Published online Jan 26, 2021. doi: 10.4330/wjc.v13.i1.21
Peer-review started: August 17, 2020
First decision: November 16, 2020
Revised: November 30, 2020
Accepted: December 13, 2020
Article in press: December 13, 2020
Published online: January 26, 2021
Processing time: 142 Days and 19.6 Hours
Patients with myasthenia gravis (MG) are at a higher risk of developing Takotsubo cardiomyopathy (TTC), particularly during a myasthenic crisis. Myasthenic crisis-associated TTC occurs predominantly in women. In this case report, we present a man with metastasized prostate carcinoma who developed TTC after new-onset MG.
An 81-year-old man with non-insulin dependent diabetes mellitus and metastasized prostate carcinoma presented with dyspnea. During primary assessment examination at the emergency department, there was evident blepharoptosis of his right eye. His electrocardiograms were suggestive of an acute anterior wall myocardial infarction, for which he underwent emergency coronary angiography. No obstructive coronary artery disease was found. During the coronary angiography, the patient developed respiratory failure and was admitted to the Intensive Care Unit for non-invasive respiratory support. The following day, diagnostic neostigmine test revealed a myasthenic crisis. Bedside echocardiography revealed left ventricular apical ballooning with a typical appearance of TTC. Despite the potentially reversible character of both MG and TTC, the patient and family requested an end of support in the Intensive Care Unit due to age and chronic malignancy with reduced quality of life in recent months after non-chemo-responding prostate carcinoma. The patient died soon after treatment withdrawal.
Elderly men should be carefully evaluated for TTC when new-onset MG is diagnosed.
Core Tip: An elderly man presented with dyspnea and neurological symptoms, including blepharoptosis. Simultaneously, the patient had signs of an acute myocardial infarction, but obstructive coronary artery disease was ruled out by coronary angiogram. Due to respiratory failure, the patient was admitted to the Intensive Care Unit for non-invasive support. The next day, bedside echocardiography revealed left ventricular apical ballooning, typical for Takotsubo cardiomyopathy. Meanwhile, second consultation by the neurologist performing a diagnostic neostigmine test confirmed a myasthenic crisis. Altogether, the patient was diagnosed with a new-onset myasthenic crisis-induced Takotsubo cardiomyopathy. Unfortunately, in this elderly man, this combination was fatal.
- Citation: Kuo Y, Ottens TH, van der Bilt I, Keunen RW, Akin S. Myasthenic crisis-induced Takotsubo cardiomyopathy in an elderly man: A case report of an underestimated but deadly combination. World J Cardiol 2021; 13(1): 21-27
- URL: https://www.wjgnet.com/1949-8462/full/v13/i1/21.htm
- DOI: https://dx.doi.org/10.4330/wjc.v13.i1.21
Takotsubo cardiomyopathy (TTC) is a transient cardiac syndrome that typically causes mid-ventricular circumferential and apical hypokinesis mimicking an acute coronary syndrome. In TTC, acute coronary or microvascular spasms can result in stunning of the myocardium with left ventricular dysfunction caused by catecholamine release due to emotion or physical stress[1]. Until 2009, only two cases of patients who had developed TTC after plasmapheresis for a myasthenic crisis were reported[2]. A myasthenic crisis if often the first manifestation of myasthenia gravis (MG). MG is a neuromuscular disease in which autoantibodies are formed against the acetylcholine receptors in the neuromuscular junction, causing severe muscular weakness[2]. The pathophysiology of MG-associated TTC is not exactly clear. A myasthenic crisis can induce severe stress, resulting in excessive catecholamine release. Guaricci et al[3] describe a neurogenic/catecholaminergic hypothesis: Supraphysiological levels of epinephrine can paradoxically lead to negative inotropic effects by reducing myofilament contractility, therefore leading to myocardial stunning[3]. Women are 9-times more likely to develop TTC then men. Post-menopausal women are especially prone to TTC[1,4].
Diabetes mellitus is a common comorbidity. When TTC develops during myasthenic crisis, the risk of respiratory failure, intubation and mechanical ventilation (81%) and death (25%) are significant[4,5]. In patients with TTC, respiratory failure is caused by a manifestation of pulmonary edema due to acute heart failure. In MG, however, respiratory distress and failure can be explained by severe skeletal muscle weakness which can lead to hypercapnic failure[6]. However, non-invasive ventilation can successfully stabilize approximately one-third of patients suffering from each of these types of respiratory failure[7,8]. This weakens the awareness that both syndromes can arise during a serious physical and/or emotional stress event. Suspicion of MG with development of pulmonary lung edema should prompt evaluation for TTC. Awareness of the possibility that both MG and TTC may cause respiratory failure, and that an association exists between these conditions, may lead to early recognition, adequate diagnostic work-up and timely treatment.
In this case report, we sought to describe an elderly man with dyspnea who was diagnosed with new-onset MG and TTC.
An 81-year-old man with a medical history of non-insulin dependent diabetes and metastasized prostate carcinoma was referred to our emergency department (ED) by his general practitioner, with complaint of dyspnea and generalized muscle weakness.
The patient had developed walking difficulties, balance impairment and ptosis of his right eye over the past few days, and developed progressive dyspnea in the last few hours before presentation to clinic. The ptosis of the right eye, as well as generalized muscle weakness, were apparent on primary examination in the ED.
The patient was known to have ongoing non-insulin dependent diabetes and metastasized prostate carcinoma.
At the ED, the patient’s temperature was 36.5 °C, heart rate was 95 beats per min, respiratory rate 26 was breaths per min, blood pressure was 190/110 mmHg, and oxygen saturation with 3 L O2 was 96%. The initial neurological examination showed a blepharoptosis, general muscular weakness, and disbalance. As such, our differential diagnosis included peripheral neuromuscular disease related to diabetes mellitus, with considerations for further diagnosis to rule out metastatic disease or paraneoplastic neurological disease. In relation to the patient’s complaint of progressive dyspnea, his electrocardiogram (ECG) showed signs of acute coronary syndrome.
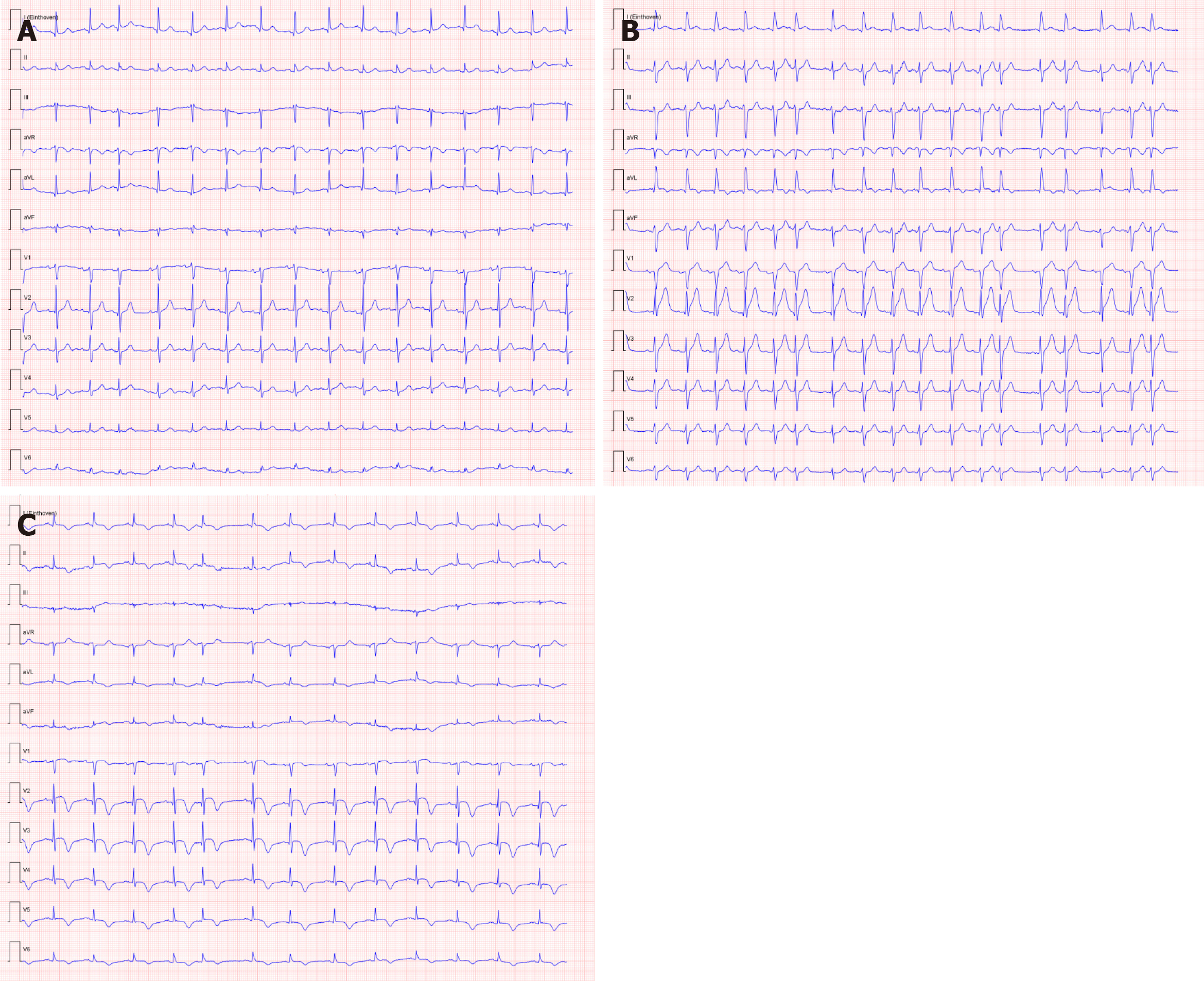
Serial ECGs revealed signs of acute anterior wall myocardial infarct, although the patient had no typical symptoms of an acute coronary syndrome (Figure 1). This finding was accompanied by raised serum high-sensitivity troponin levels (maximum 0.814 µg/L; normal range: 0-0.014 µg/L).
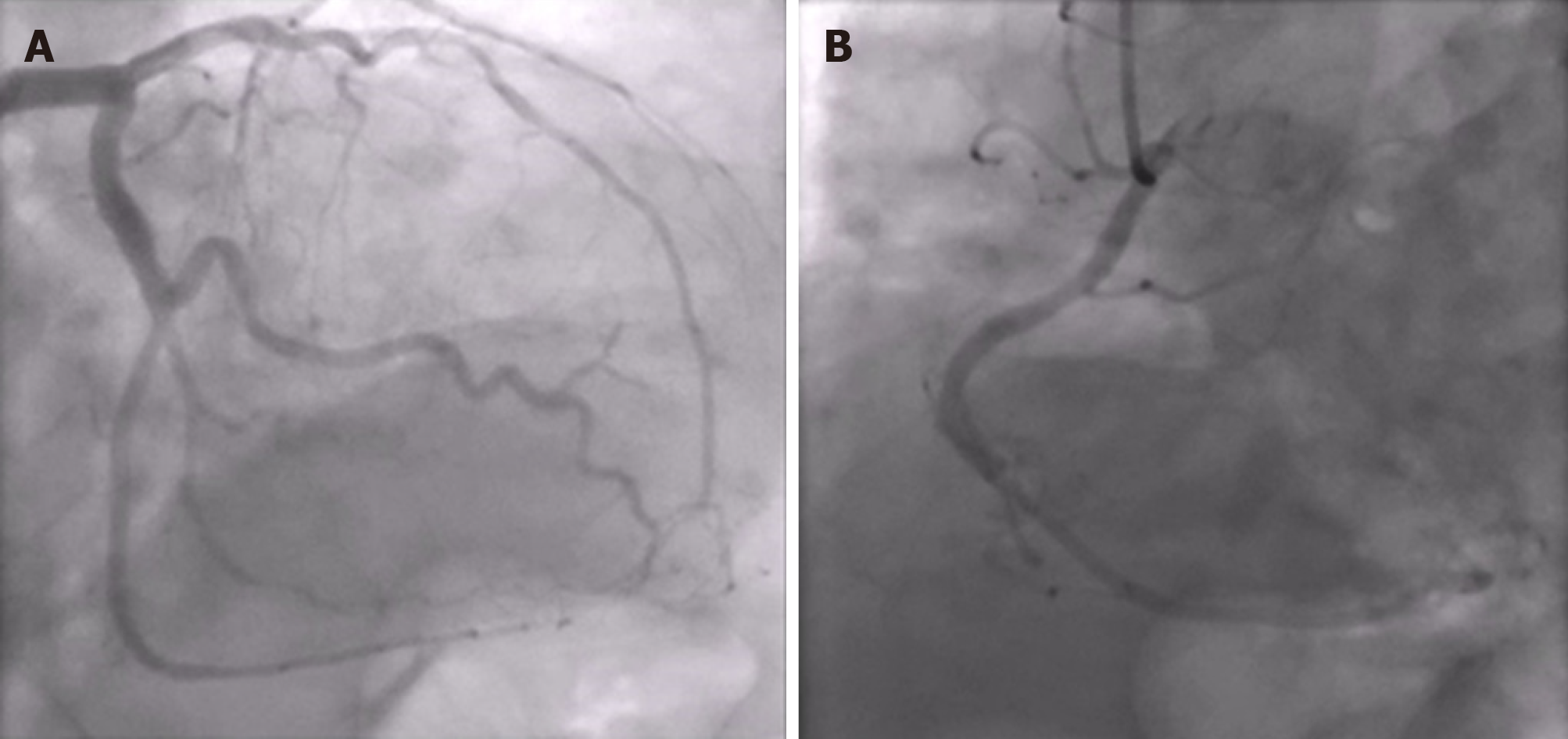
A conventional X-ray and computed tomography angiography of the chest showed no pulmonary embolism or parenchymal abnormalities, except for atelectasis of both lower lung fields. An emergency coronary angiography showed no obstructive coronary lesions (Figure 2).
The patient’s respiratory condition quickly deteriorated and he was admitted to the intensive care unit (ICU) with hypercapnic failure, where he was successfully stabilized with non-invasive ventilation (bilevel pressure support). The following day, the typical combination of ptosis and generalized weakness progressing to respiratory failure prompted re-consultation with the neurologist.
“The patient should start with mestinon and immunoglobulins treatment if the neostigmine test is positive. Further consideration of steroids has been advised.”
“The patient should continue inotropic support during continuing acute heart failure. Beside this, there should be ongoing respiratory bilevel non-invasive support to prevent further respiratory complications, accompanied by hypokinetic diaphragm, of myasthenic crisis on top of pulmonary edema by cardiac dysfunction.”
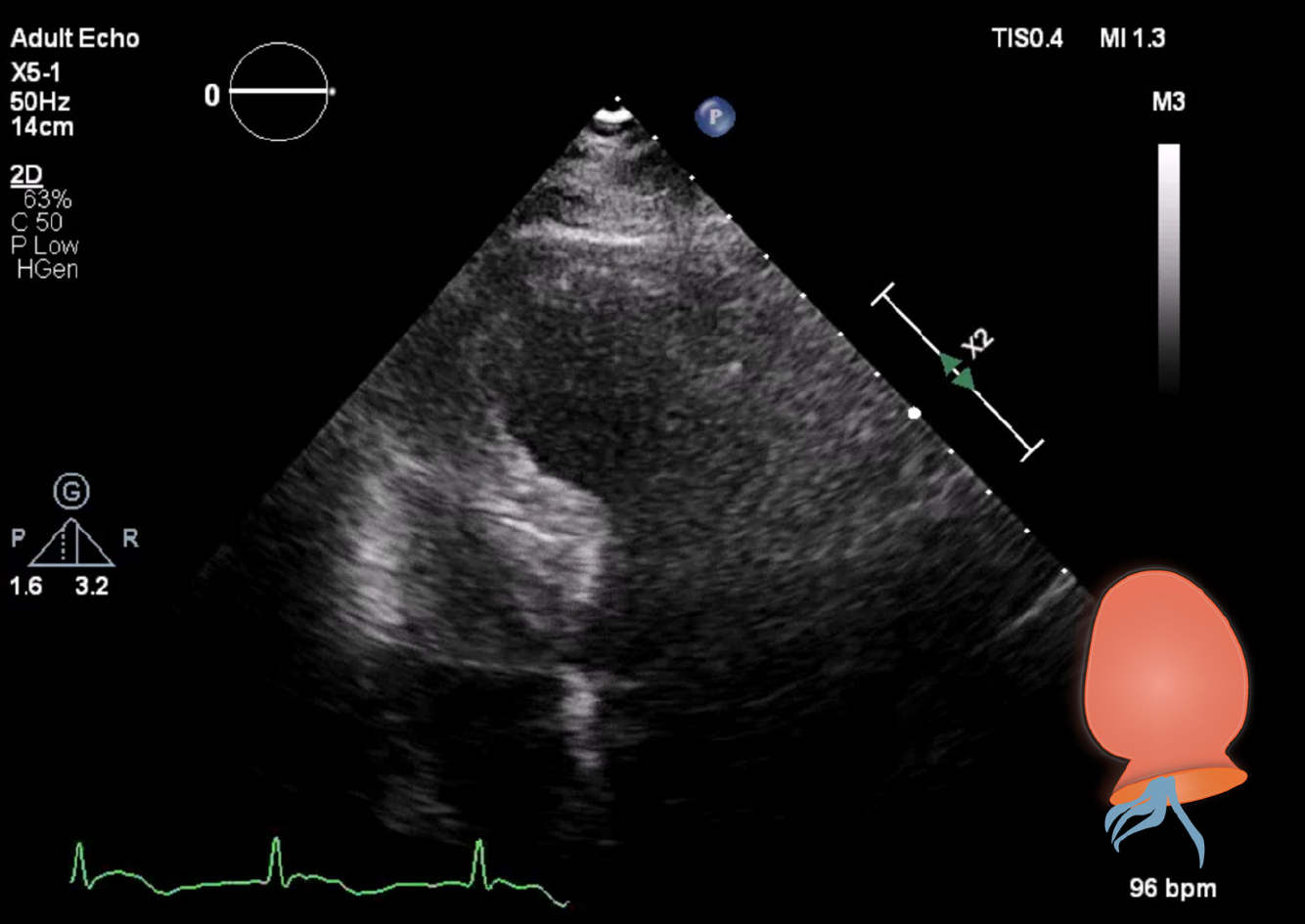
A diagnostic neostigmine test confirmed the diagnosis of myasthenic crisis with new-onset MG. The acetylcholine receptor (known as AChR) antibody was also positive. During his treatment in the ICU, the patient showed signs of progressive hemodynamic instability, requiring inotropic support with dobutamine. Although follow-up ECGs showed a persistent and evolving anterior wall myocardial infarction, the patient never developed chest pain (Figure 1). Troponin levels also decreased spontaneously. A repeat transthoracic echocardiogram showed apical ballooning of the left ventricle (resembling a Japanese octopus trap) with hypokinesia of the anterior wall, whilst the right ventricle was hyperdynamic but normal in size (Figures 3 and 4). Compared with the quick-look echo carried out in the ED, there was the same morphologic abnormality of the left ventricle. This was suggestive of stress-induced cardiomyopathy, also known as TTC. Theoretically, the myasthenic crisis may have provoked this rare cardiac complication due to excessive catecholamine release. Both MG and TTC are potentially reversible conditions. However, in this specific case of a patient of advanced age and frail physical condition due to chemotherapy-resistant metastasized prostate carcinoma, the clinical condition deteriorated quickly before the effect of intravenous immunoglobulin could occur.
The patient was supported for neuromuscular failure by non-invasive ventilation and by dobutamin to address the acute heart failure. Despite these treatments, there was ongoing deterioration of his end-organ functions.
On day 3 of ICU treatment, the patient no longer tolerated non-invasive ventilation and became agitated. The patient and his family requested withdrawal of supportive ICU treatment considering the patient’s medical history and significantly reduced quality of life in the past few months. After careful evaluation of the patient’s wishes, medical history and rapid clinical deterioration, supportive ICU treatments were withdrawn. The patient was given treatment to relieve symptoms and died quickly after withdrawal of supportive therapies.
MG is a rare disease in men and even more rarely leads to TTC[4,9]. Symptoms of respiratory failure, blepharoptosis, dysphagia and overall muscle weakness are present in more than half of the patients[5]. In MG patients, women have a higher risk of developing TTC than men, but men have a poorer prognosis. MG-associated TTC may present differently in men and women[1].
In recent years, large cohort studies have attempted to explain the association between MG and TTC. Myasthenic crisis may result in physiologic stress-induced cardiomyopathy, although direct cardiac involvement of MG cannot be ruled out. The golden standard to rule out acute coronary disease is to perform a coronary angiography (CAG). However, this is a relatively invasive and prolonged procedure in which complications can occur, as in our case where the patient became respiratory insufficient during the CAG. An alternative could have been a computed tomography angiography, which is faster and safer as no arterial access is needed, and gives reliable assessment of the coronaries[10]. Although CAG is the golden standard to distinguish acute coronary syndrome from TTC, non-invasive imaging modalities are becoming useful for TTC assessment. Even in emergent situations, echocardiography can provide a good evaluation of TTC. However, poor echo windows could still influence the visibility of ventricular walls. More accurate non-invasive cardiac imaging modalities known from the field of complex congenital anomalies could help to delineate the ventricular walls and refine the diagnosis[11].
MG-associated TTC triggered by a myasthenic crisis is rare. No more than 20 cases are described in depth in the published literature. In a Dutch national representative dataset, we found 175 cases with MC-associated TTC[4,5]. However, the case we present here is unique because of the patient’s age, male sex and comorbid malignancy. Triggers of myasthenic crisis and development of TTC seem to be heterogeneous. This rare condition should be suspected even in patients without a prior diagnosis of MG, as is demonstrated in the case we present here.
The number of cases describing the combination of these two diseases are increasing. Although, in many of them, development of TTC is a result of the treatment of MG[12,13]. In our case, there was no relation with the treatment of MG since the patient presented with ventricular dysfunction in the ED where the diagnosis of MG was not yet known. We do not consider it likely that the myasthenic crisis in our patient was related to medication use. The patient had been treated with gosereline injections every 3 mo for an extended period uneventfully.
Based on the literature, our collective experiences and experience with the case that we present as an illustration, we propose that patients with neurological symptoms together with ST-elevation myocardial infarction on the ECG without obstructive coronary lesions should be evaluated with left ventricle angiogram during coronary angiography to exclude TTC. Early initiation of a diagnostic neostigmine test can lead to more awareness of possible new-onset MG in cases of unusual presentation of circulatory instability.
We conclude there is a high need for continuous reporting of similar atypical cases to help improve the understanding of this rare entity. Further studies are needed to evaluate possible direct associations between MG and stress-induced TTC.
Manuscript source: Unsolicited manuscript
Specialty type: Critical care medicine
Country/Territory of origin: Netherlands
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Abdel Razek AAK, Ciccone MM S-Editor: Zhang L L-Editor: Filipodia P-Editor: Li JH
| 1. | Desai R, Abbas SA, Fong HK, Lodhi MU, Doshi R, Savani S, Gangani K, Sachdeva R, Kumar G. Burden and impact of takotsubo syndrome in myasthenic crisis: A national inpatient perspective on the under-recognized but potentially fatal association. Int J Cardiol. 2020;299:63-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 2. | Bijulal S, Harikrishnan S, Namboodiri N, Ajitkumar VK, Gupta D, Mathuranath PS. Tako-tsubo cardiomyopathy in a patient with myasthenia gravis crisis: a rare clinical association. BMJ Case Rep. 2009;2009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Guaricci AI, Bulzis G, Pontone G, Scicchitano P, Carbonara R, Rabbat M, De Santis D, Ciccone MM. Current interpretation of myocardial stunning. Trends Cardiovasc Med. 2018;28:263-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Pérez-Castellanos A, Martínez-Sellés M, Mejía-Rentería H, Andrés M, Sionis A, Almendro-Delia M, Martín-García A, Aguilera MC, Pereyra E, Linares Vicente JA, García de la Villa B, Núñez-Gil IJ. Tako-tsubo Syndrome in Men: Rare, but With Poor Prognosis. Rev Esp Cardiol (Engl Ed). 2018;71:703-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Rathish D, Karalliyadda M. Takotsubo syndrome in patients with myasthenia gravis: a systematic review of previously reported cases. BMC Neurol. 2019;19:281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Bedlack RS, Sanders DB. On the concept of myasthenic crisis. J Clin Neuromuscul Dis. 2002;4:40-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 7. | Neumann B, Angstwurm K, Mergenthaler P, Kohler S, Schönenberger S, Bösel J, Neumann U, Vidal A, Huttner HB, Gerner ST, Thieme A, Steinbrecher A, Dunkel J, Roth C, Schneider H, Schimmel E, Fuhrer H, Fahrendorf C, Alberty A, Zinke J, Meisel A, Dohmen C, Stetefeld HR; German Myasthenic Crisis Study Group. Myasthenic crisis demanding mechanical ventilation: A multicenter analysis of 250 cases. Neurology. 2020;94:e299-e313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 95] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 8. | Anand US, Viswanathan S, Arulneyam J. Pulmonary edema in myasthenic crisis. Case Rep Crit Care. 2013;2013:863620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Ho RYJ, Chern ZRB, Kristanto W. Takotsubo cardiomyopathy, or 'broken-heart syndrome', with concomitant myasthenic crisis. Singapore Med J. 2019;60:267-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Abdel Razek AAK, Elrakhawy MM, Yossof MM, Nageb HM. Inter-observer agreement of the Coronary Artery Disease Reporting and Data System (CAD-RADSTM) in patients with stable chest pain. Pol J Radiol. 2018;83:e151-e159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 11. | Abdel Razek AAK, Al-Marsafawy H, Elmansy M. Imaging of Pulmonary Atresia with Ventricular Septal Defect. J Comput Assist Tomogr. 2019;43:906-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Thanaviratananich S, Katirji B, Alshekhlee A. Broken heart syndrome during myasthenic crisis. J Clin Neuromuscul Dis. 2014;15:90-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Zis P, Dimopoulos S, Tavernarakis A, Nanas S. Myocardial pathology associated with myasthenia gravis. J Clin Neuromuscul Dis. 2015;16:228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |