording of Bipolar Multichannel ECGs by a Smartwatch: Modern ECG Diagnostic 100 Years after Einthoven. Sensors (Basel). 2019;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 67. | Spaccarotella CAM, Polimeni A, Migliarino S, Principe E, Curcio A, Mongiardo A, Sorrentino S, De Rosa S, Indolfi C. Multichannel Electrocardiograms Obtained by a Smartwatch for the Diagnosis of ST-Segment Changes. JAMA Cardiol. 2020;5:1176-1180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 80] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 68. | Kwon S, Lee D, Kim J, Lee Y, Kang S, Seo S, Park K. Sinabro: A Smartphone-Integrated Opportunistic Electrocardiogram Monitoring System. Sensors (Basel). 2016;16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 69. | Christiansen CS, Gjesdal K. [Implantable loop recorders in evaluation of syncope]. Tidsskr Nor Laegeforen. 2007;127:1657-1659. [PubMed] |
| 70. | Solano A, Menozzi C, Maggi R, Donateo P, Bottoni N, Lolli G, Tomasi C, Croci F, Oddone D, Puggioni E, Brignole M. Incidence, diagnostic yield and safety of the implantable loop-recorder to detect the mechanism of syncope in patients with and without structural heart disease. Eur Heart J. 2004;25:1116-1119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 64] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 71. | Francisco-Pascual J, Rodenas-Alesina E, Rivas-Gándara N, Belahnech Y, Olivella San Emeterio A, Pérez-Rodón J, Benito B, Santos-Ortega A, Moya-Mitjans À, Casas G, Cantalapiedra-Romero J, Maldonado J, Ferreira-González I. Etiology and prognosis of patients with unexplained syncope and mid-range left ventricular dysfunction. Heart Rhythm. 2021;18:597-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 72. | Rajanna RR, Natarajan S, Prakash V, Vittala PR, Arun U, Sahoo S. External Cardiac Loop Recorders: Functionalities, Diagnostic Efficacy, Challenges and Opportunities. IEEE Rev Biomed Eng. 2021;PP. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 73. | Zimetbaum PJ, Kim KY, Josephson ME, Goldberger AL, Cohen DJ. Diagnostic yield and optimal duration of continuous-loop event monitoring for the diagnosis of palpitations. A cost-effectiveness analysis. Ann Intern Med. 1998;128:890-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 71] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 74. | Schuchert A, Maas R, Kretzschmar C, Behrens G, Kratzmann I, Meinertz T. Diagnostic yield of external electrocardiographic loop recorders in patients with recurrent syncope and negative tilt table test. Pacing Clin Electrophysiol. 2003;26:1837-1840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 75. | Gladstone DJ, Spring M, Dorian P, Panzov V, Thorpe KE, Hall J, Vaid H, O'Donnell M, Laupacis A, Côté R, Sharma M, Blakely JA, Shuaib A, Hachinski V, Coutts SB, Sahlas DJ, Teal P, Yip S, Spence JD, Buck B, Verreault S, Casaubon LK, Penn A, Selchen D, Jin A, Howse D, Mehdiratta M, Boyle K, Aviv R, Kapral MK, Mamdani M; EMBRACE Investigators and Coordinators. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med. 2014;370:2467-2477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 870] [Cited by in RCA: 935] [Article Influence: 85.0] [Reference Citation Analysis (0)] |
| 76. | Pagola J, Juega J, Francisco-Pascual J, Bustamante A, Penalba A, Pala E, Rodriguez M, De Lera-Alfonso M, Arenillas JF, Cabezas JA, Moniche F, de Torres R, Montaner J, González-Alujas T, Alvarez-Sabin J, Molina CA; Crypto-AF study group. Predicting Atrial Fibrillation with High Risk of Embolization with Atrial Strain and NT-proBNP. Transl Stroke Res. 2021;12:735-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 77. | Pagola J, Juega J, Francisco-Pascual J, Bustamante A, Penalba A, Pala E, Rodriguez M, De Lera Alfonso M, Arenillas JF, Cabezas JA, Moniche F, de Torres R, Montaner J, González-Alujas T, Alvarez-Sabin J, Molina CA; Crypto-AF study group. Large vessel occlusion is independently associated with atrial fibrillation detection. Eur J Neurol. 2020;27:1618-1624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 78. | Wong GR, Lau DH, Middeldorp ME, Harrington JA, Stolcman S, Wilson L, Twomey DJ, Kumar S, Munawar DA, Khokhar KB, Mahajan R, Sanders P. Feasibility and safety of Reveal LINQ insertion in a sterile procedure room versus electrophysiology laboratory. Int J Cardiol. 2016;223:13-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 79. | Diederichsen SZ, Haugan KJ, Højberg S, Holst AG, Køber L, Pedersen KB, Graff C, Krieger D, Brandes A, Svendsen JH. Complications after implantation of a new-generation insertable cardiac monitor: Results from the LOOP study. Int J Cardiol. 2017;241:229-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 80. | Sanna T, Diener HC, Passman RS, Di Lazzaro V, Bernstein RA, Morillo CA, Rymer MM, Thijs V, Rogers T, Beckers F, Lindborg K, Brachmann J; CRYSTAL AF Investigators. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370:2478-2486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1385] [Cited by in RCA: 1539] [Article Influence: 139.9] [Reference Citation Analysis (0)] |
| 81. | Muntané-Carol G, Nombela-Franco L, Serra V, Urena M, Amat-Santos I, Vilalta V, Chamandi C, Lhermusier T, Veiga-Fernandez G, Kleiman N, Canadas-Godoy V, Francisco-Pascual J, Himbert D, Castrodeza J, Fernandez-Nofrerias E, Baudinaud P, Mondoly P, Campelo-Parada F, De la Torre Hernandez JM, Pelletier-Beaumont E, Philippon F, Rodés-Cabau J. Late arrhythmias in patients with new-onset persistent left bundle branch block after transcatheter aortic valve replacement using a balloon-expandable valve. Heart Rhythm. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 82. | Dwivedi A, Joza J, Malkani K, Mendelson TB, Priori SG, Chinitz LA, Fowler SJ, Cerrone M. Implantable Loop Recorder in Inherited Arrhythmia Diseases: A Critical Tool for Symptom Diagnosis and Advanced Risk Stratification. JACC Clin Electrophysiol. 2018;4:1372-1374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 83. | Padmanabhan D, Kancharla K, El-Harasis MA, Isath A, Makkar N, Noseworthy PA, Friedman PA, Cha YM, Kapa S. Diagnostic and therapeutic value of implantable loop recorder: A tertiary care center experience. Pacing Clin Electrophysiol. 2019;42:38-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 84. | Tsang JP, Mohan S. Benefits of monitoring patients with mobile cardiac telemetry (MCT) compared with the Event or Holter monitors. Med Devices (Auckl). 2013;7:1-5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 85. | Healey JS, Connolly SJ, Gold MR, Israel CW, Van Gelder IC, Capucci A, Lau CP, Fain E, Yang S, Bailleul C, Morillo CA, Carlson M, Themeles E, Kaufman ES, Hohnloser SH; ASSERT Investigators. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med. 2012;366:120-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1411] [Cited by in RCA: 1584] [Article Influence: 121.8] [Reference Citation Analysis (0)] |
| 86. | Stevenson WG, John RM. Ventricular arrhythmias in patients with implanted defibrillators. Circulation. 2011;124:e411-e414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 87. | Quirino G, Giammaria M, Corbucci G, Pistelli P, Turri E, Mazza A, Perucca A, Checchinato C, Dalmasso M, Barold SS. Diagnosis of paroxysmal atrial fibrillation in patients with implanted pacemakers: relationship to symptoms and other variables. Pacing Clin Electrophysiol. 2009;32:91-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 88. | Kopp DE, Wilber DJ. Palpitations and arrhythmias. Separating the benign from the dangerous. Postgrad Med. 1992;91:241-244, 247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 89. | Tsiachris D, Koutagiar I, Gatzoulis KA, Arsenos P, Rigatou A, Dilaveris P, Sideris S, Papadopoulos A, Kritikos A, Stefanadis C, Tousoulis D. Diagnosis and management of phantom tachycardias based on an electrophysiologically guided approach. Hellenic J Cardiol. 2016;57:340-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 90. | Valles E, Martí-Almor J, Bazan V, Suarez F, Cian D, Portillo L, Bruguera-Cortada J. Diagnostic and prognostic value of electrophysiologic study in patients with nondocumented palpitations. Am J Cardiol. 2011;107:1333-1337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 91. | Roca-Luque I, Rivas-Gándara N, Dos-Subirà L, Francisco-Pascual J, Pijuan-Domenech A, Pérez-Rodon J, Santos-Ortega A, Roses-Noguer F, Ferreira-Gonzalez I, García-Dorado García D, Moya Mitjans A. Predictors of Acute Failure Ablation of Intra-atrial Re-entrant Tachycardia in Patients With Congenital Heart Disease: Cardiac Disease, Atypical Flutter, and Previous Atrial Fibrillation. J Am Heart Assoc. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 92. | Pérez-Rodon J, Rodriguez-García J, Sarrias-Merce A, Rivas-Gandara N, Roca-Luque I, Francisco-Pascual J, Santos-Ortega A, Martín-Sánchez G, Ferreira-González I, Rodríguez-Palomares J, Evangelista-Masip A, García-Dorado D, Moya-Mitjans À. Predictors of acute inefficacy and the radiofrequency energy time required for cavotricuspid isthmus-dependent atrial flutter ablation. J Interv Card Electrophysiol. 2017;49:83-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 93. | Roca-Luque I, Rivas N, Francisco J, Perez J, Acosta G, Oristrell G, Terricabres M, Garcia-Dorado D, Moya A. Selective Angiography Using the Radiofrequency Catheter: An Alternative Technique for Mapping and Ablation in the Aortic Cusps. J Cardiovasc Electrophysiol. 2017;28:126-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 94. | Roca-Luque I, Francisco-Pasqual J, Oristrell G, Rodríguez-García J, Santos-Ortega A, Martin-Sanchez G, Rivas-Gandara N, Perez-Rodon J, Ferreira-Gonzalez I, García-Dorado D, Moya-Mitjans A. Flecainide Versus Procainamide in Electrophysiological Study in Patients With Syncope and Wide QRS Duration. JACC Clin Electrophysiol. 2019;5:212-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 95. | Roca-Luque I, Oristrell G, Francisco-Pasqual J, Rodríguez-García J, Santos-Ortega A, Martin-Sanchez G, Rivas-Gandara N, Perez-Rodon J, Ferreira-Gonzalez I, García-Dorado D, Moya-Mitjans A. Predictors of positive electrophysiological study in patients with syncope and bundle branch block: PR interval and type of conduction disturbance. Clin Cardiol. 2018;41:1537-1542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 96. | Wilken J. Evidence-based Recommendations for the Evaluation of Palpitations in the Primary Care Setting. Med Clin North Am. 2016;100:981-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 97. | Jelinek MV, Lown B. Exercise stress testing for exposure of cardiac arrhythmia. Prog Cardiovasc Dis. 1974;16:497-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 198] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 98. | Schaefer JR, Leussler D, Rosin L, Pittrow D, Hepp T. Improved detection of paroxysmal atrial fibrillation utilizing a software-assisted electrocardiogram approach. PLoS One. 2014;9:e89328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 99. | Feeny AK, Chung MK, Madabhushi A, Attia ZI, Cikes M, Firouznia M, Friedman PA, Kalscheur MM, Kapa S, Narayan SM, Noseworthy PA, Passman RS, Perez MV, Peters NS, Piccini JP, Tarakji KG, Thomas SA, Trayanova NA, Turakhia MP, Wang PJ. Artificial Intelligence and Machine Learning in Arrhythmias and Cardiac Electrophysiology. Circ Arrhythm Electrophysiol. 2020;13:e007952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 112] [Article Influence: 22.4] [Reference Citation Analysis (1)] |
| 100. | Yang M, Zhou R, Qiu X, Feng X, Sun J, Wang Q, Lu Q, Zhang P, Liu B, Li W, Chen M, Zhao Y, Mo B, Zhou X, Zhang X, Hua Y, Guo J, Bi F, Cao Y, Ling F, Shi S, Li YG. Artificial intelligence-assisted analysis on the association between exposure to ambient fine particulate matter and incidence of arrhythmias in outpatients of Shanghai community hospitals. Environ Int. 2020;139:105745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 101. | Serhani MA, T El Kassabi H, Ismail H, Nujum Navaz A. ECG Monitoring Systems: Review, Architecture, Processes, and Key Challenges. Sensors (Basel). 2020;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 89] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 102. | Krittanawong C, Rogers AJ, Johnson KW, Wang Z, Turakhia MP, Halperin JL, Narayan SM. Integration of novel monitoring devices with machine learning technology for scalable cardiovascular management. Nat Rev Cardiol. 2021;18:75-91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 108] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 103. | Paudel B, Paudel K. The diagnostic significance of the holter monitoring in the evaluation of palpitation. J Clin Diagn Res. 2013;7:480-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 104. | Narasimha D, Hanna N, Beck H, Chaskes M, Glover R, Gatewood R, Bourji M, Gudleski GD, Danzer S, Curtis AB. Validation of a smartphone-based event recorder for arrhythmia detection. Pacing Clin Electrophysiol. 2018;41:487-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |